Abstract
Introduction
Current production facilities for Cell-Based Health care Products (CBHPs), also referred as Advanced-Therapy Medicinal Products or Regenerative Medicine Products, are still dependent on manual work performed by skilled workers. A more robust, safer and efficient manufacturing system will be necessary to meet the expected expansion of this industrial field in the future. Thus, the ‘flexible Modular Platform (fMP)’ was newly designed to be a true “factory” utilizing the state-of-the-art technology to replace conventional “laboratory-like” manufacturing methods. Then, we built the Tissue Factory as the first actual entity of the fMP.
Methods
The Tissue Factory was designed based on the fMP in which several automated modules are combined to perform various culture processes. Each module has a biologically sealed chamber that can be decontaminated by hydrogen peroxide. The asepticity of the processing environment was tested according to a pharmaceutical sterility method. Then, three procedures, production of multi-layered skeletal myoblast sheets, expansion of human articular chondrocytes and passage culture of human induced pluripotent stem cells, were conducted by the system to confirm its ability to manufacture CHBPs.
Results
Falling or adhered microorganisms were not detected either just after decontamination or during the cell culture processes. In cell culture tests, multi-layered skeletal myoblast sheets were successfully manufactured using the method optimized for automatic processing. In addition, human articular chondrocytes and human induced-pluripotent stem cells could be propagated through three passages by the system at a yield comparable to manual operations.
Conclusions
The Tissue Factory, based on the fMP, successfully reproduced three tentative manufacturing processes of CBHPs without any microbial contamination. The platform will improve the manufacturability in terms of lower production cost, improved quality variance and reduced contamination risks. Moreover, its flexibility has the potential to adapt to the modern challenges in the business environment including employment issues, low operational rates, and relocation of facilities. The fMP is expected to become the standard design basis of future manufacturing facilities for CBHPs.
Keywords: Regenerative medicine, Automation, Cell processing facility, Manufacturing, Decontamination
Abbreviations: CBHP, cell-based health care product; fMP, flexible Modular Platform
Graphical abstract
Highlights
-
•
A novel manufacturing facility for cell-based health care products (CBHPs).
-
•
Biological isolators and industrial robots were combined in a compact, flexible facility.
-
•
Myoblasts, chondrocytes and human hiPSCs could be cultured in the facility.
-
•
The facility was named Tissue Factory.
-
•
The Tissue Factory reduces costs and risks in manufacturing CBHPs.
1. Introduction
Cell-based health care product (CBHP) is ‘health care product that contains or consists of pro- or eukaryotic cells or cell derived biological entities as an essential ingredient’ as defined in ISO 18362:2016 [1]. These products were also known as Advanced-Therapy Medicinal Products or Regenerative Medicine Products although there are some differences between their definitions. CBHPs are revolutionary new medicines that have recently been made possible and are going to form a new industrial field [2], [3]. However, current production facilities for CBHPs are still dependent on manual techniques performed by skilled workers, with ongoing problems of quality variation, microbial contamination risk, and productivity limitations [4], [5], [6]. We believe that a more robust, safer and more efficient manufacturing system will be necessary as we expect this new industrial field to expand significantly in the future. Thus, we designed a whole new manufacturing system for CBHPs, aiming for a true “factory”, to replace conventional “laboratory-like” manufacturing methods.
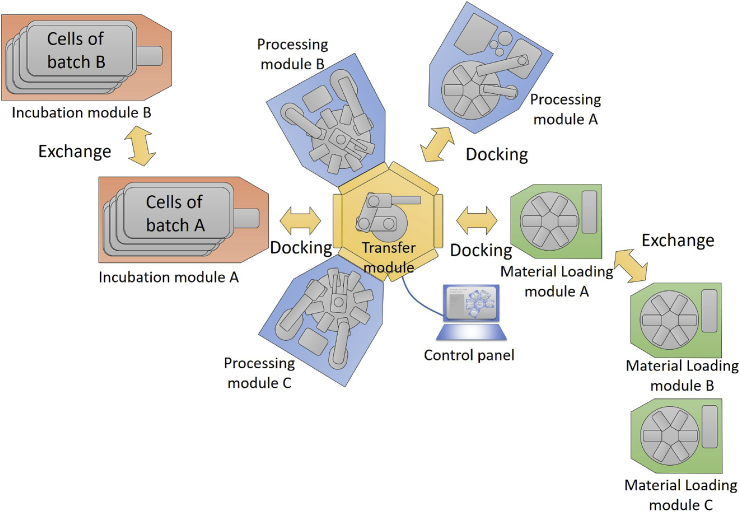
Fundamentally, the authors agreed that automation of key processes is essential in order to achieve mass production and ensure stable quality [7], [8], [9]. Generally, there are two types of automatic culture apparatus. One is a versatile type that carries out various processes with one device, and the second is a single function type that carries out only one sub process. The former is usually designed to mimic manual work by industrial robots, and it is not possible to exceed manual productivity. In contrast, the second is very efficient for a specific process although it requires manual work to bridge each process. Therefore, we decided to develop an ‘open platform’ that connects multiple single function apparatuses together and mediates the exchange of materials and information between each apparatus. The characteristics of CBHP manufacturing dictate that the time required for culturing cells is much longer than the time required for cell manipulation. This meant that the design of a CBHP production system should not be a production line method, but rather a cluster-type production method, where articles can be transferred between arbitrary modules via transfer robots. In a production line method, the entire manufacturing facility is engaged during the cell culture period, but in a cluster-type production method, the other apparatuses are separate and can be used during the cell culture period. This dramatically increases the operating efficiency of the manufacturing apparatuses. Moreover, to maximize the efficiency of this cluster production method, it is preferable that each production apparatus has the capability to be easily attached or detached based on the specific production needs.
A clean environment is critical to CBHP production, so we considered the application of biological isolators [10], [11]. Unlike biological safety cabinets, biological isolators provide a microbiologically sealed space, so the risk of infectious microorganisms being brought in from the outside is extremely low. In addition, biological isolators are often equipped with a decontamination device. Once an isolator chamber is decontaminated with evaporated disinfectants, the inner space of the chamber remains clean until the chamber is opened for maintenance or some other purpose. A drawback of biological isolators is the low manual operability owing to the need for thick gloves by operators to separate chambers. We thought that by combining automatic apparatuses and biological isolators it would compensate for any limitations that each had on its own and create a cost-effective manufacturing facility. To enable attachment and detachment of each manufacturing apparatus, they were designed to be covered with a separate isolator. Consequently, each manufacturing apparatus becomes a highly independent ‘module’, which can be attached and detached into a cluster. For this reason, we gave it the name ‘flexible Modular Platform (fMP)’ (Fig. 1). In this paper, we developed a fMP-based CBHP manufacturing facility that was named Tissue Factory, and performed the manufacturing of multi-layered myoblast sheets to demonstrate its cell- and tissue-manufacturability. In addition, we conducted the passaging culture of human articular chondrocytes and human induced pluripotent stem cells to confirm the feasibility of hydrogen peroxide decontamination and the flexibility of the system for multi-product manufacturing.
Fig. 1.
Schema of ‘flexible Modular Platform (fMP)’. The fMP system comprises four categories of modules: Processing, Transfer, Load/unload and Incubation modules. Each process, except for the cell culture process, is conducted in a combination of multiple modules. Cell culture processes are conducted by incubation modules only, apart from the Transfer Modules or other processing modules releasing them for other processes.
2. Methods
2.1. Tissue Factory
The concept for a fMP makes it possible to build a given manufacturing process by combining several modules. Here, the term ‘module’ refers to the smallest independent apparatus that can be connected to and detached from the manufacturing system. After defining the fMP concept, a demonstrative manufacturing system, named ‘Tissue Factory’, was built.
2.1.1. Modules
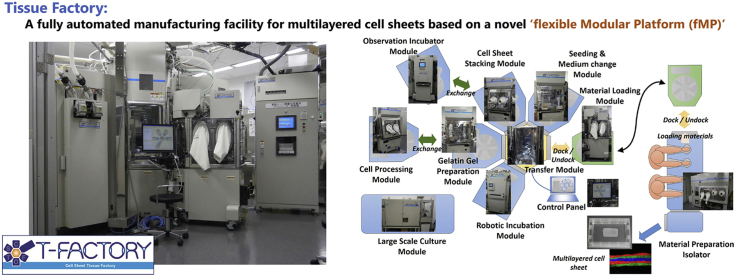
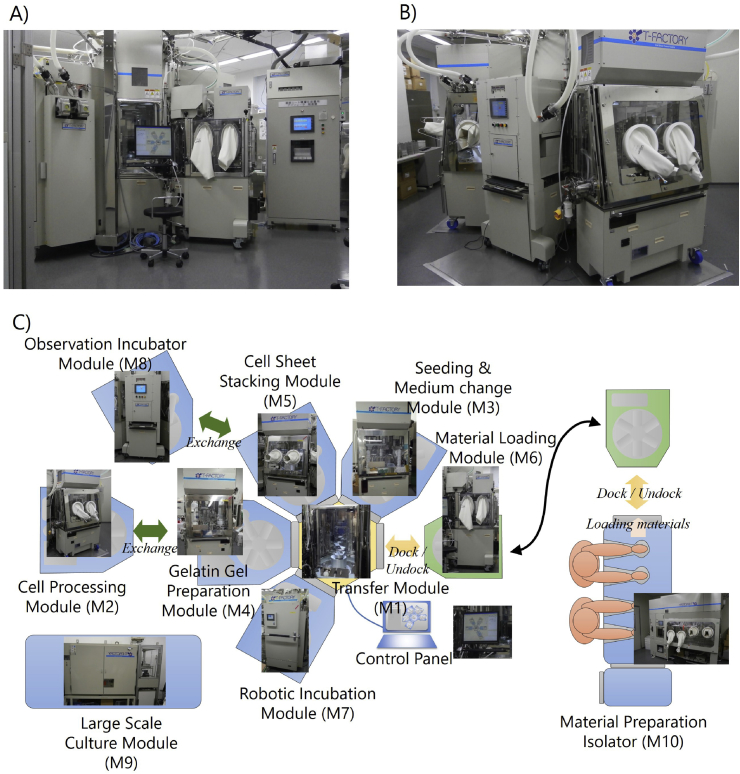
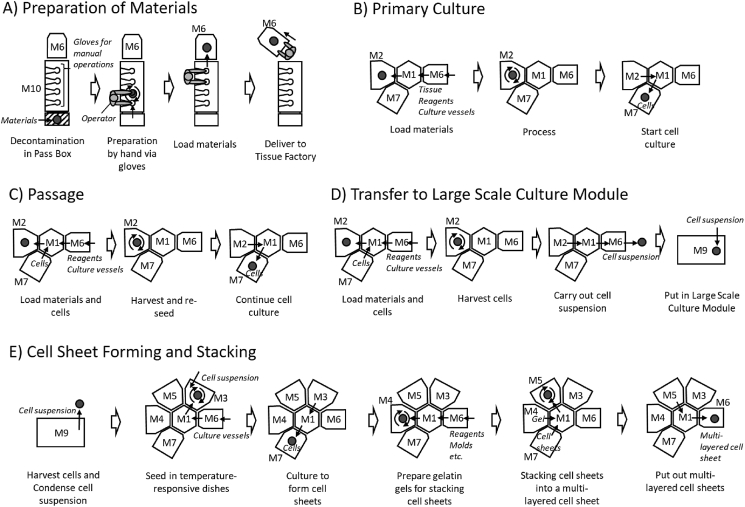
Tissue Factory was designed for manufacturing multilayered skeletal muscle myoblast sheets. A total of nine modules and a Material Preparation Isolator were assembled (Table 1 and Fig. 2). Although a system could potentially have multiple Transfer Modules, we made a hexagonal Transfer Module (M1) to serve as a hub of the manufacturing cluster. Modules can be connected to five of the six sides of the central hexagon. An industrial robot designed for silicon wafers with minimal dust emission was adopted to transfer articles between modules. Processing modules (M2–M5) commonly have connection ports to the Transfer Module (M1). Incubation Modules (M7–M9) perform long-term processes such as cell culture. The essential difference from a Processing module is the standalone operability that can run parallel to the cell culture processes. In this study, three types of Incubation Modules were assembled. In order to introduce materials into the system, a Material Preparation Isolator (M10) and a Material Loading Module (M6) were produced. The Material Loading Module (M6) has two interfaces. It can be connected both to the Transfer Module and to the Material Preparation Isolator (M10). The Material Preparation Isolator (M10) is a commercially available isolator for manual processing customized to be equipped with a port for connecting to the Material Loading Module (M6). To introduce materials into the system the materials are first prepared in the Material Preparation Isolator (M10), placed on a rack in the Material Loading Module (M6), and then, the entire Material Loading Module (M6) is moved to an empty port on the Transfer Module (M1).
Table 1.
Modules of the Tissue Factory.
| Module category | No. | Module name | Description |
|---|---|---|---|
| Transfer modules | M1 | Transfer Module | Transferring articles from module to module. |
| Processing modules | M2 | Cell Processing Module | Primary cells are isolated from a harvested tissue and subcultured. This module consists of a pipetting unit that handles liquid reagents, a unit that performs both enzymatic digestion and tissue shredding, and a centrifugal separation unit that collects liberated cells. |
| M3 | Seeding and Medium Change Module | Dispensing cell suspension into culture vessels or changing medium. This module consists of a unit that injects a specified amount of cell suspension or medium to each vessel from a temporal stock bottle, and an aspiration unit that removes the medium from culture vessels. | |
| M4 | Gelatin Gel Preparation Module | Preparing a gelatin gel for stacking cell sheets. This module has a unit for heating and stirring the gelatin solution, a pipetting unit for pouring the gelatin solution into a mold, a cooling unit for solidifying the gelatin gel, and a unit for removing the gelatin gel from the mold. | |
| M5 | Cell Sheet Stacking Module | Stacking up cell sheets into a multilayered construct using the gelatin gel by the method previously reported [21], [22]. This module has a unit to press the gelatin gel against a cell sheet while adjusting the temperature of the vessel. | |
| Material loading modules | M6 | Material Loading Module | Transferring materials from the Material Preparation Isolator to the Transfer Module. |
| Incubation modules | M7 | Robotic Incubation Module | Incubate up to 60 cell culture vessels (6 racks of 10 vessels). Each vessel can be put in and retrieved by indicating an address from 60 possible positions. |
| M8 | Observation Incubator Module | An incubator equipped with a phase contrast microscope which enables observation while culturing cells. | |
| M9 | Large Scale Culture Module | This module is a specially prepared incubator for culturing large amounts of cells at the order of 108 to 109 cells. Unlike other incubation modules, this module is not connected to Transfer Module, but cells are delivered as a cell suspension via tubing and bags. | |
| Manual operation apparatus | M10 | Material Preparation Isolator | Preparing materials by hand before starting automatic processes. |
Fig. 2.
Overview of the Tissue Factory. A total of nine modules and a manual operation isolator were built for the Tissue Factory. Each module conducts a relatively simple function but combining multiple modules via the Transfer Module enables a wide variety of cell culture processes. All modules docked to the Transfer Module are controlled from the Control Panel.
Within this system only the Transfer Module (M1), Large Scale Culture Module (M9) and Material Preparation Isolator (M10) were fixed to the floor. The height of the Transfer Module (M1) was 2460 mm. The side of each hexagon of the Transfer Module (M1) was 535 mm long. Unlike the other incubation modules, the Large Scale Culture Module (M9) was designed to be stationary, and the module had no sealed chamber because it uses only sealed culture vessels with pre-assembled tubing (a closed system). The other modules (M2-M8) were movable, and the heights were limited to under 1650 mm to ensure stability during movement. These modules were equipped with casters, allowing one operator to move and connect or detach from the Transfer Module (M1). Base plates of the Processing modules (M2-M5) and Material Loading Module (M6) were set at 750 mm above the floor. Robots and processing units were attached to each base plate and covered with a chamber that isolates its operational space from the outer environment. Clean air was provided from the ceiling of the chamber through a High Efficiency Particulate Air (HEPA) filter, and the air was evacuated from the corners of the base plate. In open air operations, such as opening the lids of culture dishes, articles were handled at least 300 mm above the base plates where stable downward airflow was formed, minimizing the risk of particle contamination.
2.1.2. Interface
In designing manufacturing facilities based on fMP, it is necessary to consider handling multiple kinds of CBHPs that may differ from its original purpose. In other words, not only does it need to produce the original product, but also be able to adapt to future functions that are not yet envisioned. Although such a way of thinking is fundamental to software development, it has not been sufficiently considered in hardware development, especially in manufacturing equipment. We defined interfaces for both software and hardware to extend the future usefulness of the system.
2.1.2.1. Transfer Module interface
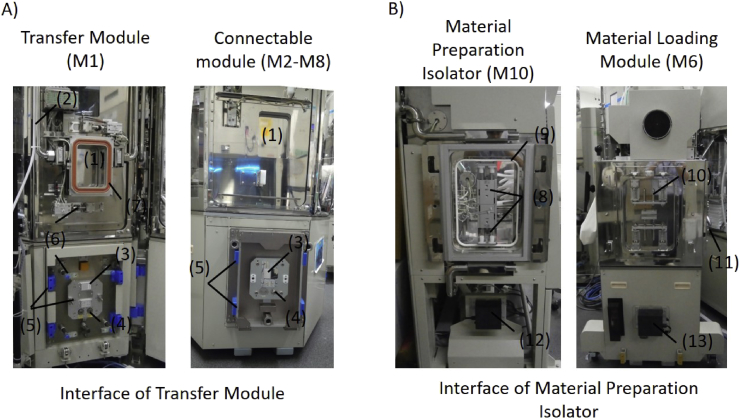
The Transfer Module (M1) has five ports that connect to the Processing Modules (M2–M5), the two Incubation Modules (M7, M8) and the Material Loading Modules (M6) (connectable modules). The interface between the Transfer Module (M1) and a connectable module (M2-M8) was standardized so that any one of them can connect to any port on the Transfer Module (M1) (Fig. 3A). When a connectable module (M2–M8) is brought into proximity to a port, the connection is made automatically. This connection includes a physical connection, power supply lines, control signal lines, a compressed air line, and a carbon dioxide gas line. The connection of hydrogen peroxide vapor was designed to be manually connected. Every port on the Transfer Module (M1) has a door and each connectable module (M2–M8) also has a door to transport articles, such as cells, medium, and other materials. The two doors face each other at a distance and the space between the two doors is sealed and decontaminated by hydrogen peroxide. Then, both doors are opened to form one enclosed space. The connectable module (M2–M8) has a specific area inside the door defined as an article standby position. The transfer robot in the Transfer Module (M1) moves articles between the article standby positions of the two joined connectable modules (M2–M8). The footprint of any articles was defined to be 128 × 86 mm with reference to the SBS standard for cell culture plates (ANSI SLAS 1-2004). Articles with a height of ca. 15 cm and a weight of 1 kg or less can be conveyed. Laser sensors are used at each article standby position to confirm the presence or absence of any articles.
Fig. 3.
Inter-module connection interface. (1) Article transfer doors. (2) H2O2 Injection port to decontaminate the interface. (3) Electrical couplings. (4) Gas connections. (5) Docking guides. (6) Clamping bars. (7) Inflation sealing. (8) Door-combining socket. (9) Sealing. (10) Door-combining pin. (11) Manual clamp. (12) Non-contact power coupling (supplier). (13) Non-contact power coupling (receiver).
2.1.2.2. Material Preparation Isolator interface
The Material Preparation Isolator (M10) has another port for inputting materials to the Material Loading Module (M6) (Fig. 3B). This port has a simpler structure than those in the Transfer Module (M1), so they need to be connected manually. It includes a physical connection and a non-contact power supply. The doors on both sides are opened manually using the gloves in the Material Preparation Isolator (M10).
2.1.2.3. Control software
The software interface for each module was designed to be independent to facilitate development of new modules. Each module has its own control software, executes a single operation according to a command from the process control software, and reports the result. The control for maintaining an aseptic environment is done by an environment controller. This allows developers of each module to concentrate on developing culture operations. The schema of the control software is shown in Supplementary Material 1. Both software and hardware handshakes are performed simultaneously at the time the modules are connected to avoid separate management of connection states for software and hardware.
2.1.3. Consumables
Consumables used in the Tissue Factory are listed in Supplementary Material 1. Several types of cell culture consumables were designed specifically for the system. A dedicated cell culture dish was designed with a SBS standard footprint so that the Transfer Module (M1) could handle them directly. The culture area is 6.5 × 10 cm and the depth is 2.46 cm. The culture surface was treated to handle either a cell culture (O2 plasma treatment) or for forming cell sheets (temperature-responsive surface) [12]. The Large Scale Culture vessel is a dedicated culture vessel for the Large Scale Culture Module (M9). Its culture area is 4200 cm2, and the Large Scale Culture Module (M9) can store a maximum of six vessels. Medium/cell suspension containers are used in the Seeding and Medium Change Module (M3) for temporary storage of large amounts of medium or cell suspension to dispense into smaller culture vessels within a short time frame. The enzyme treatment container functions as a receptacle for enzyme digesting and mechanically cutting supplied tissue for primary cell isolation. The gelatin gel mold is a container for solidifying a gelatin gel for cell sheet stacking. For other materials, commercially available products are mounted on dedicated stands with SBS standard footprints.
2.1.4. Decontamination
Decontamination refers to a process that reduces the number of viable microorganisms to an acceptable level by a validated method. The system was designed to be decontaminated by hydrogen peroxide vapor. The effectiveness was verified by placing biological indicators (Apex Laboratories, HMV – 091) containing 1 million indicator bacteria (Geobacillus stearothermophilus) in various places and confirmed that after decontamination there were no bacteria detected (10−6 reduction). Aeration time for removing residual hydrogen peroxide was based on reducing the concentration of hydrogen peroxide to less than 1 ppm.
2.2. Confirmation of aseptic environment
Aerobic bacteria, yeast, and fungi were examined by a method in accordance with the Japanese Pharmacopoeia. For falling microorganisms, agar medium dishes (BD BBL ™ gamma ray irradiated triple packaging SCDLP) were placed with the lids opened for 4 h or more. Then, these dishes were collected and cultured for five days or more at 30 °C, and the absence of any colonies was confirmed. For adhering microorganisms, agar medium dishes were used (BD BBL ™ IC – XT rocking grid gamma ray irradiation triple packaging SCDLP), and after bringing them into contact with the five fingers of the gloves, the dishes were cultured for five days or more at 30 °C, when the absence of any colonies was confirmed.
2.3. Confirmation of cell-manufacturability
2.3.1. Skeletal myoblasts
As a source of skeletal muscle myoblasts, a piece of muscle tissue was dissected from the thigh of a miniature pig (Nippon Institute for Biological Science, 9 months). Then, isolation, expansion, cell sheet preparation, and cell sheet stacking were performed in the Tissue Factory. This experiment was approved by the Institutional Animal Care and Use Committee of Tokyo Women's Medical University and was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Tokyo Women's Medical University. In optimizing and validating each process, we also used human skeletal muscle myoblasts purchased from Lonza (Basel, Switzerland).
2.3.2. Human articular chondrocytes
Normal human articular chondrocytes (NHACs) were purchased from Lonza Japan Ltd. (Tokyo, Japan; Cat. No. CC-2561), one or two passages in CGM-2 medium (Lonza, CC-3216), and then cryopreserved. Then, expansion cultures were performed by the Tissue Factory.
2.3.3. Human induced pluripotent stem cells
Human induced-pluripotent stem cells prepared from human fibroblasts (clone 201B7) [13] were provided by RIKEN (Tsukuba, Japan), and expanded by hand through several passages by a feeder-free method derived from the method previously described [14]. Then, the cells were introduced to the Tissue Factory. Characterization of passaged cells were performed by flow cytometry for undifferentiation markers: SSEA-4 (stage-specific embryonic antigen-4) [15] and rBC2LCN, a recombinant lectin probe [16].
3. Results
3.1. Asepticity
Decontamination of modules is achieved by hydrogen peroxide vapor, generated by a hydrogen peroxide decontamination apparatus (HYDEC, Shibuya Kogyo) mounted on the Material Preparation Isolator (M10), and supplied to each module by dedicated piping. Generally, the intensity of decontamination depends on the temperature, amount of hydrogen peroxide injected, holding time of hydrogen peroxide vapor, and other conditions. Accordingly, the decontamination parameters differed depending on the combinations of target modules (see Supplementary Material 1). Seven decontamination patterns were developed based on the criteria described in the Method section. Decontamination of the whole system was broken down into three successive steps because the hydrogen peroxide vaporizing device used in the system was not capable of decontaminating all modules at once.
To maintain the aseptic environment after decontamination, the chambers of all modules, except for Large Scale Culture Module (M9), are maintained at a slight positive pressure against the outer environment that prevents microorganisms from entering through potential microscopic holes. Both immediately after decontamination and during the subculture processes, no falling or adhering microorganism were detected.
3.2. Cell-manufacturability
To verify the manufacturability of CBHPs and compatibility for manual operations, three tentative CBHPs were manufactured by the Tissue Factory.
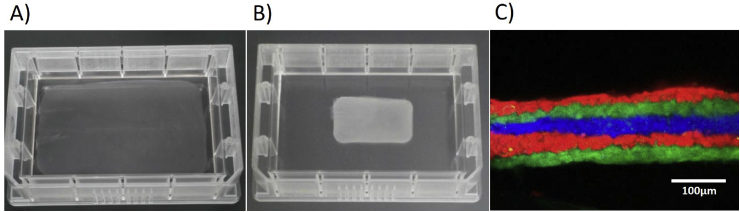
Firstly, multi-layered skeletal myoblast sheets were manufactured. It has been reported that recovery of cardiac function can be expected by transplanting multi-layered skeletal myoblast sheets to heart failure patients [17], [18], [19]. In advance preparation, a procedure for manual culture [18] was modified to be executable by the system. In the cell isolation process by the Cell Processing Module (M2), muscle tissue was subjected to enzymatic digestion and shredding at the same time in a dedicated vessel equipped with cutting blades, though the muscle tissue was minced before the enzymatic digestion in the manual procedure. The digested tissue was filtered in the same vessel to remove any undigested tissue and large debris, then the cells were isolated by centrifugation. As a result of comparing this method to the standard manual method, equivalent yield and myoblast purity were achieved [20]. Expansion of the culture process was accomplished in the Large Scale Culture Module (M9) by utilizing a dedicated culture vessel. An important feature of the vessel is its large culture area to reduce the number of vessels required. Another feature is its hermetically sealed structure with tubing. Clean air with 5% CO2 was injected directly into the vessels. Such large culture vessels are difficult to handle manually, and even a minute inclination causes a non-uniform depth in the culture medium. In the Large Scale Culture Module (M9), the depth of the culture medium is kept uniform by a mechanism controlling the inclination of the culture vessels. Concentrating the recovered cell suspension in the sealed vessel by centrifugation was difficult to automate because it required separating and reconnecting the tubing. Therefore, to solve this problem a membrane concentration method utilizing a hollow fiber module was adopted. As a result, it was possible to concentrate a large amount of cell suspension by a simple method compared with separation by centrifugation. The stacking of cell sheets had originally been carried out using tweezers and transfer membranes in manual operations, but now the system integrates a cell sheet manipulator technique [21], [22]. The Gelatin Gel Preparation Module (M4) forms a gelatin gel pad on a manipulator, and the Cell Sheet Stacking Module (M5) stacks cell sheets using the gelatin gel. A multi-layered cell sheet is then produced on a temperature-responsive dish. Each manufacturing process was comprised by a combination of several modules as shown in Fig. 4. A total of four runs were performed using swine muscle tissues, and multi-layered skeletal myoblast sheets were successfully obtained (Table 2 and Fig. 5). This procedure was performed without decontamination.
Fig. 4.
Culture processes in Tissue Factory. In the Tissue Factory, multiple modules cooperatively perform a culture process. Each module can be attached and detached according to the requirements. Materials are prepared in the Material Preparation Isolator (M10) and carried to the Tissue Factory using the Material Loading Module (M6) (A). The other processes are performed in the Tissue Factory (B–E). The Large Scale Culture Module (M9) conducts culture processes apart from the Transfer Module (M1). Module is indicated by the symbols in Table 1.
Table 2.
Results of expansion culture of swine myoblasts.
| Strain 1 | Strain 2 | Strain 3 | Strain 4 | |
|---|---|---|---|---|
| Tissue sample | 4.48 g | 5.06 g | 4.36 g | 6.9 g |
| Passage 1 | Day 7 3.1 × 105 cells |
Day 7 2.7 × 105 cells |
Day 7 1.4 × 106 cells |
Day 10 3.0 × 106 cells |
| Passage 2 | Day 13 2.8 × 106 cells |
Day 13 1.7 × 106 cells |
Day 9 3.0 × 106 cells |
Day 20a 3.7 × 107 cells |
| Passage 3 | Day 23a 1.6 × 107 cells |
Day 23a 9.3 × 106 cells |
Day 20a 4.5 × 107 cells |
Day 30a 1.4 × 108 cells |
| Passage 4 | Day 33a 4.1 × 107 cells |
Day 34a 5.3 × 107 cells |
Day 26a 7.0 × 107 cells |
– |
Passages marked by asterisks were conducted in a Large Scale Culture Module (M9) and the others were conducted in a Cell Processing Module (M1).
Fig. 5.
Multi-layered myoblast sheets. Five-layer myoblast sheets were manufactured by the Tissue Factory. A) A swine five-layer myoblast sheet adhered on a temperature-responsive culture dish. B) A swine five-layer myoblast sheet detached from the temperature-responsive culture dish by reducing the temperature. C) A cross-sectional view of a human five-layer myoblast sheet obtained by a confocal microscope. Cell sheets stained by three different fluorescent dyes before stacking.
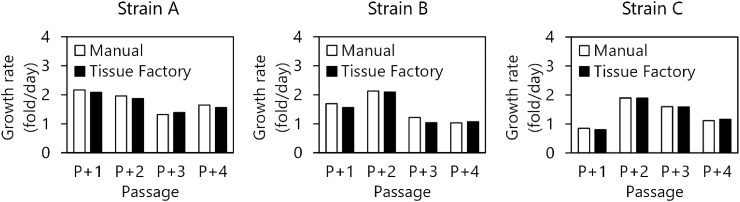
Secondary, we performed expansion culture of human chondrocytes with full decontamination process for comparison with manual operations and evaluation of any remaining hydrogen peroxide. Three strains of human articular chondrocytes were subcultured. After decontamination, the cryopreserved chondrocytes were thawed and re-suspended in CGM-2 manually, and then the cell suspension was introduced to the system. The cells were seeded in a dish in the Cell Processing Module (M2) and cultured in the Robotic Incubation Module (M7). For passaging, the dish was returned to the Cell Processing Module (M2) again. The cells were treated by TrypLE Select enzyme (Life Technologies) on a warm plate at 37 °C and collected by shaking the dish. The collected cell suspension was concentrated by a centrifuge unit and seeded in new culture dishes. In the first preliminary trials, we found that the chondrocytes in the Robotic Incubation Module (M7) were damaged, probably by residual hydrogen peroxide. Hence, the procedure was changed to start culturing at 24 h after decontamination after which time the concentration of hydrogen peroxide in the Robotic Incubation Module decreased to 0.1 ppm or less. Three passages were performed for each strain, both by hand and by the system, and the number of cells was counted at the time of each passage and again at the end of the culture. There was no apparent difference in growth rates between passage by hand and the system (Fig. 6). During the culture in the system, observation in the Observation Incubator Module (M8) was carried out as designed. Neither falling nor adhering microorganisms were detected in any of the runs.
Fig. 6.
Growth rates of human chondrocytes. Three independent strains of human articular chondrocytes were passaged both manually and by the Tissue Factory. Growth rates were calculated from seeding densities and harvested cell numbers for each passage. Passage numbers are indicated as additional passage times in the experiment.
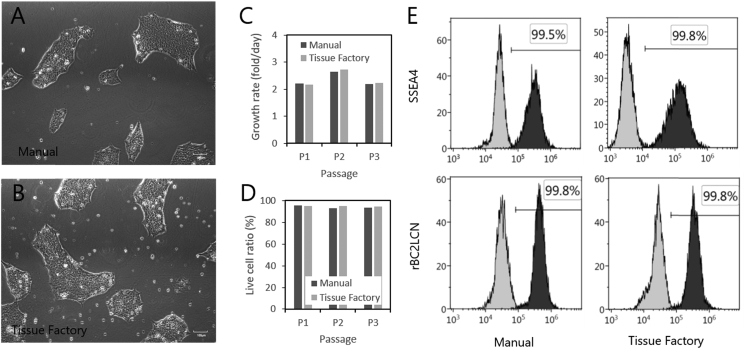
Lastly, we performed passaging culture of human induced-pluripotent stem cells (hiPSCs) to elucidate any difference in vulnerability for mechanical and/or chemical damage among cell types. The originally distributed cells were subcultured by hand in several passages and introduced to the system in a cell suspension. In the system, the cells were seeded on a dish that had been coated with iMatrix-511 Recombinant Laminin-511 E8 Fragments (Reprocell, Japan). After 4- or 5-day culture, the cells were harvested by Accutase (Innovative cell technologies) by shaking (without scraping), centrifuged, and re-seeded onto new iMatrix-511-coated dishes. In a preceding optimization of culture conditions, we had found that hiPSCs were damaged even 24 h after decontamination, and we also found that heating the Robotic Incubation Module (M7) at 50 °C for 48 h after decontamination archived sufficient hydrogen peroxide removal to successfully culture hiPSCs. This fact suggests that hiPSCs were more sensitive to any residual hydrogen peroxide than chondrocytes. After three passages, hiPSCs cultured in the system showed comparable growth rates and characteristics to the cells manually passaged by a skilled person (Fig. 7). During the culture in the system, observation in the Observation Incubator Module (M8) was carried out as designed. Neither falling nor adhering microorganisms were detected during the process.
Fig. 7.
Culture results of human iPS cells. Human induced pluripotent stem cells (hiPSCs) were passaged both manually and by the Tissue Factory. hiPSCs cultured by the Tissue Factory showed comparable growth ratios and characteristics to hiPSCs cultured manually. A) Microscopic view of hiPSCs passaged manually. B) Microscopic view of hiPSCs passaged in the Tissue Factory. C) Growth rates through three successive passages. D) Live cell ratios measured using Trypan Blue dye. E) Flow cytometric analysis of hiPSCs after three passages by stage specific embryonic antigen 4 (SSEA4) and rBC2LCN lectin. Outlined histograms shows isotype control and no-dye control in SSEA4 and rBC2LCN, respectively. Filled histograms show the measured data. Horizontal axes indicate the fluorescent intensities of fluorescein isothiocyanate (FITC) conjugated with SSEA4 and rBC2LCN. Vertical axes indicate cell numbers at each intensity. Percentages of positive (undifferentiated) cells are annotated by the thresholds where the control samples were measured as 0.5%.
4. Discussion
4.1. Conventional facility and automatic cell culture equipment
At present, most CBHP manufacturing facilities adopt a manual operation-based production system utilizing clean rooms and biological safety cabinets. This is partially based on the general idea that production by trained operators is more efficient in small-volume situations than production by automated manufacturing equipment. On the other hand, even if the scale of production increases, problems still exist that make it difficult to introduce automated devices. Firstly, the whole manufacturing process of a CBHP, including introduction of sterilized consumables and culture media is too complicated to automate because aseptic handling is required throughout the whole process. Secondly, even if automatic equipment is only used for a specific sub-process, introducing the equipment to an existing clean room can add extra-cost. For example, the equipment may need to be re-designed because manufacturing equipment used in a clean room should have dust-free properties and biological cleanliness. Alternately, a larger clean room may be necessary to install large-sized apparatus. In either case, the difficulties faced to introduce automatic equipment into CBHP manufacturing facilities have not been solved yet.
4.2. Biological isolator and biological safety cabinet
In this study, we built the Tissue Factory, which is a new CBHP production system using biological isolator technology and factory automation technology. Tissue Factory succeeded in manufacturing multilayered skeletal myoblast sheets, expanding human articular chondrocytes and passaging un-differentiated hiPSCs. The major challenge for Tissue Factory was to reduce the manufacturing cost of CBHPs by eliminating the necessity for clean rooms with strict specifications. When a CBHP is manufactured using biological safety cabinets, a clean room with high standards is typically required. Biological safety cabinets can reduce the risk of extrinsic contamination by airflow control, but floating particles in the room can be brought in with materials or carried in on the hands of workers. Therefore, the cleanliness of the installation environment may affect the cleanliness inside the cabinet. On the other hand, with biological isolators, materials to be carried in can be cleaned by a decontamination pass box. Also, workers operate through gloves fixed on the front wall of the chamber. Therefore, biological isolators are less susceptible to contamination from the installation environment than biological safety cabinets. In ISO 18362:2016, as an example of layout a manufacturing environment, the cleanliness of cell operation rooms where biological safety cabinets are installed is categorized as Class 7, and for biological isolators, it is categorized as Class 8. This difference in cleanliness levels may reduce the costs of construction and maintenance of manufacturing facilities.
4.3. Decontamination
Another merit of a biological isolator is that it can be decontaminated. In this study, decontamination processes were performed in culturing human chondrocytes and hiPSCs. In a biological safety cabinet, it is common to clean the chamber at the end of a single culture process in order to prevent cross-contamination among different batches. Decontamination of biological isolators provides a more reliable means to prevent cross-contamination than manual wiping with disinfectant. In the Tissue Factory, decontamination with a 10−6 reduction by hydrogen peroxide was achieved. Decontamination is also useful to clean the outer surface of materials before being carried in. The only disadvantage of decontamination, is that it takes a considerable amount of time to remove the hydrogen peroxide after decontamination, as shown in Supplementary Material 1. Particularly in incubation modules, even a small residual of hydrogen peroxide, under 1 ppm, may affect cell viability, and careful removal is necessary. Therefore, new technology for reducing the time required for decontamination is required.
4.4. Compatibility with manual operations
When designing the processes to apply in the Tissue Factory, the existing manual procedures were not necessarily suitable for automation. The Tissue Factory successfully reproduced three tentative manufacturing processes of CBHPs that were minimally modified from established manual methods. Although adapting a procedure to automation is advantageous for improving productivity, it is critical to ascertain whether there is any change in product quality due to the process change, after the quality assurance of the manual procedure is already established. In the concept of fMP, it is not necessary to automate everything. Manual work via gloves is also acceptable. Moreover, a simpler design by integration of a Transfer Module and processing modules is also possible.
4.5. Sealed-vessel culture system and sealed-chamber culture system
Among the modules in the Tissue Factory, Large Scale Culture Modules (M9) adopted a different approach to cleanliness control compared to the other modules that have sealed chambers. Large Scale Culture Modules (M9) use sealed culture vessels instead. The Sealed-vessel culture system (also referred as Closed system) sometimes has a smaller aseptic space than a sealed-chamber culture system (also referred as Open system) and has the advantage that the apparatus can be simplified, since airflow control and decontamination of equipment are unnecessary [23]. Like biological isolators, a sealed-vessel culture system is also less susceptible to contamination from the installation environment. However, it should be noted that there is a risk of contamination when injecting cells into a sealed-vessel or when retrieving cells from the vessel. In the Tissue Factory, the connection for transferring cell suspensions was made using a single-use sterile connector (Opta® SFT-I Connector, Sartorius stedim). The Sealed-vessel culture system has difficulty to cope with some types of culture operations, such as the cell sheet stacking process, but they can be effective general cell culture processes.
4.6. Modularity, flexibility and cost-effectiveness
As mentioned above, fMP could reduce the manufacturing cost of CBHPs by minimizing the facility scale and by changing the cleanliness management strategy in comparison with conventional clean room-based facilities. Moreover, the ‘flexibility’ of fMP may further reduce the cost. For example, in the Tissue Factory, recombination of the system is possible due to its standardized connection interface between modules. When regulatory problems can be cleared, it is possible to improve the operation rate of equipment by manufacturing multiple CBHPs and/or multiple batches in parallel. It also makes it unnecessity to completely remake the whole system for a new product. It is possible to establish a new manufacturing process by combining existing modules and adding new modules.
The impact of flexibility on manufacturing and development cost has been a major focus in the automotive industry. In order to respond to the demands of car customers for unique products and respond to rapid changes in regulations or required specifications, it is necessary to design flexible processes such as producing multiple types of vehicles on one manufacturing line. Koste and Malhotra have listed five important flexibilities in the automotive industry: machine flexibility, labor flexibility, mix flexibility, new product flexibility, and modification flexibility [24]. Additionally, Gupta and Somers have proposed volume flexibility as another important flexibility [25], [26]. Applying these concepts from the automotive industry, we examined the importance of these flexibilities in the CHBP industry.
Regarding machine flexibility, most current manufacturing processes for CHBPs have been used with commercially available biological safety cabinets and incubators, and their flexibility is sufficient for manual operations. In other words, almost all types of cells can be cultured with standard safety cabinets and incubators. Even when using automatic culture apparatuses, basic cell culture procedures such as cell seeding and expansion culture are relatively common for a wide variety of cell types. Machine flexibility is already well advanced in CHBPs.
Labor flexibility is important in CHBP manufacturing. A major difference from the automotive industry is in the heterogeneity of jobs. There are many job classes in the automotive industry according to the number of processes. Generally, a CHBP has fewer processes, thus, fewer job classes are needed. In the simplest case, there may be only two classes, Quality Control class and Manufacturing class. However, in conventional manufacturing facilities, many of the important manufacturing processes are dependent on trained technicians. Therefore, the quality variance between operators is an influential element in the quality of CHBPs (Referred as Uniformity element in Ref. [24]). In a cell culture process for CHBPs, the work date is strictly determined for consistency in the process, and it is not possible to change the work date for the convenience of workers. Therefore, it is necessary to always maintain surplus personnel to accommodate issues such as sick leave. Therefore, automation of cell culture processes is one remedy for employment redundancy.
Mix flexibility and new product flexibility requires special consideration for CHBPs concerning cross-contamination and potential mix-ups between different batches. Generally, it is not appropriate to handle two or more batches in the same place at the same time. However, suitable changeover and mix-up prevention procedures enable multiple parallel processes including different CHBPs. In this respect, fMP provides a basal technology to realize multi-product manufacturing for CHBPs.
Modification flexibility is not a current problem, but a future problem. CHBP specifications are strictly defined in the approval process, and specification options are rarely proposed. However, some quantity options, such as cell numbers or product sizes, may be allowed in the future.
Volume flexibility is the range of profitable production volume. It is important both in autologous cell products or allogeneic cell products. Since autologous cell products are manufactured according to the patient's treatment plan, the date and time of manufacture cannot be significantly changed. Therefore, the capacity of the manufacturing facility must be designed to its maximum capacity, and then volume fluctuation also increases. Allogeneic cell products on the other hand are usually cryopreserved, so the production schedule can be changed by consulting the donor. Therefore, it is theoretically possible to maximize the operational rate of the facility. However, quality testing (including viral clearance) for allogenic cell products have significant costs, so that it is more efficient to make the batch size as large as possible. When the size of a batch exceeds the number of orders in the same time period as the production time of the batch, the facility must be paused to avoid over-production. In both cases, the operational rate of facilities tends to be low, and cutting the costs during idling time is essential.
As discussed above, in manufacturing CHBPs, flexibility is required from different viewpoints in comparison with the automotive industry. Especially in CHBP manufacturing, it is difficult to maximize the operational rate of facilities and to equalize the number of required operators throughout the year. In this respect, fMP may be able to provide a better solution. In conventional manufacturing facilities, air conditioning, cleaning and monitoring are always performed regardless of the operational rates because stopping and resuming the facility incurs high costs and takes time. Conversely, because fMP can be decontaminated on demand, it is possible that non-working time costs can be reduced by simply switching off the facility when no production runs are scheduled. Also, fMP does not require a large number of workers at all times, as stated above. Therefore, fMP may be able to significantly reduce the risk of cost increases for sudden loss of production orders, either planned or unexpected.
Finally, we would like to point out that ‘portability’ is an important feature of fMP. When it is necessary to relocate a large factory due to changes in economic environments, significant costs are incurred to transfer and restart the facility. In that respect, since fMP is composed of relatively small modules, it is easy to transport and start up. Any large factory relies on many skilled workers, but when it is difficult to hire them at one location, new workers need to be hired and educated. An additional important point with fMP is that such labor costs are minimal. This is applicable not only when moving factory location but also when the owner of the factory changes. In other words, it is easy to purchase and resell the facility. Such mobility is an asset that may reduce the risks associated with changes in the financial environment (asset flexibility).
5. Conclusion
The introduction of biological isolators and industrial factory automation technology allowed us to assemble a novel CBHP manufacturing system, the Tissue Factory, based on the concept of flexible Modular Platforms, which we believe will produce cost-effective manufacturing processes for CBHPs owing to its many ‘flexibilities’.
Conflicts of interest
-
•
Teruo Okano is a shareholder and the chairman of the Scientific Advisory Board of CellSeed Inc.
-
•
Tatsuya Shimizu is a shareholder and a member of the Scientific Advisory Board of CellSeed Inc.
-
•
Tetsutaro Kikuchi was an employee of CellSeed Inc. from December 2005 to March 2015.
-
•
Tokyo Women's Medical University received the research funding from CellSeed Inc., Hitachi Ltd., and Nihon Kohden Corporation.
Acknowledgements
This study was supported in part by the Cabinet Office, Government of Japan and the Japan Society for the Promotion of Science (JSPS) through the "Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program)", and in part by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP16be0204439. We would like to thank Mr. Allan Nisbet for his useful comments and his editorial assistance.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.reth.2018.08.004.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Manufacture of cell-based health care products – control of microbial risks during processing. International Standard; 2016. ISO 18362. [Google Scholar]
- 2.Bubela T., McCabe C., Archibald P., Atkins H., Bradshaw S.E., Kefalas P. Bringing regenerative medicines to the clinic: the future for regulation and reimbursement. Regen Med. 2015;10:897–911. doi: 10.2217/rme.15.51. [DOI] [PubMed] [Google Scholar]
- 3.Mount N.M., Ward S.J., Kefalas P., Hyllner J. Cell-based therapy technology classifications and translational challenges. Philos Trans R Soc B Biol Sci. 2015;370:20150017. doi: 10.1098/rstb.2015.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez F., Di Bartolo C., Piazza T., Passannanti A., Gerlach J.C., Gridelli B. A quality risk management model approach for cell therapy manufacturing. Risk Anal. 2010;30:1857–1871. doi: 10.1111/j.1539-6924.2010.01465.x. [DOI] [PubMed] [Google Scholar]
- 5.Ratcliffe E., Thomas R.J., Williams D.J. Current understanding and challenges in bioprocessing of stem cell-based therapies for regenerative medicine. Br Med Bull. 2011;100:137–155. doi: 10.1093/bmb/ldr037. [DOI] [PubMed] [Google Scholar]
- 6.Hümmer C., Poppe C., Bunos M., Stock B., Wingenfeld E., Huppert V. Automation of cellular therapy product manufacturing: results of a split validation comparing CD34 selection of peripheral blood stem cell apheresis product with a semi-manual vs. an automatic procedure. J Transl Med. 2016;14:76. doi: 10.1186/s12967-016-0826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knoll A., Scherer T., Poggendorf I., Lutkemeyer D., Jurgen L. Flexible automation of cell culture and tissue engineering tasks. Biotechnol Prog. 2004;20:1825–1835. doi: 10.1021/bp049759v. [DOI] [PubMed] [Google Scholar]
- 8.Franscini N., Wuertz K., Patocchi-Tenzer I., Durner R., Boos N., Graf-Hausner U. Development of a novel automated cell isolation, expansion, and characterization platform. J Lab Autom. 2011;16:204–213. doi: 10.1016/j.jala.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Konagaya S., Ando T., Yamauchi T., Suemori H., Iwata H. Long-term maintenance of human induced pluripotent stem cells by automated cell culture system. Sci Rep. 2015;5:1–9. doi: 10.1038/srep16647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyslop L., Prathalingam N., Nowak L., Fenwick J., Harbottle S., Byerley S. A novel isolator-based system promotes viability of human embryos during laboratory processing. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kino-oka M., Taya M. Recent developments in processing systems for cell and tissue cultures toward therapeutic application. J Biosci Bioeng. 2009;108:267–276. doi: 10.1016/j.jbiosc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Okano T., Yamada N., Sakai H., Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J Biomed Mater Res. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa M., Taniguchi Y., Senda S., Takizawa N., Ichisaka T., Asano K. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep. 2015;4:3594. doi: 10.1038/srep03594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomson J., Itskovitz-Eldor J., Shapiro S., Waknitz M., Swiergiel J., Marshall V. Embryonic stem cell lines derived from human blastocysts. Science. 1989;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 16.Onuma Y., Tateno H., Hirabayashi J., Ito Y., Asashima M. RBC2LCN, a new probe for live cell imaging of human pluripotent stem cells. Biochem Biophys Res Commun. 2013;431:524–529. doi: 10.1016/j.bbrc.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Sawa Y., Miyagawa S., Sakaguchi T., Fujita T., Matsuyama A., Saito A. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg Today. 2012;42:181–184. doi: 10.1007/s00595-011-0106-4. [DOI] [PubMed] [Google Scholar]
- 18.Sawa Y., Yoshikawa Y., Toda K., Fukushima S., Yamazaki K., Ono M. Safety and efficacy of autologous skeletal myoblast sheets (TCD-51073) for the treatment of severe chronic heart failure due to ischemic heart disease. Circ J. 2015;79:991–999. doi: 10.1253/circj.CJ-15-0243. [DOI] [PubMed] [Google Scholar]
- 19.Sekiya N., Matsumiya G., Miyagawa S., Saito A., Shimizu T., Okano T. Layered implantation of myoblast sheets attenuates adverse cardiac remodeling of the infarcted heart. J Thorac Cardiovasc Surg. 2009;138:985–993. doi: 10.1016/j.jtcvs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Kubo H., Shioyama T., Oura M., Suzuki A., Ogawa T., Makino H. Development of automated 3-dimensional tissue fabrication system tissue factory – automated cell isolation from tissue for regenerative medicine. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:358–361. doi: 10.1109/EMBC.2013.6609511. [DOI] [PubMed] [Google Scholar]
- 21.Haraguchi Y., Shimizu T., Sasagawa T., Sekine H., Sakaguchi K., Kikuchi T. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat Protoc. 2012;7:850–858. doi: 10.1038/nprot.2012.027. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi T., Shimizu T., Wada M., Yamato M., Okano T. Automatic fabrication of 3-dimensional tissues using cell sheet manipulator technique. Biomaterials. 2014;35:2428–2435. doi: 10.1016/j.biomaterials.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen K. Process automation in manufacturing of mesenchymal stromal cells. Transfusion. 2016;56:26S–28S. doi: 10.1111/trf.13567. [DOI] [PubMed] [Google Scholar]
- 24.Koste L.L., Malhotra M.K. Trade-offs among the elements of flexibility: a comparison from the automotive industry. Omega. 2000;28:693–710. [Google Scholar]
- 25.Gupta Y., Goyal S. Flexibility of manufacturing systems; concept and measurements. Eur J Oper Res. 1989;43:119–135. [Google Scholar]
- 26.Diffner, B., Björkman, M., Johansen, K. To stay competitive in future automotive assembly – some challenges related to flexibility. Proc 2011 Int Conf Ind Eng Oper Manag :62–67.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.