A stereoselective synthesis of (2)-tetrahydrolipstatin has been accomplished utilizing olefin metathesis of an acrylate ester as the key step.
Tetrahydrolipstatin 1, a member of the lipstatin class of blactone microbial agents, is a potent and irreversible inhibitor of pancreatic lipase.1 The lipase enzyme is responsible for the digestion of fat in the diet of humans.2 The strained b-lactone functionality of 1 is critical to its lipase inhibitory properties. The inactivation mechanism involves an irreversible acylation of the active site serine residue of pancreatic lipase by the blactone moiety.3 Recent clinical studies have revealed that treatment with 1 along with diet modifications have led to sustained weight loss in humans.4 Indeed, Hoffman-La Roche Laboratories have now introduced (2)-tetrahydrolipstatin under the trade name Xenical® as an anti-obesity agent. The important biological properties along with its unique structural features have stimulated interest in the synthesis of 1 and its structural variants.5 Herein we report an asymmetric synthesis of (2)-tetrahydrolipstatin. The key synthetic strategy involves a stereoselective construction of a syn-1,3-diol synthon by olefin metathesis, stereoselective epoxidation and regioselective epoxide reduction followed by its elaboration to 1.
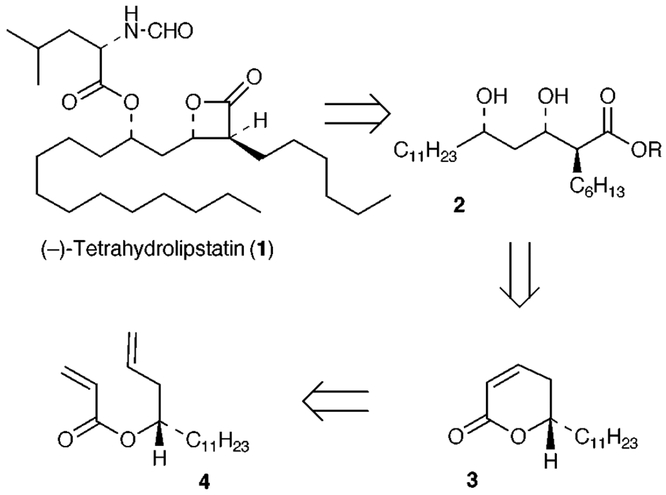
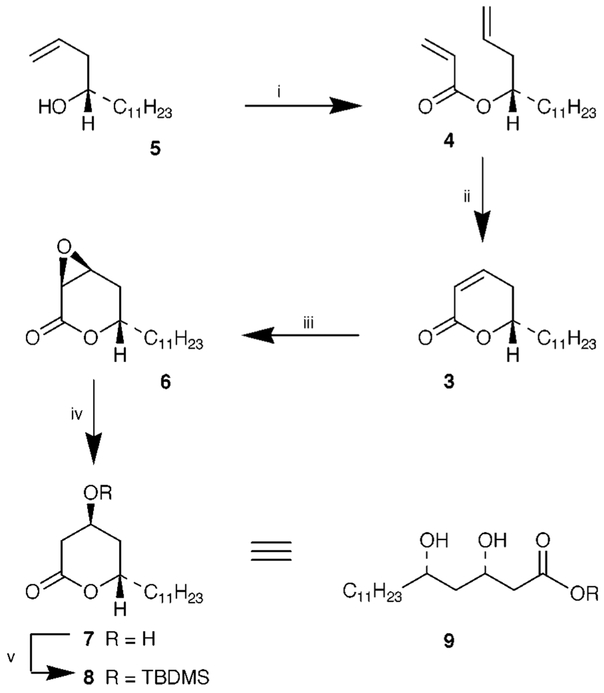
As depicted in Scheme 1, we planned to construct the blactone ring from the corresponding β-hydroxy acid derivative 2. The elaboration of the syn-1,3-diol functionality and stereoselective introduction of the C-2 alkyl chain in 2 would be achieved from the α, β -unsaturated δ-lactone 3. The intermediate 3 would be derived from ring-closing metathesis of the corresponding acrylate ester 4. Recently, a number of convenient syntheses of various α,β-unsaturated γ- and δ-lactones have been reported involving ring closing metathesis of acrylates utilizing Grubbs’ catalyst.6 The broad synthetic utility of Grubbs’ catalyst is now well established.7 The key starting material, homoallylic alcohol 5 was prepared in multigram quantities by Keck’s enantioselective allylation of dodecanal employing a catalytic amount (10 mol%) of (R)-BINOL and Ti(OPri)4 to furnish 5 in 90% yield.8 The optical purity of the alcohol 5 [92% ee, (c 1.23, CHCl3)] was obtained by formation of the Mosher ester and 19F NMR analysis.9 Reaction of 5 with acryloyl chloride (1.2 equiv.) and Et3N (3 equiv.) in the presence of a catalytic amount of DMAP in CH2Cl2 provided the acrylate ester 4 in 91% yield after silica gel chromatography (Scheme 2). Olefin metathesis of 4 with commercially available Grubbs’ catalyst (10 mol%) in the presence of Ti(OPri)4 (0.3 equiv.) in refluxing CH2Cl2 (0.007 M solution) for 15 h afforded the α,β-unsaturated δ-lactone 3 in 93% yield. Consistent with our earlier report, exposure of acrylate ester 4 to Grubbs’ catalyst (10 mol%) in CH2Cl2 for 15 h in the absence of Ti(OPri)4 resulted in low conversion of lactone 3 (50% by 1H NMR) with a substantial amount of unreacted starting material remaining.6b Epoxidation of lactone 3 was carried out with alkaline H2O2 in MeOH at 23 °C for 1 h. Acidification, extractive work-up followed by azeotropic removal of the water by heating in benzene furnished the epoxide 6 as a single isomer. Epoxidation of 3 proceeded stereoselectively from the less hindered β-face.10 Exposure of epoxide 6 to PhSeSePh and NaBH4 in PriOH at 0 °C in the presence of AcOH resulted in regioselective reduction of the epoxide to afford the β-hydroxy-lactone 7 in 83% yield (from 3) after silica gel chromatography.11 Thus, the sequence of reactions involving olefin metathesis, stereoselective epoxidation and regioselective epoxide reduction constitute an effective protocol for the syn-1,3-diol synthon 9. For introduction of the C-2 alkyl chain, attempted direct alkylation of the β-hydroxylactone 7 under a variety of reaction conditions was unsuccessful. Therefore, the elaboration of the C-2 side chain was carried out by an alternate route using Seebach’s asymmetric alkylation of β-hydroxy esters.12
Scheme 1.
Scheme 2.
Reagents and conditions: i, CH2=CHCOCl, Et3N, DMAP, 23 °C (91%); ii, (PCy3)2Cl2Ru=CHPh (10 mol%), Ti(OPri)4 (0.3 equiv.), CH2Cl2, 40 °C (93%); iii, aq. NaOH, H2O2, 23 °C; iv, PhSeSePh, NaBH4, PriOH, AcOH, 0 °C (83%); v, TBDMSCl, Pri2NEt, DMF, 25 °C (98%).
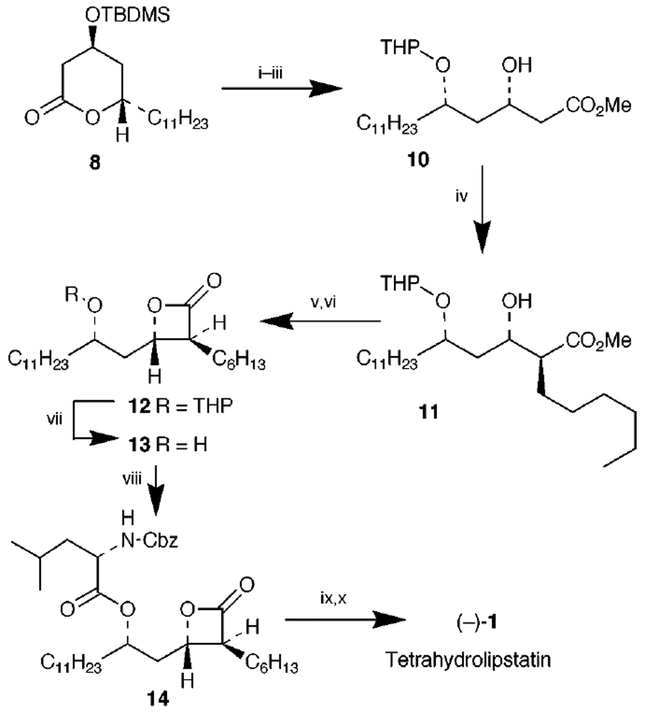
The β-hydroxy lactone 7 was first protected as a TBDMS ether 8 by treatment with TBDMSCl and Pri2NEt in DMF at 23 °C for 12 h. Lactone 8 was converted to β-hydroxy ester 10 in a three step sequence involving (i) opening of the lactone ring by exposure to Et3N in MeOH at 23 °C for 12 h, (ii) protection of the resulting δ-hydroxy methyl ester as THP ether, and (iii) removal of the TBDMS group by treatment with Bu4NF in THF in the presence of AcOH at 23 °C for 5 h (60% from 7). The C(2) hexyl side chain was then introduced by an asymmetric alkylation of the β-hydroxy ester 10 (Scheme 3). Thus, methyl ester 10 was treated with LDA (2.2 equiv.) in the presence of HMPA (5 equiv.) in THF at −78 °C and the reaction mixture was warmed to −50 °C for 2 h. The resulting dianion was cooled to −78 °C and reacted with hexyl iodide (2 equiv.) at −78 to 0 °C for 6 h to afford the alkylated product 11 in 85% yield (based upon 30% recovery of starting material). The removal of the THP ether group in 11 revealed excellent diastereoselectivity (ratio 22:1 by 13C NMR).13 The stereochemical course of such alkylation processes has been well-established previously.12
Scheme 3.
Reagents and conditions: i, Et3N, MeOH, 23 °C, 12 h (75%); ii, DHP, PPTS, 8 h; iii, Bu4NF, THF, AcOH, 25 °C, 5 h (60% from 7); iv, LDA, HMPA, C6H13I, THF, −78 to 0 °C, 6 h (70% conversion, 85%); v, aq. LiOH, 25 °C, 12 h, H+; vi, PhSO2Cl, Py, 0 °C, 8 h (84% from 11); vii, PPTS, EtOH, reflux, 3 h (90%); viii, Cbz-Leu, DCC, DMAP (95%); ix, H2, Pd-C, 12 h; x, AcOCHO, THF, 25 °C, (87%).
Saponification of ester 11 with aqueous LiOH followed by exposure of the resulting acid to PhSO2Cl in pyridine at 0 °C for 8 h, as described by Barbier and Schneider, afforded the β-lactone 12 in 84% yield (from 11).5k The removal of the THP group by treatment with PPTS in EtOH at reflux furnished the (5S)-hydroxy β-lactone 13 [ (c 1.2, CHCl3)] as a single isomer. Attempted esterification with N-formylleucine under a variety of conditions failed to provide satisfactory results. To complete the synthesis, N-formylleucine was introduced by an alternate protocol as described by Uskokovic et al.5g Esterification of 13 with Cbz-leu and DCC in the presence of DMAP provided the Cbz derivative 14 in 95% yield.14 Catalytic hydrogenation of 14 over 10% Pd-C followed by N-formylation of the resulting amine with formic acetic anhydride in THF at 23 °C for 1 h furnished the synthetic (2)-tetrahydrolipstatin 1 [ (c 1.4, CHCl3); lit.,1 , (c 1, CHCl3)]. Spectral data (IR and 400 MHz 1H NMR) for the synthetic tetrahydrolipstatin are identical to those reported for the natural product.1
In summary, an asymmetric synthesis of (—)-tetrahydrolipstatin has been accomplished. A number of key features of this synthesis are noteworthy; a Keck enantioselective allylation of dodecanal, olefin metathesis of an acrylate ester to an unsaturated δ-lactone, elaboration of this lactone to a syn-1,3-diol synthon and Seebach’s asymmetric alkylation of a β-hydroxy ester.
Acknowledgments
Financial support for this work was provided by the National Institutes of Health (GM 55600).
Notes and references
- 1.Weibel EK, Hadvary P, Hochuli E, Kupfer E and Lengsfeld H,J. Antibiot, 1987, 40, 1081; [DOI] [PubMed] [Google Scholar]; Hadvary P, Lengsfeld H and Wolfer H, Biochem. J, 1988, 256, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogan S, Fleury A, Hadvary P, Lengsfeld H, Meier MK, Triscari J and Sullivan AC, Int. J. Obes, 1987, 11, 35 (suppl. 3). [PubMed] [Google Scholar]
- 3.Hadvary P, Sidler W, Meister W, Vetter W and Wolfer H, J. Biol. Chem, 1991, 266, 2021. [PubMed] [Google Scholar]
- 4.Drent ML and van der Veen EA, Obes. Res, 1995, 3 (suppl. 4), 623S. [DOI] [PubMed] [Google Scholar]
- 5.(a) Paterson I and Doughty VA, Tetrahedron Lett, 1999, 40, 393; [Google Scholar]; (b) Fleming I and Lawrence NJ, J. Chem. Soc., Perkin Trans 1,, 1998, 2679; [Google Scholar]; (c) Giese B and Roth MJ, J. Braz. Chem. Soc, 1996, 7, 243; [Google Scholar]; (d) Pommier A, Pons JM, Kocienski PJ and Wong L, Synthesis, 1994, 1294; [Google Scholar]; (e) Hanessian S, Tehim A and Chen P, J. Org. Chem, 1993, 58, 7768; [Google Scholar]; (f) Case-Green SC, Davies SG and Hedgecock CJR, Synlett, 1991, 781; [Google Scholar]; (g) Chadha NK, Batcho AD, Tang PC, Courtney LF, Cook CM, Wovkulich PM and Uskokovic MR, J. Org. Chem, 1991, 56, 4714; [Google Scholar]; (h) Fleming I and Lawrence NJ, Tetrahedron Lett, 1990, 31, 3645; [Google Scholar]; (i) Pons J and Kocienski PJ, Tetrahedron Lett, 1989, 30, 1833; [Google Scholar]; (j) Barbier P and Schneider F, J. Org. Chem, 1988, 53, 1218; [Google Scholar]; (k) Barbier P and Schneider F, Helv. Chim. Acta, 1987, 70, 196; [Google Scholar]; (l) Barbier P, Schneider F and Widmer U, Helv. Chim. Acta, 1987, 70, 1412. [Google Scholar]
- 6.(a) Nicolaou KC, Rodriguez RM, Mitchell HJ and van Delft FL, Angew. Chem., Int. Ed, 1998, 37, 1874; [Google Scholar]; (b) Ghosh AK, Cappiello J and Shin D, Tetrahedron Lett, 1998, 39, 4651; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cossy J, Bauer D and Bellosta V, Tetrahedron Lett, 1999, 40, 4187 and references cited therein. [Google Scholar]
- 7.Wright DL, Curr. Org. Chem, 1999, 3, 211;Grubbs RH and Chang S, Tetrahedron, 1998, 54, 4413;Amstrong SK, J. Chem. Soc., Perkin Trans 1, 1998, 371;Schuster M and Blechert S, Angew. Chem., Int. Ed. Engl, 1997, 36, 2037 and references cited therein.For recent reviews, see:
- 8.Keck GE, Tarbet KH and Geraci LS, J. Am. Chem. Soc, 1993, 115, 8467; Keck GE and Krishnamurthy D, Org. Synth, 1997, 75, 12. [Google Scholar]
- 9.Dale JA, Dull DL and Mosher HS, J. Org. Chem, 1969, 34, 2543. [Google Scholar]
- 10.Takano S, Shimazaki Y, Sekiguchi Y and Ogasawara K, Synthesis, 1989, 539. [Google Scholar]
- 11.Miyashita M, Suzuki T and Yoshikoshi A, Tetrahedron Lett., 1987, 28, 4293; [Google Scholar]; Miyashita M, Hoshino M, Suzuki T and Yoshikoshi A, Chem. Lett, 1988, 507. [Google Scholar]
- 12.Seebach D, Aebi J and Wasmuth D, Org. Synth, 1985, 63, 109. [Google Scholar]
- 13.Alkylation of the corresponding b-hydroxy ester containing an anti-dbenzyloxy group proceeded with excellent diastereoselectivity (40+1). [ref. 5(e)]. Thus, the stereochemistry of the remote alkoxy group has little influence on the stereochemical outcome of this alkylation process.
- 14.All new compounds gave satisfactory spectroscopic and analytical results.