Abstract
The endogenous kappa opioid system has primarily been shown to be involved with a state of dysphoria and aversion. Stress and exposure to drugs of abuse, particularly alcohol, can produce similar states of unease and anxiety, implicating the kappa opioid system as a target of stress and alcohol. Numerous behavioral studies have demonstrated reduced sensitivity to manipulations of the kappa opioid system in early-life relative to adulthood, and recent reports have shown that the kappa opioid system is functionally different across ontogeny. Given the global rise in early-life stress and alcohol consumption, understanding how the kappa opioid system responds and adapts to stress and/or alcohol exposure differently in early-life and adulthood is imperative. Therefore, the objective of this review is to highlight and discuss studies examining the impact of early-life stress and/or alcohol on the kappa opioid system, with focus on the documented neuroadaptations that may contribute to future vulnerability to stress and/or increase the risk of relapse. We first provide a brief summary of the importance of studying the effects of stress and alcohol during early-life (prenatal, neonatal/juvenile, and adolescence). We then discuss the literature on the effects of stress or alcohol during early-life and adulthood on the kappa opioid system. Finally, we discuss the few studies that have shown interactions between stress and alcohol on the kappa opioid system and provide some discussion about the need for studies investigating the development of the kappa opioid system.
Introduction
Mental disorders, particularly anxiety and depression, can manifest at various stages of life. There are numerous factors that can contribute to the development of anxiety and depression, including genetic predisposition (Craske & Stein, 2016), chronic stress and/or trauma (Enoch, 2011), and alcohol exposure (Koob, 2014; Schmidt, Buckner, & Keough, 2007). While the onset of mental disorders in genetically predisposed individuals is difficult to predict and prevent, there is growing evidence that stress and/or alcohol exposure during early life can have more devastating and long-term effects than exposure in adulthood (Enoch, 2011; Romeo, 2017; Tottenham & Galvan, 2016). Nevertheless, negative outcomes of stress and/or alcohol have been attributed to a multitude of neurobiological alterations in humans and animal models (Enoch, 2011). There has been a recent rise in interest in the kappa opioid system as a key target of stress and alcohol due to its role in aversion and dysphoria, a common phenotype associated with exposure to stress and/or alcohol (Anderson & Becker, 2017; Chavkin & Ehrich, 2014; Crowley & Kash, 2015; Schwarzer, 2009; Tejeda, Shippenberg, & Henriksson, 2012; Van’t Veer & Carlezon, 2013). However, there have been a number of paradoxical effects reported following manipulations of the kappa opioid system for which the underlying mechanisms have yet to be determined [highlighted in these reviews (Crowley & Kash, 2015; Hang, Wang, He, & Liu, 2015)]. Importantly, age-dependent changes in kappa opioid system function have recently been identified that may begin to explain some of these paradoxical effects of kappa opioid receptor (KOR) activation. However, this potential link between kappa opioid system function and differential age-dependent vulnerabilities to stress and/or alcohol has not been exclusively examined. Based on these understudied interactions, this review will focus on the impact of stress and/or alcohol on the kappa opioid system as a function of age. Since there is a wealth of literature on stress and alcohol alone, we will only briefly describe the importance of studying them from an ontogenetic perspective.
Early-life stress
Stress is an inevitable phenomenon that all organisms experience from in utero through late-stages of life. Depending on the type of stressor(s) (i.e. physical or psychological), stress can result in numerous deleterious outcomes. While physical stressors, such as exhaustion from exercise, may resolve relatively quickly, psychological stressors can result in life-long consequences, such as various forms of mental illness, including anxiety, depression, and drug/alcohol abuse (Lopez, Turner, & Saavedra, 2005; Low, Lee, Johnson, Williams, & Harris, 2008; Rutledge & Sher, 2001; Schmidt et al., 2007). Additionally, the duration of stress may also significantly contribute to long-term consequences (Lucassen et al., 2014). Importantly, growing evidence indicates that age of exposure to stress can significantly influence the neurodevelopmental trajectory of the organism, such that early-life stress may be more detrimental and irreversible than stress exposure in adulthood (Enoch, 2011; Romeo, 2017; Tottenham & Galvan, 2016). This is becoming increasingly evident as the rates of anxiety (Merikangas et al., 2010), depression (Carrellas, Biederman, & Uchida, 2017), and alcohol use disorder (Patrick et al., 2013) in adolescents are steadily rising. The kappa opioid system has been established as a primary target of stress and growing evidence indicates that alterations in the kappa opioid system can arise from stress exposure through the life-span, increasing the predisposition to future stress vulnerability, among other psychopathologies.
Early-life alcohol exposure
Alcohol consumption is socially accepted on a global scale, and although our awareness of the deleterious consequences of alcohol has grown, there is still a significant issue with excessive alcohol use worldwide. In the U.S, rates of heavy alcohol consumption in youth are high, with an estimated 2% of adolescents (ages 12–17) meeting the criteria for diagnosis of an alcohol use disorder ((SAMHSA)-1, 2016), and 50% of adolescents reporting consumption of alcohol before the age of 18 ((SAMHSA)-2, 2016). Furthermore, in 2016, 57.1% of U.S. college students (ages 18–25) reported drinking alcohol in the previous month; an additional 38.4% reported binge drinking (reaching a blood alcohol concentration of 0.08 g/dL) and 10.1% reported heavy alcohol use (binge drinking on 5+ days in the past month) ((SAMHSA)-3, 2016). Equally staggering, the rate of fetal alcohol spectrum disorders (FASDs) resulting from alcohol consumption during pregnancy is 2–5% (May et al., 2009), which is likely an underestimate due, in part, to the stigma of disclosing drinking habits during pregnancy (Corrigan et al., 2017). Hence, and relevant to this review, exposure to alcohol early in development is decidedly prevalent, and can result in a multitude of harmful effects to the developing central nervous system, as similarly noted with early-life stress. Furthermore, there is a long-standing literature indicating that the kappa opioid system is a major target of alcohol throughout ontogeny.
Kappa opioid system: a target of stress and ethanol
The kappa opioid system is one of three classical endogenous opioid systems in the central nervous system (CNS). Dynorphin is the endogenous ligand for KORs (Chavkin, James, & Goldstein, 1982), which are G-protein receptors that couple with Gi (Bruchas et al., 2007), although a few studies have also demonstrated coupling with Gs in cell culture lines from various brain regions, including catecholaminergic cells from brainstem (Baraban, Lothman, Lee, & Guyenet, 1995), hippocampus (Hampson, Mu, & Deadwyler, 2000), and dorsal root ganglia (Shen & Crain, 1990a, 1990b, 1994). Historically, activation of KORs has been shown to be involved in numerous functions, including learning and memory, emotional control, stress response, and pain (Schwarzer, 2009). Although the mechanisms by which KORs modulate cellular activity are important, discussion of these mechanisms are outside of the scope of this review; however, for further information on this subject, see the excellent review by Bruchas and colleagues (Bruchas et al., 2007).
Despite the various roles of KORs, it is well established that activation of the kappa opioid system can produce dysphoric and aversive effects in humans (Pfeiffer, Brantl, Herz, & Emrich, 1986) and animal models (Carlezon et al., 2006; Mague et al., 2003; Todtenkopf, Marcus, Portoghese, & Carlezon, 2004). Given that stress and alcohol dependence also result in dysphoria and aversion, there has been a surge in interest attempting to determine whether the consequences of stress and alcohol dependence are mediated, in part, through alterations in the kappa opioid system (Anderson & Becker, 2017; Bruchas, Land, & Chavkin, 2010; Crowley & Kash, 2015; Knoll & Carlezon, 2010; Tejeda, Shippenberg, et al., 2012; Van’t Veer & Carlezon, 2013; Walker, Valdez, McLaughlin, & Bakalkin, 2012; Wee & Koob, 2010). However, despite the wealth of literature which has demonstrated that activation of the kappa opioid system produces dysphoria and aversion in stress- and alcohol-naïve animals, there have been a number of studies that have reported the opposite effect [see review (Hang et al., 2015)]. For example, several studies have shown that systemic administration of KOR agonists can produce anxiolytic effects (Alexeeva, Nazarova, & Sudakov, 2012; Bilkei-Gorzo et al., 2008; Braida et al., 2009; Kudryavtseva, Gerrits, Avgustinovich, Tenditnik, & Van Ree, 2006; Privette & Terrian, 1995). Similar anxiolytic effects have been reported following site-specific activation of KORs within the infralimbic cortex (Wall & Messier, 2000b), while blockade of KORs within the infralimbic cortex with the KOR-selective antagonist nor-BNI produced anxiogenesis (Wall & Messier, 2000a). Various factors have been suggested to contribute to these paradoxical effects of the kappa opioid system, such as species/strain, dose of KOR agonist/antagonist, timing of drug administration, and behavioral test. However, while noted in a subset of studies, the influence of age is largely ignored within this literature.
Although only few studies have examined the role of the kappa opioid system in early-life (i.e. neonatal through adolescence), they have provided some interesting observations. In one study, infant rats (~postnatal day (P) 0) exhibited increased appetitive responding for water following KOR activation (Petrov, Nizhnikov, Varlinskaya, & Spear, 2006), an effect opposite to what has been reported in adults (Bals-Kubik, Herz, & Shippenberg, 1989; Shippenberg & Herz, 1986). While not directly testing age as a factor, anxiolytic effects of KOR activation have also been reported in presumably younger animals (based on reported weight – 170–210 grams) (Alexeeva et al., 2012; Privette & Terrian, 1995). Reduced sensitivity to the aversive effects of KOR activation was also demonstrated in drug-naïve adolescents (P30–35) compared to adults (P72–77) (Anderson, Morales, Spear, & Varlinskaya, 2014). Another study similarly found that adolescents (P28) did not show an aversive response to KOR activation relative to nicotine-treated adults (P60), who exhibited robust KOR-mediated aversion (Tejeda, Natividad, Orfila, Torres, & O’Dell, 2012). Interestingly, KOR activation in juveniles (P21) reduced social play, although there was not a direct comparison to adults (Vanderschuren, Niesink, Spruijt, & Van Ree, 1995). In addition to behavioral evidence supporting ontogenetic differences in the kappa opioid system, we and others have recently demonstrated functional evidence for age-dependent changes in the kappa opioid system in brain regions associated with emotional processing and drug addiction. Specifically, we found that KOR activation in the basolateral amygdala increases GABA transmission in adolescents (P30–45), but not in adults (>P60), and this age-dependent effect was not observed in the central amygdala where KOR activation reduces GABA transmission (Przybysz, Werner, & Diaz, 2017). Another study has shown that dynorphin-induced hyperpolarization in neurons of the paraventricular nucleus of the thalamus increases through puberty (~4 weeks of age) followed by a return to levels similar to those observed at 2 weeks of age (Chen et al., 2015). Interestingly, the previously described study by Tejeda and colleagues found that although the KOR-mediated decrease in dopamine levels in the nucleus accumbens was exacerbated in nicotine-treated adults relative to saline controls, the KOR-mediated decrease in dopamine was not observed in adolescents (Tejeda, Natividad, et al., 2012). While these studies provide compelling evidence for age-dependent functions in the kappa opioid system across specific brain regions, it speaks to the difficulty of interpreting results from systemic administration of KOR-selective drugs where potentially opposing processes are engaged that may negate each other. Because of this, there is a clear need to investigate the development of the kappa opioid system in other brain structures and to understand how these systems may interact, particularly following exposure to stress and/or alcohol.
Impact of stress on kappa opioid system
Prenatal
While our understanding of the role of the kappa opioid system in early-life is limited, studies that have examined the impact of early-life stress on this system are scarcer. To our knowledge, only one study has examined the long-term neuroadaptations in the kappa opioid system following prenatal stress. Peters and colleagues found that offspring from dams that were stressed during the last week of gestation (gestational days (G) 14–20) showed robust anxiety-like behavior on the elevated plus maze in adulthood, which was completely abolished when adult offspring were pretreated with two novel short-acting KOR antagonists, AZ-MTAB and LY-DMPF (Peters et al., 2011). Based on this study, it is plausible that prenatal stress leads to hyper-activation of the kappa opioid system in adult offspring; however, the mechanisms driving this potential hyper-activation have yet to be determined.
It is surprising that the long-term neuroadaptations in the kappa opioid system following early-life stress have not been studied given the well-established literature in adults demonstrating stress-induced upregulation of kappa opioid function (Knoll & Carlezon, 2010). In addition to long-term alterations in the kappa opioid system following prenatal stress, it is worth noting that dynorphin can induce release of adrenocorticotropin in the fetal brain through opioid and non-opioid mechanisms (Szeto, 2003). Specifically, through the classical opioid-dependent mechanism, dynorphin activation of hypothalamic KORs in the fetal brain leads to release of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) which can trigger adrenocorticotropin release. In contrast, the non-opioid mechanism involves NMDA receptors and prostaglandins. Interestingly, unlike the classical opioid mechanism that is active throughout the lifespan, the non-opioid mechanism peaks a few days prior to parturition and is only transiently active during the perinatal period. While currently unknown, based on our knowledge of the impact of stress on the kappa opioid system, these transient periods in kappa opioid function are likely highly vulnerable developmental stages.
Early-life/adolescence
Unlike research in the prenatal stress field, there has been a recent rise in studies investigating the impact of early-life stress on the kappa opioid system. Models of neonatal stress have revealed several interesting observations. Michaels and Holtzman demonstrated that following maternal separation (P1–12), adult offspring exhibited attenuated KOR-induced place aversion (Michaels & Holtzman, 2008). Conversely, another study using a series of neonatal stressors from P5–9 (maternal separation, gavage feedings and/or hypoxia/hyperoxia) found a reduction in cocaine conditioned-place preference, which was reversed by a KOR agonist only in the stressed adult offspring, but not in the non-handled offspring (Hays et al., 2012). These data suggest that neonatal stress may sensitize the kappa opioid system, since the change in conditioned-place preference was reversed following KOR activation only in the neonatally stressed animals. Therefore, it is possible that the ‘normal’ neurochemical balance is shifted following early-life stress, which can ultimately result in brain dysfunction and psychopathology. Together, these demonstrate that early-life stress results in various long-term alterations in the kappa opioid system, but the location of these effects within the brain, as well as the identity of the respective mechanisms contributing to the observed behavioral alterations, are unknown. However, a study by Ploj and Nylander examined KOR levels in adults that underwent maternal separation as neonates (age unknown) and found that KOR levels were unchanged in all of the brain regions tested (Ploj & Nylander, 2003), suggesting that neonatal stress may not alter KOR levels. It is possible, though, that prenatal stress alters KOR function or dynorphin release, but this has yet to be tested.
As previously discussed, adolescent stress has become a hot topic due to the increased vulnerability to stress during that developmental stage. While not much work has been done to investigate the effects of adolescent stress on the kappa opioid system, recently, a group using a model of social isolation during adolescence has demonstrated robust and persistent effects on various behaviors, including anxiety, fear conditioning, and ethanol consumption (Butler, Ariwodola, & Weiner, 2014; Butler, Karkhanis, Jones, & Weiner, 2016). Interestingly, it was found that social isolation from P28–110 leads to a hypodopaminergic state in the nucleus accumbens that could be a result of increased KOR sensitivity (i.e. KOR-mediated inhibition of dopamine release) (Karkhanis, Rose, Weiner, & Jones, 2016). It is also possible that early-life stress permanently increases KOR levels, as a long-lasting increase in KOR binding throughout the CNS of adults was shown following juvenile isolation from P22–35 (Van den Berg, Van Ree, Spruijt, & Kitchen, 1999). Regardless of the mechanisms that are engaged by early-life stress, since adolescence is a period when the kappa opioid system is functionally changing in various brain regions (Chen et al., 2015; Przybysz et al., 2017), studies aimed at better understanding potential neuroadaptations in the kappa system following adolescent stress are warranted.
Adulthood
Most of our understanding of the effects of stress on the kappa opioid system is derived from studies done in adults. While the alterations are vast and have been discussed numerous times (Crowley & Kash, 2015; Hang et al., 2015; Knoll & Carlezon, 2010; Van’t Veer & Carlezon, 2013), there are several consistent effects that have been reported following exposure to stress in adulthood. Initially, stress induces release of dynorphin which activates the HPA axis (Hayes & Stewart, 1985; Iyengar, Kim, & Wood, 1986; Pascoe et al., 2008; Ur, Wright, Bouloux, & Grossman, 1997), likely through both stimulation of CRH release in the hypothalamus and CRH-independent mechanisms (Buckingham & Cooper, 1986; Carboni et al., 2010; Nikolarakis, Almeida, & Herz, 1986). Given this effect, stress-induced increases in corticosterone levels are blunted in animals injected with norBNI and prodynorphin knockouts (Wittmann et al., 2009). Although this HPA axis-centered stress response is important as a general marker of stress activation, we know that stress induces dynorphin release throughout the CNS, not just within the hypothalamus. Based on this, it is hypothesized that dynorphin activation of KORs can disinhibit brain areas involved in the physiological and psychological manifestations of aversion, dysphoria, and anxiety (Van’t Veer & Carlezon, 2013). Much of this work has demonstrated that KOR stimulation inhibits dopamine release in the nucleus accumbens (Carlezon et al., 2006), thereby disinhibiting medium spiny neurons. Recently, it was also shown that KORs differentially modulate synaptic glutamatergic and GABAergic inputs onto D1 and D2 dopamine receptor-expressing medium spiny neurons (Tejeda et al., 2017). Furthermore, where dynorphin release occurs within the nucleus accumbens can also differentially modulate circuits involved in either reward or aversion (Al-Hasani et al., 2015). Similar mechanisms have been suggested to occur in the basolateral amygdala, whereby a reciprocal interaction between dynorphin and CRH has been reported (Bruchas, Land, Lemos, & Chavkin, 2009; Land et al., 2008); however, although the specific neurophysiological consequences of these interactions in the basolateral amygdala have not been examined, a potentially similar mechanism in the central amygdala was recently investigated using KOR knockout mice (Kang-Park, Kieffer, Roberts, Siggins, & Moore, 2015). Additionally, KORs within the medial prefrontal cortex have been shown to gate inputs from the basolateral amygdala (Tejeda et al., 2015), with a similar mechanism regulating basolateral amygdala, but not prefrontal cortical, inputs into the bed nucleus of the stria terminalis (Crowley et al., 2016). This suggests that stress could lead to neuroadaptations at various levels of neuronal regulation that are associated with anxiety/depression and alcohol consumption.
Although stress exposure leads to acute activation of the kappa opioid system throughout the CNS, an important question to consider is what the neuroadaptations in the kappa opioid system are in adults following repeated stress exposure. This is particularly important as the sustained consequences of stress are thought to be due, in part, to alterations in the kappa opioid system. Several studies have attempted to block stress-induced anxiogenic and prodepressive-like effects in adults through administration of KOR antagonists (Knoll, Meloni, Thomas, Carroll, & Carlezon, 2007; McLaughlin, Conron, Koenen, & Gilman, 2010). However, these studies have primarily demonstrated that the long-lasting stress-induced neuroadaptations of the kappa opioid system can be prevented by blocking KORs before the exposure to stress. Therefore, while it is not clear whether kappa opioid system function is upregulated following repeated exposure to stress based on behavioral studies, we do know that the kappa opioid system continues to be activated following repeated exposure to forced swim stress in the paraventricular nucleus (Lemos et al., 2012) or fear conditioning in the basolateral amygdala (Knoll et al., 2011) in adulthood. This suggests that hyper-activation of the kappa opioid system contributes to the long-term behavioral consequences of stress (Knoll et al., 2011; Lemos et al., 2012). Taken together, it is highly probable that different mechanisms are engaged acutely following stress than those that result from chronic exposure to stress, a topic that is comprehensively covered by some of the leaders in the field (Knoll & Carlezon, 2010). Although these elegant studies have provided us an important basis regarding the neuroadaptations that the kappa opioid system undergoes following exposure to stress, our current understanding of the relationship between stress and KORs is still lacking.
Kappa Opioid Receptors and Ethanol
Ethanol and the kappa opioid system have a bidirectional relationship, such that ethanol exposure activates and alters the kappa opioid system, while manipulation of KORs alters responding for ethanol. For example, studies have shown that acute ethanol leads to release of dynorphin in several brain regions of adult animals (Jarjour, Bai, & Gianoulakis, 2009; Lam, Marinelli, Bai, & Gianoulakis, 2008; Marinelli, Lam, Bai, Quirion, & Gianoulakis, 2006). Additionally, manipulations of the kappa opioid system have been shown to influence ethanol intake in studies using KOR agonists/antagonists and KOR/dynorphin knockout animals (Blednov, Walker, Martinez, & Harris, 2006; Femenia & Manzanares, 2012; Kovacs et al., 2005; Miranda-Morales, Nizhnikov, & Spear, 2014; Morales, Anderson, Spear, & Varlinskaya, 2014). The KOR system also plays a role in the reinforcing properties of ethanol, although the results from available studies indicate that this may only occur following ethanol dependence (Wee & Koob, 2010). Given the complex interactions between the kappa opioid system in the ethanol-naïve versus the ethanol-exposed brain (Wee & Koob, 2010), a critical consideration is that ethanol exposure at certain developmental points may differentially alter the function of the kappa opioid system. This is further supported by our increasing knowledge of developmental regulation of the kappa opioid system, as previously discussed, in the absence of exposure to ethanol. Interestingly, KOR activation has been shown to facilitate expression of appetitive ethanol reinforcement in ethanol-naïve infant pups (P14–15) (Pautassi, Nizhnikov, Acevedo, & Spear, 2012) and adults (8–12 weeks old) (Sperling, Gomes, Sypek, Carey, & McLaughlin, 2010). However, prenatal ethanol exposure may result in different effects than adult exposure. For example, Diaz-Cenzano et al. recently demonstrated that prenatal ethanol exposure on G19–20 likely activates the kappa opioid system in the fetus, resulting in increased palatability for ethanol on P14. Specifically, when prenatal ethanol was administered along with the KOR antagonist, nor-BNI, the increased palatability for ethanol was not observed (Diaz-Cenzano, Gaztanaga, & Gabriela Chotro, 2014), implicating an association with prenatal ethanol and activation of the kappa opioid system in utero. However, blocking of KORs during prenatal ethanol treatment did not affect ethanol intake, as both the prenatal ethanol-exposed group and the nor-BNI-paired group had increased ethanol intake compared with controls, with no difference between these two experimental groups (Diaz-Cenzano et al., 2014). Similar findings were found with prenatal ethanol exposure on G19–20 (Gaztanaga, Aranda-Fernandez, & Chotro, 2015). Thus, understanding the ontogenetic regulation of the kappa opioid system has important implications for the role that ethanol exposure plays in further altering its function, especially during developmental periods in which shifts in function of the kappa opioid system may occur. Here, we review the available information about the effects that ethanol exposure at different developmental points has on the kappa opioid system.
Prenatal Ethanol Exposure
While an abundance of work has been dedicated to investigating changes to the KOR system following ethanol exposure in adulthood (to be discussed later), very few studies have examined the consequences of early life ethanol exposure. This is surprising, given that a study in 2006 examining human post-mortem brain tissue from aborted fetuses at mid-gestation found significant alterations in KOR mRNA in various brain structures involved with anxiety/depression and alcohol consumption (i.e. amygdala, striatum, and hippocampus) following prenatal alcohol exposure (Wang, Dow-Edwards, Anderson, Minkoff, & Hurd, 2006). In animal models, most of the work investigating the effects of prenatal ethanol exposure has been limited to exposure during gestational days 17–20, a time at which the kappa opioid system is already functional (De Vries, Hogenboom, Mulder, & Schoffelmeer, 1990) and during which transient processes involving the kappa opioid system occur (Szeto, 2003). However, ethanol exposure during this short prenatal window nevertheless alters the expression of KORs, as well as their associated proteins and genes, in specific brain regions similar to what has been reported in humans. For instance, following exposure to 1 or 2 g/kg ethanol on G17–20, KOR and prodynorphin mRNA and protein levels were differentially affected in the ventral tegmental area, nucleus accumbens, and hypothalamus, with a specific reduction in KOR protein in the nucleus accumbens at P14 (Bordner & Deak, 2015). The timing of this reduction is consistent with another study within the same gestational exposure period, which shows that KOR protein is reduced in the amygdala, nucleus accumbens, and hippocampus on P14 (Nizhnikov et al., 2014), although no other neonatal points were examined. Another study extended their investigation of the effects of prenatal ethanol exposure on gestational days 17–20 to mid-late adolescence (P35–39), and found that while prenatal ethanol exposed animals have higher ethanol intake, there were no differences in KOR mRNA in the nucleus accumbens, infralimbic cortex, or ventral tegmental area (Fabio, Macchione, Nizhnikov, & Pautassi, 2015), suggesting that alterations in the kappa opioid system may be transient. Nevertheless, it is clear that prenatal ethanol exposure alters the development of the kappa opioid system and that the timing of these neuroadaptations is brain region-dependent and may be ontogenetically unique.
The literature regarding functional alterations in the kappa opioid system following prenatal ethanol exposure is also limited, but consistently demonstrates that prenatal alcohol exposure either activates or leads to alterations in the kappa opioid system. One study by Nizhnikov et al. found that prenatal ethanol exposure on gestational days 17–20 results in alterations in the kappa opioid system associated with increased ethanol intake in pre-weanlings. Specifically, while blocking KORs in control animals increases intake, blocking KORs in prenatal ethanol-exposed animals reduces ethanol intake, presumably as a result of decreased KOR protein expression in the above mentioned brain regions (Nizhnikov et al., 2014). Furthermore, KOR activation in control animals results in conditioned place aversion, while ethanol-exposed animals exhibit conditioned place preference (Nizhnikov et al., 2014). However, the potential effects of prolonged prenatal ethanol exposure, whether or not these alterations persist beyond juvenility, and the outcomes resulting from these alterations have yet to be investigated.
Adolescent Ethanol Exposure
As with the lack of studies examining the impact of prenatal alcohol exposure on this system, there is also a lack of studies that have examined interactions between ethanol and the kappa opioid system during adolescence. This is particularly shocking considering 1) adolescence is a period of significant neural development and maturation, 2) the kappa opioid system plays a role in stress and anxiety, which are typically high during adolescence, and 3) adolescence is the period during which experimentation with ethanol often begins and when ethanol use is high (Spear, 2000, 2014). Interestingly, one study found that while systemic inhibition of KORs with norBNI increases ethanol intake (limited access to 10% ethanol/supersaccarin solution every other day) in adult males and decreases intake in adult females (P70–80), there was no effect of norBNI on ethanol intake in adolescents (P28–38) of either sex (Morales et al., 2014). While this study provides additional evidence for an ontogenetic difference in function of the kappa opioid system, it is unknown why adolescents are unresponsive to norBNI, given that adolescents have functional KORs in brain regions involved in modulating ethanol intake, such as the basolateral and central amygdala (Przybysz et al., 2017) and the paraventricular nucleus (Chen et al., 2015). One potential explanation is that since KOR activation has opposing effects in inter-connected brain structures (i.e. KOR activation ↑ GABA in the basolateral and ↓ GABA in the central amygdala (Przybysz et al., 2017)), systemic blockade of KORs may result in no net effect. However, since other brain regions are also involved in mediating ethanol consumption, this is likely not the primary contribution to the observed lack of effect in adolescents. Another possibility is that there may be little to no tonic release of dynorphin in the adolescent brain, particularly during ethanol consumption. In contrast, in the adult brain, ethanol consumption and/or a history of ethanol intake may induce a tonic release of dynorphin whose response is attenuated by norBNI. These are just a few examples of questions that demonstrate the need for further research investigating the neurobiological mechanisms that underlie insensitivity to inhibition of the kappa opioid system in adolescents, particularly in the context of ethanol.
Acute & Chronic Ethanol Exposure in Adulthood
As has been emphasized, the majority of work investigating ethanol-induced alterations to the kappa opioid system has been done in adults. Studies have repeatedly shown that acute ethanol administration elevates dynorphin levels, particularly in the ventral tegmental area (Jarjour et al., 2009), central amygdala (Lam et al., 2008), nucleus accumbens (Marinelli et al., 2006), and paraventricular nucleus (Chang et al., 2007). Additionally, prodynorphin gene expression was also shown to be upregulated in the prefrontal cortex and amygdala following a single exposure to ethanol (D’Addario et al., 2013).
As alluded to earlier, several studies have examined whether manipulations of the kappa opioid system can alter ethanol consumption in adults. Systemic administration of a KOR agonist has consistently been shown to decrease ethanol consumption (Henderson-Redmond & Czachowski, 2014; Lindholm, Werme, Brene, & Franck, 2001; Logrip, Janak, & Ron, 2009; Morales et al., 2014), although see (Anderson, Lopez, & Becker, 2016). Based on this general observation, the proposed hypothesis is that KOR-mediated dysphoria results in an aversion to consume ethanol. Along the same lines, blocking KORs in non-dependent adults increases ethanol consumption (Mitchell, Liang, & Fields, 2005), as this relieves KOR-dependent dysphoria. Interestingly, in alcohol preferring strains, such as the Alko Alcohol (AA) rat, activation of ventral pallidal KORs does not affect ethanol self-administration (Kemppainen, Raivio, Suo-Yrjo, & Kiianmaa, 2012). Conversely, manipulation of the kappa opioid system in ethanol-dependent animals produces the opposite effect of that seen in non-dependent animals. For example, KOR blockade significantly reduces voluntary drinking (Kissler et al., 2014; Nealey, Smith, Davis, Smith, & Walker, 2011; Walker & Koob, 2008; Walker, Zorrilla, & Koob, 2011). Other studies have also demonstrated that during withdrawal from chronic ethanol exposure, a period that is highly vulnerable to relapse (Burish, Maisto, Cooper, & Sobell, 1981; Heilig, Egli, Crabbe, & Becker, 2010; Sinclair, 1972), KOR agonists potentiate ethanol intake (Holter, Henniger, Lipkowski, & Spanagel, 2000), while KOR antagonists decrease anxiety-like behavior (Valdez & Harshberger, 2012). Interestingly, these findings are similar to the previously discussed effects seen following prenatal exposure to alcohol, whereby KOR activation in ethanol-exposed pups results in conditioned place preference for ethanol (Nizhnikov et al., 2014). Taken together, these studies demonstrate that chronic ethanol exposure induces changes in the kappa opioid system that, when engaged, promote further ethanol consumption associated with increased anxiety-like behavior. Importantly, this suggests that the organism’s perception of an activated kappa opioid system changes from non-dependence to dependence.
Along similar lines, several studies have very elegantly demonstrated some of the neuroadaptations in the kappa opioid system arising from chronic ethanol exposure in adulthood [for more information, see (Anderson & Becker, 2017)]. Following induction of ethanol dependence, dynorphin A immunoreactivity was shown to increase in the central amygdala, as did dynorphin A-stimulated coupling of KORs (Kissler et al., 2014), consistent with our proposed potential mechanism of increased tonic dynorphin release following a history of ethanol exposure. Increased cFos immunoreactivity was also observed in the paraventricular nucleus, and this was demonstrated to be mediated by the kappa opioid system using prodynorphin knock-out animals (Racz, Markert, Mauer, Stoffel-Wagner, & Zimmer, 2013). Additionally, after 7 days of repeated intra-gastric administration of 40% ethanol, increased dynorphin release in the hippocampus was detected which was accompanied by deficits in learning and memory (Kuzmin et al., 2013). The relationship between the kappa opioid system and dopamine is also altered by chronic ethanol exposure, such that KOR-induced decrease in dopamine release in the nucleus accumbens is augmented in animals chronically exposed to ethanol (Karkhanis, Huggins, Rose, & Jones, 2016; Rose et al., 2016). Given the differential role of KOR modulation of dopamine in various parts of the nucleus accumbens in regulating reward and aversion (Al-Hasani et al., 2015), this may have the potential to influence and alter the perception of the rewarding properties of ethanol.
In total, ethanol exposure at every stage in life can impact both the expression and function of the kappa opioid system. However, the direction and long-term impact of ethanol on the kappa opioid system may differ across ontogeny. Given potential developmental switches in KOR function, it is important to highlight and address the lack of attention paid to ethanol exposure during early life and its impact on the kappa opioid system. Furthermore, it is important to note that nearly all of the studies that have examined the interactions between ethanol and the kappa opioid system have used males. Interestingly, Morales and colleagues further expanded their research to include tests of females (which have been largely ignored in the field), and found the opposite effect, with non-dependent adult females drinking more ethanol following administration of the KOR agonist – males showed decreased drinking (Morales et al., 2014). These findings suggest that, besides age-dependent differences in kappa opioid function, there are likely sex-differences that should be further investigated. In support of this, the few studies that have examined alcohol- and stress-naïve females have consistently shown that adult females are less sensitive to KOR agonists (Rasakham and Liu-Chen 2011, Chartoff and Mavrikaki 2015). Whether or not young females will be similarly insensitive has yet to be determined.
Stress and alcohol interactions on kappa system
The interactions between stress and alcohol are highly complex, given that exposure to stress can increase alcohol consumption, while alcohol exposure itself, and particularly withdrawal from alcohol, can also be a stressor. As we have discussed, the kappa opioid system is a target of both stress and alcohol; how this system increases vulnerability to future stress or produces a predisposition to consume more alcohol, particularly following pre-exposure to either one in early-life, is of significant importance given the high societal prevalence of early-life exposure to stress and/or alcohol. Equally important to understand are the interactions of these exposures (stress and alcohol) in adulthood. Studies have begun to elucidate these interactions and have demonstrated somewhat consistent findings, as described below.
Early-life/adolescence
A few studies have examined the interactions between early-life stress and alcohol on the kappa opioid system using maternal separation as a model of early-life stress. One of these studies found that, although maternally separated offspring exhibit potentiated ethanol-induced locomotor activity and sedation as infants (P15), systemic KOR antagonism has similar effects in both maternally separated (P1–13) and control offspring (Fernandez et al., 2014). In contrast, another study found that maternally separated offspring (age unknown) show increased ethanol intake that is negatively correlated with KOR binding in the nucleus accumbens (Ploj & Nylander, 2003). Additionally, a different study found that a single two-hour session of voluntary ethanol drinking is sufficient to increase dynorphin B immunoreactivity in the hippocampus and amygdala of adult animals that underwent maternal separation from P0–21 (Palm, Daoura, Roman, & Nylander, 2013). These studies demonstrate the complexity of the kappa opioid system and the various ways in which early-life manipulations can alter this system.
Some studies have tested the effects of adolescent stress or ethanol exposure on subsequent stress and/or ethanol responding and the role of the kappa opioid system. One of these studies used restraint stress on adolescent and adult rats to test ethanol consumption and found that, while stress does not alter conditioned taste aversion (CTA) to ethanol at either age, KOR antagonism blunts CTA in stressed adults compared to control adults (P72–77), an effect not observed in stressed adolescents (P30–35) (Anderson, Agoglia, Morales, Varlinskaya, & Spear, 2013). However, another study (that only reported rat weight but not age) showed that in Sprague Dawley rats weighing 170–220 g (presumably mid-late adolescents), a KOR agonist dose-dependently reduced ethanol conditioned-place preference, and the dose-response curve was shifted to the left in fear-conditioned rats (Matsuzawa, Suzuki, Misawa, & Nagase, 1999), suggesting hyperactive kappa opioid modulation of ethanol response following adolescent stress. As previously mentioned, males have primarily been used for these studies; however, a recent study found that stress differentially alters ethanol intake in adolescent (P30–34) and adult (P70–74) females (Wille-Bille et al., 2017). Specifically, stress leads to increased ethanol intake in adolescent females, but decreased ethanol intake in adult females, and the effect of stress on ethanol intake in adolescents is blocked by the KOR antagonist. While some of these findings are consistent with reduced KOR function in naïve adolescents, others suggest that the commonly used measures/tests may not be sufficient to unmask an equally important role for the kappa opioid system in adolescence. Nonetheless, these studies do not directly address the neurobiological effects of stress and/or alcohol on the kappa opioid system during adolescence. Only a couple of studies have begun to assess the neuroadaptations in the kappa opioid system that link stress and alcohol interactions following early-life perturbations. For instance, using social isolation to model stress, Palm and Nylander found that, while dynorphin levels are reduced in the amygdala of adolescents (isolation began on P21 or P28) and adults (isolation began on P70) and in the nucleus accumbens of socially isolated-adults, ethanol consumption restores dynorphin to control (group-housed) levels (Palm & Nylander, 2014). Interestingly, dynorphin levels are unaffected by social isolation in the nucleus accumbens of adolescents, and ethanol consumption has no additional effect (Palm & Nylander, 2014). This study indicates the importance of examining different brain structures, as they could be differentially affected by stress and/or alcohol.
Adult
Surprisingly, there are also few studies examining the interactions between stress and alcohol and its impact on the kappa opioid system in adults. A recent study by Anderson and colleagues demonstrated that forced swim stress increases ethanol intake in animals with a history of chronic ethanol exposure, and this effect is blocked by the pre-treatment with a KOR antagonist (Anderson et al., 2016). Similarly, another study found that yohimbine-induced ethanol reinstatement is blocked by a KOR antagonist (Funk, Coen, & Le, 2014). Interestingly, forced swim stress-induced potentiation of ethanol place preference is attenuated with a KOR antagonist, and a KOR agonist mimics the influence of forced swim stress (Sperling et al., 2010).
Conclusion
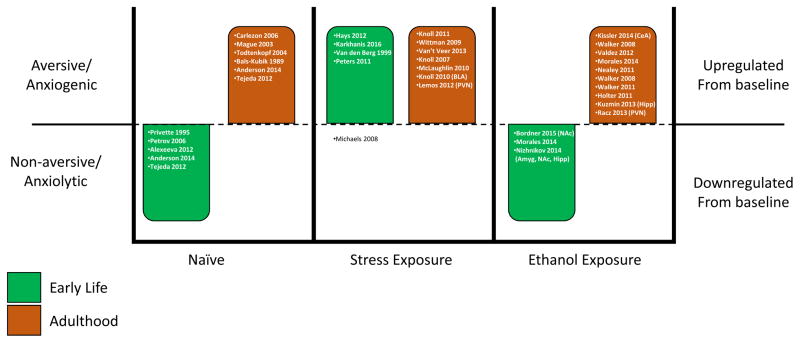
Overall, the studies reviewed here clearly converge on the kappa opioid system as a target for stress and alcohol interactions regardless of age. While some similarities have been found between early-life and adult exposure to stress and/or alcohol, there are numerous specified differences, particularly in the context of ethanol. Given the number of studies discussed, we have constructed a diagram summarizing the main effects (aversive/anxiogenic, non-aversive/anxiolytic, upregulated from baseline, or downregulated from baseline) reported in these various studies, separated by relative age (early-life or adulthood) (Fig. 1). Overall, it is apparent that in naïve animals during early life, KOR activation is either anxiolytic or non-aversive, whereas in adults it is aversive and/or anxiogenic. Surprisingly, regardless of when stress exposure occurs (early-life or adulthood), the kappa opioid system is upregulated and KOR activation produces aversion and/or anxiogenesis, with the exception of one study that found reduced conditioned-place aversion following maternal separation (Michaels & Holtzman, 2008). Strikingly and in contrast to stress, ethanol exposure during early-life appears to result in downregulation of the kappa opioid system and associated anxiolytic effect or a lack of effect in response to KOR activation. However, ethanol exposure in adulthood produces similar effects to stress, in that the kappa opioid system is upregulated and KOR activation is aversive and/or anxiogenic. Unfortunately, due to the limited number of studies systemically utilizing subjects at both ages, there is a clear gap in our current understanding of both the short- and the long-term consequences on the kappa opioid system across ontogeny. Additionally, the means by which these potential ontogenetic differences in vulnerability to stress and/or alcohol influence subsequent exposure to either is still to be determined. This lack of information is particularly critical given the abundance of work indicating the rise in stress-related neuropathologies and alcohol use in early-life.
Figure 1.
Summary of the role of the kappa opioid system in early-life and adulthood at baseline (naïve) and in response to stress or ethanol exposure. Within each bar are citations for the studies demonstrating the reported effects.
It is worth noting that while preclinical studies have clearly demonstrated the involvement of the kappa opioid system in stress and alcohol use, human clinical trials have not been as successful (Buda, Carroll, Kosten, Swearingen, & Walters, 2015; Chavkin & Martinez, 2015). One evident issue is that the pharmacological tools that are used in animal models have been shown to exert variable effects, depending on timing of drug administration relative to testing. Additionally, some of the effects of commonly used KOR antagonists, such as nor-BNI, appear to be long-lasting, up to 20–30 days and may initially have actions on mu opioid receptors. Several reports have suggested that these effects result from shared signaling pathways between KOR agonists and antagonists (Bruchas et al., 2010; Melief et al., 2011). Although this issue/topic is not directly the focus of this review [but see review by (Anderson & Becker, 2017) for further discussion on this topic], an important consideration that this current review raises is the age of when stress or alcohol use began, as the timing of exposure may differentially alter the kappa opioid system (Fig. 1).
Highlights.
The kappa opioid system is a primary target of stress and alcohol
Age may contribute to paradoxical effects of kappa opioid system
Stress and/or alcohol alter the kappa opioid system differently across ontogeny
Acknowledgments
This work was supported by R03-AA024890, P50 AA017823 and the NIAAA Conference Grant AA017581.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (SAMHSA)-1, S. A. a. M. H. S. A. National Survey on Drug Use and Health (NSDUH). Table 5.5B—Substance Use Disorder in Past Year among Persons Aged 12 to 17, by Demographic Characteristics: Percentages, 2015 and 2016 2016 [Google Scholar]
- (SAMHSA)-2, S. A. a. M. H. S. A. Tobacco Product and Alcohol Use in Lifetime, Past Year, and Past Month among Persons Aged 16 or 17: Percentages, 2015 and 2016 2016 [Google Scholar]
- (SAMHSA)-3, S. A. a. M. H. S. A. National Survey on Drug Use and Health (NSDUH). Table 2.7B—Tobacco Product and Alcohol Use in Lifetime, Past Year, and Past Month among Persons Aged 18 to 25: Percentages, 2015 and 2016 2016 [Google Scholar]
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, et al. Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron. 2015;87(5):1063–1077. doi: 10.1016/j.neuron.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeeva EV, Nazarova GA, Sudakov SK. Effects of peripheral mu, delta, and Kappa-opioid receptor agonists on the levels of anxiety and motor activity of rats. Bull Exp Biol Med. 2012;153(5):720–721. doi: 10.1007/s10517-012-1809-2. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Agoglia AE, Morales M, Varlinskaya EI, Spear LP. Stress, kappa manipulations, and aversive effects of ethanol in adolescent and adult male rats. Neuroscience. 2013;249:214–222. doi: 10.1016/j.neuroscience.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Becker HC. Role of the Dynorphin/Kappa Opioid Receptor System in the Motivational Effects of Ethanol. Alcohol Clin Exp Res. 2017;41(8):1402–1418. doi: 10.1111/acer.13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, Becker HC. Stress-Induced Enhancement of Ethanol Intake in C57BL/6J Mice with a History of Chronic Ethanol Exposure: Involvement of Kappa Opioid Receptors. Front Cell Neurosci. 2016;10:45. doi: 10.3389/fncel.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Morales M, Spear LP, Varlinskaya EI. Pharmacological activation of kappa opioid receptors: aversive effects in adolescent and adult male rats. Psychopharmacology (Berl) 2014;231(8):1687–1693. doi: 10.1007/s00213-013-3095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals-Kubik R, Herz A, Shippenberg TS. Evidence that the aversive effects of opioid antagonists and kappa-agonists are centrally mediated. Psychopharmacology (Berl) 1989;98(2):203–206. doi: 10.1007/BF00444692. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Lothman EW, Lee A, Guyenet PG. Kappa opioid receptor-mediated suppression of voltage-activated potassium current in a catecholaminergic neuronal cell line. J Pharmacol Exp Ther. 1995;273(2):927–933. [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Michel K, Mauer D, Zimmer A, Klingmuller D, et al. Control of hormonal stress reactivity by the endogenous opioid system. Psychoneuroendocrinology. 2008;33(4):425–436. doi: 10.1016/j.psyneuen.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Harris RA. Reduced alcohol consumption in mice lacking preprodynorphin. Alcohol. 2006;40(2):73–86. doi: 10.1016/j.alcohol.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordner K, Deak T. Endogenous opioids as substrates for ethanol intake in the neonatal rat: The impact of prenatal ethanol exposure on the opioid family in the early postnatal period. Physiol Behav. 2015;148:100–110. doi: 10.1016/j.physbeh.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Capurro V, Zani A, Rubino T, Vigano D, Parolaro D, et al. Potential anxiolytic-and antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br J Pharmacol. 2009;157(5):844–853. doi: 10.1111/j.1476-5381.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, et al. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci. 2007;27(43):11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Lemos JC, Chavkin C. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One. 2009;4(12):e8528. doi: 10.1371/journal.pone.0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham JC, Cooper TA. Pharmacological characterization of opioid receptors influencing the secretion of corticotrophin releasing factor in the rat. Neuroendocrinology. 1986;44(1):36–40. doi: 10.1159/000124618. [DOI] [PubMed] [Google Scholar]
- Buda JJ, Carroll FI, Kosten TR, Swearingen D, Walters BB. A Double-Blind, Placebo-Controlled Trial to Evaluate the Safety, Tolerability, and Pharmacokinetics of Single, Escalating Oral Doses of JDTic. Neuropsychopharmacology. 2015;40(9):2059–2065. doi: 10.1038/npp.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burish TG, Maisto SA, Cooper AM, Sobell MB. Effects of voluntary short-term abstinence from alcohol on subsequent drinking patterns of college students. J Stud Alcohol. 1981;42(11):1013–1020. doi: 10.15288/jsa.1981.42.1013. [DOI] [PubMed] [Google Scholar]
- Butler TR, Ariwodola OJ, Weiner JL. The impact of social isolation on HPA axis function, anxiety-like behaviors, and ethanol drinking. Front Integr Neurosci. 2014;7:102. doi: 10.3389/fnint.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Karkhanis AN, Jones SR, Weiner JL. Adolescent Social Isolation as a Model of Heightened Vulnerability to Comorbid Alcoholism and Anxiety Disorders. Alcohol Clin Exp Res. 2016;40(6):1202–1214. doi: 10.1111/acer.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Barros VG, Ibba M, Silvagni A, Mura C, Antonelli MC. Prenatal restraint stress: an in vivo microdialysis study on catecholamine release in the rat prefrontal cortex. Neuroscience. 2010;168(1):156–166. doi: 10.1016/j.neuroscience.2010.03.046. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, et al. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316(1):440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carrellas NW, Biederman J, Uchida M. How prevalent and morbid are subthreshold manifestations of major depression in adolescents? A literature review. J Affect Disord. 2017;210:166–173. doi: 10.1016/j.jad.2016.12.037. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ, et al. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: relationship with circulating triglycerides. Alcohol Clin Exp Res. 2007;31(2):249–259. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Ehrich JM. How does stress-induced activation of the kappa opioid system increase addiction risk? Biol Psychiatry. 2014;76(10):760–762. doi: 10.1016/j.biopsych.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215(4531):413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Martinez D. Kappa Antagonist JDTic in Phase 1 Clinical Trial. Neuropsychopharmacology. 2015;40(9):2057–2058. doi: 10.1038/npp.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Tang Y, Tao H, Li C, Zhang X, Liu Y. Dynorphin activation of kappa opioid receptor reduces neuronal excitability in the paraventricular nucleus of mouse thalamus. Neuropharmacology. 2015;97:259–269. doi: 10.1016/j.neuropharm.2015.05.030. [DOI] [PubMed] [Google Scholar]
- Corrigan PW, Lara JL, Shah BB, Mitchell KT, Simmes D, Jones KL. The Public Stigma of Birth Mothers of Children with Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res. 2017;41(6):1166–1173. doi: 10.1111/acer.13381. [DOI] [PubMed] [Google Scholar]
- Craske MG, Stein MB. Anxiety. Lancet. 2016;388(10063):3048–3059. doi: 10.1016/S0140-6736(16)30381-6. [DOI] [PubMed] [Google Scholar]
- Crowley NA, Bloodgood DW, Hardaway JA, Kendra AM, McCall JG, Al-Hasani R, et al. Dynorphin Controls the Gain of an Amygdalar Anxiety Circuit. Cell Rep. 2016;14(12):2774–2783. doi: 10.1016/j.celrep.2016.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley NA, Kash TL. Kappa opioid receptor signaling in the brain: Circuitry and implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2015 doi: 10.1016/j.pnpbp.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Addario C, Caputi FF, Rimondini R, Gandolfi O, Del Borrello E, Candeletti S, et al. Different alcohol exposures induce selective alterations on the expression of dynorphin and nociceptin systems related genes in rat brain. Addict Biol. 2013;18(3):425–433. doi: 10.1111/j.1369-1600.2011.00326.x. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Hogenboom F, Mulder AH, Schoffelmeer AN. Ontogeny of mu-, delta- and kappa-opioid receptors mediating inhibition of neurotransmitter release and adenylate cyclase activity in rat brain. Brain Res Dev Brain Res. 1990;54(1):63–69. doi: 10.1016/0165-3806(90)90065-7. [DOI] [PubMed] [Google Scholar]
- Diaz-Cenzano E, Gaztanaga M, Gabriela Chotro M. Exposure to ethanol on prenatal days 19–20 increases ethanol intake and palatability in the infant rat: involvement of kappa and mu opioid receptors. Dev Psychobiol. 2014;56(6):1167–1178. doi: 10.1002/dev.21162. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 2011;214(1):17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabio MC, Macchione AF, Nizhnikov ME, Pautassi RM. Prenatal ethanol increases ethanol intake throughout adolescence, alters ethanol-mediated aversive learning, and affects mu but not delta or kappa opioid receptor mRNA expression. Eur J Neurosci. 2015;41(12):1569–1579. doi: 10.1111/ejn.12913. [DOI] [PubMed] [Google Scholar]
- Femenia T, Manzanares J. Increased ethanol intake in prodynorphin knockout mice is associated to changes in opioid receptor function and dopamine transmission. Addict Biol. 2012;17(2):322–337. doi: 10.1111/j.1369-1600.2011.00378.x. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Fabio MC, Nizhnikov ME, Spear NE, Abate P, Pautassi RM. Maternal isolation during the first two postnatal weeks affects novelty-induced responses and sensitivity to ethanol-induced locomotor activity during infancy. Dev Psychobiol. 2014;56(5):1070–1082. doi: 10.1002/dev.21192. [DOI] [PubMed] [Google Scholar]
- Funk D, Coen K, Le AD. The role of kappa opioid receptors in stress-induced reinstatement of alcohol seeking in rats. Brain Behav. 2014;4(3):356–367. doi: 10.1002/brb3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaztanaga M, Aranda-Fernandez PE, Chotro MG. Prenatal exposure to vanilla or alcohol induces crawling after these odors in the neonate rat: The role of mu and kappa opioid receptor systems. Physiol Behav. 2015;148:58–64. doi: 10.1016/j.physbeh.2014.12.046. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Mu J, Deadwyler SA. Cannabinoid and kappa opioid receptors reduce potassium K current via activation of G(s) proteins in cultured hippocampal neurons. J Neurophysiol. 2000;84(5):2356–2364. doi: 10.1152/jn.2000.84.5.2356. [DOI] [PubMed] [Google Scholar]
- Hang A, Wang YJ, He L, Liu JG. The role of the dynorphin/kappa opioid receptor system in anxiety. Acta Pharmacol Sin. 2015;36(7):783–790. doi: 10.1038/aps.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AG, Stewart BR. Effect of mu and kappa opioid receptor agonists on rat plasma corticosterone levels. Eur J Pharmacol. 1985;116(1–2):75–79. doi: 10.1016/0014-2999(85)90186-4. [DOI] [PubMed] [Google Scholar]
- Hays SL, McPherson RJ, Juul SE, Wallace G, Schindler AG, Chavkin C, et al. Long-term effects of neonatal stress on adult conditioned place preference (CPP) and hippocampal neurogenesis. Behav Brain Res. 2012;227(1):7–11. doi: 10.1016/j.bbr.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15(2):169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson-Redmond A, Czachowski C. Effects of systemic opioid receptor ligands on ethanol- and sucrose seeking and drinking in alcohol-preferring (P) and Long Evans rats. Psychopharmacology (Berl) 2014;231(22):4309–4321. doi: 10.1007/s00213-014-3571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter SM, Henniger MS, Lipkowski AW, Spanagel R. Kappa-opioid receptors and relapse-like drinking in long-term ethanol-experienced rats. Psychopharmacology (Berl) 2000;153(1):93–102. doi: 10.1007/s002130000601. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Kim HS, Wood PL. Kappa opiate agonists modulate the hypothalamic-pituitary-adrenocortical axis in the rat. J Pharmacol Exp Ther. 1986;238(2):429–436. [PubMed] [Google Scholar]
- Jarjour S, Bai L, Gianoulakis C. Effect of acute ethanol administration on the release of opioid peptides from the midbrain including the ventral tegmental area. Alcohol Clin Exp Res. 2009;33(6):1033–1043. doi: 10.1111/j.1530-0277.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- Kang-Park M, Kieffer BL, Roberts AJ, Siggins GR, Moore SD. Interaction of CRF and kappa opioid systems on GABAergic neurotransmission in the mouse central amygdala. J Pharmacol Exp Ther. 2015;355(2):206–211. doi: 10.1124/jpet.115.225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Huggins KN, Rose JH, Jones SR. Switch from excitatory to inhibitory actions of ethanol on dopamine levels after chronic exposure: Role of kappa opioid receptors. Neuropharmacology. 2016;110(Pt A):190–197. doi: 10.1016/j.neuropharm.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Weiner JL, Jones SR. Early-Life Social Isolation Stress Increases Kappa Opioid Receptor Responsiveness and Downregulates the Dopamine System. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen H, Raivio N, Suo-Yrjo V, Kiianmaa K. Opioidergic modulation of ethanol self-administration in the ventral pallidum. Alcohol Clin Exp Res. 2012;36(2):286–293. doi: 10.1111/j.1530-0277.2011.01611.x. [DOI] [PubMed] [Google Scholar]
- Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, et al. The one-two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors. Biol Psychiatry. 2014;75(10):774–782. doi: 10.1016/j.biopsych.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323(3):838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG, et al. Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry. 2011;70(5):425–433. doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurocircuitry of alcohol addiction: synthesis from animal models. Handb Clin Neurol. 2014;125:33–54. doi: 10.1016/B978-0-444-62619-6.00003-3. [DOI] [PubMed] [Google Scholar]
- Kovacs KM, Szakall I, O’Brien D, Wang R, Vinod KY, Saito M, et al. Decreased oral self-administration of alcohol in kappa-opioid receptor knock-out mice. Alcohol Clin Exp Res. 2005;29(5):730–738. doi: 10.1097/01.alc.0000164361.62346.d6. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva N, Gerrits MA, Avgustinovich DF, Tenditnik MV, Van Ree JM. Anxiety and ethanol consumption in victorious and defeated mice; effect of kappa-opioid receptor activation. Eur Neuropsychopharmacol. 2006;16(7):504–511. doi: 10.1016/j.euroneuro.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Chefer V, Bazov I, Meis J, Ogren SO, Shippenberg T, et al. Upregulated dynorphin opioid peptides mediate alcohol-induced learning and memory impairment. Transl Psychiatry. 2013;3:e310. doi: 10.1038/tp.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MP, Marinelli PW, Bai L, Gianoulakis C. Effects of acute ethanol on opioid peptide release in the central amygdala: an in vivo microdialysis study. Psychopharmacology (Berl) 2008;201(2):261–271. doi: 10.1007/s00213-008-1267-8. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28(2):407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Roth CA, Messinger DI, Gill HK, Phillips PE, Chavkin C. Repeated stress dysregulates kappa-opioid receptor signaling in the dorsal raphe through a p38alpha MAPK-dependent mechanism. J Neurosci. 2012;32(36):12325–12336. doi: 10.1523/JNEUROSCI.2053-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm S, Werme M, Brene S, Franck J. The selective kappa-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav Brain Res. 2001;120(2):137–146. doi: 10.1016/s0166-4328(00)00368-5. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Blockade of ethanol reward by the kappa opioid receptor agonist U50,488H. Alcohol. 2009;43(5):359–365. doi: 10.1016/j.alcohol.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez B, Turner RJ, Saavedra LM. Anxiety and risk for substance dependence among late adolescents/young adults. J Anxiety Disord. 2005;19(3):275–294. doi: 10.1016/j.janxdis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Low NC, Lee SS, Johnson JG, Williams JB, Harris ES. The association between anxiety and alcohol versus cannabis abuse disorders among adolescents in primary care settings. Fam Pract. 2008;25(5):321–327. doi: 10.1093/fampra/cmn049. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Pruessner J, Sousa N, Almeida OF, Van Dam AM, Rajkowska G, et al. Neuropathology of stress. Acta Neuropathol. 2014;127(1):109–135. doi: 10.1007/s00401-013-1223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305(1):323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of dynorphin A(1–8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2006;30(6):982–990. doi: 10.1111/j.1530-0277.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M, Nagase H. Different roles of mu-, delta-and kappa-opioid receptors in ethanol-associated place preference in rats exposed to conditioned fear stress. Eur J Pharmacol. 1999;368(1):9–16. doi: 10.1016/s0014-2999(99)00008-4. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15(3):176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Conron KJ, Koenen KC, Gilman SE. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychol Med. 2010;40(10):1647–1658. doi: 10.1017/S0033291709992121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Carroll FI, Beguin C, Carlezon WA, Jr, Cohen BM, et al. Duration of action of a broad range of selective kappa-opioid receptor antagonists is positively correlated with c-Jun N-terminal kinase-1 activation. Mol Pharmacol. 2011;80(5):920–929. doi: 10.1124/mol.111.074195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels CC, Holtzman SG. Early postnatal stress alters place conditioning to both mu- and kappa-opioid agonists. J Pharmacol Exp Ther. 2008;325(1):313–318. doi: 10.1124/jpet.107.129908. [DOI] [PubMed] [Google Scholar]
- Miranda-Morales RS, Nizhnikov ME, Spear NE. Prenatal exposure to ethanol during late gestation facilitates operant self-administration of the drug in 5-day-old rats. Alcohol. 2014;48(1):19–23. doi: 10.1016/j.alcohol.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology (Berl) 2005;182(3):384–392. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- Morales M, Anderson RI, Spear LP, Varlinskaya EI. Effects of the kappa opioid receptor antagonist, nor-binaltorphimine, on ethanol intake: impact of age and sex. Dev Psychobiol. 2014;56(4):700–712. doi: 10.1002/dev.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM. kappa-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology. 2011;61(1–2):35–42. doi: 10.1016/j.neuropharm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolarakis KE, Almeida OF, Herz A. Stimulation of hypothalamic beta-endorphin and dynorphin release by corticotropin-releasing factor (in vitro) Brain Res. 1986;399(1):152–155. doi: 10.1016/0006-8993(86)90610-4. [DOI] [PubMed] [Google Scholar]
- Nizhnikov ME, Pautassi RM, Carter JM, Landin JD, Varlinskaya EI, Bordner KA, et al. Brief prenatal ethanol exposure alters behavioral sensitivity to the kappa opioid receptor agonist (U62,066E) and antagonist (Nor-BNI) and reduces kappa opioid receptor expression. Alcohol Clin Exp Res. 2014;38(6):1630–1638. doi: 10.1111/acer.12416. [DOI] [PubMed] [Google Scholar]
- Palm S, Daoura L, Roman E, Nylander I. Effects of rearing conditions on behaviour and endogenous opioids in rats with alcohol access during adolescence. PLoS One. 2013;8(10):e76591. doi: 10.1371/journal.pone.0076591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm S, Nylander I. Alcohol-induced changes in opioid peptide levels in adolescent rats are dependent on housing conditions. Alcohol Clin Exp Res. 2014;38(12):2978–2987. doi: 10.1111/acer.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe JE, Williams KL, Mukhopadhyay P, Rice KC, Woods JH, Ko MC. Effects of mu, kappa, and delta opioid receptor agonists on the function of hypothalamic-pituitary-adrenal axis in monkeys. Psychoneuroendocrinology. 2008;33(4):478–486. doi: 10.1016/j.psyneuen.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Schulenberg JE, Martz ME, Maggs JL, O’Malley PM, Johnston LD. Extreme binge drinking among 12th-grade students in the United States: prevalence and predictors. JAMA Pediatr. 2013;167(11):1019–1025. doi: 10.1001/jamapediatrics.2013.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov ME, Acevedo MB, Spear NE. Early role of the kappa opioid receptor in ethanol-induced reinforcement. Physiol Behav. 2012;105(5):1231–1241. doi: 10.1016/j.physbeh.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MF, Zacco A, Gordon J, Maciag CM, Litwin LC, Thompson C, et al. Identification of short-acting kappa-opioid receptor antagonists with anxiolytic-like activity. Eur J Pharmacol. 2011;661(1–3):27–34. doi: 10.1016/j.ejphar.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Petrov ES, Nizhnikov ME, Varlinskaya EI, Spear NE. Dynorphin A (1–13) and responsiveness of the newborn rat to a surrogate nipple: immediate behavioral consequences and reinforcement effects in conditioning. Behav Brain Res. 2006;170(1):1–14. doi: 10.1016/j.bbr.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233(4765):774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Ploj K, Nylander I. Long-term effects on brain opioid and opioid receptor like-1 receptors after short periods of maternal separation in rats. Neurosci Lett. 2003;345(3):195–197. doi: 10.1016/s0304-3940(03)00515-9. [DOI] [PubMed] [Google Scholar]
- Privette TH, Terrian DM. Kappa opioid agonists produce anxiolytic-like behavior on the elevated plus-maze. Psychopharmacology (Berl) 1995;118(4):444–450. doi: 10.1007/BF02245945. [DOI] [PubMed] [Google Scholar]
- Przybysz KR, Werner DF, Diaz MR. Age-dependent regulation of GABA transmission by kappa opioid receptors in the basolateral amygdala of Sprague-Dawley rats. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz I, Markert A, Mauer D, Stoffel-Wagner B, Zimmer A. Long-term ethanol effects on acute stress responses: modulation by dynorphin. Addict Biol. 2013;18(4):678–688. doi: 10.1111/j.1369-1600.2012.00494.x. [DOI] [PubMed] [Google Scholar]
- Romeo RD. The impact of stress on the structure of the adolescent brain: Implications for adolescent mental health. Brain Res. 2017;1654(Pt B):185–191. doi: 10.1016/j.brainres.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC, et al. Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. Int J Neuropsychopharmacol. 2016;19(5) doi: 10.1093/ijnp/pyv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge PC, Sher KJ. Heavy drinking from the freshman year into early young adulthood: the roles of stress, tension-reduction drinking motives, gender and personality. J Stud Alcohol. 2001;62(4):457–466. doi: 10.15288/jsa.2001.62.457. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Buckner JD, Keough ME. Anxiety sensitivity as a prospective predictor of alcohol use disorders. Behav Modif. 2007;31(2):202–219. doi: 10.1177/0145445506297019. [DOI] [PubMed] [Google Scholar]
- Schwarzer C. 30 years of dynorphins--new insights on their functions in neuropsychiatric diseases. Pharmacol Ther. 2009;123(3):353–370. doi: 10.1016/j.pharmthera.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KF, Crain SM. Cholera toxin-A subunit blocks opioid excitatory effects on sensory neuron action potentials indicating mediation by Gs-linked opioid receptors. Brain Res. 1990a;525(2):225–231. doi: 10.1016/0006-8993(90)90868-c. [DOI] [PubMed] [Google Scholar]
- Shen KF, Crain SM. Cholera toxin-B subunit blocks excitatory effects of opioids on sensory neuron action potentials indicating that GM1 ganglioside may regulate Gs-linked opioid receptor functions. Brain Res. 1990b;531(1–2):1–7. doi: 10.1016/0006-8993(90)90751-v. [DOI] [PubMed] [Google Scholar]
- Shen KF, Crain SM. Nerve growth factor rapidly prolongs the action potential of mature sensory ganglion neurons in culture, and this effect requires activation of Gs-coupled excitatory kappa-opioid receptors on these cells. J Neurosci. 1994;14(9):5570–5579. doi: 10.1523/JNEUROSCI.14-09-05570.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res Monogr. 1986;75:563–566. [PubMed] [Google Scholar]
- Sinclair JD. The alcohol-deprivation effect. Influence of various factors. Q J Stud Alcohol. 1972;33(3):769–782. [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescents and alcohol: acute sensitivities, enhanced intake, and later consequences. Neurotoxicol Teratol. 2014;41:51–59. doi: 10.1016/j.ntt.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl) 2010;210(2):199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- Szeto HH. Dynorphin and the hypothalamo-pituitary-adrenal axis during fetal development. Life Sci. 2003;73(6):749–758. doi: 10.1016/s0024-3205(03)00407-7. [DOI] [PubMed] [Google Scholar]
- Tejeda HA, Hanks AN, Scott L, Mejias-Aponte C, Hughes ZA, O’Donnell P. Prefrontal Cortical Kappa Opioid Receptors Attenuate Responses to Amygdala Inputs. Neuropsychopharmacology. 2015;40(13):2856–2864. doi: 10.1038/npp.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Natividad LA, Orfila JE, Torres OV, O’Dell LE. Dysregulation of kappa-opioid receptor systems by chronic nicotine modulate the nicotine withdrawal syndrome in an age-dependent manner. Psychopharmacology (Berl) 2012;224(2):289–301. doi: 10.1007/s00213-012-2752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Shippenberg TS, Henriksson R. The dynorphin/kappa-opioid receptor system and its role in psychiatric disorders. Cell Mol Life Sci. 2012;69(6):857–896. doi: 10.1007/s00018-011-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Wu J, Kornspun AR, Pignatelli M, Kashtelyan V, Krashes MJ, et al. Pathway-and Cell-Specific Kappa-Opioid Receptor Modulation of Excitation-Inhibition Balance Differentially Gates D1 and D2 Accumbens Neuron Activity. Neuron. 2017;93(1):147–163. doi: 10.1016/j.neuron.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172(4):463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Galvan A. Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci Biobehav Rev. 2016;70:217–227. doi: 10.1016/j.neubiorev.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ur E, Wright DM, Bouloux PM, Grossman A. The effects of spiradoline (U-62066E), a kappa-opioid receptor agonist, on neuroendocrine function in man. Br J Pharmacol. 1997;120(5):781–784. doi: 10.1038/sj.bjp.0700971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Harshberger E. kappa opioid regulation of anxiety-like behavior during acute ethanol withdrawal. Pharmacol Biochem Behav. 2012;102(1):44–47. doi: 10.1016/j.pbb.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl) 2013;229(3):435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg CL, Van Ree JM, Spruijt BM, Kitchen I. Effects of juvenile isolation and morphine treatment on social interactions and opioid receptors in adult rats: behavioural and autoradiographic studies. Eur J Neurosci. 1999;11(9):3023–3032. doi: 10.1046/j.1460-9568.1999.00717.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Spruijt BM, Van Ree JM. Mu- and kappa-opioid receptor-mediated opioid effects on social play in juvenile rats. Eur J Pharmacol. 1995;276(3):257–266. doi: 10.1016/0014-2999(95)00040-r. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33(3):643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Valdez GR, McLaughlin JP, Bakalkin G. Targeting dynorphin/kappa opioid receptor systems to treat alcohol abuse and dependence. Alcohol. 2012;46(4):359–370. doi: 10.1016/j.alcohol.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol. 2011;16(1):116–119. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PM, Messier C. Concurrent modulation of anxiety and memory. Behav Brain Res. 2000a;109(2):229–241. doi: 10.1016/s0166-4328(99)00177-1. [DOI] [PubMed] [Google Scholar]
- Wall PM, Messier C. U-69,593 microinjection in the infralimbic cortex reduces anxiety and enhances spontaneous alternation memory in mice. Brain Res. 2000b;856(1–2):259–280. doi: 10.1016/s0006-8993(99)01990-3. [DOI] [PubMed] [Google Scholar]
- Wang X, Dow-Edwards D, Anderson V, Minkoff H, Hurd YL. Discrete opioid gene expression impairment in the human fetal brain associated with maternal marijuana use. Pharmacogenomics J. 2006;6(4):255–264. doi: 10.1038/sj.tpj.6500375. [DOI] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210(2):121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille-Bille A, Ferreyra A, Sciangula M, Chiner F, Nizhnikov ME, Pautassi RM. Restraint stress enhances alcohol intake in adolescent female rats but reduces alcohol intake in adolescent male and adult female rats. Behav Brain Res. 2017;332:269–279. doi: 10.1016/j.bbr.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, et al. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology. 2009;34(3):775–785. doi: 10.1038/npp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]