Abstract
Ecosystem metabolism is an important determinant of trophic structure, nutrient cycling, and other critical ecosystem processes in streams. Whereas watershed- and local-scale controls on stream metabolism have been independently investigated, little is known about how controls exerted at different scales interact to determine stream metabolic rates, particularly in urban streams and across seasons. To address this knowledge gap, we measured ecosystem metabolism in four urban and four reference streams in northern Kentucky, USA, with paired closed and open riparian canopies, during each of the four seasons. Gross primary production (GPP), ecosystem respiration, and net ecosystem production (NEP) were all best predicted by models with season as a main effect, but interactions between season, canopy, and watershed varied for each response. Urban streams exhibited higher GPP during most seasons, likely due to elevated nutrient loads. Open canopy reaches in both urban and forested streams, supported higher rates of GPP than the closed canopy which reaches during the summer and fall, when the overhead vegetation shaded the closed reaches. The effect of canopy cover on GPP was similar among urban and forested streams. The combination of watershed and local-scale controls resulted in urban streams that alternated between net heterotrophy (NEP <0) and net autotrophy (NEP >0) at the reach-scale during seasons with dense canopy cover. This finding has management relevance because net production can lead to accumulation of algal biomass and associated issues like nighttime hypoxia. Our study suggests that although watershed urbanization fundamentally alters ecosystem function, the preservation and restoration of canopied riparian zones can provide an important management tool at the local scale, with the strongest impacts on stream metabolism during summer.
Keywords: stream metabolism, gross primary production (GPP), ecosystem respiration (ER), urban streams, riparian land cover, phenology
Introduction
Encompassing both gross primary production (GPP) and ecosystem respiration (ER), stream ecosystem metabolism is an integrated measure of stream function and trophic state (Izagirre and others 2008). Stream ecosystem GPP quantifies the total amount of carbon fixed via photosynthesis by in-stream autotrophs, whereas ER is sum of autotrophic and heterotrophic respiration (Fisher and Likens 1973; Bott and others 1985; Lovett and others 2006). These processes control the rates of carbon and organic matter cycling, and are likely to regulate numerous aspects of ecosystem function, including nutrient processing and secondary production (Meyer and others 2007).
Critical drivers of stream metabolism include light availability (Dodds and others 1999; Mulholland and others 2001), nutrient availability (Guasch and others 1995), organic matter quantity, and hydrology (Acuna and others 2004; Roberts and others 2007). However, the controlling factors are expected to differ somewhat between GPP and ER. GPP, for instance, is driven mainly by nutrient and light availability to stream autotrophs (Hill and others 1995, 2000; Mulholland and others 2001), and may be further regulated by the availability of stable habitat (Grimm and Fisher 1989; Uehlinger 2000). ER, on the other hand, is influenced most strongly by organic matter availability and hydrology, with nutrient availability having a less important role (Sinsabaugh 1997; Mulholland and others 2001; Roberts and others 2007). Given these controlling factors, both watershed-scale (for example, land use/land-cover influencing hydrology and nutrient inputs) and local-scale (for example, riparian canopy influencing light and OM availability) characteristics should exert control over GPP and ER. Within the confines of the regional template (that is, climate zone, biome, ecoregion), many of these influences are also likely to vary seasonally with changes in temperature, light, and vegetation (Figure 1).
Figure 1.
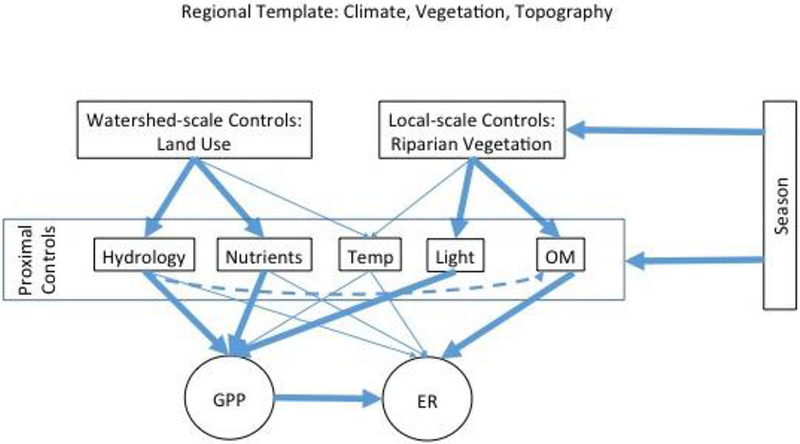
Conceptual diagram of drivers of stream ecosystem metabolism by spatial scale. The regional template is expected to shape climate, vegetation, and topography. Watershed-scale factors are an important determinant of nutrient availability and the hydrologic regime, while terrestrial organic matter (OM) and light conditions are most strongly controlled by local-scale riparian canopy characteristics. Seasonality is expected to influence all of these factors, leading to potential shifts in the interaction between watershed and local scale controls on GPP and ER. Dashed line reflects that hydrology is expected to influence the retention of terrestrial organic matter within stream channels. “Temp” represents stream-water temperature. Arrow size roughly represents the magnitude of influence.
Streams and their associated terrestrial environment are tightly linked, and human development patterns in the landscape can affect stream ecosystem function. At the local scale, riparian land use or management practices largely dictate light availability at the stream surface. The riparian zone also regulates thermal regime which exerts an important control over a wide range of stream functions (for example, GPP, ER, denitrification, nitrification) (Roberts and others 2007; Demars and others 2011). Riparian buffers can also reduce non-point source pollution by intercepting nutrients (Hession and others 2003; Sweeney and others 2004) and can stabilize aquatic ecosystem functions by providing in-stream habitat and allochthonous organic matter. Leaf-fall from streamside trees, for instance, is an important energy subsidy to aquatic macroinvertebrates, particularly in small streams with low in situprimary production (Roy and others 2005), and may act as a driver of heterotrophic respiration [discussed in Tank and others (2010)].
Stream metabolism may also be influenced by human development patterns at the larger watershed-scale. The proliferation of urban/exurban development and the associated changes in hydrology and nutrient loading has become a critical stressor in many stream and river networks. As of 2007, urban land cover accounted for nearly 3% of the land area of the United States, equivalent to approximately 61 million acres (USDA 2011). Even in areas with relatively small urban centers, however, the reach of urban influence on ecosystems can be quite large (Folke and others 1997), and may present a host of problems for aquatic systems. Specifically, increasing impervious cover and loss of watershed vegetation associated with human development drives a cascade of changes in stream ecosystems. Collectively, these changes are dubbed as the Urban Stream Syndrome (Meyer and others 2005; Walsh and others 2005), and are broadly characterized by the disruption of natural hydrological patterns (Paul and Meyer 2001), resulting in changes in water chemistry (Carpenter and others 1998), and degradation of biological communities (Snyder and others 2003). Land use changes associated with development and the resulting altered hydrology can affect nutrient cycling, decrease organic matter retention, simplify in-stream habitat, and physically remove biota from urban streams via scour (Konrad and Booth 2005). Elevated concentrations of nutrients, including phosphorous and nitrogen, are often found in connection with watershed urbanization (Jordan and others 1997; Vitousek and others 1997; Carpenter and others 1998), and thermal regimes in urban streams are also expected to differ from natural systems (LeBlanc and others 1997). Such substantial changes in stream characteristics have the potential to manifest dramatic change in stream metabolism.
As the understanding of the impacts of urbanization on aquatic ecosystems has grown, ecological restoration has become an important tool of environmental managers. While the specific goals of individual restoration projects may vary, the general purpose is typically to restore the ecological condition to a desirable state, often moving towards a reference condition similar to that of unperturbed watersheds in the same region (Palmer and others 1997; Bond and Lake 2003; Beauchamp and others 2015). Riparian replanting is one of the most common restoration techniques in the United States (Bernhardt and others 2005, 2007; Bernhardt and Palmer 2007). Given the dramatic influence of watershed urbanization on stream ecosystems, however, it is unclear to what degree local-scale factors, such as riparian zone condition, retain control over important processes like primary production. Furthermore, nutrient and light availability have been shown to have a synergistic effect on stream periphyton, where the combined effects of increased nutrient and light availability is greater than the sum of their individual effects, likely because the increased availability of one resource leads to more efficient utilization of the other (Hill and others 2011). As a result, the degree to which riparian zone management can ameliorate the effects of altered hydrological regimes and increased anthropogenic nutrient inputs in urbanized watersheds remains in question (Roy and others 2005).
Relatively few studies have examined how stream metabolism responds to land use at multiple spatial scales, particularly in urbanized watersheds, and fewer still have taken seasonal variability into account, in spite of its likely importance. Most multi-stream studies estimate stream metabolism estimates from one or two seasons (for example, Meyer and others 2005; Sudduth and others 2011), though a handful of continuous studies highlight the potential for variability throughout the year (for example, Roberts and others 2007; Beaulieu and others 2013). These, and other studies (for example, Acuna and others 2004; Reichert and others 2009), have provided valuable insights into stream metabolism controls and processes, yet have been focused on detailed assessments of multiple reaches within a single stream, making it difficult to interpret how results might be applicable with variation in watershed land use. Seasonal changes in abiotic (that is, photoperiod, temperature) and biotic (that is, leaf-out, leaf-fall) factors are likely to substantially mediate the effects of watershed- and local-scale land cover on stream metabolism. Roberts and others (2007), for instance, observed that both stream GPP and ER were minimal in seasons when riparian canopy was most dense, that ER increased in response to leaf-fall, and that both ER and GPP were relatively high during early spring when light availability was greatest, suggesting a strong influence of riparian canopy. Similarly, Beaulieu and others (2013) report that both GPP and ER rates were highest during winter, when canopy cover was minimal. However, ER did not increase in response to leaf-fall, and the investigators suggested that altered hydrology due to watershed development may have flushed organic matter from the system. The lack of consistent seasonal patterns in stream metabolism suggests that the interaction of drivers may be complex.
The seasonally varying interplay between watershed- and local-scale factors is likely to have a central role in the function of stream ecosystems, and may have important implications for mitigating anthropogenic disturbance. Addressing the associated knowledge gap will improve our ability to model and predict changes in important stream processes in the face of land use change or proposed restoration activities. A better understanding of how these interactions shape variation in ecosystem metabolism and trophic state is also crucial for the management of riparian zones, the spatial scale at which most conservation and restoration efforts take place.
The objectives of this work are to examine: (1) how stream metabolic rates respond to the individual and interactive effects of watershed-scale land cover and local-scale riparian canopy cover and (2) how seasonal variation influences these relationships. To meet these objectives, we measured rates of metabolism on a seasonal basis from paired open and closed canopy reaches, within streams draining forested (reference) and urbanized watersheds. We hypothesized the following: (1) GPP would be greater in open than closed reaches during seasons exhibiting full canopy cover due to greater irradiance driving more photosynthesis, (2) GPP would be elevated in urban streams for each canopy treatment due to elevated nutrient availability, (3) riparian canopy influence on ER rates would differ by watershed land use and season based on differences in organic matter dynamics, and (4) net ecosystem production (NEP = GPP − ER) would be determined by the interaction of seasonal, watershed-, and local-scale influence on GPP and ER.
Specifically, we anticipated that while GPP would correspond to light availability regulated by riparian canopy, differences in nutrient levels associated with watershed land use would affect the magnitude of canopy cover influence. We also predicted that although canopy inputs (that is, leaf litter) would drive ER rates in reference streams, the combination of decreased retention of allochthonous inputs and elevated algal biomass in urban streams would result in autochthonous organic matter being a more important driver of ER in urban streams. Finally, we anticipated that a synergistic relationship between nutrient and light availability, along with seasonal variation in organic matter dynamics, temperature, and photoperiod, would result in local-scale variation in NEP. Specifically, we anticipated that urban open reaches would be net autotrophic (NEP >0) during most of the year, whereas all other reaches would be net heterotrophic (NEP <0).
Methods
Study Area
This study took place within eight streams in northern Kentucky, U.S.A., located within the Outer Bluegrass Physiographic Region, and underlain primarily with Ordovician limestone and shale (Ray and others 1994). This area is also located within the Outer Bluegrass Ecoregion, characterized by open savannah deciduous woodlands before human habitation (Bailey 1995). Average precipitation is 104.6 cm annually, with the wettest months occurring during spring and early summer. The hottest and coldest months are July and January, with mean temperatures of 30.4 and 4.0°C, respectively (NCDC 2008). Commonly observed riparian tree species include American sycamore (Plantanus occidentalis), red maple (Acer rubrum), pin oak (Quercus palustris), white oak (Quercus alba), and eastern cottonwood (Populus deltoids).
Watersheds were chosen to be similar in size, and ranged from 5.7 to 11.4 km2 (Table 1), and all samplings were completed during four distinct periods meant to capture among-season differences: October 2013 and February, May, and July 2014 (Fall, Winter, Spring, and Summer, respectively). Four streams were classified as urban, based on high watershed impervious surface coverage ranging from 18.8 to 31.6% of the watershed area, whereas four were classified as forested reference with impervious cover ranging from 1.7 to 2.2% of the watershed area. Given the difficulty in locating truly pristine systems for reference, predominantly pasture/hay agricultural land cover was present in all watersheds and ranged from 8.5 to 27.8% of the catchment area, below the threshold at which impacts on the stream might be expected (discussed in Allan 2004). Land cover was assessed using the 2011 National Land Cover Dataset (Homer and others 2015). Within each stream, paired reaches (~100 m) exhibiting open (summer cover = 14.5 ± 4.2%; mean ± SD) and closed (summer cover = 86.9 ± 3.6%; mean ± SD) canopies were selected. Canopy cover was estimated using a spherical convex densitometer (Forestry Suppliers, Inc, Jackson, MS, U.S.A.) following Riley and Dodds (2012). The percentage of canopy cover visible in the densiometer facing upstream, downstream, right bank, and left bank was recorded every ten meters, and the mean value was used to represent local-scale canopy cover. The paired reaches chosen had similar in-stream habitat (that is, substrate type, embeddedness) as assessed using protocols described by the Kentucky Division of Water (2008), and there were no tributary confluences between the paired reaches.
Table 1.
Study stream drainage area and watershed % coverage of impervious surface and major land use types.
| Stream | Watershed | Drainage Area (km2) | Watershed Impervious Surface Cover (%) | Watershed Forest Cover (%) | Watershed Agriculture Cover (%) |
|---|---|---|---|---|---|
| Ashby Fk | Reference | 11.4 | 2.2 | 69.1 | 23.1 |
| Cruise Ck | Reference | 11.1 | 1.7 | 67.2 | 27.8 |
| Middle Ck | Reference | 8.5 | 2.1 | 73.5 | 22.1 |
| Steep Ck | Reference | 9.6 | 1.7 | 66.4 | 25.3 |
| Elijah Ck | Urban | 10.1 | 31.6 | 22.3 | 16.7 |
| Oakbrook | Urban | 10.1 | 26.5 | 18.2 | 26.7 |
| Sunnybrook | Urban | 5.7 | 27.3 | 13.4 | 13.3 |
| Threemile Ck | Urban | 8.2 | 18.8 | 28.6 | 8.5 |
Water Quality Analyses
Grab samples of water were collected seasonally from all reaches during periods of base and elevated flow using 500 mL HDPE containers, for a total of 128 samples (8 streams × 2 canopy treatments × 4 seasons × 2 flow regimes). Elevated flow samples were collected as soon as possible after a rain event of large enough size to result in substantial increase in runoff to the streams—in practice, these were events of greater than 10–15 mm of precipitation, depending on antecedent moisture conditions. Samples were transported to the laboratory on ice where they were stored at 4°C until additional processing within 48 h of collection. Water samples were analyzed for pH (Orion Ross Ultra Combination pH, Thermo Fisher Scientific, Waltham, MA) and conductivity (Orion Conductivity Cell; Thermo Fisher Scientific, Waltham, MA). The remaining sample was filtered through a 0.45-μm filter (Millipore MF™ membrane filter; Millipore, Billerica, MA) and subdivided for phosphate (PO4 3−), ammonium (NH4 +), and nitrate (NO3 −). All subsamples were frozen until they could be analyzed for PO4 3− using the ascorbic acid method (Murphy and Riley 1962) adapted for a microplate reader (Biotek®Synergy H1 Hybrid Microplate reader; Biotek, Winooski, VT); NH4 + using the phenol-hypochlorite reaction (Weatherburn 1967) adapted for a microplate reader; and NO3 − using a spectrophotometric method (Doane and Horwath 2003) adapted for a microplate reader. Mean nutrient concentrations for each reach from each sampling season were used in the statistical analyses.
Leaf Litter, Organic Matter, and Algal Biomass
Leaf litter traps (0.25 m2 polypropylene container; L: 0.60 m × W: 0.43 m × H: 0.15 m; Sterilite Corp, Townsend, MA, U.S.A.) were used to measure direct litterfall into streams. Traps were staked into the center of the streambed at the upstream, middle, and downstream thirds of each reach, and left in place for ten days during each seasonal sampling period. Benthic organic matter, including leaves and algae, was collected from five locations within each reach to a depth of 5 cm using 0.09 m2 Surber samplers (Wildco, Yulee, FL, U.S.A.). Once collected, samples were dried at 60 °C for 48 h, and then combusted to determine loss on ignition and ash-free dry mass (AFDM).
Algal biomass was quantified from chlorophyll-a analyses of both periphyton (Reavie and others 2010) and water column samples. Periphyton was scraped from one randomly selected rock in each of five separate riffles separated by at least ten meters, and composited into a single reach sample. Two subsamples of the reach composite were then filtered on 47 mm GF/F glass fiber filters, extracted for 24 h in 95% ethanol, and used for spectrophotometric chlorophyll-a analyses in the laboratory (SM 10200 H, APHA 2005).
Stream Metabolism
Stream metabolism rates were estimated using the single-station, open-channel method, which assesses diel variation in oxygen concentrations within the stream. Calibrated dissolved oxygen and temperature loggers (MiniDOT loggers, Precision Measurement Engineering, Vista, California, U.S.A.) were deployed simultaneously in all monitoring reaches, ensuring that weather patterns were similar among stream reaches during the monitoring periods. Loggers were placed at the downstream boundary of each reach to estimate GPP and ER within the reach located upstream.
Dissolved oxygen and temperature measurements were taken every ten minutes for a period of two days each sampling season, when the streams were at base flow. GPP, ER, and k (reaeration coefficient) were estimated using the Bayesian Metabolic Model (BaMM), an oxygen mass-balance model (Holtgrieve and others 2010). The model estimates GPP using dissolved oxygen saturation and light availability, whereas ER is modeled as a function of temperature and nighttime dissolved oxygen under-saturation. The ER estimate includes all oxygen-consuming reactions, and does not incorporate potential changes in autotrophic respiration rates during the day. Only estimates from successful model fits were included in our statistical analysis. Model results were deemed acceptable when the autocorrelation of all parameters was less than 5% (BaMM user manual; Holtgrieve and others 2010), and the Geweke diagnostic from the CODA package (Plummer and others 2006) in R (R Core Development Team 2014) indicated model convergence (Grace and others 2015). We verified that reach lengths were sufficiently separated, and that logger readings were not representing spatially overlapped stream segments, by calculating the length of stream affecting the DO measurements at each logger. This integration distance is determined by the balance between water velocity (v) and gas exchange and is calculated as 3v/k (Chapra and Ditoro 1991; Reichert and others 2009; Demars and others 2015). Based on this analysis, the integration distance ranged from 43 to 88 m upstream of the given logger, always smaller than our canopy treatment reach lengths.
To minimize the influence of day-to-day variation in cloud cover on the study results, we used metabolism data collected only on days when the integrated daily photosynthetically active radiation (PAR) was above the 75th percentile for all days in the sampling month (that is, relatively clear days). PAR measurements for the region were obtained from a nearby weather station at the University of Cincinnati Center for Field Studies, Harrison, Ohio.
Statistical Approach
Linear mixed effects models were used to analyse the metabolism data, accounting for the nesting of canopy treatments (that is, open and closed reaches) within streams, and allowing season to enter the model as a fixed effect. The models were constructed using the lme4 package (Bates and Maechler 2010) in R and the full model took the following form:
where × indicated that all possible combinations of main effect and interaction terms are assessed. This random error structure nests canopy treatment within streams, therefore comparisons between open and closed reaches are made among paired reaches within one stream. All possible models were assessed using Akaike Information Criteria (AIC) (Burnham and Anderson 2002). Models that exhibited the lowest AIC scores, and explained significantly more variation than the null model, were then subjected to model diagnostics assessing residual variance, residual distribution, and model convergence. This model selection approach was used for GPP, ER, and NEP. In addition, water chemistry and organic matter variables were subjected to the same statistical tests in order to determine whether urban watersheds did indeed have significantly higher nutrient concentrations as anticipated.
Results
Site Characterization
Specific conductivity, PO4 3−, NH4 +, and NO3 − concentrations were elevated in the urban streams relative to reference streams (Table 2). Benthic chlorophyll-a was also elevated in urban streams, and in open reaches relative to closed reaches. Although leaf litter inputs were higher in reference streams than urban, and closed canopies than open, benthic detritus standing stock did not differ by watershed or canopy, with the exception of urban streams exhibiting significantly greater biomass than reference streams during winter.
Table 2.
Physicochemical properties and organic matter estimates by watershed, reach, and season. Values are Mean (SD).
| Watershed | Reach | Season | Specific Conductivity (μS/cm) | pH | Mean Temperature (°C) | NH4+ (mg/L) | NO3− (mg/L) | PO43− (mg/L) | Benthic Chl a (mg/m2) | Benthic Detritus (g AFDM /m2) | Leaf litterfall (AFDM g m−2 season−1) | Canopy Cover (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Open | Fall | 487.2 (117.0) | 8.1 (0.5) | 4.2 (1.1) | 0.02 (0.01) | 0.12 (0.07) | 0.06 (0.04) | 71.6 (61.6) | 5.0 (2.9) | 79.8 (38.6) | 9.4 (1.2) |
| Winter | 497.6 (112.2) | 8.2 (0.2) | 1.6 (0.1) | 0.04 (0.03) | 0.33 (0.12) | 0.06 (0.02) | 57.8 (20.6) | 1.8 (0.8) | 1.9 (1.5) | 6.75 (0.8) | ||
| Spring | 344.4 (162.8) | 8.8 (0.4) | 17.9 (1.7) | 0.05 (0.03) | 0.36 (0.17) | 0.07 (0.05) | 63.2 (28.1) | 2.0 (0.7) | 1.5 (1.1) | 7.2 (2.3) | ||
| Summer | 504.0 (56.7) | 8.0 (0.3) | 22.5 (1.6) | 0.06 (0.04) | 0.10 (0.04) | 0.07 (0.04) | 60.9 (39.1) | 1.8 (0.9) | 4.1 (1.9) | 18.3 (3.5) | ||
| Closed | Fall | 486.8 (116.4) | 8.1 (0.4) | 4.2 (1.1) | 0.03 (0.01) | 0.13 (0.07) | 0.07 (0.04) | 21.4 (13.7) | 6.2 (0.7) | 196.9 (76.2) | 31.0 (4.9) | |
| Winter | 494.5 (101.0) | 8.2 (0.3) | 1.5 (0.1) | 0.04 (0.01) | 0.30 (0.08) | 0.05 (0.02) | 47.3 (37.3) | 1.4 (0.4) | 2.1 (1.6) | 17.1 (3.3) | ||
| Spring | 333.0 (163.5) | 8.8 (0.3) | 17.8 (1.7) | 0.05 (0.04) | 0.37 (0.16) | 0.08 (0.05) | 48.0 (18.5) | 2.3 (0.8) | 2.0 (0.7) | 18.3 (4.8) | ||
| Summer | 508.2 (60.7) | 7.9 (0.4) | 20.8 (0.9) | 0.08 (0.09) | 0.10 (0.05) | 0.06 (0.04) | 20.2 (6.4) | 2.8 (3.1) | 8.5 (3.2) | 86.0 (0.6) | ||
| Urban | Open | Fall | 682.2 (236.8) | 8.0 (0.5) | 2.6 (0.5) | 0.03 (0.01) | 0.15 (0.06) | 0.14 (0.11) | 171.5 (49.8) | 5.4 (1.5) | 35.9 (6.9) | 7.3 (2.6) |
| Winter | 1126.5 (266.2) | 8.1 (0.4) | 1.8 (1.3) | 0.07 (0.05) | 0.48 (0.13) | 0.03 (0.02) | 124.8 (43.2) | 3.1 (0.5) | 1.3 (0.6) | 6.3 (2.2) | ||
| Spring | 757.2 (347.7) | 8.5 (0.1) | 17.7 (3.2) | 0.16 (0.18) | 0.37 (0.20) | 0.06 (0.05) | 166.3 (53.6) | 2.4 (0.6) | 1.8 (0.4) | 8.2 (3.1) | ||
| Summer | 783.8 (119.1) | 8.1 (0.2) | 23.9 (0.3) | 0.14 (0.06) | 0.26 (0.37) | 0.19 (0.11) | 245.7 (110.9) | 2.9 (2.6) | 3.4 (0.6) | 14.7 (3.9) | ||
| Closed | Fall | 682.6 (237.5) | 8.0 (0.5) | 2.7 (0.5) | 0.02 (0.01) | 0.15 (0.06) | 0.15 (0.11) | 189.4 (85.4) | 4.5 (1.2) | 98.7 (24.8) | 28.2 (4.0) | |
| Winter | 1116.4 (274.2) | 8.1 (0.4) | 1.9 (1.3) | 0.06 (0.05) | 0.49 (0.13) | 0.02 (0.02) | 158.5 (28.8) | 3.4 (1.1) | 2.7 (1.2) | 14.3 (2.1) | ||
| Spring | 738.4 (333.4) | 8.5 (0.2) | 17.6 (3.2) | 0.14 (0.17) | 0.37 (0.22) | 0.07 (0.05) | 172.4 (68.8) | 2.1 (0.6) | 1.5 (0.5) | 31.3 (5.2) | ||
| Summer | 772.8 (122.0) | 8.0 (0.1) | 23.3 (0.6) | 0.12 (0.03) | 0.12 (0.06) | 0.20 (0.10) | 54.8 (16.3) | 1.2 (0.3) | 5.1 (1.8) | 82.1 (0.7) | ||
Metabolism Modeling
Diel oxygen and temperature profiles were measured at each study reach on two consecutive days at base flows each season, resulting in 128 diel observations. Of these, eight were rejected based on our PAR criteria (that is, 75th percentile of the sampling month). Another 35 that failed to pass model convergence diagnostics, resulting in 85 total measurement days were included in the analysis. Each study reach was represented by at least one measurement (day) per season. For those study reaches with more than one day available, mean rates from the two days were used in the final analysis. Reaeration coefficients (k) ranged from 5.11 to 88.0 cm h−1, with a mean of 27.7 cm h−1.
GPP
GPP ranged from 0.0 to 16.0 g O2 m−2 day−1, with a mean of 5.9 g O2 m−2 day−1, and was best predicted by a model including season as a main effect, as well as the season × watershed and season × watershed × canopy interaction terms (Table 3). GPP for all treatment combinations was greatest in spring (Figure 2). The interaction of season and watershed was mostly attributable to closed reaches in reference streams exhibiting elevated GPP in the fall season relative to closed reaches in urban streams, which was suppressed relative to other seasons. Urban GPP was higher than reference GPP in the other three seasons for both canopy treatments. In contrast, spring GPP in urban closed reaches was actually higher than in urban open reaches, whereas the opposite pattern was evident between canopy treatments in reference streams. During summer, when riparian canopy cover reaches its annual peak (Table 2), GPP was higher in open reaches for both urban and reference streams (Figure 2).
Table 3.
Best fit models for linear mixed model of stream metabolic rates. For each model, the table shows predictors included, marginal R2 (variance explained by fixed factors) and conditional R2 (variance explained by both fixed and random factors). For all models, canopy was treated as a fixed effect nested within the random effect of stream. Each best fit model explained significantly more variation in GPP, ER, or NEP than the null model.
| Response | Main Effect | Interaction | Marginal R2 | Combined R2 |
|---|---|---|---|---|
| GPP | season | season×watershed season×watershed×canopy |
0.60 | 0.66 |
| ER | season | season×watershed | 0.36 | 0.39 |
| NEP | season watershed |
season×watershed | 0.46 | 0.48 |
Figure 2.
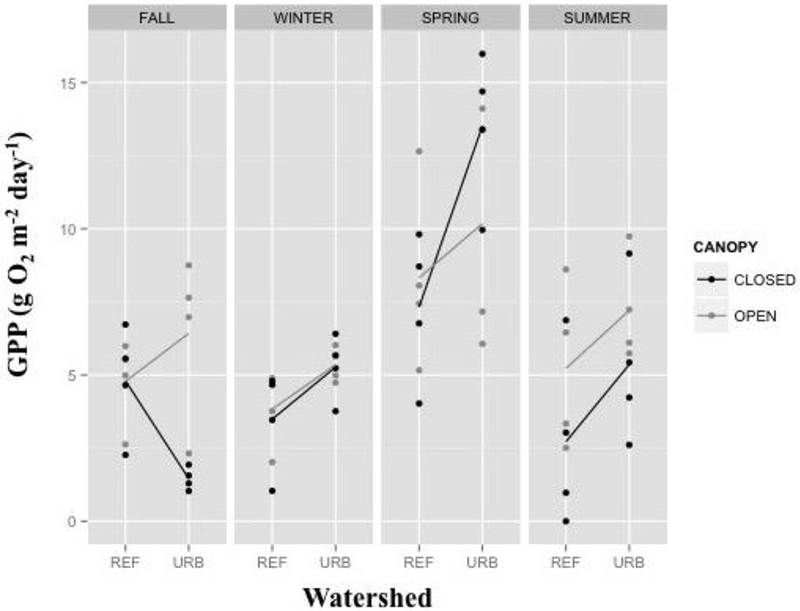
Rates of stream Gross Primary Productivity (GPP) by watershed, canopy, and season. Dots represent the rate for individual study reaches during the specific sampling season. Lines connect the mean value for each canopy treatment by watershed type.
For each model, the table shows predictors included, marginal R 2 (variance explained by fixed factors) and conditional R 2 (variance explained by both fixed and random factors). For all models, canopy was treated as a fixed effect nested within the random effect of stream. Each best-fit model explained significantly more variation in GPP, ER, or NEP than the null model.
ER
ER ranged from 0.9 to 19.8 g O2 m−2 day−1, with a mean of 7.3 g O2 m−2 day−1. ER was best predicted by a model including season as a main effect, and the season × watershed interaction term (Table 3). The interaction between season and watershed indicates that seasonal patterns in ER varied by watershed land use. ER in urban streams was highest in the spring and lowest in the fall, whereas in reference streams, ER was greatest during the summer and lowest during the winter (Figure 3). There was no significant effect of canopy cover on ER.
Figure 3.
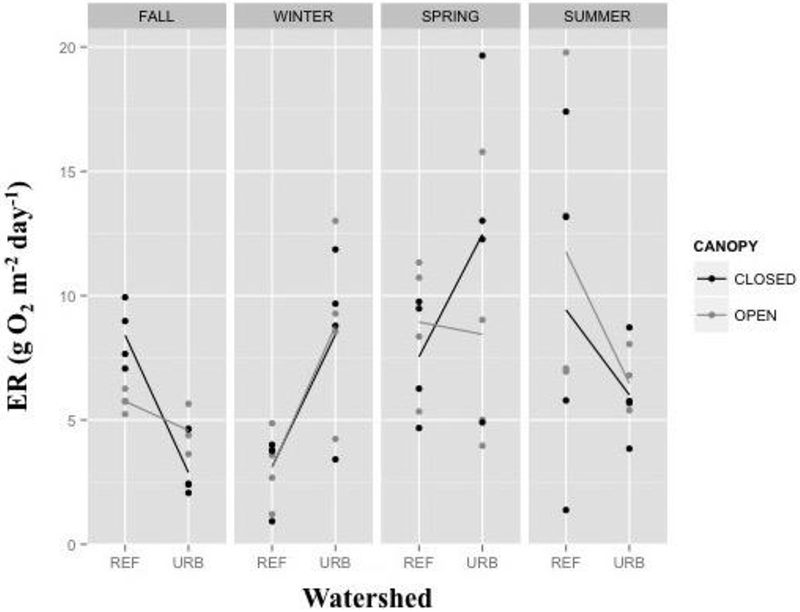
Rates of stream Ecosystem Respiration (ER) by watershed, canopy, and season. Dots represent the rate for individual study reaches during the specific sampling season. Lines connect the mean value for each canopy treatment by watershed type.
NEP
NEP ranged from −11.2 to 7.3 g O2 m−2 day−1, with a mean of −1.3 g O2 m−2 day−1. NEP was best predicted by a model including both season and watershed as main effects, as well as the season × watershed interaction term (Table 3). Urban reaches tended to exhibit more positive NEP in urban reaches than their reference counterparts, except during winter (Figure 4).
Figure 4.
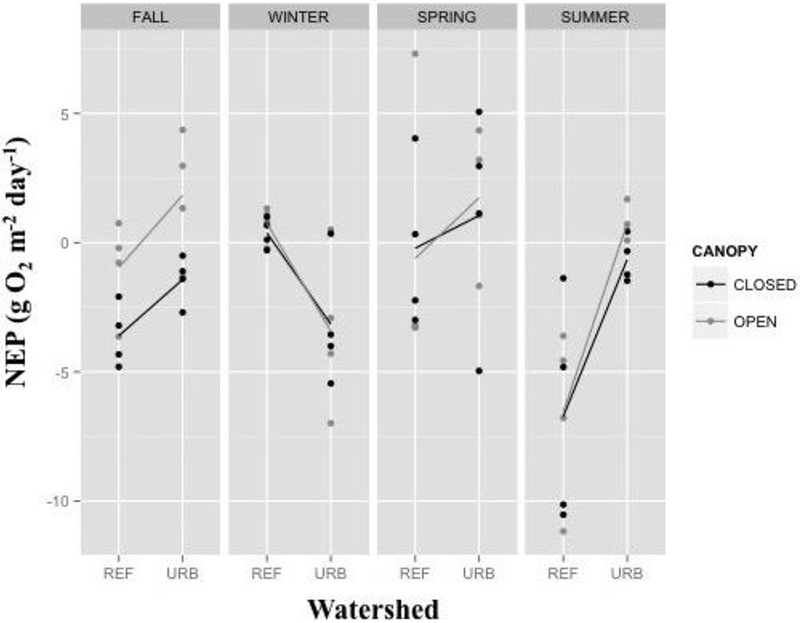
Rates of stream Net Ecosystem Productivity (NEP) by watershed, canopy, and season. Dots represent the rate for individual study reaches during the specific sampling season. Lines connect the mean value for each canopy treatment by watershed type.
NEP rates in reference streams were most positive in winter, highly variable during the spring, and were negative (net heterotrophic) in summer and fall, regardless of canopy treatment. In urban streams, NEP was negative during winter and positive (net autotrophic) during the spring, but differed by canopy treatment during fall and summer. Although not significant in our model, the impact of canopy cover was evident during the times of year when riparian canopy shaded the stream.
Discussion
Our study is among the first to examine how stream metabolism is influenced by the interaction of watershed-scale land use, local-scale riparian canopy, and season. As a result, we observed distinct patterns in stream GPP, ER, and NEP, and our findings were consistent with our conceptual model of interacting controls on stream metabolism (Figure 1). Although watershed factors and seasonality appeared to have a strong influence on stream metabolism in this study, we have also shown that the restoration and/or preservation of riparian canopy cover may still have value as a mitigation technique. It should be noted that the closed canopy reaches in this study were not recently replanted, and may not necessarily reflect the specific characteristics of a restored riparian zone. However, the impact of canopy in our urban streams suggests that preservation of a riparian corridor during development could be an important mechanism to minimize the deleterious impacts of urbanization on some key aspects of stream ecosystem function.
The observation that GPP varied with season was consistent with our expectations and observations from previous studies, (for example, Roberts and others 2007; Izagirre and others 2008). Our finding that GPP by reach type in urban streams was higher than their reference counterparts, with the exception of closed reaches in the fall, also generally conforms with the previous studies reporting elevated GPP rates in urban watersheds compared to reference watersheds (for example, Bernot and others 2010). The lack of a consistently strong canopy effect over GPP in the present study was unexpected, given the important role often attributed to the impacts of light limitation on algal production (Young and Huryn 1999; Mulholland and others 2001; Bott and others 2006) as well community composition (Hill and others 1995). However, shading from leaves in the canopy may be more variable among streams during spring and fall and greatly lessened during winter compared to other seasons (Table 2), so the canopy’s influence would be expected to vary accordingly.
During seasons when a canopy effect on stream metabolism was statistically detectable (that is, summer), the effect size was similar across watershed land use (parallel lines in Figure 2-SUMMER). Contrary to our expectations, this suggests that canopy (that is, light availability) does not interact synergistically with watershed land use. A synergistic response might be expected if background nutrient concentrations determined the degree to which GPP can respond to additional light, as suggested by the findings of Hill and others (2011). The potential for a synergistic relationship between light and nutrient loading, factors likely to be controlled at different spatial scales, could lead to an undesirable state of excess algal biomass. Rather, it appears that in our study system, the canopy and watershed effects on GPP were additive.
Although light is often cited as one of the strongest controls on in-stream GPP (Bott and others 1985; Young and Huryn 1999), our findings indicate the effects of watershed urbanization may be equally as strong. With the exception of closed reaches in fall, GPP was greater in urban streams than their reference counterparts. In fact, GPP in urban closed reaches was approximately equal to or greater than that of reference open reaches in winter, spring, and summer. This result indicates that the magnitude of the watershed urbanization and riparian cover effects may be similar and that shaded streams in urban watersheds can support GPP rates similar to those observed in high-light streams draining lesser-developed watersheds.
ER varied seasonally (Figure 3), which was anticipated in response to changes in the temperature and organic matter sources (that is, leaf-fall, GPP rates). ER was highest in reference streams during the summer, which could be a result of elevated water temperatures. In urban streams, on the other hand, ER was highest in spring, corresponding to a period of elevated GPP. The high spring ER could reflect high rates of autotrophic respiration, which is included in the ER estimate, or may be a result of heterotrophic respiration fueled by accumulated algal biomass (as discussed in Hall and Beaulieu 2013). Urban stream ER was lowest in fall, a period when increased leaf litter inputs are expected to elevate heterotrophic respiration rates (Roberts and others 2007), possibly because flashy hydrology flushed the leaves downstream (Konrad and Booth 2005; Walsh and others 2005). On the other hand, estimated autumn litterfall in the urban streams was approximately half that of the reference streams, regardless of canopy cover (Table 2). Our urban stream litterfall estimate was also substantially less than that observed by Mulholland (1997), who estimated that litterfall totaled 459 g/m2 on an annual basis in a forested watershed. The combination of lower leaf litter inputs and flashy hydrology may result in a minimal heterotrophic response in urban streams, and thus no measurable increase in ER during the fall. This result is consistent with that described in Beaulieu and others (2013), where ER in an urban stream was reported to not peak during the fall season when terrestrial inputs are likely highest.
Canopy did not significantly affect ER in our model, possibly because canopy effects on ER varied by season and watershed and were therefore highly variable through time and space. Despite the lack of a statistically significant canopy effect, there were differences between reaches under certain conditions. For example, closed reference reaches supported greater ER during the fall than the corresponding open reaches, which is consistent with numerous reports that elevated leaf litter inputs can stimulate ER (Roberts and others 2007; Riley and Dodds 2012).
Taken together, the data suggest that urban stream ER is related more closely linked to GPP than to terrestrial inputs, signifying a potential shift in energetic reliance from allochthonous to autochthonous carbon sources in urban streams. Walsh and others (2005) suggest that the residence time of terrestrial inputs in urban streams is likely to be relatively short, due to frequent scouring associated with hydrological alterations of urban streams. In urbanized watersheds, potential flushing of terrestrial organic matter inputs due to flashy hydrology, combined with observed elevated algal biomass, and decreased leaf litter in our study systems, might be expected to shift small streams away from their more natural reliance on allochthonous matter. This is in agreement with Hagen and others (2010) who noted a similar shift in trophic state in agricultural watersheds. The impact of such a fundamental alteration of the trophic basis of production could have strong implications on food web structure and secondary production (Marcarelli and others 2011).
As a function of both GPP and ER, NEP was influenced strongly by both watershed and season (Figure 4). These NEP patterns were anticipated based on expected differences in GPP and ER due to temperature, photoperiod, and temporal shifts in organic matter inputs associated with seasonality, as well as elevated nutrient loads attributed to watershed urbanization (Figure 1). Although urban NEP was higher than reference NEP for much of the year, reference streams were net autotrophic in winter, whereas urban streams were strongly net heterotrophic. Our observations regarding reference stream NEP in winter is consistent with a forested stream in Tennessee being net autotrophic only during late winter and early spring (Roberts and others 2007). As for the urban streams, it is likely that ER was elevated by the algal biomass that accumulated in the summer and fall and senesced during the short and cold winter days. This conclusion is supported by our benthic detritus data that indicate significantly higher detrital standing stocks in urban than reference streams during winter. In summer, on the other hand, reference streams exhibited high levels of net heterotrophy, whereas urban streams were more balanced. These differences appear to be driven mainly by relatively high rates of ER in the reference streams during summer and in the urban streams during winter, rather than by variation in GPP (Figures 2, 3).
Canopy had no consistent influence on NEP, presumably because there was less difference in light levels between open and closed reaches during the winter and spring when the riparian vegetation had few or no leaves. In fact, winter canopy cover in closed reaches was roughly equal to that of summer cover in open reaches (Table 2). The canopy effect was, however, apparent during the summer and fall, when the open reaches supported higher rates of GPP, and our data suggest that for much of the year, the existence of a riparian canopy may be enough to maintain urban streams in a heterotrophic state, similar to reference conditions. This observation has substantial management relevance, as net autotrophy is likely to lead to an accumulation of algal biomass and nighttime hypoxia (Carpenter and others 1998). Dodds and Welch (2000) state that nuisance algal levels in streams are likely to occur between 100 and 200 mg/m2 chlorophyll. Algae in the urban reaches in this study were within this range, with the exception of closed reaches during summer when canopy was densest, highlighting the potential importance of riparian cover.
The difference in GPP between open and closed reaches resulted in urban streams that alternated between net autotrophy and net heterotrophy at a local reach-scale during seasons when riparian canopy was dense. Open reaches in urban streams were net autotrophic during summer and fall, whereas closed reaches were net heterotrophic. During these seasons, because the average NEP of the closed-urban reaches was only slightly negative, reduced riparian canopy shading had a strong enough effect to shift the urban open reaches into a state of net production. How these changes in trophic state might also affect primary and secondary consumers is unknown, particularly considering the relatively small spatial and temporal scale at which these differences are observed.
Whereas stream metabolism has been discussed as a potential measure of ecological health (Mulholland and others 2005; Young and others 2008), our study suggests that sampling timeframe and riparian condition can wield strong influence over such assessment measures. Differences between reference and urban conditions varied dramatically depending on the time of year and specific location assessed. For example, urban streams appear to exhibit higher GPP than reference streams for much of the year, whereas the trophic state, measured as NEP, of urban streams, varied at the reach-scale. It is also important to note that differences in NEP appear to be especially stark between watersheds and reaches during summer, the timeframe during which most sampling is likely to occur.
In the face of ongoing human development, impacts on stream ecosystems are likely to become more widespread. This study provides evidence that stream metabolism, an integrative measure of stream function, is controlled by factors operating at both watershed and local riparian scales, but that the type and degree of control vary on a seasonal basis. Although it is clear that watershed land use affects stream metabolism, this study indicates that the preservation and restoration of vegetated riparian zones management could still provide an important management tool in urbanized watersheds.
Supplementary Material
Acknowledgments
The research support was provided by the University of Cincinnati and the Wieman Wendel Benedict Award program. We would like to thank Jennifer Davisson, Mark Mitchell, Kate Johnson, Kevin Ley, Steven Doyle, Vivian Miller, Matt Wooten, and Elizabeth Fet for their assistance in the field and the laboratory. We would also like to thank Ken Fritz for helpful reviews that greatly improved the earlier versions of this manuscript. The views expressed in this paper are those of the author[s] and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
References
- 1.Acuna V, Giorgi A, Munoz I, Uehlinger U, Sabater S. 2004. Flow extremes and benthic organic matter shape the metabolism of a headwater Mediterranean stream. Freshwater Biology 49: 960–971. [Google Scholar]
- 2.Allan JD. 2004. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annual Review of Ecology Evolution and Systematics 35: 257–284. [Google Scholar]
- 3.American Public Health Administration. 2005. Standard methods for the examination of water and wastewater. American Public Health Administration, Washington DC. [Google Scholar]
- 4.Bates D, Maechler M. 2010. Lme4: Linear mixed-effects models using S4 classes. R package version 0.999375–37. [Google Scholar]
- 5.Bailey RG. 1995. Description of the ecoregions of the United States. 2nd edition U.S. Department of Agriculture, Forest Service; Washington, D.C., U.S.A. [Google Scholar]
- 6.Beauchamp VB, Swan CM, Szlavecz K, Hu J. 2015. Riparian community structure and soil properties of restored urban streams. Ecohydrology 8: 880–895. [Google Scholar]
- 7.Beaulieu JJ, Arango CP, Balz DA, Shuster WD. 2013. Continuous monitoring reveals multiple controls on ecosystem metabolism in a suburban stream. Freshwater Biology 58: 918–937. [Google Scholar]
- 8.Bernhardt ES, Likens GE, Hall RO, Buso DC, Fisher SG, Burton TM, Meyer JL, McDowell WH, Mayer MS, Bowden WB, Findlay SEG, Macneale KH, Stelzer RS, Lowe WH. 2005. Can’t see the forest for the stream? - In-stream processing and terrestrial nitrogen exports. Bioscience 55: 219–230. [Google Scholar]
- 9.Bernhardt ES, Palmer MA. 2007. Restoring streams in an urbanizing world. Freshwater Biology 52: 738–751. [Google Scholar]
- 10.Bernhardt ES, Sudduth EB, Palmer MA, Allan JD, Meyer JL, Alexander G, Follastad-Shah J, Hassett B, Jenkinson R, Lave R, Rumps J, Pagano L. 2007. Restoring rivers one reach at a time: Results from a survey of US river restoration practitioners. Restoration Ecology 15: 482–493. [Google Scholar]
- 11.Bernot MJ, Sobota DJ, Hall RO, Mulholland PJ, Dodds WK, Webster JR, Tank JL, Ashkenas LR, Cooper LW, Dahm CN, Gregory SV, Grimm NB, Hamilton SK, Johnson SL, McDowell WH, Meyer JL, Peterson B, Poole GC, Valett HM, Arango C, Beaulieu JJ, Burgin AJ, Crenshaw C, Helton AM, Johnson L, Merriam J, Niederlehner BR, O’Brien JM, Potter JD, Sheibley RW, Thomas SM, Wilson K. 2010. Inter-regional comparison of land-use effects on stream metabolism. Freshwater Biology 55: 1874–1890. [Google Scholar]
- 12.Bond NR, Lake PS. 2003. Characterizing fish-habitat associations in streams as the first step in ecological restoration. Austral Ecology 28: 611–621. [Google Scholar]
- 13.Bott TL, Brock JT, Dunn CS, Naiman RJ, Ovink RW, Petersen RC. 1985. Benthic community metabolism in 4 temperate stream systems - an inter-biome comparison and evaluation of the river continuum concept. Hydrobiologia 123: 3–45. [Google Scholar]
- 14.Bott TL, Newbold JD, Arscott DB. 2006. Ecosystem metabolism in piedmont streams: Reach geomorphology modulates the influence of riparian vegetation. Ecosystems 9: 398–421. [Google Scholar]
- 15.Burnham KP, Anderson DR. 2002. Model selection and multi-model inference: a practical information theoretic approach. Second edition Springer-Verlag, New York, NY, U.S.A. [Google Scholar]
- 16.Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH. 1998. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecological Applications 8: 559–568. [Google Scholar]
- 17.Chapra SC, Ditoro DM. 1991. Delta method for estimating primary production, respiration, and reaeration in streams. Journal of Environmental Engineering-Asce 117: 640–655. [Google Scholar]
- 18.Demars BOL, Manson JR, Olafsson JS, Gislason GM, Gudmundsdottir R, Woodward G, Reiss J, Pichler DE, Rasmussen JJ, Friberg N. 2011. Temperature and the metabolic balance of streams. Freshwater Biology 56: 1106–1121. [Google Scholar]
- 19.Demars BOL, Thompson J, Manson JR. 2015. Stream metabolism and the open diel oxygen method: Principles, practice, and perspectives. Limnology and Oceanography-Methods 13: 356–374. [Google Scholar]
- 20.Doane TA, Horwath W. 2003. Spectrophotometric Determination of Nitrate with a Single Reagent. Analytical Letters 36: 2713–2722. [Google Scholar]
- 21.Dodds WK, Biggs BJF, Lowe RL. 1999. Photosynthesis-irradiance patterns in benthic microalgae: Variations as a function of assemblage thickness anc community structure. Journal of Phycology 35: 42–53. [Google Scholar]
- 22.Dodds WK, Welch EB. 2000. Establishing nutrient criteria in streams. Journal of the North American Benthological Society 19: 186–196. [Google Scholar]
- 23.Fisher SG, Likens GE. 1973. Energy flow in Bear Brook, New Hampshire - integrative approach to stream ecosystem metabolism. Ecological Monographs 43: 421–439. [Google Scholar]
- 24.Folke C, Jansson A, Larsson J, Costanza R. 1997. Ecosystem appropriation by cities. Ambio 26: 168–172. [Google Scholar]
- 25.Grace MR, Giling DP, Hladyz S, Caron V, Thompson RM, Mac Nally R. 2015. Fast processing of diel oxygen curves: Estimating stream metabolism with BASE (BAyesian Single-station Estimation). Limnology and Oceanography-Methods 13: 103–114. [Google Scholar]
- 26.Grimm NB, Fisher SG. 1989. Stability of periphyton and macroinvertebrates to disturbance by flash floods in a desert stream. Journal of the North American Benthological Society 8: 293–307. [Google Scholar]
- 27.Guasch H, Marti E, Sabater S. 1995. Nutrient enrichment effects on biofilm metabolism in a Mediterranean stream. Freshwater Biology 33: 373–383. [Google Scholar]
- 28.Hagen EM, McTammany ME, Webster JR, Benfield EF. 2010. Shifts in allochthonous input and autochthonous production in streams along an agricultural land-use gradient. Hydrobiologia 655: 61–77. [Google Scholar]
- 29.Hall RO, Beaulieu JJ. 2013. Estimating autotrophic respiration in streams using daily metabolism data. Freshwater Science 32: 507–516. [Google Scholar]
- 30.Hession WC, Pizzuto JE, Johnson TE, Horwitz RJ. 2003. Influence of bank vegetation on channel morphology in rural and urban watersheds. Geology 31: 147–150. [Google Scholar]
- 31.Hill BH, Hall RK, Husby P, Herlihy AT, Dunne M. 2000. Interregional comparisons of sediment microbial respiration in streams. Freshwater Biology 44: 213–222. [Google Scholar]
- 32.Hill WR, Roberts BJ, Francoeur SN, Fanta SE. 2011. Resource synergy in stream periphyton communities. Journal of Ecology 99: 454–463. [Google Scholar]
- 33.Hill WR, Ryon MG, Schilling EM. 1995. Light limitation in a stream ecosystem - responses by primary producers and consumers. Ecology 76: 1297–1309. [Google Scholar]
- 34.Homer CG, Dewitz JA, Yang L, Jin S, Danielson P, Xian G, Coulston J, Herold ND, Wickham JD, Megown K 2015. Completion of the 2011 National Land Cover Database for the conterminous United States-Representing a decade of land cover change information. Photogrammetric Engineering and Remote Sensing 81: 345–354 [Google Scholar]
- 35.Holtgrieve GW, Schindler DE, Branch TA, A’Mar ZT. 2010. Simultaneous quantification of aquatic ecosystem metabolism and reaeration using a Bayesian statistical model of oxygen dynamics. Limnology and Oceanography 55: 1047–1063. [Google Scholar]
- 36.Izagirre O, Agirre U, Bermejo M, Pozo J, Elosegi A. 2008. Environmental controls of whole-stream metabolism identified from continuous monitoring of Basque streams. Journal of the North American Benthological Society 27: 252–268. [Google Scholar]
- 37.Jordan TE, Correll DL, Weller DE. 1997. Relating nutrient discharges from watersheds to land use and streamflow variability. Water Resources Research 33: 2579–2590. [Google Scholar]
- 38.Kentucky Division of Water (KDOW). 2008. Standard Methods for Assessing Biological Integrity of Surface Waters in Kentucky . Environmental and Public Protection Cabinet, Department for Environmental Protection, Frankfort, KY. [Google Scholar]; Odum HT. 1956. Primary production in flowing waters. Limnology and Oceanography. 2:102–117. [Google Scholar]
- 39.Konrad CP, Booth DB. 2005. Hydrologic changes in urban streams and their ecological significance Brown LR, Gray RH, Hughes RM, Meador MR editors. Effects of Urbanization on Stream Ecosystems. [Google Scholar]
- 40.LeBlanc RT, Brown RD, FitzGibbon JE. 1997. Modeling the effects of land use change on the water temperature in unregulated urban streams. Journal of Environmental Management 49: 445–469. [Google Scholar]
- 41.Lovett G, Cole J, Pace M. 2006. Is net ecosystem production equal to ecosystem carbon accumulation? Ecosystems 9: 152–155. [Google Scholar]
- 42.Marcarelli AM, Baxter CV, Mineau MM, Hall RO. 2011. Quantity and quality: unifying food web and ecosystem perspectives on the role of resource subsidies in freshwaters. Ecology 92:1215–1225. [DOI] [PubMed] [Google Scholar]
- 43.Meyer JL, Paul MJ, Taulbee WK. 2005. Stream ecosystem function in urbanizing landscapes. Journal of the North American Benthological Society 24: 602–612. [Google Scholar]
- 44.Meyer JL, Strayer DL, Wallace JB, Eggert SL, Helfman GS, Leonard NE. 2007. The contribution of headwater streams to biodiversity in river networks. Journal of the American Water Resources Association 43: 86–103. [Google Scholar]
- 45.Mulholland PJ. 1997. Organic matter dynamics in the West Fork of Walker Branch, Tennessee, USA. Journal of the North American Benthological Society 16: 61–67. [Google Scholar]
- 46.Mulholland PJ, Fellows CS, Tank JL, Grimm NB, Webster JR, Hamilton SK, Marti E, Ashkenas L, Bowden WB, Dodds WK, McDowell WH, Paul MJ, Peterson BJ. 2001. Inter-biome comparison of factors controlling stream metabolism. Freshwater Biology 46: 1503–1517. [Google Scholar]
- 47.Mulholland PJ, Houser JN, Maloney KO. 2005. Stream diurnal dissolved oxygen profiles as indicators of in-stream metabolism and disturbance effects: Fort Benning as a case study. Ecological Indicators 5: 243–252. [Google Scholar]
- 48.Murphy J, Riley JP. 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica 27: 31–36. [Google Scholar]
- 49.National Climatic Data Center (NCDC), NOAA Satellite and Information Service 2008. Data obtained for Cincinnati Northern KY Airport, Covington/Cincinnati, KY, United States. [Google Scholar]
- 50.Palmer MA, Ambrose RF, Poff NL. 1997. Ecological theory and community restoration ecology. Restoration Ecology 5: 291–300. [Google Scholar]
- 51.Paul MJ, Meyer JL. 2001. Streams in the urban landscape. Annual Review of Ecology and Systematics 32: 333–365. [Google Scholar]
- 52.Plummer M, Best N, Cowles K, Vines K. 2006. CODA: Convergence diagnosis and output analysis for MCMC. R News 6:7–11. [Google Scholar]; R Development Core Team. 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: ISBN 3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- 53.Ray JA, Webb JS, O’Dell PW, 1994. Groundwater Sensitivity Regions of Kentucky Kentucky Department of Environmental Protection, Division of Water, Groundwater Branch, Lexington, Kentucky, U.S.A. [Google Scholar]
- 54.Reavie ED, Jicha TM, Angradi TR, Bolgrien DW, Hill BH. 2010. Algal assemblages for large river biomonitoring: Comparison among biovolume, absolute, and relative abundance metrics. Ecological Indicators 10: 167–177. [Google Scholar]
- 55.Reichert P, Uehlinger U, Acuna V. 2009. Estimating stream metabolism from oxygen concentrations: Effect of spatial heterogeneity. Journal of Geophysical Research-Biogeosciences 114: 2156–2202. [Google Scholar]
- 56.Riley AJ, Dodds WK. 2012. The expansion of woody riparian vegetation, and subsequent stream restoration, influences the metabolism of prairie streams. Freshwater Biology 57: 1138–1150. [Google Scholar]
- 57.Roberts BJ, Mulholland PJ, Hill WR. 2007. Multiple scales of temporal variability in ecosystem metabolism rates: Results from 2 years of continuous monitoring in a forested headwater stream. Ecosystems 10: 588–606. [Google Scholar]
- 58.Roy AH, Faust CL, Freeman MC, Meyer JL. 2005. Reach-scale effects of riparian forest cover on urban stream ecosystems. Canadian Journal of Fisheries and Aquatic Sciences 62: 2312–2329. [Google Scholar]
- 59.Sinsabaugh RL. 1997. Large-scale trends for stream benthic respiration. Journal of the North American Benthological Society 16: 119–122. [Google Scholar]
- 60.Snyder CD, Young JA, Villella R, Lemarie DP. 2003. Influences of upland and riparian land use patterns on stream biotic integrity. Landscape Ecology 18: 647–664. [Google Scholar]
- 61.Sudduth EB, Hassett BA, Cada P, Bernhardt ES. 2011. Testing the Field of Dreams Hypothesis: functional responses to urbanization and restoration in stream ecosystems. Ecological Applications 21: 1972–1988. [DOI] [PubMed] [Google Scholar]
- 62.Sweeney BW, Bott TL, Jackson JK, Kaplan LA, Newbold JD, Standley LJ, Hession WC, Horwitz RJ. 2004. Riparian deforestation, stream narrowing, and loss of stream ecosystem services. Proceedings of the National Academy of Sciences of the United States of America 101: 14132–14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tank JL, Rosi-Marshall EJ, Griffiths NA, Entrekin SA, Stephen ML. 2010. A review of allochthonous organic matter dynamics and metabolism in streams. Journal of the North American Benthological Society 29: 118–146. [Google Scholar]
- 64.Uehlinger U 2000. Resistance and resilience of ecosystem metabolism in a flood-prone river system. Freshwater Biology 45: 319–332. [Google Scholar]
- 65.United States Department of Agriculture. 2011. Major land uses of the United States, 2007. Economic Information Bulleting No. EIB-89, 67 pp. [Google Scholar]
- 66.Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman D. 1997. Human alteration of the global nitrogen cycle: Sources and consequences. Ecological Applications 7: 737–750. [Google Scholar]
- 67.Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan RP. 2005. The urban stream syndrome: current knowledge and the search for a cure. Journal of the North American Benthological Society 24: 706–723. [Google Scholar]
- 68.Weatherburn MW. 1967. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 39: 971–974. [Google Scholar]
- 69.Young RG, Huryn AD. 1999. Effects of land use on stream metabolism and organic matter turnover. Ecological Applications 9: 1359–1376. [Google Scholar]
- 70.Young RG, Matthaei CD, Townsend CR. 2008. Organic matter breakdown and ecosystem metabolism: functional indicators for assessing river ecosystem health. Journal of the North American Benthological Society 27: 605–625. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


