Abstract
Acute respiratory distress syndrome (ARDS) is a life-threatening medical emergency. The etiology of ARDS can involve various causes. ARDS associated with the use of iodinated contrast media is rarely reported, and the literature includes only one case of ARDS due to gadobutrol. A 46-year-old female patient presented to our emergency department with shortness of breath, wheezing, swelling of the lips, and difficulty swallowing about 30 minutes after undergoing magnetic resonance imaging with 6.5 ml (0.1 ml/kg) gadobutrol (Gadovist) contrast for a submandibular mass. She was treated for anaphylaxis, then immediately evaluated using chest x-ray and arterial blood gas analysis. Based on the findings, she was diagnosed with ARDS and started on continuous positive airway pressure (CPAP) ventilatory support and methylprednisolone at a dose of 1 mg/kg/day. On day 3 of follow-up, all symptoms had completely regressed.
Keywords: ARDS, Contrast agent, Gadolinium
1. Introduction
Gadolinium has been in use for approximately 30 years due to its high reliability and low rates of adverse effects. Gadobutrol is a second-generation non-ionic macrocyclic gadolinium-based contrast agent with high thermostability. Having twice higher ion concentration compared to other contrast media enables the acquisition of high-quality images at low doses of it [1].
Adverse reaction due to Gadolinium-based contrast media and also noncardiogenic pulmonary edema following any contrast-media injection is extremely rare. So far, to our best knowledge, two contrast-media induced noncardiogenic edema due to iothalamate meglumine and sodium diatrizoate [2,3], and one acute respiratory distress syndrome (ARDS) due to Gadolinium-based contrast media [4] have been reported. Herein, we present an ARDS case developed following Gadobutrol use.
2. Case report
46-year-old female patient with no known medical history exhibited swelling in the submandibular region and underwent contrast magnetic resonance imaging (MRI) for a submandibular mass. Nothing remarkable was observed in the laboratory tests. Approximately 30 minutes after receiving an injection of 6.5 ml (0.1 ml/kg) gadobutrol (Gadovist, Bayer Inc., Toronto, Canada) during MRI, the patient presented to the emergency department with shortness of breath, wheezing, swelling of the lips, and difficulty swallowing. Her respiratory rate was 28/min, pulse was 130/min (radial, rhythmic), body temperature was 37.6 °C, and blood pressure was 130/75 mmHg. Her oxygen saturation was 58% while breathing room air. Edema of the uvula was noted on physical examination. On auscultation, bilateral rales that were more pronounced in the lung bases were noted. The patient was treated for anaphylaxis with intramuscular injection of 0.5 mg 1/1000 epinephrine and intravenous 5 mg dexamethasone; ventilatory support with 5 l/min oxygen was provided via nasal mask. Arterial blood gas analysis showed pH = 7.34, p02 = 56.8 mmHg, and Pa02/Fi02 = 138.5. Posterior-anterior (PA) x-ray revealed extensive ground glass opacity with areas of consolidation in all zones of both lungs (Fig. 1). In unenhanced chest CT, increased complete and incomplete density was observed in all lobes bilaterally, starting from the hilar region and extending to the periphery, with complete density being more pronounced. Areas of unaffected lung and air bronchograms were observed in the periphery (Fig. 2). The patient was admitted to intensive care with a diagnosis of moderate ARDS. Continuous positive airway pressure (CPAP) was administered at 10 cmH2O and methylprednisolone was initiated at a dose of 1 mg/kg/day. In transthoracic echocardiography, ejection fraction was 60% and left ventricular function was normal. On day 3 of follow-up, oxygen saturation in room air was 92% and near complete regression was observed on PA chest x-ray (Fig. 3).
Fig. 1.
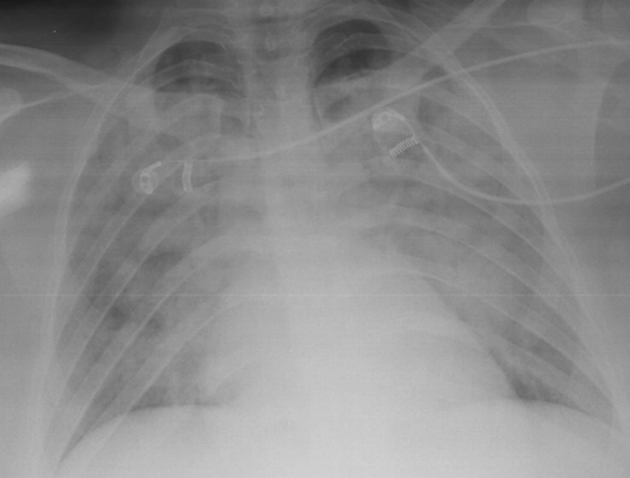
Ground glass opacity with areas of consolidation in all zones of both lungs.
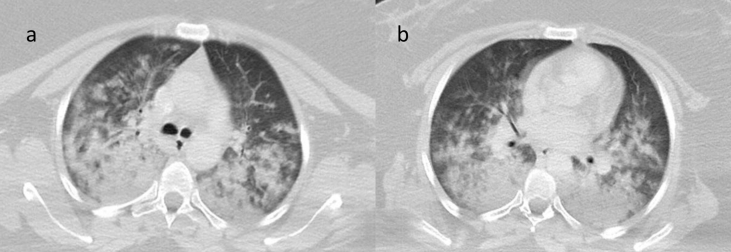
Fig. 2.
Increased complete and incomplete density was observed in all lobes bilaterally (a:upper zone, b: lower zone), starting from the hilar region and extending to the periphery, with complete density being more pronounced. Areas of unaffected lung and air bronchograms were observed in the periphery.
Fig. 3.
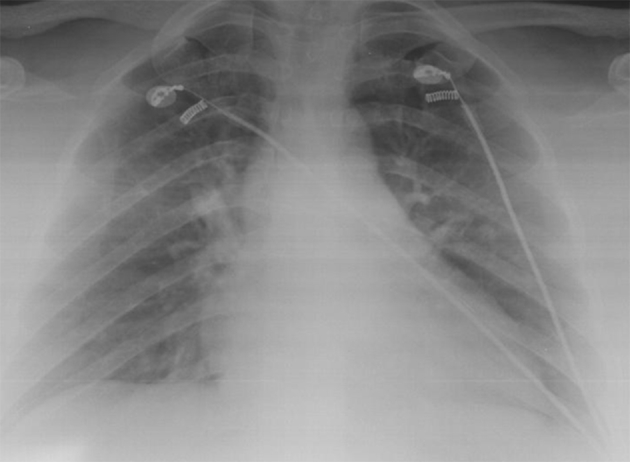
On day 3 of follow-up, near complete regression was observed.
3. Discussion
Previous studies have confirmed that the contrast agent used in MRI causes fewer adverse reactions compared to the contrast agent used in CT. Therefore, MRI is used safely in patients with allergies to iodinated contrast agents [5]. However, as seen in our case, adverse reaction due to use of contrast agent in MRI can be seen.
With the increasing accessibility of imaging devices, the number of MRI procedures performed has risen significantly over the past 10 years. Studies have shown that adverse reactions and anaphylaxis due to gadobutrol, a commonly used contrast agent in MRI, occur at rates of 0.55% and 0.01%, respectively [6]. Minor adverse reactions such as changes in taste and temperature perception have been reported, and very rarely, it can induce nephrogenic systemic fibrosis [7]. In addition to the one previously reported case, ours is the second case of gadobutrol-associated ARDS in the literature.
In drug-induced ARDS, vascular endothelial and epithelial damage caused by the drug leads to the accumulation of protein-rich material in the alveoli, causing hypoxia and a subsequent increase in pulmonary vascular resistance. A complex series of events including neutrophil-mediated damage, cytokine-induced inflammation, oxidant-mediated damage, and dysregulation of the coagulation and fibrinolytic pathways also play a role in the development of ARDS [8,9].
In previous studies on anaphylactic reactions to gadobutrol, which is frequently used in MRI, 82.7% were observed after the first 5 minutes and 95.7% within the first 10 minutes [10]. The presence of dyspnea, uvular edema, and respiratory symptoms with wheezing in our patient initially suggested anaphylaxis. However, symptom onset occurred 30 minutes after injection and her blood pressure remained normal after symptoms appeared, which was inconsistent with anaphylaxis. The clinical and radiologic presentation of our patient was similar to previously reported cases of anaphylactoid pulmonary edema associated with the use of ionic iodinated contrast media and ARDS associated with the use of Gadobutrol [[2], [3], [4]]. To detect these rare complications of gadolinium use, a 1:10 dilution of gadobutrol is injected intradermally and tests for IgE-mediated allergy to gadolinium contrast agent is performed. However, the positive and negative predictive value of these tests for ARDS development has not been determined [11].
Consistent with a previously reported case of gadobutrol-associated ARDS, our patient had no known history of allergy to any drug. In the previous case [4], the patient has undergone abdominal CT in the emergency department after presenting with abdominal pain. CT imaging has revealed an ovarian cyst, and contrast-enhanced abdominal MRI has been performed using gadobutrol (7.5 ml) 83 hours later. Fifty minutes after MRI, the patient has exhibited symptoms similar to those in our case, and has been evaluated as having severe ARDS and supported with mechanical ventilation. In the case presented here, the patient first encountered the contrast agent during MRI for a submandibular mass. Onset of the adverse reaction occurred 30 minutes after injection. The amount of contrast agent administered was lower than in the other case (6.5 ml). She was diagnosed with moderate ARDS and supported with CPAP.
The use of contrast-enhanced MRI has increased in recent years due to greater accessibility and growing need. Despite being considered a safer option in terms of contrast material-induced allergic reactions, we present this case to highlight the fact that gadolinium can lead to life-threatening conditions such as ARDS and should be used with caution.
Conflict of interest
All authors disclosure no Conflict of Interest.
References
- 1.Scott L.J. Gadobutrol: a review of its use for contrast-enhanced magnetic resonance imaging in adults and children. Clin. Drug Investig. 2013;33(4):303–314. doi: 10.1007/s40261-013-0066-0. [DOI] [PubMed] [Google Scholar]
- 2.Boden W.E. Anaphylactoid pulmonary edema (“shock lung”) and hypotension after radiologic contrast media injection. Chest. 1982;81(6):759–761. doi: 10.1378/chest.81.6.759. [DOI] [PubMed] [Google Scholar]
- 3.Solomon D.R. Anaphylactoid reaction and non-cardiac pulmonary edema following intravenous contrast injection. Am. J. Emerg. Med. 1986;4(2):146–149. doi: 10.1016/0735-6757(86)90161-0. [DOI] [PubMed] [Google Scholar]
- 4.Park J., Byun I.H., Park K.H., Lee J.-H., Nam E.J., Park J.-W. Acute respiratory distress syndrome after the use of gadolinium contrast media. Yonsei Med. J. 2015;56(4):1155–1157. doi: 10.3349/ymj.2015.56.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brockow K., Sánchez-Borges M. Hypersensitivity to contrast media and dyes. Immunol. Allergy Clin. 2014;34(3):547–564. doi: 10.1016/j.iac.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Herborn C.U., Honold E., Wolf M., Kemper J., Kinner S., Adam G., Barkhausen J. Clinical safety and diagnostic value of the gadolinium chelate gadoterate meglumine (Gd-DOTA) Investig. Radiol. 2007;42(1):58–62. doi: 10.1097/01.rli.0000248893.01067.e5. [DOI] [PubMed] [Google Scholar]
- 7.Nicholas B., Vricella G.J., Smith M., Passalacqua M., Gulani V., Ponsky L.E. Contrast-induced nephropathy and nephrogenic systemic fibrosis: minimizing the risk. Can. J. Urol. 2012;19(1):6074–6080. [PubMed] [Google Scholar]
- 8.Hussain M., Xu C., Ahmad M., Majeed A., Lu M., Wu X., Tang L., Wu X. Clinical Pharmacology & Therapeutics; 2018. Acute Respiratory Distress Syndrome: Bench‐to‐Bedside Approaches to Improve Drug Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerget B., Araz O., Ucar E.Y., Akgun M., Sağlam L. Acute respiratory distress syndrome; A rare complication caused by usage of ruxolitinib. Respir. Med. Case Rep. 2017;22:243–245. doi: 10.1016/j.rmcr.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsting M., Palkowitsch P. Prevalence of acute adverse reactions to gadobutrol—a highly concentrated macrocyclic gadolinium chelate: review of 14,299 patients from observational trials. Eur. J. Radiol. 2010;74(3):e186–e192. doi: 10.1016/j.ejrad.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Hasdenteufel F., Luyasu S., Renaudin J.-M., Paquay J.-L., Carbutti G., Beaudouin E., Moneret-Vautrin D.A., Kanny G. Anaphylactic shock after first exposure to gadoterate meglumine: two case reports documented by positive allergy assessment. J. Allergy Clin. Immunol. 2008;121(2):527–528. doi: 10.1016/j.jaci.2007.08.027. [DOI] [PubMed] [Google Scholar]