Abstract
Nanoparticle delivery systems offer advantages over free drugs, in that they increase solubility and biocompatibility. Nanoparticles can deliver a high payload of therapeutic molecules while limiting off target side effects. Therefore, delivery of an existing drug with a nanoparticle frequently results in an increased therapeutic index. Whether of synthetic or biologic origin, nanoparticle surface coatings are often required to reduce immune clearance and thereby increase circulation times allowing the carriers to reach their target site. To this end, polyethylene glycol (PEG) has long been used, with several PEGylated products reaching clinical use. Unfortunately, the growing use of PEG in consumer products has led to an increasing prevalence of PEG-specific antibodies in the human population, which in turn has fueled the search for alternative coating strategies. This review highlights alternative bio-inspired nanoparticle shielding strategies, which may be more beneficial moving forward than PEG and other synthetic polymer coatings.
Keywords: nanoparticles, shielding, coatings, anti-fouling, PEG, bio-inspired
Graphical Abstract
Introduction
Nanoparticles have potential for drug delivery by solubilizing hydrophobic drug molecules and allowing for targeted delivery of toxic compounds, therefore limiting off-target effects. They offer hope for new therapies and provide opportunities to revitalize drugs taken off the market due to toxicity. Nanoparticles of diverse materials can be produced in many different shapes and sizes, and can be loaded with drugs, contrast agents, targeting ligands, and combinations thereof. One of the many advantages that nanoparticle engineering provides is the possibility of tuning the carrier to a specific biological problem. Nanoparticle research has rapidly grown in recent years, with over 21,000 papers published in 2017 alone. While the development pipeline is moving rapidly, many nanoparticle formulations fail to translate to the clinic.
Engineered nanoparticles face numerous biological barriers upon administration (reviewed in1). One of the first challenges is avoiding recognition and clearance by the immune system. Nanoparticles associate with serum proteins and other molecules (termed opsonization), leading to formation of a ‘protein corona’ which alters their in vivo properties.2–3 This corona is more prominent for synthetic nanoparticles than proteinaceous viral-based nanocarriers, but in both cases the corona can include immune proteins such as immunoglobulins and complement proteins.4–5 These proteins tag nanoparticles for clearance by phagocytic cells of the mononuclear phagocyte system (MPS), thereby preventing nanocarriers from ever reaching their target sites. Once enveloped with a protein corona, nanoparticles are sequestered in MPS organs, including the liver, spleen, and kidney.6 These interactions with the innate immune system can also trigger the adaptive immune system, which can then lead to the production of neutralizing antibodies and/or carrier-specific cellular immune responses, which can be especially challenging for nanotherapeutic or imaging strategies that require repeat administrations. Presence of carrier specific antibodies leads to accelerated blood clearance (ABC) of the nanoparticle and increased accumulation in organs such as the liver, reducing the efficacy of nanoparticle formulations.7 This event is often referred to as the ABC phenomenon.
PEG and other synthetic polymeric shielding strategies
Nanoparticles are frequently PEGylated to avoid immune recognition and to provide better pharmacokinetic profiles. PEG is a flexible hydrophilic polymer that was originally considered to have little to no immunogenicity and few biological interactions, and therefore was expected to be advantageous as a shielding agent.8 By grafting PEG to the surface of a nanoparticle formulation, protein adsorption and antibody binding are decreased, as is uptake by phagocytic cells. Reduced uptake occurs because the PEG layer forms a hydrophilic barrier on the surface of nanoparticles and blocks receptor interactions via steric hindrance.9 The effectiveness of PEG to increase circulation times has been demonstrated on nanoparticles with a range of shapes, sizes, and composition.10–14 In fact, several clinically approved nanoparticle therapies such as Doxil® include PEGylation. During the design of Doxil adding PEG to doxorubicin containing liposomes was found to increase the circulating half-life from approximately 10 min to over 40 hours.15 Nevertheless, choosing the correct PEG formulation can be tricky. PEG polymers can have different physical properties by varying characteristics such as chain length and number of branch arms. The conjugation density of the PEG molecules on the nanoparticle surface can alter the PEG conformation as well, allowing it to adopt either a mushroom like globular conformation at low density or a more extended brush like conformation at high density. The effectiveness of PEG as a shielding agent is dependent on the chosen physical characteristics,12–14 implying that optimization of the PEG shield is usually necessary. In practice nanoparticles have been optimized with various PEG formulations, for example 10 kDa molecular weight PEG best reduced clearance for chitosan/siRNA nanoparticles delivered intravenously, while 1 kDa molecular weight PEG was best for oral administration of prodrug-based micelles.16–17
Despite the successes with PEG as a shielding agent, there have been recent challenges. While initial reports on PEG suggested low immunogenicity, more recent reports indicate a significant level of immunogenicity as PEG-specific antibodies have been found in the general population. Data from 2012 indicate that up to 25% of the population have anti-PEG antibodies, up from 0.2% in 1984.18–19 This is likely due to the prevalence of PEG in everyday products. Not only is PEG used as an anti-fouling agent in biomedical applications, but it is also found in cosmetics and food products.19 Development of anti-PEG antibodies can be especially detrimental for nanoparticle formulations that require repeat administration. Newer nanoparticle formulations that rely on PEG shielding have struggled in clinical trials mainly because of the immunogenicity of PEG.
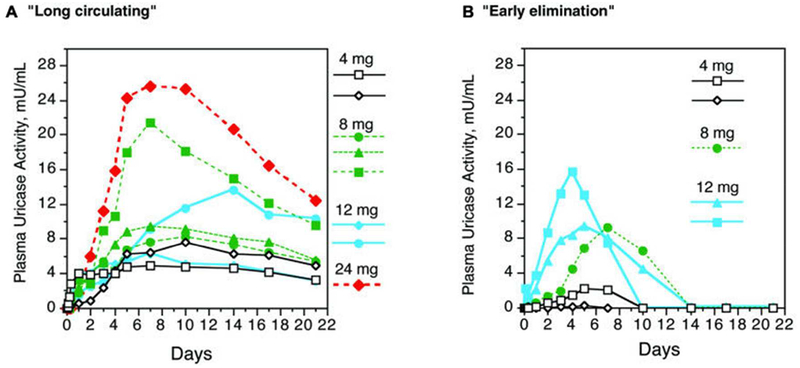
A Phase 1 clinical trial published in 2006 found that treatment of hyperuricemia in gout patients by PEGylated uricase led to induction of PEG-specific IgM and IgG antibodies in 5 of 13 patients.20 PEG-uricase was cleared in these subjects by 10 days post injection, whereas the 8 patients who did not show measurable antibodies against PEG had circulating PEG uricase 21 days post injection (Figure 1). Another study demonstrated that antibodies against PEG affected the efficacy of a PEGylated asparaginase therapy for treatment of acute lymphoblastic leukemia patients.21 Rapid clearance of PEG-asparaginase was observed for 15 patients, of whom 12 had measurable antibodies against PEG. Since serum asparaginase levels need to remain elevated for 21 days to achieve a response in acute lymphoblastic leukemia, the authors concluded that rapid clearance by PEG-specific antibodies could render the treatment ineffective. Furthermore, a recent clinical trial of pegnivacogin, a PEGylated RNA aptamer, was halted after three patients displayed rapid allergic reactions minutes after administration of the therapy, which was correlated with pre-existing PEG-specific antibodies.22 There are several other examples from pre-clinical and clinical studies which concluded that PEGylated nanoparticles are rapidly cleared upon repeat administrations because of a PEG-specific immune response.23–25 These data highlight the importance to revisit nanotechnology shielding strategies and to adapt to the increased prevalence of anti-PEG antibodies in the population.
Figure 1.
Uricase activity in plasma over 21 days in subjects with and without PEG antibodies. A) Subjects without PEG antibodies had “long circulating” uricase after a single injection as indicated by detectable plasma uricase activity for 21 days. B) In contrast, subjects with anti-PEG antibodies displayed “early elimination” of uricase. Each line in panels A and B represents an individual patient receiving either 4 (black), 8 (green), 12 (blue), or 24 (red) mg uricase. Reproduced with permission from20 from Biomed Central.
One strategy to develop next-generation shielding strategies is to modify the PEG polymer or backbone to configurations not recognized by anti-PEG antibodies. To avoid antibody recognition by PEG-specific antibodies, one study, which investigated the effect of ethylene glycol (EG) oligomer length on antigenicity, found that the antigenicity of PEG can be reduced using nine side-chain EG repeats on a poly(methyl methacrylate) backbone.26 Furthermore, using three side-chain EG repeats completely eliminated the antigenicity. Thus using these newly defined chemical design strategies, the next generation of EG oligomers combined with other polymer backbones could yield the next-generation of PEG-based shielding strategies.
While PEG is the most well characterized shielding polymer, other hydrophilic synthetic polymers have been studied. These alternative polymers are less commercially available and not used in as many products, but this may change over time. Based on the limited exposure of the public to these novel and alternative polymers, the risk of pre-existing antibodies against them in the general population is small. Nevertheless, their immunogenicity needs to be assessed in detail considering that many therapeutics require repeat administration. While a patient may not present with pre-existing antibodies, antibodies could develop over the course of treatment. While a diverse group of polymers with anti-fouling properties exists, each with their own advantages, to date there is sporadic data on their immunogenic properties after repeat exposure.
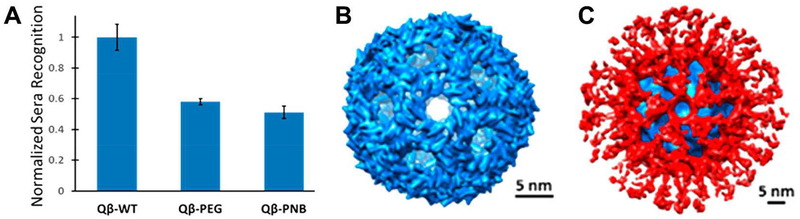
One example from our group in collaboration with Drs. Pokorski and Hore focused on the investigation of polynorbornene (PNB) polymer shields clicked to virus-like particle (VLPs) nanotechnologies. Similar to PEG, PNB was shown to reduce carrier-specific antibody recognition when grafted to VLPs from the bacteriophage Qβ, as shown in Figure 2A.27 Cryo-electron microscopy was used in this study to reveal the nearly complete coverage of the viral surface with a compact PNB layer (Figure 2B, C).
Figure 2.
Polynorbornene shielding effects on icosahedral Qβ nanoparticles. A) ELISA response to Qβ immunized sera with nanoparticles coated with PEG or PNB. B) Structure of Qβ by cryo-electron microscopy. C) Structure of PNB-shielded Qβ by cryo-electron microscopy, with Qβ density in blue and PNB density in red. Adapted with permission from27. Copyright 2017 American Chemical Society.
Additional polymers have also been investigated for their anti-fouling and shielding effects, including polyoxazolines (POX/POZ), poly(N-vinylpyrrolidone) (PVP), polybetaines, poly(phosphoesters), poloxamers, poly(glycerols), polyacrylamides, and many others.28–38 Nevertheless, as chemistries and products are being made available to wider audiences, the risk exists that these polymers are also formulated into everyday products thus leading to generation of acquired immunity against them. Furthermore, as pointed out above, the risk also exists that patients could develop antibodies after repeated exposure, as has been observed for PEG. Therefore, a promising alternative approach may be to explore shielding agents based on biologically relevant molecules, including lipids, carbohydrates, and proteins – in particular those that the body would recognize as ‘self’.
Bio-inspired shielding strategies
The in vivo environment is diverse, with a plethora of cells, proteins, and small molecules circulating at any given moment. Bio-inspired shielding strategies harness the body’s complexity, by cloaking nanoparticles in biodegradable polymers and components normally found in circulation. Thus, nanoparticles are not just hidden from clearance mechanisms through ‘passive shielding,’ but are camouflaged within their environment through ‘active stealthing.’ In this section, various bio-inspired shielding strategies are discussed, including those based on carbohydrates, lipids, and proteins.
Carbohydrate-based shielding strategies
While carbohydrates can be found on foreign pathogens, such as lipopolysaccharides (LPS) in gram negative bacteria, they are also critical in the human body. They are used as energy sources and are found in extracellular matrices and on cell surfaces. For example, eukaryotic cells are coated with glycosaminoglycans (GAGs), a class of negatively charged polysaccharides which are frequently conjugated to proteins on the cell surface or within the extracellular matrix. Research has shown that these hydrophilic polysaccharides can provide shielding effects for nanoparticles by mimicking naturally occurring GAGs. Carbohydrate-based shields have limited toxicity and immunogenicity. Furthermore, carbohydrate shielding mechanisms can also benefit from the natural functions of carbohydrates in the body by targeting certain carbohydrate-binding receptors (see discussion and references in the following section).
Polymers of sialic acid, a monosaccharide that frequently modifies amino acids in the body, have been investigated as a shielding strategy for proteins such as insulin, catalase, and asparaginase.39–42 In the case of asparaginase, conjugation of a polysialic acid (PSA) was shown to increase circulation times and reduce antigenicity to the protein in mice, even in mice with pre-existing antibodies against asparaginase (raised through immunization with PSA-coated asparaginase).43 Based on these promising results, more recent studies have investigated PSAs as a shielding agent in nanoparticle formulations, however these studies are still in early stages. One study developed PSA-coated micelles as an alternative to PEG coated micelles for the delivery of hydrophobic drugs. It was shown that PSA coated micelles did not affect the viability of synovial fibroblast cells in culture while allowing for uptake.44 It remains to be seen how these nanoparticles compare to PEG-coated nanoparticles in vivo. Another study investigated the chemotherapeutic doxorubicin loaded into nanoparticles composed of hydrophobically-modified PSA, and found that the nanoparticles had low toxicity in non-cancer tissue in mice, while demonstrating an antitumor effect similar to free doxorubicin.45 The authors attributed these results to the biodegradable and biocompatible nature of PSA.
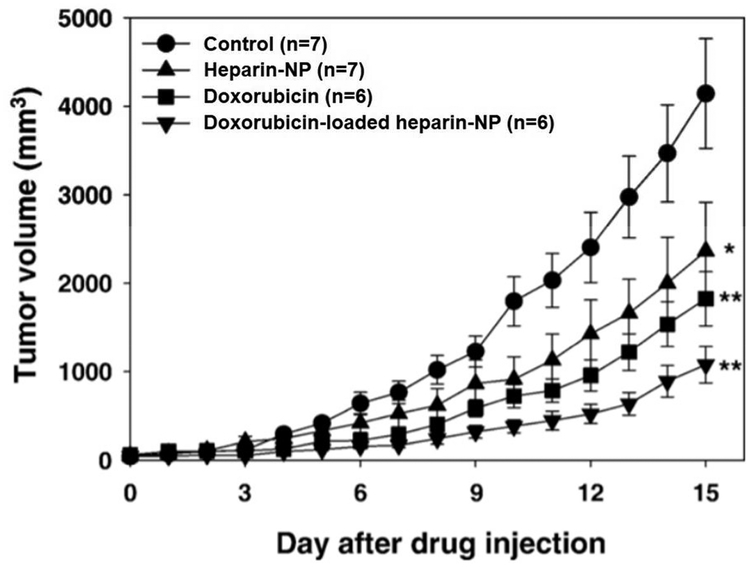
Heparin is a GAG known for its anticoagulant properties, as well as many other functions including inhibition of angiogenesis and inflammation.46 Not only that, but it can also provide shielding properties when used to coat numerous types of nanoparticles.47–51 One study found that low molecular weight heparin coating improved the biological properties of mesoporous nanoparticles by preventing recognition and uptake by macrophages.47 Heparin-coated nanoparticles have also been shown to prevent angiogenesis and metastasis, making the carbohydrate an especially beneficial shielding agent for nanoparticles in cancer therapy.52–53 It has been demonstrated that heparin-coated nanoparticles loaded with the chemotherapy doxorubicin suppressed tumor growth in a mouse model of squamous cell carcinoma.54 These nanoparticles showed reduced tumor growth compared to free doxorubicin or heparin coated nanoparticles alone (Figure 3). The authors concluded this was due to the extended circulation times, along with a combination of the cytotoxicity from doxorubicin and the anti-proliferative effects of heparin.
Figure 3.
Antitumor effects of heparin nanoparticle (NP) formulation, doxorubicin, and doxorubicin-loaded heparin-NP in mice model of subcutaneous squamous cell carcinoma. Reproduced with permission from54.
Another GAG, hyaluronic acid (HA), is ubiquitous within the body and found in high levels within the extracellular matrix.55 Stealth coatings based on HA have been shown to prevent cell interactions.56–57 One study revealed that HA-coated liposomes did not result in accelerated blood clearance and hypersensitivity upon repeat administrations. PEG-coated liposomes, on the other hand, had increased accumulation in the liver after repeat administrations due to the ABC phenomenon.58 Others have used HA not only to shield nanoparticles but also as a targeting ligand, as HA interacts with CD44 receptors, which are upregulated in some tumor tissues, including colon and ovarian cancers.59–61
Lipid membrane shielding strategies
Because lipid membranes coat all cells, nanoparticles enveloped with cell membrane-like shields can pass through circulation incognito. A significant portion of research into lipid shielding strategies has focused on membranes of blood particles, such as red blood cells, and platelets. However, studies have also investigated the use of membranes from other cell types, synthesized lipid coatings such as artificial membrane ‘wraps,’ and lipopeptide formulations.
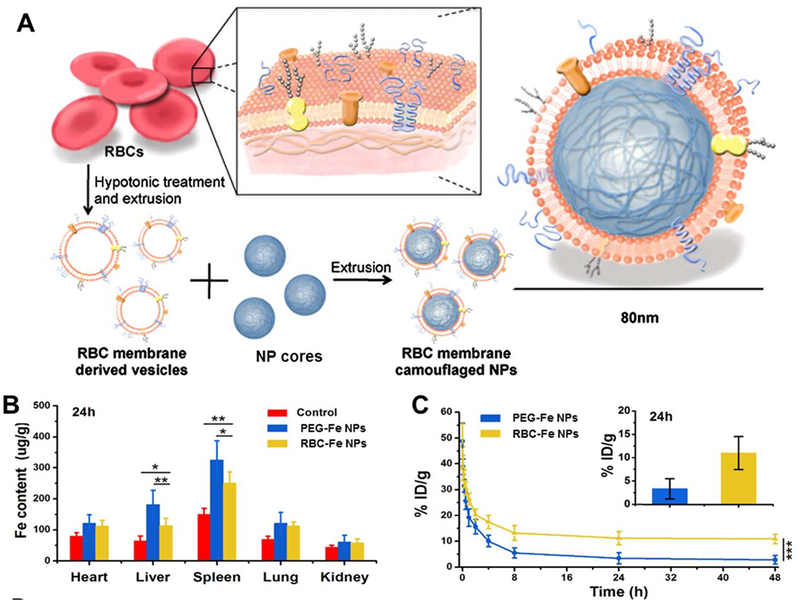
A 2011 study first investigated the use of red blood cell (RBC) membranes as an alternative shielding strategy to polyethylene glycol. RBC-vesicles were derived from natural red blood cells harvested from mice, and used to coat polymeric nanoparticles (Figure 4A).62 By doing so, the RBC-coated particles retained the lipid bilayer structure and membrane proteins from the original cells. RBC coated particles exhibit reduced uptake into MPS organs and have longer circulation times compared to bare and PEGylated particles (Figure 4B, C).62–63 The biomimetic coating decreases macrophage uptake of nanoparticles and does not result in toxicity or accelerated blood clearance upon repeat administrations in vivo.63–64 RBC-coating has also been shown to result in increased blood retention times and enhanced tumor uptake of nanoparticles.65–66 Based on the success of RBC coatings, many other nanoparticle formulations have begun to explore membrane-based shielding strategies.
Figure 4.
RBC-membranes for use as camouflage for nanoparticles. A) Schematic of extraction of membranes from RBCs and method for coating nanoparticle surfaces. Adapted from62, copyright 2011 National Academy of Sciences, USA. B) Biodistribution of Fe nanoparticles coated with PEG or RBCs in mice 24 hrs post-injection. Adapted with permission from63, copyright 2016 IOP Publishing. C) Pharmacokinetics of Fe nanoparticles coated with PEG or RBCs and blood retention at 24 hrs post-injection in mice. Adapted with permission from63, copyright 2016 IOP Publishing.
Along the same vein, white blood cell membranes have also been harnessed for stealth coatings. Leukolike vectors (LLVs) are those coated with cellular membranes harvested from mouse macrophages or human monocytes. In doing so, LLVs avoid opsonization and have reduced uptake by the phagocytic immune cells from which they were derived.67 LLVs can activate lymphocyte receptor-mediated pathways in vitro and have been shown to have enhanced vascular permeability in vivo.68 Macrophage cell membrane coated nanoparticles have also been found to have increased circulation times and reduced retention in the organs of the MPS.69 Another study showed that coating nanoparticles with cytotoxic T-lymphocyte membranes not only served to provide shielding properties such as reduced macrophage uptake, but also enhanced localization to gastric tumor tissue in vivo.70 This is likely because cytotoxic T-cells localize in gastric tissue, suggesting that the nanoparticle’s lipid-based coating provides targeting as well as shielding.
Platelets have the ability to marginate to the vascular wall and interact with injury sites in the vasculature, thus it has been hypothesized that nanoparticles stealth-coated with platelet membranes could be beneficial in the treatment of diseases such as atherosclerosis and thrombosis-related diseases. In one study nanoparticles coated with platelet membranes reduced particle uptake and complement activation, which the authors attributed to the platelet membrane bound complement regulator proteins.71 The authors also found that the platelet cloaked particles benefitted from platelet like properties such as adhesion to damaged vasculature and binding to platelet-adhering pathogens.
Other cellular membranes have been explored for cloaking nanoparticles as well. Cancer cells, stem cells, and others have been used to provide membranes for nanoparticle cloaking for biomedical applications.72–74 Researchers have also investigated the possibility of designing synthetic lipid coatings that emulate biological membranes. For example, membrane ‘wraps’ are an artificial platform for shielding nanoparticles that can be tailored to target specific host organs.75 Lipopeptides can be inserted into liposomal nanoparticles to create a more biomimetic exterior, which helps limit serum protein adsorption.76
Protein and polypeptide shielding strategies
Proteins are essential for numerous functions in the cell, serving as transporters, enzymes, signaling molecules, scaffolds, and many other important roles. Because they are so diverse and are found universally throughout the body, protein-based strategies hold great promise for stealth coating. Some of these strategies are based on naturally occurring proteins, while others involve synthetically derived polypeptides. Much of the work in protein stealth-coating strategies focuses on extending the half-life of protein-based therapies, but this approach holds promise for other nanoscale material applications as well. In the following section, we highlight some examples.
First, CD47 is a cell membrane glycoprotein that has been reported to be a ‘marker of self,’ preventing clearance signaling through CD172a, a phagocytic cell receptor.77–78 When used to coat nanoparticles, peptides derived from CD47 have been shown to mimic the CD47 CD172a pathway, thus preventing macrophage mediated clearance.79 CD47 coating preferentially reduces nanoparticle uptake by M1 macrophages, as compared to PEG which decreases clearance by all macrophage phenotypes.80 This selective evasion may allow for a more rationalized approach for nanoparticle shielding control.
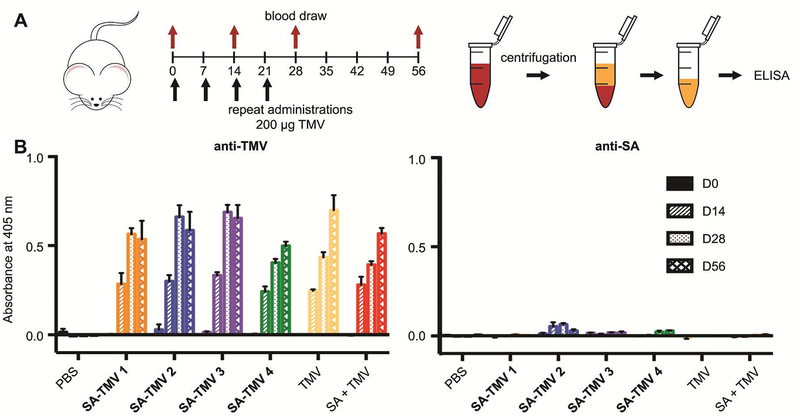
Second, serum albumin (SA) is the most abundant protein in blood plasma. As such, it is a good candidate for camouflaging nanoparticles in the vascular environment. For example, we investigated SA-coatings on the plant viral nanotechnology platform derived from tobacco mosaic virus (TMV). SA-coated TMV nanoparticles exhibited reduced antibody recognition and increased circulation times compared to PEGylated TMV.81 Structural studies of SA-conjugated particles revealed that SA camouflage is likely due to steric hindrance as the SA molecules would prevent antibodies from reaching the nanoparticle surface.82 Immune studies of these particles revealed that antibodies to the SA-coating are not produced after repeat injections (Figure 5), which highlights the benefits of this biologic coating over traditional PEGylation.83
Figure 5.
Repeated administration of SA-coated TMV NPs does not produce an appreciable immune response to the SA coating. A) SA-coated TMV NP administration schedule and assay protocol. B) Production of antibodies generated against the NP and the SA coating after repeat administrations of four differing SA-coated TMV constructs (SA-TMV 1, 2, 3, and 4) at days 0, 14, 28, and 56 (D0, D14, D28, D56). Constructs varied the amount of SA coating and linker length. PBS, TMV, and a non-conjugated mixture of SA and TMV (SA+TMV) served as controls. Adapted from83 with permission from Royal Society of Chemistry.
Third, elastin like peptides (ELPs) are a class of genetically engineered protein based polymers. They are based on a pentapeptide repeat of Val-Pro-Gly-X-Gly found in human elastin, where X can be any amino acid except proline. ELPs are thermally responsive. Below their transition temperature they are soluble in aqueous solutions. Above their transition temperature, ELPs transition into an insoluble colloid-rich “coacervate” phase.84–85 ELPs have long circulation half-lives, which can be controlled based on their composition and chain length.86–87 Furthermore, ELP-protein conjugates in clinical trials did not induce a significant immune response in most subjects.88 However, while the potential application of ELP-based nanoparticle formulations has been reported, the use of ELPs as nanoparticle coatings has yet to be explored.
Fourth, PASylation is a synthetic peptide based alternative to PEGylation that uses small amino acids to create a hydrophilic uncharged polypeptide chain with properties similar to PEG, and provides prolonged pharmacokinetics and other advantageous in vivo properties.89 These chains are comprised of prolines, alanines, and serines and can be genetically encoded into protein-based therapies. Recombinant proteins are typically cleared via the kidney due to their small size.90 PAS-fusion proteins have increased hydrodynamic radii, thus prolonging circulation times in vivo.89, 91–92 For example, intravital injection studies with a PASylated recombinant form of erythropoietin, a hormone that regulates the production of red blood cells, led to extended circulation times compared to non-shielded erythropoietin. However, it was noted that the half-life of the PASylated form was shorter than that of the PEGylated form of the protein.91 Nevertheless, PASylated proteins have been observed to have more activity than their PEGylated counterparts.92 It remains to be seen if PASylation can provide similar benefits for larger nanoparticles that are primarily cleared through the liver and spleen.
And lastly, a zwitterionic synthetic peptide with a sequence of Glu-Lys repeats has been developed to prevent nonspecific protein adsorption and mimic naturally occurring protein surfaces, as the amino acids used are the two most prevalent amino acids on protein surfaces.93–94 Using this peptide based strategy also allows for easy addition of targeting sequences to the end of the stealth peptide.95 The zwitterionic peptide coating was shown to prevent nonspecific cell uptake of gold nanoparticles in both macrophage and endothelial cell lines.95 However, the shielding effects of the zwitterionic peptide remain to be demonstrated in vivo.
Together, these examples highlight the opportunities of protein and peptide-based shielding strategies and area with room for exploration.
Beyond shielding – Immune editing
For proteinaceous nanoparticles, an alternative to coating with shielding agents is to immune-engineer and render the particles themselves less immunogenic. Immune editing strategies have been explored for the application of viral vectors for gene therapy. For example, the antigenic epitopes of adeno-associated virus (AAV)-based gene delivery vectors have been mapped by a variety of techniques, including but not limited to directed evolution, peptide scanning, mutagenesis studies, and cryo-electron microscopy (cryoEM).96 Mapping of antigenic regions can then be used to develop a new generation of vectors that are antigenically distinct. In one study, cryoEM guided antigenic footprinting was used to guide directed evolution and led to the production of AAV vectors with unique capsid antigenic motifs (CAMs) that evade anti-AAV antibodies in sera from mice, nonhuman primates, and humans without additional shielding molecules.97 The strategy of developing new non antigenic nanoparticles has drawbacks, however. Editing of immunogenic epitopes can only be implemented for proteinaceous nanoparticles. Modifying the vectors to be antigenically distinct may alter the ability of the virus to deliver as efficiently or alter which cells it can infect. Also, while second-generation vectors could evade pre-existing antibodies, there is a risk that newly engineered epitopes could be produced, limiting their use in repeated administrations. Nevertheless, this strategy shows promise for specific applications.
Conclusions
Nanoparticle formulations offer great opportunities in drug delivery. However, after administration immune recognition and clearance create challenges for nanoparticles remaining in circulation long enough to reach the site of disease. This can be especially detrimental upon repeat administration, when blood clearance can be accelerated. To address this issue, shielding strategies are frequently employed to coat nanoparticle formulations and to help them evade immune recognition. Typically, nanoparticles are coated with synthetic polymers, most commonly PEG. Polymeric coatings can effectively reduce nanoparticle-protein interactions. However, the widespread use of PEG has limited its effectiveness because of the prevalence of PEG-specific antibodies that allow for recognition and clearance of PEGylated nanoparticles. Other polymeric coatings could suffer the same fate with increasing use. Biopolymers, namely carbohydrates, lipids, and proteins, can provide similar shielding effects to PEG, while also camouflaging nanoparticles as ‘self.’
Bio-inspired shielding strategies have general advantages over their synthetic counterparts: they are biocompatible, biodegradable, and may be chosen so that they have low immunogenicity. Furthermore, because they are derived from natural components, they provide not just passive evasion of immune recognition but an active mechanism of camouflage. In other words, by appearing as if they belong carbohydrate-based shielding strategies can mimic cell-surface polysaccharides, lipid-based strategies camouflage nanoparticles as cell-like entities, and protein-based nanoparticle coatings blend in with the proteins found universally within the body.
As the shielding requirements of different nanoparticle formulations will vary depending on their application, several bio-inspired strategies have been developed. These shielding strategies offer immune evasion while also providing the ability of the shielding molecule to interact productively with other biological macromolecules. Carbohydrate-, lipid-, and protein-based coatings are generally biodegradable and thus do not lead to persistence of materials within the body. Alternative approaches also exist, such as making the nanoparticle itself less immunogenic, and these may have advantages in specific circumstances.
In moving towards translation for nanoparticle therapies, it will be important to consider the balance between immune evasion and nanoparticle targeting. Since they are derived from natural components that sometimes have built-in targeting ability, bio-inspired coatings may have the ability to deliver nanoparticles to specific sites in the body. However, redirecting bio-shielded nanoparticles may be an issue. For example, targeting platelet membrane-coated nanoparticles away from vascular injury could prove difficult. The appropriate coating will need to be chosen based on the requirements of the nanoparticle application. Separate nanoparticle modifications may be needed for shielding and for targeting. It will be important to investigate whether the effects of targeting interfere with the stealth coating mechanism. It will also be important to consider the function of the nanoparticle cargo. For drug delivery, long-circulating nanoparticles are often the goal. However, when delivering contrast agents, it is not beneficial to have excessively long circulation times as imaging can be done within a matter of hours. Thus, having a way to control the half-life of a nanoparticle in the body by altering the coating would be advantageous, which could potentially be accomplished with a bio-inspired approach to shielding. Because they offer immune evasion with many other inherent advantages, bio-inspired shielding strategies will likely play a significant role in future nanoparticle drug delivery applications.
Acknowledgement
This work was funded in part by grants from the National Institute of Health, R01HL137674 (to N.F.S.), R01CA202814 (to N.F.S), and T32GMS008803 (to N.M.G).
Footnotes
Publisher's Disclaimer: Just Accepted
Publisher's Disclaimer: “Just Accepted” manuscripts have been peer-reviewed and accepted for publication. They are posted online prior to technical editing, formatting for publication and author proofing. The American Chemical Society provides “Just Accepted” as a service to the research community to expedite the dissemination of scientific material as soon as possible after acceptance. “Just Accepted” manuscripts appear in full in PDF format accompanied by an HTML abstract. “Just Accepted” manuscripts have been fully peer reviewed, but should not be considered the official version of record. They are citable by the Digital Object Identifier (DOI®). “Just Accepted” is an optional service offered to authors. Therefore, the “Just Accepted” Web site may not include all articles that will be published in the journal. After a manuscript is technically edited and formatted, it will be removed from the “Just Accepted” Web site and published as an ASAP article. Note that technical editing may introduce minor changes to the manuscript text and/or graphics which could affect content, and all legal disclaimers and ethical guidelines that apply to the journal pertain. ACS cannot be held responsible for errors or consequences arising from the use of information contained in these “Just Accepted” manuscripts.
References
- 1.Blanco E; Shen H; Ferrari M, Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 2015, 33 (9), 941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kokkinopoulou M; Simon J; Landfester K; Mailander V; Lieberwirth I, Visualization of the protein corona: towards a biomolecular understanding of nanoparticle cell interactions. Nanoscale 2017, 9 (25), 8858–8870. [DOI] [PubMed] [Google Scholar]
- 3.Lee YK; Choi EJ; Webster TJ; Kim SH; Khang D, Effect of the protein corona on nanoparticles for modulating cytotoxicity and immunotoxicity. Int J Nanomedicine 2015, 10, 97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Docter D; Distler U; Storck W; Kuharev J; Wunsch D; Hahlbrock A; Knauer SK; Tenzer S; Stauber RH, Quantitative profiling of the protein coronas that form around nanoparticles. Nat Protoc 2014, 9 (9), 2030–44. [DOI] [PubMed] [Google Scholar]
- 5.Pitek AS; Wen AM; Shukla S; Steinmetz NF, The Protein Corona of Plant Virus Nanoparticles Influences their Dispersion Properties, Cellular Interactions, and In Vivo Fates. Small 2016, 12 (13), 1758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pallardy MJ; Turbica I; Biola Vidamment A, Why the Immune System Should Be Concerned by Nanomaterials? Front Immunol 2017, 8, 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu Lila AS; Kiwada H; Ishida T, The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J Control Release 2013, 172 (1), 38–47. [DOI] [PubMed] [Google Scholar]
- 8.Abuchowski A; McCoy JR; Palczuk NC; van Es T; Davis FF, Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem 1977, 252 (11), 3582–6. [PubMed] [Google Scholar]
- 9.Drobek T; Spencer ND; Heuberger M, Compressing PEG brushes. Macromolecules 2005, 38 (12), 5254–5259. [Google Scholar]
- 10.Hatakeyama H; Akita H; Harashima H, The polyethyleneglycol dilemma: advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol Pharm Bull 2013, 36 (6), 892–9. [DOI] [PubMed] [Google Scholar]
- 11.Bruckman MA; Randolph LN; VanMeter A; Hern S; Shoffstall AJ; Taurog RE; Steinmetz NF, Biodistribution, pharmacokinetics, and blood compatibility of native and PEGylated tobacco mosaic virus nano-rods and-spheres in mice. Virology 2014, 449, 163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jokerst JV; Lobovkina T; Zare RN; Gambhir SS, Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond) 2011, 6 (4), 715–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KL; Shukla S; Wu M; Ayat NR; El Sanadi CE; Wen AM; Edelbrock JF; Pokorski JK; Commandeur U; Dubyak GR; Steinmetz NF, Stealth filaments: Polymer chain length and conformation affect the in vivo fate of PEGylated potato virus X. Acta Biomater 2015, 19, 166–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry JL; Reuter KG; Kai MP; Herlihy KP; Jones SW; Luft JC; Napier M; Bear JE; DeSimone JM, PEGylated PRINT nanoparticles: the impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett 2012, 12 (10), 5304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Northfelt DW; Martin FJ; Working P; Volberding PA; Russell J; Newman M; Amantea MA; Kaplan LD, Doxorubicin encapsulated in liposomes containing surface bound polyethylene glycol: pharmacokinetics, tumor localization, and safety in patients with AIDS related Kaposi’s sarcoma. J Clin Pharmacol 1996, 36 (1), 55–63. [DOI] [PubMed] [Google Scholar]
- 16.Li ZB; Han XP; Zhai YL; Lian H; Zhang D; Zhang WJ; Wang YJ; He ZG; Liu Z; Sun J, Critical determinant of intestinal permeability and oral bioavailability of pegylated all trans retinoic acid prodrug based nanomicelles: Chain length of poly (ethylene glycol) corona. Colloid Surface B 2015, 130, 133–140. [DOI] [PubMed] [Google Scholar]
- 17.Yang C; Gao S; Dagnaes Hansen F; Jakobsen M; Kjems J, Impact of PEG Chain Length on the Physical Properties and Bioactivity of PEGylated Chitosan/siRNA Nanoparticles in Vitro and in Vivo. ACS Appl Mater Interfaces 2017, 9 (14), 12203–12216. [DOI] [PubMed] [Google Scholar]
- 18.Richter AW; Akerblom E, Polyethylene glycol reactive antibodies in man: titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors. Int Arch Allergy Appl Immunol 1984, 74 (1), 36–9. [DOI] [PubMed] [Google Scholar]
- 19.Garay RP; El Gewely R; Armstrong JK; Garratty G; Richette P, Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG conjugated agents. Expert Opin Drug Deliv 2012, 9 (11), 1319–23. [DOI] [PubMed] [Google Scholar]
- 20.Ganson NJ; Kelly SJ; Scarlett E; Sundy JS; Hershfield MS, Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res Ther 2006, 8 (1), R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong JK; Hempel G; Koling S; Chan LS; Fisher T; Meiselman HJ; Garratty G, Antibody against poly(ethylene glycol) adversely affects PEG asparaginase therapy in acute lymphoblastic leukemia patients. Cancer 2007, 110 (1), 103–11. [DOI] [PubMed] [Google Scholar]
- 22.Ganson NJ; Povsic TJ; Sullenger BA; Alexander JH; Zelenkofske SL; Sailstad JM; Rusconi CP; Hershfield MS, Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J Allergy Clin Immunol 2016, 137 (5), 1610–1613 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamad I; Hunter AC; Szebeni J; Moghimi SM, Poly(ethylene glycol)s generate complement activation products in human serum through increased alternative pathway turnover and a MASP-2-dependent process. Mol Immunol 2008, 46 (2), 225–32. [DOI] [PubMed] [Google Scholar]
- 24.Dams ET; Laverman P; Oyen WJ; Storm G; Scherphof GL; van Der Meer JW; Corstens FH; Boerman OC, Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther 2000, 292 (3), 1071–9. [PubMed] [Google Scholar]
- 25.Ishida T; Ichihara M; Wang X; Yamamoto K; Kimura J; Majima E; Kiwada H, Injection of PEGylated liposomes in rats elicits PEG specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J Control Release 2006, 112 (1), 15–25. [DOI] [PubMed] [Google Scholar]
- 26.Qi Y; Simakova A; Ganson NJ; Li X; Luginbuhl KM; Ozer I; Liu W; Hershfield MS; Matyjaszewski K; Chilkoti A, A brush polymer/exendin-4 conjugate reduces blood glucose levels for up to five days and eliminates poly(ethylene glycol) antigenicity. Nature Biomedical Engineering 2016, 1 (0002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee PW; Isarov SA; Wallat JD; Molugu SK; Shukla S; Sun JE; Zhang J; Zheng Y; Lucius Dougherty M; Konkolewicz D; Stewart PL; Steinmetz NF; Hore MJ; Pokorski JK, Polymer Structure and Conformation Alter the Antigenicity of Virus like Particle Polymer Conjugates. J Am Chem Soc 2017, 139 (9), 3312–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J; Dong X; Feng T; Lin L; Guo Z; Xia J; Tian H; Chen X, Charge conversional zwitterionic copolymer as pH sensitive shielding system for effective tumor treatment. Acta Biomater 2015, 26, 45–53. [DOI] [PubMed] [Google Scholar]
- 29.Muller J; Bauer KN; Prozeller D; Simon J; Mailander V; Wurm FR; Winzen S; Landfester K, Coating nanoparticles with tunable surfactants facilitates control over the protein corona. Biomaterials 2017, 115, 1–8. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y; Sundaram HS; Liu S; Zhang L; Xu X; Yu Q; Xu J; Jiang S, A robust graft to strategy to form multifunctional and stealth zwitterionic polymer coated mesoporous silica nanoparticles. Biomacromolecules 2014, 15 (5), 1845–51. [DOI] [PubMed] [Google Scholar]
- 31.Ye L; Zhang Y; Yang B; Zhou X; Li J; Qin Z; Dong D; Cui Y; Yao F, Zwitterionic-Modified Starch Based Stealth Micelles for Prolonging Circulation Time and Reducing Macrophage Response. ACS Appl Mater Interfaces 2016, 8 (7), 4385–98. [DOI] [PubMed] [Google Scholar]
- 32.Cao J; Zhai S; Li C; He B; Lai Y; Chen Y; Luo X; Gu Z, Novel pH-sensitive micelles generated by star-shape copolymers containing zwitterionic sulfobetaine for efficient cellular internalization. J Biomed Nanotechnol 2013, 9 (11), 1847–61. [DOI] [PubMed] [Google Scholar]
- 33.Knop K; Hoogenboom R; Fischer D; Schubert US, Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl 2010, 49 (36), 6288–308. [DOI] [PubMed] [Google Scholar]
- 34.Mehanny M; Hathout RM; Geneidi AS; Mansour S, Studying the effect of physically-adsorbed coating polymers on the cytotoxic activity of optimized bisdemethoxycurcumin loaded PLGA nanoparticles. J Biomed Mater Res A 2017, 105 (5), 1433–1445. [DOI] [PubMed] [Google Scholar]
- 35.Lakshmikuttyamma A; Sun Y; Lu B; Undieh AS; Shoyele SA, Stable and efficient transfection of siRNA for mutated KRAS silencing using novel hybrid nanoparticles. Mol Pharm 2014, 11 (12), 4415–24. [DOI] [PubMed] [Google Scholar]
- 36.Gaucher G; Asahina K; Wang J; Leroux JC, Effect of poly(N-vinyl-pyrrolidone)-block-poly(D,L-lactide) as coating agent on the opsonization, phagocytosis, and pharmacokinetics of biodegradable nanoparticles. Biomacromolecules 2009, 10 (2), 408–16. [DOI] [PubMed] [Google Scholar]
- 37.Kaneda Y; Tsutsumi Y; Yoshioka Y; Kamada H; Yamamoto Y; Kodaira H; Tsunoda S; Okamoto T; Mukai Y; Shibata H; Nakagawa S; Mayumi T, The use of PVP as a polymeric carrier to improve the plasma half-life of drugs. Biomaterials 2004, 25 (16), 3259–66. [DOI] [PubMed] [Google Scholar]
- 38.Bludau H; Czapar AE; Pitek AS; Shukla S; Jordan R; Steinmetz NF, POxylation as an alternative stealth coating for biomedical applications. Eur Polym J 2017, 88, 679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gregoriadis G; Jain S; Papaioannou I; Laing P, Improving the therapeutic efficacy of peptides and proteins: a role for polysialic acids. Int J Pharm 2005, 300 (1–2), 125–30. [DOI] [PubMed] [Google Scholar]
- 40.Jain S; Hreczuk-Hirst DH; McCormack B; Mital M; Epenetos A; Laing P; Gregoriadis G, Polysialylated insulin: synthesis, characterization and biological activity in vivo. Biochim Biophys Acta 2003, 1622 (1), 42–9. [DOI] [PubMed] [Google Scholar]
- 41.Fernandes AI; Gregoriadis G, Synthesis, characterization and properties of sialylated catalase. Biochim Biophys Acta 1996, 1293 (1), 90–6. [DOI] [PubMed] [Google Scholar]
- 42.Fernandes AI; Gregoriadis G, Polysialylated asparaginase: preparation, activity and pharmacokinetics. Biochim Biophys Acta 1997, 1341 (1), 26–34. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes AI; Gregoriadis G, The effect of polysialylation on the immunogenicity and antigenicity of asparaginase: implication in its pharmacokinetics. Int J Pharm 2001, 217 (1–2), 215–24. [DOI] [PubMed] [Google Scholar]
- 44.Wilson DR; Zhang N; Silvers AL; Forstner MB; Bader RA, Synthesis and evaluation of cyclosporine A-loaded polysialic acid-polycaprolactone micelles for rheumatoid arthritis. Eur J Pharm Sci 2014, 51, 146–56. [DOI] [PubMed] [Google Scholar]
- 45.Jung B; Shim MK; Park MJ; Jang EH; Yoon HY; Kim K; Kim JH, Hydrophobically modified polysaccharide-based on polysialic acid nanoparticles as carriers for anticancer drugs. Int J Pharm 2017, 520 (1–2), 111–118. [DOI] [PubMed] [Google Scholar]
- 46.Cassinelli G; Naggi A, Old and new applications of non-anticoagulant heparin. Int J Cardiol 2016, 212 Suppl 1, S14–21. [DOI] [PubMed] [Google Scholar]
- 47.Bellido E; Hidalgo T; Lozano MV; Guillevic M; Simon-Vazquez R; Santander-Ortega MJ; Gonzalez-Fernandez A; Serre C; Alonso MJ; Horcajada P, Heparin-engineered mesoporous iron metal-organic framework nanoparticles: toward stealth drug nanocarriers. Adv Healthc Mater 2015, 4 (8), 1246–57. [DOI] [PubMed] [Google Scholar]
- 48.Jaulin N; Appel M; Passirani C; Barratt G; Labarre D, Reduction of the uptake by a macrophagic cell line of nanoparticles bearing heparin or dextran covalently bound to poly(methyl methacrylate). J Drug Target 2000, 8 (3), 165–72. [DOI] [PubMed] [Google Scholar]
- 49.Socha M; Lamprecht A; El Ghazouani F; Emond E; Maincent P; Barre J; Hoffman M; Ubrich N, Increase in the vascular residence time of propranolol loaded nanoparticles coated with heparin. J Nanosci Nanotechnol 2008, 8 (5), 2369–76. [DOI] [PubMed] [Google Scholar]
- 50.Socha M; Bartecki P; Passirani C; Sapin A; Damge C; Lecompte T; Barre J; El Ghazouani F; Maincent P, Stealth nanoparticles coated with heparin as peptide or protein carriers. J Drug Target 2009, 17 (8), 575–85. [DOI] [PubMed] [Google Scholar]
- 51.Wuang SC; Neoh KG; Kang ET; Pack DW; Leckband DE, Heparinized magnetic nanoparticles: In-vitro assessment for biomedical applications. Adv Funct Mater 2006, 16 (13), 1723–1730. [Google Scholar]
- 52.Ono K; Ishihara M; Ishikawa K; Ozeki Y; Deguchi H; Sato M; Hashimoto H; Saito Y; Yura H; Kurita A; Maehara T, Periodate-treated, non-anticoagulant heparin carrying polystyrene (NAC-HCPS) affects angiogenesis and inhibits subcutaneous induced tumour growth and metastasis to the lung. Br J Cancer 2002, 86 (11), 1803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lundin L; Larsson H; Kreuger J; Kanda S; Lindahl U; Salmivirta M; Claesson-Welsh L, Selectively desulfated heparin inhibits fibroblast growth factor-induced mitogenicity and angiogenesis. J Biol Chem 2000, 275 (32), 24653–60. [DOI] [PubMed] [Google Scholar]
- 54.Park K; Lee GY; Kim YS; Yu M; Park RW; Kim IS; Kim SY; Byun Y, Heparin-deoxycholic acid chemical conjugate as an anticancer drug carrier and its antitumor activity. J Control Release 2006, 114 (3), 300–6. [DOI] [PubMed] [Google Scholar]
- 55.Toole BP, Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 2004, 4 (7), 528–39. [DOI] [PubMed] [Google Scholar]
- 56.Upadhyay KK; Bhatt AN; Castro E; Mishra AK; Chuttani K; Dwarakanath BS; Schatz C; Le Meins JF; Misra A; Lecommandoux S, In vitro and in vivo evaluation of docetaxel loaded biodegradable polymersomes. Macromol Biosci 2010, 10 (5), 503–12. [DOI] [PubMed] [Google Scholar]
- 57.Peer D; Park EJ; Morishita Y; Carman CV; Shimaoka M, Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science 2008, 319 (5863), 627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Q; Deng C; Fu Y; Sun X; Gong T; Zhang Z, Repeated Administration of Hyaluronic Acid Coated Liposomes with Improved Pharmacokinetics and Reduced Immune Response. Mol Pharm 2016, 13 (6), 1800–8. [DOI] [PubMed] [Google Scholar]
- 59.Ebbesen MF; Olesen MT; Gjelstrup MC; Pakula MM; Larsen EK; Hansen IM; Hansen PL; Mollenhauer J; Malle BM; Howard KA, Tunable CD-44 specific cellular retargeting with hyaluronic acid nanoshells. Pharm Res 2015, 32 (4), 1462–74. [DOI] [PubMed] [Google Scholar]
- 60.Negi LM; Jaggi M; Joshi V; Ronodip K; Talegaonkar S, Hyaluronic acid decorated lipid nanocarrier for MDR modulation and CD-44 targeting in colon adenocarcinoma. Int J Biol Macromol 2015, 72, 569–74. [DOI] [PubMed] [Google Scholar]
- 61.Wickens JM; Alsaab HO; Kesharwani P; Bhise K; Amin M; Tekade RK; Gupta U; Iyer AK, Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discov Today 2017, 22 (4), 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu CM; Zhang L; Aryal S; Cheung C; Fang RH; Zhang L, Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A 2011, 108 (27), 10980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rao L; Xu JH; Cai B; Liu H; Li M; Jia Y; Xiao L; Guo SS; Liu W; Zhao XZ, Synthetic nanoparticles camouflaged with biomimetic erythrocyte membranes for reduced reticuloendothelial system uptake. Nanotechnology 2016, 27 (8), 085106. [DOI] [PubMed] [Google Scholar]
- 64.Gao W; Hu CM; Fang RH; Luk BT; Su J; Zhang L, Surface functionalization of gold nanoparticles with red blood cell membranes. Adv Mater 2013, 25 (26), 3549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piao JG; Wang L; Gao F; You YZ; Xiong Y; Yang L, Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano 2014, 8 (10), 10414–25. [DOI] [PubMed] [Google Scholar]
- 66.Ren X; Zheng R; Fang X; Wang X; Zhang X; Yang W; Sha X, Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials 2016, 92, 13–24. [DOI] [PubMed] [Google Scholar]
- 67.Parodi A; Quattrocchi N; van de Ven AL; Chiappini C; Evangelopoulos M; Martinez JO; Brown BS; Khaled SZ; Yazdi IK; Enzo MV; Isenhart L; Ferrari M; Tasciotti E, Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol 2013, 8 (1), 61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palomba R; Parodi A; Evangelopoulos M; Acciardo S; Corbo C; de Rosa E; Yazdi IK; Scaria S; Molinaro R; Furman NE; You J; Ferrari M; Salvatore F; Tasciotti E, Biomimetic carriers mimicking leukocyte plasma membrane to increase tumor vasculature permeability. Sci Rep 2016, 6, 34422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xuan M; Shao J; Dai L; He Q; Li J, Macrophage Cell Membrane Camouflaged Mesoporous Silica Nanocapsules for In Vivo Cancer Therapy. Adv Healthc Mater 2015, 4 (11), 1645–52. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L; Li R; Chen H; Wei J; Qian H; Su S; Shao J; Wang L; Qian X; Liu B, Human cytotoxic T-lymphocyte membrane camouflaged nanoparticles combined with low-dose irradiation: a new approach to enhance drug targeting in gastric cancer. Int J Nanomedicine 2017, 12, 2129–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu CM; Fang RH; Wang KC; Luk BT; Thamphiwatana S; Dehaini D; Nguyen P; Angsantikul P; Wen CH; Kroll AV; Carpenter C; Ramesh M; Qu V; Patel SH; Zhu J; Shi W; Hofman FM; Chen TC; Gao W; Zhang K; Chien S; Zhang L, Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526 (7571), 118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao C; Lin Z; Jurado Sanchez B; Lin X; Wu Z; He Q, Stem Cell Membrane-Coated Nanogels for Highly Efficient In Vivo Tumor Targeted Drug Delivery. Small 2016, 12 (30), 4056–62. [DOI] [PubMed] [Google Scholar]
- 73.Fang RH; Hu CM; Luk BT; Gao W; Copp JA; Tai Y; O’Connor DE; Zhang L, Cancer cell membrane coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett 2014, 14 (4), 2181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang P; Chen Y; Zeng Y; Shen C; Li R; Guo Z; Li S; Zheng Q; Chu C; Wang Z; Zheng Z; Tian R; Ge S; Zhang X; Xia NS; Liu G; Chen X, Virus-mimetic nanovesicles as a versatile antigen-delivery system. Proc Natl Acad Sci U S A 2015, 112 (45), E6129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu F; Reiser M; Yu X; Gummuluru S; Wetzler L; Reinhard BM, Lipid-Mediated Targeting with Membrane-Wrapped Nanoparticles in the Presence of Corona Formation. ACS Nano 2016, 10 (1), 1189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ranalli A; Santi M; Capriotti L; Voliani V; Porciani D; Beltram F; Signore G, Peptide-Based Stealth Nanoparticles for Targeted and pH-Triggered Delivery. Bioconjug Chem 2017, 28 (2), 627–635. [DOI] [PubMed] [Google Scholar]
- 77.Brown EJ; Frazier WA, Integrin-associated protein (CD47) and its ligands. Trends Cell Biol 2001, 11 (3), 130–5. [DOI] [PubMed] [Google Scholar]
- 78.Oldenborg PA; Zheleznyak A; Fang YF; Lagenaur CF; Gresham HD; Lindberg FP, Role of CD47 as a marker of self on red blood cells. Science 2000, 288 (5473), 2051–4. [DOI] [PubMed] [Google Scholar]
- 79.Rodriguez PL; Harada T; Christian DA; Pantano DA; Tsai RK; Discher DE, Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 2013, 339 (6122), 971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qie Y; Yuan H; von Roemeling CA; Chen Y; Liu X; Shih KD; Knight JA; Tun HW; Wharen RE; Jiang W; Kim BY, Surface modification of nanoparticles enables selective evasion of phagocytic clearance by distinct macrophage phenotypes. Sci Rep 2016, 6, 26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pitek AS; Jameson SA; Veliz FA; Shukla S; Steinmetz NF, Serum albumin ‘camouflage’ of plant virus based nanoparticles prevents their antibody recognition and enhances pharmacokinetics. Biomaterials 2016, 89, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gulati NM; Pitek AS; Steinmetz NF; Stewart PL, Cryo-electron tomography investigation of serum albumin-camouflaged tobacco mosaic virus nanoparticles. Nanoscale 2017, 9 (10), 3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gulati NM; Pitek AS; Czapar AE; Stewart PL; Steinmetz NF, The in vivo fates of plant viral nanoparticles camouflaged using self-proteins: overcoming immune recognition. J Mater Chem B 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meyer DE; Chilkoti A, Quantification of the effects of chain length and concentration on the thermal behavior of elastin-like polypeptides. Biomacromolecules 2004, 5 (3), 846–51. [DOI] [PubMed] [Google Scholar]
- 85.Urry DW; Pattanaik A, Elastic protein based materials in tissue reconstruction. Ann N Y Acad Sci 1997, 831, 32–46. [DOI] [PubMed] [Google Scholar]
- 86.Liu W; Dreher MR; Chow DC; Zalutsky MR; Chilkoti A, Tracking the in vivo fate of recombinant polypeptides by isotopic labeling. J Control Release 2006, 114 (2), 184–92. [DOI] [PubMed] [Google Scholar]
- 87.Liu W; Dreher MR; Furgeson DY; Peixoto KV; Yuan H; Zalutsky MR; Chilkoti A, Tumor accumulation, degradation and pharmacokinetics of elastin-like polypeptides in nude mice. J Control Release 2006, 116 (2), 170–8. [DOI] [PubMed] [Google Scholar]
- 88.Gilroy CA; Luginbuhl KM; Chilkoti A, Controlled release of biologics for the treatment of type 2 diabetes. J Control Release 2016, 240, 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schlapschy M; Binder U; Borger C; Theobald I; Wachinger K; Kisling S; Haller D; Skerra A, PASylation: a biological alternative to PEGylation for extending the plasma half-life of pharmaceutically active proteins. Protein Eng Des Sel 2013, 26 (8), 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ali A; Suhail M; Mathew S; Shah MA; Harakeh SM; Ahmad S; Kazmi Z; Alhamdan MA; Chaudhary A; Damanhouri GA; Qadri I, Nanomaterial Induced Immune Responses and Cytotoxicity. J Nanosci Nanotechnol 2016, 16 (1), 40–57. [DOI] [PubMed] [Google Scholar]
- 91.Hedayati MH; Norouzian D; Aminian M; Teimourian S; Ahangari Cohan R; Sardari S; Khorramizadeh MR, Molecular Design, Expression and Evaluation of PASylated Human Recombinant Erythropoietin with Enhanced Functional Properties. Protein J 2017, 36 (1), 36–48. [DOI] [PubMed] [Google Scholar]
- 92.Kuhn N; Schmidt CQ; Schlapschy M; Skerra A, PASylated Coversin, a C5-Specific Complement Inhibitor with Extended Pharmacokinetics, Shows Enhanced Anti-Hemolytic Activity in Vitro. Bioconjug Chem 2016, 27 (10), 2359–2371. [DOI] [PubMed] [Google Scholar]
- 93.Chen S; Cao Z; Jiang S, Ultra-low fouling peptide surfaces derived from natural amino acids. Biomaterials 2009, 30 (29), 5892–6. [DOI] [PubMed] [Google Scholar]
- 94.White AD; Nowinski AK; Huang WJ; Keefe AJ; Sun F; Jiang SY, Decoding nonspecific interactions from nature. Chem Sci 2012, 3 (12), 3488–3494. [Google Scholar]
- 95.Nowinski AK; White AD; Keefe AJ; Jiang S, Biologically inspired stealth peptide-capped gold nanoparticles. Langmuir 2014, 30 (7), 1864–70. [DOI] [PubMed] [Google Scholar]
- 96.Tseng YS; Agbandje McKenna M, Mapping the AAV Capsid Host Antibody Response toward the Development of Second Generation Gene Delivery Vectors. Front Immunol 2014, 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tse LV; Klinc KA; Madigan VJ; Rivera RMC; Wells LF; Havlik LP; Smith JK; Agbandje-McKenna M; Asokan A, Structure-Guided Iterative Evolution of Antigenically Advanced AAV Variants for Therapeutic Gene Transfer. Mol Ther 2017, 25 (5), 232–232.28129117 [Google Scholar]