Abstract
Background
Postpartum dysgalactia syndrome (PDS) in sows is difficult to diagnose and the pathogenesis is obscure. Hormonal changes related to the disease are often difficult to distinguish from those found in the normal transition period from gestation to lactation. The study aimed to investigate metabolic and hormonal changes related to PDS with the goal of identifying potential biomarkers in sows suffering from PDS (PDS+). Selected biomarkers were examined by comparing 38 PDS+ sows with 38 PDS negative (PDS-) sows. The sows were sampled every 24 h from 60 h ante partum (a.p.) to 36 h post partum (p.p.).
Results
Compared to the baseline (60 to 36 h a.p.), cortisol in serum and saliva and fasting blood glucose concentrations increased in PDS+ as well as PDS- sows. C-peptide decreased relative to the baseline in PDS+ sows, and prolactin and 8-epi prostaglandin F2 alpha (8-epi-PGF2α) decreased in PDS- sows. Concentrations of cortisol in serum and saliva, salivary chromogranin A (CgA), fasting blood glucose, C-peptide, and 8-epi-PGF2α differed significantly between PDS+ and PDS- sows, with levels of cortisol in serum and saliva, salivary CgA, and 8-epi-PGF2α in serum being different in the two groups already before parturition. Concentrations of salivary CgA were significantly lower in PDS- sows than in PDS+ sows during the entire study period.
Conclusions
The results suggest that salivary CgA, cortisol and serum 8-epi-PGF2α may potentially serve as early diagnostic indicators for PDS. The consistently higher salivary CgA concentration in PDS+ sows compared to PDS- sows may indicate that homeostatic disturbances are present between 36 to 60 h before parturition in sows developing PDS. The higher serum and saliva cortisol concentration in PDS+ sows compared to PDS- sows could reflect an early sign of inflammation or stress. The significantly lower C-peptide in PDS+ sows compared to PDS- sows may reflect a lower food intake. Our results contribute to the understanding of the pathogenesis of PDS, and the homeostatic disturbances detected before parturition warrants further investigation. The diagnostic potential of the markers identified in this study should be investigated further in a larger population of sows.
Electronic supplementary material
The online version of this article (10.1186/s12917-018-1649-z) contains supplementary material, which is available to authorized users.
Keywords: PPDS, Chromogranin A, Prostaglandin F2α, Glucose metabolism, Cortisol
Background
The pathogenesis of postpartum dysgalactia syndrome (PDS) in sows is not fully understood, and the clinical diagnosis is often difficult. Commonly used indicators for PDS include fever, reduced appetite, mastitis, and signs of piglet starvation. These signs, however, may vary considerably [1–4]. Improved knowledge about the pathogenesis of PDS, including disturbances in hormonal and metabolic processes at parturition, may thus be relevant for future diagnosis, treatment and prophylaxis.
In the transition from gestation to lactation, mammals undergo extensive hormonal changes [5, 6], including a rapid shift from an anabolic to a catabolic state [6]. Serum concentrations of progesterone and estradiol decrease, and cortisol concentrations increase temporarily [7]. Prolactin concentrations starts to increase close to parturition and increase further at the onset of lactation [8, 9]. Glucose is the most important nutrient for milk production [10, 11], as it is a precursor for lactose [12]. Cortisol serves to mobilize glucose from glycogen stores [13, 14], and the peripartal cortisol release [15, 16] thus leads to increased blood glucose levels [17] in healthy sows. A number of other biological events are also associated with increased levels of cortisol, for example stress [18] and inflammation [19, 20].
Factors like inadequate body condition of the sow [21, 22], improper feeding strategy in late gestation [23], or improper feed composition [22, 24] may negatively affect the production of colostrum and milk, thus limiting piglet growth and survival [25]. Reduced appetite and feed intake are normal peri-parturient features in healthy sows [26], but excessive anorexia may be a sign of illness. Decreased feed intake decreases glucose uptake from the gastrointestinal tract, but under normal circumstances adequate blood glucose levels are also maintained in sows ingesting less feed than intended [27]. C-peptide concentrations reflect the insulin response to glucose [28], and the excretion is correlated to insulin [29]. Measuring C-peptide concentrations instead of insulin is advantageous, since C-peptide is more stable [30, 31]. C-peptide has not been investigated in sows before.
Cromogranin A (CgA) reflects activation of the sympatho-adrenal medullary system (SAM) [32, 33] and is secreted into saliva. It is necessary for the regulation of vascular homeostasis in humans [34] and concentrations are increased in individuals with neuroendocrine tumors [35] and endocrine cells of the human gastrointestinal tract [36, 37]. In humans, CgA levels can be elevated by hypertension, inflammatory bowel disease, sepsis, and other inflammatory diseases [34]. It has been linked to oxidative stress in humans [38] and shown to be a trigger of free radical production in rats [39]. CgA has only been studied to a limited extent in pigs, but it was recently shown by Escribano and coworkers [40] to be a marker of stress in a pig model.
Eight-epi prostaglandin F2 alpha (8-epi-PGF2α) is considered to be a biomarker of oxidative stress in humans [41], and it is released when free oxygen radicals are produced in excess or when antioxidants are lacking [42]. Vitamin E and selenium deficiencies have been shown to inhibit the immune function in sows [43], and antioxidants seem to reduce the risk of sow agalactia [44]. To our knowledge, 8-epi-PGF2α has not been studied in sows before.
The aims of this study were to describe the changes of cortisol, CgA, glucose, C-peptide, prolactin, 8-epi-PGF2α, sodium, and potassium levels in healthy sows (PDS-) and sows suffering from PDS (PDS+) during the periparturient period. Furthermore, we aimed to evaluate the potential of these biomarkers to identify affected animals in the early stage of disease.
Results
Body condition score
A body condition score of 2 was noted in 12 PDS+ sows and 17 PDS- sows, and score 3 was assigned to 26 PDS+ sows and 21 PDS- sows (p = 0.09).
Obstetric aid, parturition duration and feeding time
Obstetric aid was provided more often in PDS+ sows (18 PDS+ sows and 11 PDS- sows; p < 0.05) and parturition lasted longer for PDS+ sows (mean length 654.2 min; SD 444.0) compared to PDS- sows (mean length 432.3 min; SD 267.5) (p < 0.01). Feeding time relative to parturition (less than or more than 4 h before parturition) had no influence on whether sows were categorized as PDS+ sows or PDS- sows (p = 0.16).
Piglet weight gain
The effect of PDS status on litter weight gain was dependent on litter weight at first weighing (a significant interaction was noted between PDS status and weight at first weighing; p < 0.01). For the PDS+ sows, the litter weight gain decreased with increasing litter weight at first weighing, but for the PDS- sows, the litter weight gain was not dependent on weight at first weighing (Fig. 1).
Fig. 1.
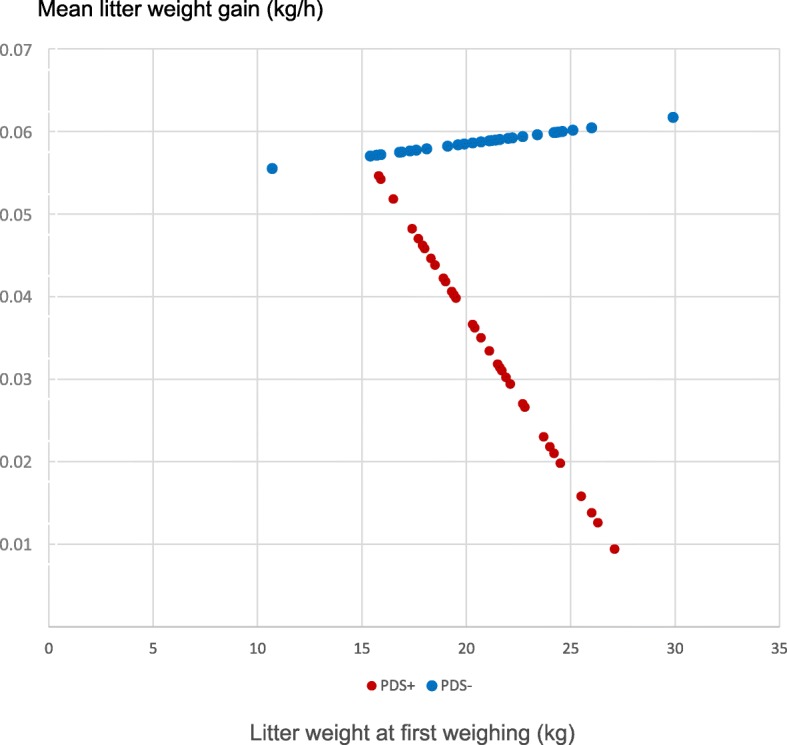
Mean weight gain (kg/h) in litters from 38 PDS+ sows and litters from 38 PDS- sows. In PDS+ sows, the litter weight gain depended on the litter weight (kg) at first weighing, where litters with the highest weight had the smallest weight gain. This interdependency was not apparent in litters born to PDS- sows
Changes in hormonal and metabolic indicators in relation to parturition
Changes over time for PDS+ and PDS- sows are illustrated by raw data in Fig. 2 for CgA (Fig. 2A), saliva cortisol (Fig. 2B), serum cortisol (Fig. 2C), fasting blood glucose (Fig. 2D), C-peptide (Fig. 2E) and 8-epi-PGF2α (Fig. 2F).
Fig. 2.
Raw data for A. chromogranin A (CgA; 10− 5 g/L), B. saliva cortisol (10− 5 g/L), C. serum cortisol (10− 5 g/L), D. fasting blood glucose (10− 3 mol/L), E. C-peptide (10− 12 mol/L) and F. 8-epi prostaglandin F2 alpha (8-epi-PGF2α; 10− 9 g/L) assessed from 60 h ante partum (time interval A) until 36 h post partum (time interval G) in sows suffering from postpartum dysgalactia syndrome (PDS+, red) and healthy sows (PDS-, blue). Each point represents the precise sample time of each observation relative to parturition of piglet number one (0 h). The line represents the mean value at each sampling time point
When compared to baseline A (60 to 36 h a.p.), hormonal and metabolic changes occurred over time in both PDS+ and PDS- sows. Differences between time interval A and intervals B to G are reported with lowercase letters in Table 1; In PDS+ sows, cortisol (serum and saliva) and fasting blood glucose increased significantly over time (B to G) relative to baseline A, while C-peptide decreased over time. In PDS- sows, increases over time in cortisol (serum and saliva) and fasting blood glucose concentrations were also noted relative to the baseline. In contrast to PDS+ sows, serum cortisol levels in PDS- sows were only significantly increased relative to the baseline at − 12 to 0 h p.p, and significantly decreased 24 to 36 h p.p. Compared to the baseline, no significant changes were noted for C-peptide in PDS- sows. Concentrations of prolactin (Additional file 1) and 8-epi-PGF2α decreased significantly after parturition in PDS- sows but remained unaltered in PDS+ sows (Table 1). Concentrations of Na and K did not change over time in any of the groups (Additional files 2 and 3).
Table 1.
Changes in concentrations of hormones and metabolic biomarkers in 38 sows suffering from postpartum dysgalactia syndrome (PDS+) and 38 healthy (PDS-) sows
| Parameter | Group | n | A. (−60 to − 36 h) | B.(− 36 to − 24 h) | C. (− 24 to − 12 h) | D. (− 12 to 0 h) | E. (0 to 12 h) | F. (12 to 24 h) | G. (24 to 36 h) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LSMEANS | ±SD | LSMEANS | ±SD | LSMEANS | ±SD | LSMEANS | ±SD | LSMEANS | ±SD | LSMEANS | ±SD | LSMEANS | ±SD | |||
| Serum cortisol | PDS+ | 38 | 3.14 | 1.10 | 3.07 * | 1.08 | 3.34 | 1.15 | 4.84 b | 1.08 | 6.36 ***a | 1.15 | 5.55 ****a | 1.09 | 5.43 ***c | 1.19 |
| PDS- | 38 | 3.18 | 1.10 | 3.89 * | 1.09 | 3.63 | 1.15 | 4.58 c | 1.08 | 2.79 *** | 1.17 | 3.14 **** | 1.09 | 2.12 ***d | 1.19 | |
| Saliva cortisol | PDS+ | 38 | 0.35 | 1.12 | 0.37 | 1.10 | 0.50 | 1.16 | 0.61 *b | 1.10 | 0.71 c | 1.21 | 1.09 a | 1.13 | 0.82 d | 1.39 |
| PDS- | 38 | 0.28 | 1.11 | 0.32 | 1.10 | 0.44 d | 1.16 | 0.44 *c | 1.10 | 0.84 a | 1.19 | 0.95 a | 1.11 | 0.66 b | 1.23 | |
| † Chromogranin A *** | PDS+ | 38 | 0.39 | 1.19 | 0.46 | 1.16 | 0.68 | 1.25 | 0.98 | 1.16 | 0.97 | 1.31 | 1.07 | 1.19 | 1.18 | 1.62 |
| PDS- | 38 | 0.26 | 1.18 | 0.35 | 1.16 | 0.58 | 1.25 | 0.56 | 1.15 | 0.70 | 1.28 | 0.61 | 1.17 | 0.34 | 1.37 | |
| Fasting blood glucose | PDS+ | 38 | 3.76 | 0.37 | 3.91 | 0.36 | 4.07 | 0.50 | 4.10 | 0.36 | 4.53 | 0.49 | 4.89 d | 0.40 | 4.64 **** | 0.69 |
| PDS- | 38 | 3.85 | 0.37 | 3.74 | 0.35 | 4.76 | 0.60 | 4.09 | 0.35 | 5.35 d | 0.57 | 4.98 d | 0.36 | 9.55 ****a | 0.63 | |
| C-peptid | PDS+ | 38 | 301.9 | 1.21 | 326.42 | 1.17 | 222.29 | 1.33 | 240.95 | 1.18 | 109.22 c | 1.35 | 161.14 **d | 1.20 | 220.37 | 1.42 |
| PDS- | 38 | 267.25 | 1.20 | 285.60 | 1.18 | 303.87 | 1.33 | 182.97 | 1.18 | 238.13 | 1.35 | 349.32 ** | 1.18 | 412.03 | 1.41 | |
| Prolactin | PDS+ | 38 | 11,553.00 | 1.13 | 12,358.51 | 1.11 | 12,891.20 | 1.18 | 12,356.04 | 1.11 | 9075.19 | 1.18 | 11,896.50 | 1.12 | 7873.04 | 1.22 |
| PDS- | 38 | 12,610.69 | 1.12 | 13,355.72 | 1.11 | 12,187.91 | 1.18 | 12,410.52 | 1.11 | 11,292.57 | 1.18 | 11,443.76 | 1.12 | 8304.06 d | 1.21 | |
| 8-epi prostaglandin F2 α | PDS+ | 38 | 41.71 | 4.91 | 40.78 | 4.08 | 44.02 | 7.18 | 42.60 * | 4.19 | 31.87 | 7.15 | 38.41 | 4.62 | 25.37 | 8.75 |
| PDS- | 38 | 44.39 | 4.70 | 38.10 | 4.12 | 44.07 | 7.06 | 30.07 *d | 4.09 | 34.39 | 7.42 | 30.23 d | 4.28 | 29.36 | 8.53 | |
| Natrium | PDS+ | 38 | 148.58 | 1.02 | 148.68 | 1.01 | 150.63 | 1.02 | 147.00 | 1.01 | 149.64 | 1.02 | 153.62 | 1.02 | 152.23 | 1.03 |
| PDS- | 38 | 147.41 | 1.02 | 151.64 | 1.01 | 144.68 | 1.03 | 151.40 | 1.01 | 148.96 | 1.03 | 151.28 | 1.01 | 155.18 | 1.03 | |
| Potassium | PDS+ | 38 | 4.47 | 1.03 | 4.55 | 1.03 | 4.35 | 1.05 | 4.34 | 1.03 | 4.69 | 1.05 | 4.50 | 1.03 | 4.27 | 1.06 |
| PDS- | 38 | 4.37 | 1.03 | 4.53 | 1.03 | 4.24 | 1.04 | 4.45 | 1.03 | 4.47 | 1.05 | 4.44 | 1.03 | 4.89 | 1.05 | |
Significant differences for each time interval are given as least-squares means (LSMEANS) and standard deviations (SD). Lowercase letters represent the level of significance between time interval A and the following time intervals (B to G, respectively): a p < 0.0001, b p < 0.001, c p < 0.01, d p < 0.05. Bold letters and asterisk symbols represent the level of significance between PDS+ and PDS- sows: **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05
†No significant interaction between time and case-control was found for chromogranin A. Therefore, an all over significant difference for chromogranin A between PDS+ and PDS- sows (p < 0.001) was estimated using the statistic model B (OUTCOME PARAMETERij = μ + TIMEi + GROUPj + ε)
Differences between hormonal and metabolic parameters in PDS+ and PDS- sows
Concentrations of cortisol (serum and saliva), CgA, fasting blood glucose, C-peptide and 8-epi-PGF2α differed between PDS- and PDS+ sows (bold letters and asterisk symbols; Table 1). From − 36 to − 24 h, serum cortisol concentration was significantly lower in the PDS+ sows compared to the PDS- sows, while concentrations were significantly higher in the PDS+ sows compared to the PDS- sows 0–36 h p.p. Before parturition, significantly higher concentrations of salivary cortisol and 8-epi-PGF2α were demonstrated in the PDS+ sows (− 12 h to 0 h) compared to the PDS- sows. CgA concentration was significantly higher in the PDS+ sows compared to the PDS- sows throughout the whole study period. Fasting blood glucose was significantly lower at 24–36 h p.p. and C-peptide was significantly lower at 12–24 h p.p. in the PDS+ sows compared to the PDS- sows (Table 1).
Discussion
To our knowledge, this is the first study that compares hormonal and metabolic alterations in sows suffering from PDS and healthy sows in the immediate periparturient period.
Previously established criteria were selected to identify sows suffering from PDS [4, 45–51], but as already pointed out by others [52–54], these vary greatly and are associated with uncertainty. However, the present study, and previous results showing significant differences between PDS+ and PDS- sows for several inflammatory parameters [55] support the usefulness of our PDS definition.
Lactogenesis is influenced by local mammary factors (e.g. hormonal receptors), by the removal of milk through suckling, and by circulating nutrients and hormones [56]. Weight loss was more pronounced in heavy litters, which are expected to suckle with most intensity, and the limiting factor for milk production in PDS+ sows was thus most likely related to insufficient nutrient supply or hormonal disturbances.
Obstetric aid was more frequently given in sows diagnosed with mastitis, metritis and agalactia [57], coliform mastitis [58] and post-farrowing discharge [59], but the significance of the parturition duration in PDS is obscure [4, 50, 57]. Whether extended parturition and increased obstetric aid cause PDS or vice versa remains to be elucidated.
Chromogranin A and cortisol
The continuously increased CgA concentration among the PDS+ sows at all time intervals may indicate a disturbance in the homeostasis of these sows. Interestingly, this disturbance seemed to occur before the systemic inflammation that, based on clinical signs and blood biochemical changes, became evident from 12 to 36 h p.p. in the PDS+ sows [55]. The homeostatic function of CgA in humans includes the endocrine, cardiovascular, and immune systems, and the glucose and calcium balances [60]. Salivary CgA is regulated by a neuronal pathway [61] and is a reliable marker of stress in humans [62, 63]. In the human gastrointestinal tract, CgA is released from enterochromaffin cells and from neurons of submucosal and myenteric ganglia [64, 65], and may modulate colonic motility in response to inflammation [66]. To our knowledge, CgA has never been studied in the porcine gastrointestinal tract, but the present results warrant further investigation, since constipation is considered to be a major feature of PDS [67].
The higher serum and salivary cortisol and higher serum CgA concentrations demonstrated in the PDS+ sows may reflect differences in stress level [40] in the two groups, but could also be related to inflammation caused for example by a bacterial infection [20]. CgA has been investigated to a limited extent in swine, but a previous study demonstrated CgA release in response to experimentally induced transportation stress [40]. To determine the potential occurrence of stress in PDS+ sows, detailed behavioral observations are needed.
An increased cortisol level during the periparturient period is a normal physiological occurrence [15, 16] as also suggested by the results in our study, where PDS- sows showed increased cortisol concentrations (compared to the baseline) from − 24 h a.p. (Table 1). Inflammation [20] and stress [18] have also been shown to cause cortisol release in pigs. We have previously demonstrated a significant inflammatory response 12–36 h p.p. in the PDS+ sows [55], which could have contributed to the higher cortisol concentrations observed in sows developing PDS. Normal physiological alterations, inflammation, and stress may all have contributed to the increased cortisol concentration observed in the PDS+ sows, but stress was not further assessed in our study populations.
8-epi-PGF2α
The increased 8-epi-PGF2α concentrations in PDS+ sows suggest that oxidative stress may be a feature of PDS, caused either by antioxidant deficiency or excessive production of free radicals [42]. Cells of the immune system are particularly susceptible to oxidation [68] and oxidative stress may thus affect the immune responses. 8-epi-PGF2α is released after stimulation of the adrenal cortex [69], thus explaining the coinciding 8-epi-PGF2α, CgA and salivary cortisol responses 0–12 h after parturition. Further research is required to determine the role of 8-epi-PGF2α and its potential as an early biomarker for PDS.
Glucose and C-peptide
Compared to the baseline, a significantly increased fasting blood glucose concentration was demonstrated in both groups after parturition (Table 1). Increased blood glucose and reduced insulin responsiveness have previously been described in late gestation and early lactation in healthy sows [17, 70]. Reduced insulin responsiveness is believed to support the transportation of glucose into the udder [11, 71]. The changes observed in PDS- sows (unaltered C-peptide concentration in conjunction with increased blood glucose) are consistent with the reduced insulin responsiveness normally observed in the transition period. Interestingly, three individual sows displayed extremely high glucose values ranging from 12.7 to 24.3 × 10− 3 mol/L (Fig. 2D). In these sows, the high glucose concentrations were accompanied by high C-peptide values (Additional file 4). A similar syndrome, referred to as “physiological insulin resistance”, is described in humans after fasting [72]. The significantly decreased levels of C-peptide in the PDS+ sows compared to the baseline may reflect insufficient feed intake.
The sow’s ability to mobilize glucose might be crucial for the development of PDS, and post-feeding blood glucose and insulin concentrations can affect piglet growth [73]. The poor litter weight gain and lower C-peptide concentration 12–24 h p.p. in the PDS+ sows could reflect a reduced feed intake and low lipid and protein metabolism. Indeed, glucose homeostasis is challenged during parturition, where sows may be depleted of energy if the onset of farrowing starts more than 3 h after the last meal was consumed [74]. However, we were not able to demonstrate an association between PDS and feeding time relative to parturition.
Prolactin
Reduced prolactin concentration has previously been found in sows suffering from metritis-mastitis-agalactia syndrome [75], and experimental lipopolysaccharide (LPS) administration has also been shown to cause lowered serum prolactin [76]. In contrast to these previous studies, prolactin decreased in PDS- sows, but remained unchanged in PDS+ sows (Table 1). How prolactin may be involved in the reduced milk production observed in PDS+ sows was not clear from our results.
Conclusions
Salivary CgA, cortisol and serum 8-epi-PGF2α are increased in PDS+ sows before parturition, reflecting a situation of activation of the adrenergic system (CgA), adrenal system (cortisol) and oxidative stress (8-epi-PGF2α), and these analytes may potentially serve as biomarkers in the early detection of PDS. In addition, PDS+ sows showed metabolic changes consisting of decreased glucose and C-peptide concentrations that may be caused by a lower energy intake due to sickness.
The interrelationship between the metabolites and hormones assessed in the current study are not entirely clear. Our results suggest that the homeostasis of PDS+ sows may be affected before parturition and that the normal periparturient glucose metabolism is disrupted in PDS+ sows. The persistently high CgA concentration in PDS+ sows is a highly interesting finding that warrants further investigation.
Methods
Experimental design
The study was carried out as described in detail in our previous publication [55]. Briefly, a case-cohort study was performed in one herd that bought sows from a conventional breeder. In all, 109 sows were included, from which 38 (34.9%) were categorized as PDS positive (PDS+) and retrospectively matched with the 38 healthy sows (PDS-).
All sows (n = 109) were systematically observed and sampled every 24 h from at least 60 h before expected parturition until PDS occurred or until 36 h p.p. Due to animal welfare considerations it was necessary to treat sick sows. Therefore, a clinical definition of PDS were defined and sows was deemed PDS+ if at least two of the following clinical criteria were fulfilled: 1. Reduced feed intake, defined as “trough not empty within 30 min after feeding”, 2. General inflammation of the udder, characterized by a subjective assessment of redness, swelling and increased skin temperature, 3. rectal temperature ≥ 39.5 °C. Sows farrowing prematurely or sows that were treated for other reasons were excluded from the study. All observations were performed by a veterinarian (the first author). The sows remained in the farm and were kept according to common management routines after completion of the study.
Prior to parturition, monitoring included: 1. samples of saliva and capillary blood taken before the morning feeding as described below, 2. clinical examinations and 3. blood sampling from v. jugularis as described below. Except for 1., all recordings were done after the morning feeding.
Body condition was evaluated based on a Danish 4-point scoring system, where score was 1 considered thin, 2) lean, 3) medium and optimal body condition, and 4) fat [77]. Sows that were considered PDS+ (n = 38) were treated immediately after the veterinary clinical examination and sampling. All sows continued in the production at the farm after they exited the study.
Litters were equalized to 15 piglets and subsequently weighed on two occasions: within 24 h p.p. and again when the sows left the study. The weight of dead piglets was registered daily and included in the total litter weight. Cross-fostering was not allowed after litter standardization. The electronic scale (WEDO S/N 45705, Werner Dorsch GmbH, Germany) was calibrated daily.
Sampling
Saliva was collected by a cotton swab without additives (Salivette® Cortisol, Haunisen, Denmark). The sows were allowed to chew on the swab for 3 min. The swabs were centrifuged for 5 min at 1000×g and immediately stored on ice. The saliva was tested for CgA by a time-resolved immunofluorometric assay (TR-IFMA) previously described by Escribano and coworkers [40]. Saliva cortisol was tested by a solid-phase, competitive chemiluminescent enzyme immunoassay using an automated biochemistry analyzer (IMMULITE 1000 Immunoassay System cortisol, Siemens, California, US) as previously described [18] and according to the manufacturer’s instructions. Blood sample droplets for fasting blood glucose were collected from v. auricularis after administration of cutaneous lidocaine spray on the dorsal area of the ear (Xylocain 100 mg/mL, AstraZeneca, UK). The blood glucose concentration was immediately measured using the Accu-Chek Aviva system (Roche Diagnostics, Basel, Switzerland; [78]). Following sampling, each sow was given a small lump of sugar as a “reward”. Blood samples were collected from v. jugularis in tubes without additives (BD, New Jersey, US) for preparation of serum and kept at room temperature for a maximum of 30 min. before being processed. The tubes were centrifuged for 10 min. at 3000×g and sera and saliva were stored at -80 °C until analysis. Potassium (K) and sodium (Na) were analyzed by the Hematology System Complete Blood Count method using an automated biochemistry analyzer (ADIVA 2120/2120i, Siemens Healthcare A/S, Denmark). Plasma prolactin was analyzed by a commercially available porcine ELISA kit (#SEA846Po, Cloud-Clone Corp. Texas, US) as described elsewhere [79]. The absorbance was read at 450 nm (Polar Star/Galaxy, BMG Labtech, Germany). Concentrations of C-peptide in serum were determined by a porcine-specific C-peptide ELISA (#10-1256-01, Mercodia AB, Sweden) as described previously [80–82] in accordance with the instructions given by the manufacturer, and the absorbance was read at 450 nm (Polar Star/Galaxy, BMG Labtech, Germany). The concentration of 8-epi prostaglandin F2 Alpha (8-epi-PGF2α) was analyzed by a pan-308 species commercial ELISA (#CEA701Ge, Cloud-Clone Corp., Texas, US) as described in humans by Haxhi and coworkers [83].
Statistical analyses
The exact sampling times (date:hour:min.) were retrospectively related to the exact time of the birth of the first piglet as revealed by the video records.
The sampling time points were grouped into time intervals where 0 h was the time of birth of the first piglet: A. -60 to − 36 h; B. -36 to − 24 h; C. -24 to − 12 h; D. -12 to 0 h; E. 0 to 12 h; F. 12 to 24 h, and G. 24 to 36 h. The number of observations within each interval may vary because of the individual sampling times relative to parturition (0 h).
For statistical evaluation, two autoregressive linear regression models (A and B) were performed in the PROC MIXED procedure of Statistic Analytical Software, Enterprise Guide 7.1 (SAS® Institute, Cary, North Carolina, USA). Least-squares means (LSMEANS) and standard deviations (SD) were included in the statistical model A. Model A included OUTCOME PARAMETERij = μ + TIMEi + GROUPj + TIME*GROUPij + εij, where OUTCOME PARAMETERij was the measured value of the hormone or metabolic parameters; μ was the value of the observations at time 0; TIMEi was the explanatory variable “time intervals A-G”; GROUPj was the explanatory variable “PDS+/PDS-”; TIME*GROUPij was the interaction between the two groups and time, and εij was the random residual error term. When significant interaction was identified using model A, differences between the relevant groups and time intervals were accepted. In case of non-significant interaction, model A was replaced with model B consisting of the OUTCOME PARAMETERij = μ + TIMEi + GROUPj + εij. If non-significant changes in TIMEi occurred in model B, the OUTCOME PARAMETERij was considered non-significant. Significance was considered for p < 0.05. Parity and body condition score were included as explanatory variables. From preliminary analyses, obstetric aid and farrowing duration were not found to be associated with any of the outcome variables. Logarithmic transformation was used for serum and saliva cortisol, CgA, prolactin, Na and K, in order to improve normality of residuals plots. The back-transformed SD for these variables is not normally distributed and cannot be interpreted directly. Fischer’s exact test was used to examine associations between PDS and body condition, obstetric aid and the possible impact of feeding time relative to parturition (less than or more than 4 h) in the PROC FREQ procedure of Statistic Analytical Software, Enterprise Guide 7.1 (SAS® Institute, Cary, North Carolina, USA). An average weight gain per hour (kg/h) was calculated for each litter. These were compared for PDS+ and PDS- piglets using the PROC MIXED procedure of Statistic Analytical Software, Enterprise Guide 7.1 (SAS® Institute, Cary, North Carolina, USA). The association between parturition duration and PDS was tested by a simple t-test in the PROC TTEST procedure of Statistic Analytical Software, Enterprise Guide 7.1 (SAS® Institute, Cary, North Carolina, USA).
Additional files
Raw data of prolactin (10− 9 g/L) assessed from 60 h ante partum (time interval A) until 36 h post partum (time interval G) in sows suffering from postpartum dysgalactia syndrome (PDS+, red) and healthy sows (PDS-, blue). Each point represents the precise sample time of each observation relative to parturition of piglet number one (0 h). The line represents the mean value at each sampling time point. (DOCX 33 kb)
Raw data of sodium (Na; 10− 3 mol/L) assessed from 60 h ante partum (time interval A) until 36 h post partum (time interval G) in sows suffering from postpartum dysgalactia syndrome (PDS+, red) and healthy sows (PDS-, blue). Each point represents the precise sample time of each observation relative to parturition of piglet number one (0 h). The line represents the mean value at each sampling time point. (DOCX 35 kb)
Raw data of potassium (K; 10− 3 mol/L) assessed from 60 h ante partum (time interval A) until 36 h post partum (time interval G) in sows suffering from postpartum dysgalactia syndrome (PDS+, red) and healthy sows (PDS-, blue). Each point represents the precise sample time of each observation relative to parturition of piglet number one (0 h). The line represents the mean value at each sampling time point. (DOCX 31 kb)
Glucose and C-peptide concentrations obtained in three sows on repeated sampling occasions. (DOCX 14 kb)
Acknowledgments
The authors thank the staff and the vet at the farm Jørn Skov Jensen, Anna Bech and Christian Pårup Nielsen for providing access to the barn and helping with practical work; Karen Bach-Mose, Hanne Præstgaard, Jens-Ove Hansen, Peter Nøddebo Hansen, Kristina Nøddebo Balle, Sarah Mortensen, Peter Hansen, Hanne Nissen are thanked for technical work during data collection; Susana Ros, Josefa Hernandez, Isabel Rodriguez, Claus Sivertsen Stjernegaard, Birgitta Svensmark, Torben Larsen for helping with the laboratory work; Ken Steen Pedersen for professional sparring; Henrik Thoning for parts of the statistical analysis.
Funding
The study was funded by Innovation Fund Denmark (IFD) under File No. 1355-00121 and the Danish Agriculture & Food Council/SEGES, Copenhagen, Denmark. The study design was approved by SEGES and IFD. SEGES contributed with technical support during data collection and statistical analysis.
Availability of data and materials
The corresponding author can provide access to the datasets upon request.
Abbreviations
- 8-epi-PGF2α
8-epi prostaglandin F2 Alpha
- a.p.
Ante partum
- CgA
Chromogranin A
- K
Potassium
- LPS
Lipopolysaccharide
- LSMEANS
Least-squares means
- Na
Sodium
- p.p.
Post partum
- PDS
Postpartum dysgalactia syndrome (PDS+ is cases and PDS- is healthy sows)
- SAM
Sympathico-adrenal medullary system
- SD
Standard deviation
Authors’ contributions
The study was designed by MK, MJ, PHA, PKT, PB and SJ. Data and samples were collected by MK and MJ. JC, DE and MK performed laboratory analyses. MK, JD and SJ performed statistical analyses. MK and MJ drafted the manuscript. All authors interpreted, read, revised, and approved the final manuscript.
Ethics approval and consent to participate
The experimental protocol was approved in advance by the Danish Animal Experiments Inspectorate. All procedures were carried out in agreement with the Danish Animal Testing Act and approved by the Danish Animal Experiments Inspectorate. The animals originated from a private owner, who gave his written consent for the use of the animals for scientific purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marianne Kaiser, Email: marianne.kaiser@outlook.dk.
Stine Jacobsen, Email: stj@sund.ku.dk.
Pia Haubro Andersen, Email: pia.haubro.andersen@slu.se.
Poul Bækbo, Email: pb@seges.dk.
José Joaquin Cerón, Email: jjceron@um.es.
Jan Dahl, Email: jd@lf.dk.
Damián Escribano, Email: det20165@um.es.
Peter Kappel Theil, Email: peter.theil@anis.au.dk.
Magdalena Jacobson, Email: Magdalena.Jacobson@slu.se.
References
- 1.Papadopoulos GA, Vanderhaeghe C, Janssens GPJ, Dewulf J, Maes DGD. Risk factors associated with postpartum dysgalactia syndrome in sows. Vet J. 2010;184(2):167–171. doi: 10.1016/j.tvjl.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Preissler R, Gerjets I, Reiners K, Looft H, Kemper N. International Society for Animal Hygiene (ISAH): 2011; Vienna, Austria. 2011. Prevalence of postpartun dysgalactia syndrome in sows; pp. 63–65. [Google Scholar]
- 3.Stiehler T, Heuwieser W, Pfuetzner A, Burfeind O. The course of rectal and vaginal temperature in early postpartum sows. J Swine Health Prod. 2015;23:72–83. doi: 10.1016/j.theriogenology.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Tummaruk P, Pearodwong P. Postparturient disorders and backfat loss in tropical sows associated with parity, farrowing duration and type of antibiotic. Trop Anim Health Prod. 2015;47(8):1457–1464. doi: 10.1007/s11250-015-0883-7. [DOI] [PubMed] [Google Scholar]
- 5.Bell AW, Bauman DE. Adaptations of glucose metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia. 1997;2(3):265–278. doi: 10.1023/A:1026336505343. [DOI] [PubMed] [Google Scholar]
- 6.Collier RJ, McNamara JP, Wallace CR, Dehoff MH. A review of endocrine regulation of metabolism during lactation. J Anim Sci. 1984;59:498–510. doi: 10.2527/jas1984.592498x. [DOI] [PubMed] [Google Scholar]
- 7.Willcox DL, Arthur PG, Hartmann PE, Whitely JL. Perinatal changes in plasma oestradiol-17 beta, cortisol and progesterone and the initiation of lactation in sows. Aust J Biol Sci. 1983;36(2):173–181. doi: 10.1071/BI9830173. [DOI] [PubMed] [Google Scholar]
- 8.Dusza L, Krzymowska H. Plasma prolactin levels in sows during pregnancy, parturition and early lactation. J Reprod Fertil. 1981;61(1):131–134. doi: 10.1530/jrf.0.0610131. [DOI] [PubMed] [Google Scholar]
- 9.Dlamini B. Hormonal control of late pregnancy and parturition in beef cattle and pigs. 1994. [Google Scholar]
- 10.Chen F, Chen B, Guan W, Chen J, Lv Y, Qiao H, Wang C, Zhang Y. Metabolic transition of milk lactose synthesis and up-regulation by AKT1 in sows from late pregnancy to lactation. Cell Biochem Biophys. 2017;75(1):131–138. doi: 10.1007/s12013-016-0778-x. [DOI] [PubMed] [Google Scholar]
- 11.Farmer C, Trottier NL, Dourmad JY. Review: current knowledge on mammary bood flow, mammary uptake of energetic precursors and their effects on sow milk yield. Can J Anim Sci. 2008;88(2):195–204. doi: 10.4141/CJAS07074. [DOI] [Google Scholar]
- 12.Klobasa F, Werhahn E, Butler JE. Composition of sow milk during lactation. J Anim Sci. 1987;64(5):1458–1466. doi: 10.2527/jas1987.6451458x. [DOI] [PubMed] [Google Scholar]
- 13.Munck A, Koritz SB. Studies on the mode of action of glucocorticoids in rats. I. Early effects of cortisol on blood glucose and on glucose entry into muscle, liver and adipose tissue. Biochim Biophys Acta. 1962;57(1):310–317. doi: 10.1016/0006-3002(62)91124-1. [DOI] [PubMed] [Google Scholar]
- 14.Miller WL, Tyrell JB. The adrenal cortex. In: Felig P, Baxter J, Frohman L, editors. Endocrinology and metabolism. 3. New York: McGraw-Hill; 1995. pp. 555–711. [Google Scholar]
- 15.Magnusson U, Fossum C. Numerical variations among blood mononuclear cells during the peripartal period in the gilt. J Veterinary Med Ser B. 1990;37(1–10):459–467. doi: 10.1111/j.1439-0450.1990.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 16.Osterlundh I, Holst H, Magnusson U. Hormonal and immunological changes in blood and mammary secretion in the sow at parturition. Theriogenology. 1998;50(3):465–477. doi: 10.1016/S0093-691X(98)00153-8. [DOI] [PubMed] [Google Scholar]
- 17.Mosnier E, Etienne M, Ramaekers P, Père MC. The metabolic status during the peri partum period affects the voluntary feed intake and the metabolism of the lactating multiparous sow. Livest Sci. 2010;127:127–136. doi: 10.1016/j.livsci.2009.06.023. [DOI] [Google Scholar]
- 18.Escribano D, Fuentes-Rubio M, Ceron JJ. Validation of an automated chemiluminescent immunoassay for salivary cortisol measurements in pigs. J Vet Diagn Investig. 2012;24(5):918–923. doi: 10.1177/1040638712455171. [DOI] [PubMed] [Google Scholar]
- 19.Nachreiner RF, Garcia MC, Ginther OJ. Clinical, hematologic, and blood chemical changes in swine given endotoxin (Escherichia coli) during the immediate postpartum period. Am J Vet Res. 1972;33(12):2489–2499. [PubMed] [Google Scholar]
- 20.Zhu Y, Osterlundh I, Hulten F, Magnusson U. Tumor necrosis factor-alpha, interleukin-6, serum amyloid A, haptoglobin, and cortisol concentrations in sows following intramammary inoculation of Escherichia coli. Am J Vet Res. 2004;65(10):1434–1439. doi: 10.2460/ajvr.2004.65.1434. [DOI] [PubMed] [Google Scholar]
- 21.Klaver J, van Kempen GJM, de Lange PGB, Verstegen MWA, Boer H. Milk composition and daily yield of different milk components as affected by sow condition and lactation/feeding regimen. J Anim Sci. 1981;52(5):1091–1097. doi: 10.2527/jas1981.5251091x. [DOI] [Google Scholar]
- 22.Hansen AV, Lauridsen C, Sorensen MT, Bach Knudsen KE, Theil PK. Effects of nutrient supply, plasma metabolites, and nutritional status of sows during transition on performance in the next lactation. J Anim Sci. 2012;90(2):466–480. doi: 10.2527/jas.2011-3984. [DOI] [PubMed] [Google Scholar]
- 23.Persson A, Pedersen AE, Göransson L, Kuhl W. A long term study of the health status and performance of sows on different feed allowances during late pregnancy. I. Clinical observations, with special reference to agalactia post partum. Acta Vet Scand. 1989;30(1):9–17. doi: 10.1186/BF03548063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliviero C, Heinonen M, Valros A, Halli O, Peltoniemi OA. Effect of the environment on the physiology of the sow during late pregnancy, farrowing and early lactation. Anim Reprod Sci. 2008;105(3–4):365–377. doi: 10.1016/j.anireprosci.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Theil PK, Lauridsen C, Quesnel H. Neonatal piglet survival: impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal. 2014;8(7):1021–1030. doi: 10.1017/S1751731114000950. [DOI] [PubMed] [Google Scholar]
- 26.Verstegen MWA, Moughan PJ, Schrama JW. The lactating sow. Wageningen: Wageningen Pers; 1998. [Google Scholar]
- 27.Quesnel H, Meunier-Salaun MC, Hamard A, Guillemet R, Etienne M, Farmer C, Dourmad JY, Pere MC. Dietary fiber for pregnant sows: influence on sow physiology and performance during lactation. J Anim Sci. 2009;87(2):532–543. doi: 10.2527/jas.2008-1231. [DOI] [PubMed] [Google Scholar]
- 28.Meier JJ, Menge BA, Breuer TGK, Müller CA, Tannapfel A, Uhl W, Schmidt WE, Schrader H. Functional assessment of pancreatic beta-cell area in humans. Diabetes. 2009;58(7):1595. doi: 10.2337/db08-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shuai W, Wei W, Yadong Z, Junling H, Yongxi D, Shaohua Z, Xuenong L, Xuepeng C. The role of insulin C-peptide in the coevolution analyses of the insulin signaling pathway: a hint for its functions. PLoS One. 2012;7(12):e52847. doi: 10.1371/journal.pone.0052847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark PM. Assays for insulin, proinsulin(s) and C-peptide. Ann Clin Biochem. 1999;36(Pt 5):541. doi: 10.1177/000456329903600501. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro E, Tillil H, Rubenstein A, Polonsky K. Peripheral insulin parallels changes in insulin secretion more closely than C-peptide after bolus intravenous glucose administration. J Clin Endocrinol Metab. 1988;67(5):1094–1099. doi: 10.1210/jcem-67-5-1094. [DOI] [PubMed] [Google Scholar]
- 32.Sato F, Kanno T, Nagasawa S, Yanaihara N, Ishida N, Hasegawa T, Iwanaga T. Immunohistochemical localization of chromogranin A in the acinar cells of equine salivary glands contrasts with rodent glands. Cells Tissues Organs. 2002;172(1):29–36. doi: 10.1159/000064389. [DOI] [PubMed] [Google Scholar]
- 33.Saruta J, Tsukinoki K, Sasaguri K, Ishii H, Yasuda M, Osamura YR, Watanabe Y, Sato S. Expression and localization of chromogranin A gene and protein in human submandibular gland. Cells Tissues Organs. 2005;180(4):237–244. doi: 10.1159/000088939. [DOI] [PubMed] [Google Scholar]
- 34.Helle KB, Corti A. Chromogranin A: a paradoxical player in angiogenesis and vascular biology. Cell Mol Life Sci. 2015;72(2):339–348. doi: 10.1007/s00018-014-1750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nobels FRE, Kwekkeboom DJ, Coopmans W, Schoenmakers CHH, Lindemans J, De Herder WW, Krenning EP, Bouillon R, Lamberts SWJ. Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the α-subunit of glycoprotein hormones. J Clin Endocrinol Metab. 1997;82(8):2622–2628. doi: 10.1210/jcem.82.8.4145. [DOI] [PubMed] [Google Scholar]
- 36.Bishop AE, Facer P, Lloyd RV, Wilson BS, Polak JM. Chromogranin A as a marker for the endocrine cells of human gut. Regul Pept. 1984;9(4):326. doi: 10.1016/0167-0115(84)90098-3. [DOI] [Google Scholar]
- 37.Facer P, Bishop AE, Lloyd RV, Wilson BS, Hennessy RJ, Polak JM. Chromogranin: a newly recognized marker for endocrine cells of the human gastrointestinal tract. Gastroenterology. 1985;89(6):1366–1373. doi: 10.1016/0016-5085(85)90657-2. [DOI] [PubMed] [Google Scholar]
- 38.Castoldi G, Antolini L, Bombardi C, Perego L, Mariani P, Viganò MR, Torti G, Casati M, Corti A, Zerbini G, et al. Oxidative stress biomarkers and chromogranin A in uremic patients: effects of dialytic treatment. Clin Biochem. 2010;43(18):1387–1392. doi: 10.1016/j.clinbiochem.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 39.Kingham PJ, Cuzner ML, Pocock JM. Apoptotic pathways mobilized in microglia and neurones as a consequence of chromogranin A-induced microglial activation. J Neurochem. 1999;73(2):538–547. doi: 10.1046/j.1471-4159.1999.0730538.x. [DOI] [PubMed] [Google Scholar]
- 40.Escribano D, Soler L, Gutierrez AM, Martinez-Subiela S, Ceron JJ. Measurement of chromogranin A in porcine saliva: validation of a time-resolved immunofluorometric assay and evaluation of its application as a marker of acute stress. Animal. 2013;7(4):640–647. doi: 10.1017/S1751731112002005. [DOI] [PubMed] [Google Scholar]
- 41.Erve v ‘t, TJ LFB, Kadiiska MB, Deterding LJ, Eling TE, Mason RP. Reinterpreting the best biomarker of oxidative stress: the 8-iso-PGF2α/PGF2α ratio distinguishes chemical from enzymatic lipid peroxidation. Free Radic Biol Med. 2015;83:245–251. doi: 10.1016/j.freeradbiomed.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diplock AT, Charleux JL, Crozier-Willi G, Kok FJ, Rice-Evans C, Roberfroid M, Stahl W, Vina-Ribes J. Functional food science and defence against reactive oxidative species. Br J Nutr. 1998;80(Suppl 1):S77–112. doi: 10.1079/BJN19980106. [DOI] [PubMed] [Google Scholar]
- 43.Wuryastuti H, Stowe HD, Bull RW, Miller ER. Effects of vitamin E and selenium on immune responses of peripheral blood, colostrum, and milk leukocytes of sows. J Anim Sci. 1993;71(9):2464–2472. doi: 10.2527/1993.7192464x. [DOI] [PubMed] [Google Scholar]
- 44.Mahan DC. Assessment of the influence of dietary vitamin E on sows and offspring in three parities: reproductive performance, tissue tocopherol, and effects on progeny. J Anim Sci. 1991;69(7):2904–2917. doi: 10.2527/1991.6972904x. [DOI] [PubMed] [Google Scholar]
- 45.Preissler R, Tetens J, Reiners K, Looft H, Kemper N. International Society for Animal Hygiene (ISAH): 2011; Vienna, Austria. 2011. Biological pathway analysis of postpartum dysgalactia syndrome in sows via a genome-wide association study; pp. 67–69. [Google Scholar]
- 46.CP M, G B. Acute phase proteins, serum cortisol and prewearing litter performance in sows suffering from periparturient disease. Acta Vet (Beograd) 2004;54(2–3):153–161. [Google Scholar]
- 47.Persson A. Mastitis in sows. Clinical, bacteriological and cytological examinations in assessing udder health during early lactation and at weaning. 1997. [Google Scholar]
- 48.Osterlundh I, Hulten F, Johannisson A, Magnusson U. Sows intramammarily inoculated with Escherichia coli at parturition: I. Functional capacity of granulocytes in sows affected or non-affected by clinical mastitis. Vet Immunol Immunopathol. 2002;90(1–2):35–44. doi: 10.1016/S0165-2427(02)00204-0. [DOI] [PubMed] [Google Scholar]
- 49.Göransson L. The effect of dietary crude fibre content on the frequency of post partum agalactia in the sow. Zentralbl Veterinarmed A. 1989;36(6):474–479. doi: 10.1111/j.1439-0442.1989.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 50.Backstrom L, Morkoc AC, Connor J, Larson R, Price W. Clinical study of mastitis-metritis-agalactia in sows in Illinois. J Am Vet Med Assoc. 1984;185(1):70–73. [PubMed] [Google Scholar]
- 51.Gerjets I, Traulsen I, Reiners K, Kemper N. Comparison of virulence gene profiles of Escherichia coli isolates from sows with coliform mastitis and healthy sows. Vet Microbiol. 2011;152(3–4):361–367. doi: 10.1016/j.vetmic.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Maes D, Papadopoulos G, Cools A, Janssens GPJ. Postpartum dysgalactia in sows: pathophysiology and risk factors. Tierarztl Prax. 2010;38(Suppl 1):15–20. [Google Scholar]
- 53.Gerjets I, Kemper N. Coliform mastitis in sows: a review. J Swine Health Prod. 2009;17(2):97–105. [Google Scholar]
- 54.Martineau GP. Postpartum dysgalactia syndrome and mastitis in sows. In: Aiello SE, editor. The Merck Veterinary Manual. 8. New Jersey: Merck & Co., Inc; 1998. pp. 1020–1024. [Google Scholar]
- 55.Kaiser M, Jacobson M, Andersen PH, Bækbo P, Cerón JJ, Dahl J, Escribano D, Jacobsen S. Inflammatory markers before and after farrowing in healthy sows and in sows affected with postpartum dysgalactia syndrome. BMC Vet Res. 2018;14(1):83. doi: 10.1186/s12917-018-1382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Theil PK, Sørensen MT, Lauridsen C, Olaf M, Nielsen BM. Nutritional Physiology of Pigs - With emphasis on Danish production conditions. Copenhagen: Danish Pig Research Centre; 2012. Lactation, milk and suckling; pp. 1–47. [Google Scholar]
- 57.Jorsal SE. The International Pig Veterinary Society Congress: 1986; Barcelona. 1986. Epidemiology of the MMA-syndrom. A field survey in Danish sow herds; p. 93. [Google Scholar]
- 58.Gerjets I, Traulsen I, Reiners K, Kemper N. Assessing individual sow risk factors for coliform mastitis: a case-control study. Prev Vet Med. 2011;100(3–4):248–251. doi: 10.1016/j.prevetmed.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 59.Bará MR, Cameron RDA. International Pig Veterinary Society Congress: 7–10 July 1996; Bologna, Italy. 1996. The effect of faecal accumulation in farrowing crates and hand farrowing on the incidence of post-farrowing discharges and reproductive performance in sows; p. 574. [Google Scholar]
- 60.Amico MA, Ghinassi B, Izzicupo P, Manzoli L, Di Baldassarre A. Biological function and clinical relevance of chromogranin A and derived peptides. Endocr Connect. 2014;3(2):R45–R54. doi: 10.1530/EC-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chatterton RT, Jr, Vogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin Physiol (Oxford, England) 1996;16(4):433–448. doi: 10.1111/j.1475-097X.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 62.Allgrove JE, Gomes E, Hough J, Gleeson M. Effects of exercise intensity on salivary antimicrobial proteins and markers of stress in active men. J Sports Sci. 2008;26(6):653–661. doi: 10.1080/02640410701716790. [DOI] [PubMed] [Google Scholar]
- 63.Kawada S, Fukusaki C, Ohtani M, Kobayashi K. Effects of hyperoxic inhalation on psychological stress-induced salivary biomarkers. Biomed Res (Tokyo, Japan) 2009;30(4):245–249. doi: 10.2220/biomedres.30.245. [DOI] [PubMed] [Google Scholar]
- 64.Portela-Gomes GM, Stridsberg M, Johansson H, Grimelius L. Complex co-localization of chromogranins and neurohormones in the human gastrointestinal tract. J Histochem Cytochem. 1997;45(6):815–822. doi: 10.1177/002215549704500606. [DOI] [PubMed] [Google Scholar]
- 65.Shen Z, Larsson LT, Malmfors G, Oberg K, Eriksson B, Sundler F. Chromogranin A and B in neuronal elements in Hirschsprung’s disease: an immunocytochemical and radioimmunoassay study. J Pediatr Surg. 1994;29(10):1293–1301. doi: 10.1016/0022-3468(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 66.Ghia J-E, Crenner F, Rohr S, Meyer C, Metz-Boutigue M-H, Aunis D, Angel F. A role for chromogranin A (4–16), a vasostatin-derived peptide, on human colonic motility. An in vitro study. Regul Pept. 2004;121(1):31–39. doi: 10.1016/j.regpep.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 67.Martineau GP, Le Treut Y, Guillou D, Waret-Szkuta A. Postpartum dysgalactia syndrome: a simple change in homeorhesis? J Swine Health Prod. 2013;21(2):85–93. [Google Scholar]
- 68.Libetta C, Sepe V, Esposito P, Galli F, Dal Canton A. Oxidative stress and inflammation: implications in uremia and hemodialysis. Clin Biochem. 2011;44(14–15):1189–1198. doi: 10.1016/j.clinbiochem.2011.06.988. [DOI] [PubMed] [Google Scholar]
- 69.Aizawa T, Ishizaka N, Usui S-I, Ohashi N, Ohno M, Nagai R. Angiotensin II and catecholamines increase plasma levels of 8-Epi-prostaglandin F2α with different pressor dependencies in rats. Hypertension. 2002;39(1):149–154. doi: 10.1161/hy1201.097301. [DOI] [PubMed] [Google Scholar]
- 70.Pére MC, Etienne M. Insulin sensitivity during pregnancy, lactation, and postweaning in primiparous gilts. J Anim Sci. 2007;85(1):101–110. doi: 10.2527/jas.2006-130. [DOI] [PubMed] [Google Scholar]
- 71.Baer C, Bilkei G. Ultrasonographic and gross pathological findings in the mammary glands of weaned sows having suffered recidiving mastitis metritis agalactia. Reprod Domest Anim. 2005;40(6):544–547. doi: 10.1111/j.1439-0531.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- 72.Warade JP, Pandey A. Fasting blood glucose level higher than post-meal in healthy subjects: a study of 738 subjects. World J Pharm Res. 2014;3(4):1121–1128. [Google Scholar]
- 73.Valros A, Rundgren M, Špinka M, Saloniemi H, Rydhmer L, Hultén F, Uvnäs-Moberg K, Tománek M, Krejcí P, Algers B. Metabolic state of the sow, nursing behaviour and milk production. Livest Prod Sci. 2003;79(2–3):155–167. doi: 10.1016/S0301-6226(02)00154-9. [DOI] [Google Scholar]
- 74.Feyera T, Pedersen TF, Krogh U, Foldager L, Theil PK. Impact of sow energy status during farrowing on farrowing kinetics, frequency of stillborn piglets and farrowing assistance. J Anim Sci. 2018;96:2320. doi: 10.1093/jas/sky141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Threlfall WR, Dale HE, Martin CE. Serum and adenohypophyseal concentrations of prolactin in sows affected with agalactia. Am J Vet Res. 1974;35(2):313–315. [PubMed] [Google Scholar]
- 76.Smith BB, Wagner WC. Effect of Escherichia coli endotoxin and thyrotropin-releasing hormone on prolactine in lactating sows. Am J Vet Res. 1985;46(1):175–180. [PubMed] [Google Scholar]
- 77.Sørensen G. SE-number: 3419 9329. vsp-info@seges.dk: SEGES, Axelborg, Axeltorv 3, 1609 Copenhagen V. 2013. Huldvurdering af søer. [Google Scholar]
- 78.Jensen-Waern M, Andersson M, Kruse R, Nilsson B, Larsson R, Korsgren O, Essén-Gustavsson B. Effects of streptozotocin-induced diabetes in domestic pigs with focus on the amino acid metabolism. Lab Anim. 2009;43:249–254. doi: 10.1258/la.2008.008069. [DOI] [PubMed] [Google Scholar]
- 79.Roy AK, Singh M, Kumar P, Kumar BS. Effect of extended photoperiod during winter on growth and onset of puberty in Murrah buffalo heifers. Vet World. 2016;9(2):216–221. doi: 10.14202/vetworld.2016.216-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nalin L, Selvaraju RK, Velikyan I, Berglund M, Andréasson S, Wikstrand A, Rydén A, Lubberink M, Kandeel F, Nyman G, et al. Positron emission tomography imaging of the glucagon-like peptide-1 receptor in healthy and streptozotocin-induced diabetic pigs. Eur J Nucl Med Mol Imaging. 2014;41(9):1800–1810. doi: 10.1007/s00259-014-2745-3. [DOI] [PubMed] [Google Scholar]
- 81.Manell EAK, Rydén A, Hedenqvist P, Jacobson M, Jensen-Waern M. Insulin treatment of streptozotocin-induced diabetes re-establishes the patterns in carbohydrate, fat and amino acid metabolisms in growing pigs. Lab Anim. 2014;48(3):261–269. doi: 10.1177/0023677213517683. [DOI] [PubMed] [Google Scholar]
- 82.Provo N, Englund H, Gunnarsson R. Development and evaluation of a novel porcine C-peptide specific ELISA. 2011. [Google Scholar]
- 83.Haxhi J, Leto G, Di Palumbo AS, Sbriccoli P, Guidetti L, Fantini C, Buzzetti R, Caporossi D, Di Luigi L, Sacchetti M. Exercise at lunchtime: effect on glycemic control and oxidative stress in middle-aged men with type 2 diabetes. Eur J Appl Physiol. 2016;116(3):573–582. doi: 10.1007/s00421-015-3317-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data of prolactin (10− 9 g/L) assessed from 60 h ante partum (time interval A) until 36 h post partum (time interval G) in sows suffering from postpartum dysgalactia syndrome (PDS+, red) and healthy sows (PDS-, blue). Each point represents the precise sample time of each observation relative to parturition of piglet number one (0 h). The line represents the mean value at each sampling time point. (DOCX 33 kb)
Raw data of sodium (Na; 10− 3 mol/L) assessed from 60 h ante partum (time interval A) until 36 h post partum (time interval G) in sows suffering from postpartum dysgalactia syndrome (PDS+, red) and healthy sows (PDS-, blue). Each point represents the precise sample time of each observation relative to parturition of piglet number one (0 h). The line represents the mean value at each sampling time point. (DOCX 35 kb)
Raw data of potassium (K; 10− 3 mol/L) assessed from 60 h ante partum (time interval A) until 36 h post partum (time interval G) in sows suffering from postpartum dysgalactia syndrome (PDS+, red) and healthy sows (PDS-, blue). Each point represents the precise sample time of each observation relative to parturition of piglet number one (0 h). The line represents the mean value at each sampling time point. (DOCX 31 kb)
Glucose and C-peptide concentrations obtained in three sows on repeated sampling occasions. (DOCX 14 kb)
Data Availability Statement
The corresponding author can provide access to the datasets upon request.



