Abstract
Objective:
To report preliminary results of a cutting edge extreme hypofractionated treatment with concomitant boost to the dominant lesion for patients with early stage prostate cancer (PCa).
Methods:
AIRC-IG-13218 is a prospective Phase II trial started in June 2015. Patients with low and intermediate risk PCa who met the inclusion criteria underwent extreme hypofractionated radiotherapy to the prostate (36.25 Gy in 5 fractions) and a simultaneous integrated boost to the dominant intraprostatic lesion (DIL) to 37.5 Gy. The DIL was identified by a multiparamentric MRI (mpMRI) co-registered with planning CT. Toxicity was assessed according to CTCAE v4.0 and RTOG/EORTC criteria. The preliminary evaluation of the first 13 patients was required to confirm the feasibility of the treatment before completing the enrollment of 65 patients.
Results:
The first 13 patients completed the treatment between June 2015 and February 2016. With a median clinical follow-up of 17 months (range 11–26), no Grade 3 or 4 early toxicity was reported.
Conclusions:
Our preliminary data about early toxicity of an extreme hypofractionated schedule with concomitant boost on the DIL are encouraging. The higher number of patients expected for the trial and a longer follow-up are needed to confirm these results.
Advances in knowledge:
The use of mpMRI to identify and boost the DIL is an innovative and interesting approach to PCa. Our preliminary findings suggest that dose escalation using DIL boost and extremely hypofractionated radiotherapy regimens might be a safe approach, allowing for short and effective treatment of organ-confined PCa.
Introduction
Prostate cancer (PCa) is the most common tumor in European males. Nowadays though, due to improvement of screening programs and diagnostic techniques, the majority of patients are diagnosed in early stage (95% are non-metastatic). Treatment options for low and intermediate risk diseases usually consist of active surveillance, surgery, external beam radiation therapy and brachytherapyBRT). Due to PCa peculiar radiobiological features, that is a very low α/β ratio, in the past few years a great interest has been focused on hypofractionated radiotherapy. This may lead to an improvement of the therapeutic ratio, with higher probability of local control and decrease of toxicity. Moreover, hypofractionated regimens are often a better choice both for the patient, who can be treated in few days, with better quality of life and lower economic impact, and for the Center, which can reduce waiting lists. Some studies have been performed and are still ongoing with the aim of assessing feasibility, toxicity outcome and effectiveness of extreme hypofractionated schedules, which require advanced techniques and technologies. These trials have been conducted using a stereotactic approach, delivering high doses (6.7–10 Gy) in 5 fractions, pointing out the feasibility of such regimens.1
In parallel, dose escalation has been investigated, showing benefit in terms of tumor control. The majority of dose escalation studies include conventional fractionation regimes. Most recently, dose escalation with boost to the dominant intraprostatic lesion (DIL) within conventional fractionation has been studied.2, 3
The aim of this prospective Phase II is to assess the feasibility of an extreme hypofractionated schedule with a simultaneous integrated boost on the DIL. Our study combines dose escalation (limited to DIL) and extreme hypofractionated radiotherapy concepts. This is the report of the ad interim analysis planned after treatment of the first 13 patients.
METHODS AND PATIENTS
Study design
The study protocol has been previously published,4 so will be briefly described.
The primary endpoint of the study is feasibility, in terms of incidence of early effects, evaluated at the end of treatment and within a month after, according to Radiotherapy Oncology Group/European Organization for Research and Treatment of Cancer (RTOG/EORTC) scale: every Grade 3 or 4 event is considered as “failure”. Late toxicity, as a secondary endpoint, is evaluated at 6, 12 and 24 months after treatment completion, according to Common Terminology Criteria for Adverse Events (CTCAE) v 4.0 and RTOG/EORTC scale. Cumulative toxicity is defined as the worst event experienced by the patient.
Further secondary endpoints, investigating efficacy, include biochemical response (through 3-monthly prostatic specific antigen evaluation), time to biochemical failure, disease-free survival (both local and distant), cause-specific survival and overall survival.
Treatment-related quality of life, according to EORTC questionnaires QLQ-C30 and QLQ-PR25, International Prostatic Symptoms Score (IPSS) and International Index of Erectile Function (IEEF-5), is also evaluated.
Furthermore, we decided to examine the role of multiparametric MRI (mp-MRI) in identifying the tumor and in guiding treatment planning, given the increasing use of MRI in PCa staging before treatments.
The last part of our work is addressed to the identification of molecular prognostic factors for aggressive PCa, through the implementation of MRI-guided biopsy of intraprostatic lesions.
Inclusion and exclusion criteria are reported in Table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Histologically confirmed prostate adenocarcinoma |
| Very low, low and intermediate NCCN risk categories | |
| Age >18 years | |
| Good performance status (ECOG <2) | |
| No previous pelvic radiotherapy | |
| No previous prostatectomy | |
| Good urinary flow (peak flow >10 ml s−1) | |
| Ability to complete questionnaires about quality of life | |
| Written informed consent signed | |
| Exclusion criteria | Extracapsular tumor or locally advanced disease (cT3-cT4) |
| Nodal involvement or distant metastasis (cN1 or cM1) | |
| IPSS questionnaire score >20 | |
| Concomitant inflammatory bowel diseases | |
| Important systemic diseases or oral anticoagulant therapy ongoing | |
| Non-conformity to dose constraints at the treatment planning | |
| Previous invasive cancer (apart from non-melanoma skin), unless the patient has been free from disease for at least 3 years | |
| Mental diseases that cannot ensure a valid informed consent |
ECOG, Eastern Cooperative Oncology Group; IPSS, International Prostatic Symptoms Score; NCCN, National Comprehensive Cancer Network.
Ethical aspects
The study has been approved by the Ethical Committee of the Institute and registered at ClinicalTrials.gov.
Treatment planning
The DIL was identified by using all images available in the mp-MRI of the prostate: high spatial resolution small field of view T2 weighted images on three planes (axial, sagittal, coronal), diffusion weighted images with high b value and ADC maps, as well as dynamic contrast-enhanced images.
The mp-MRI protocol was PI-RADS compliant and involved: sagittal, coronal and axial T2 weighted images, axial diffusion weighted and pre-contrast T1 weighted images, and a dynamic series of axial T1 weighted images obtained before, during and after injection of gadopentetate dimeglumine (Magnevist, Bayer HealthCare, Berlin, Germany). The contrast agent was administered with a 0.1 mmol kg–1 injection through a peripheral vein at a flow rate of 3 ml s−1, followed by a 10 ml bolus of saline at the same flow rate, using a mechanical injector (Spectris MR Injection System, Medrad, Leverkusen, Germany).
The mp-MRI was then co-registered with the planning CT for the treatment planning: the high spatial resolution small field of view T2 weighted images on the axial plane were used for the delineation of the DIL, representing the gross tumor volume (GTV).
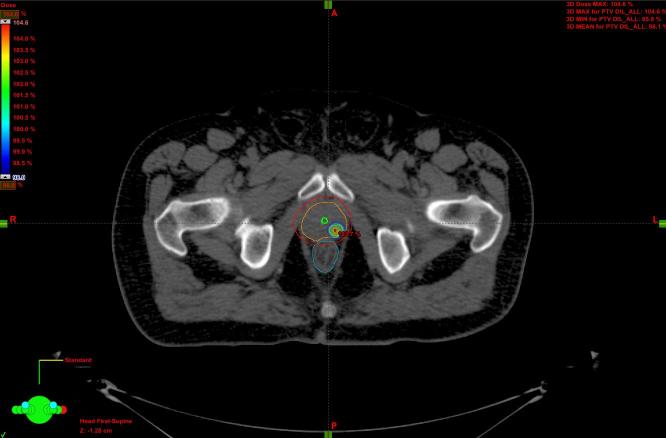
The clinical target volume (CTV) was represented by the whole prostate, identified on co-registered imaging, and the proximal third of the seminal vesicles in intermediate risk PCa. A margin of 3 mm posteriorly and 5 mm in all the other directions was added to create the planning target volume for the prostate (PTV-P) and 3 mm in all directions for the DIL (PTV-DIL). The planning dose to PTV-P was 36.25 Gy in 5 fractions of 7.25 Gy each, while the PTV-DIL received 37.5 Gy in 7.5 Gy per fraction as a simultaneous integrated boost. PTVs had to be covered at least by 95% of the prescription dose (D95% ≥ 95% for PTV-P and D95% ≥ 98% for PTV-DIL); maximum dose (up to 110%) had to be inside the DIL. The treatment has been delivered every other day, with a total treatment time of 10 days. Figures 1 and 2 show an example of the dose distribution.
Figure 1.
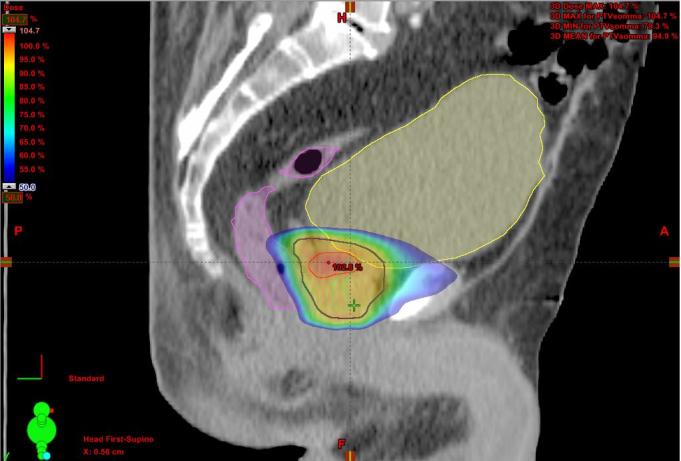
Color wash dose distribution in sagittal plane.
Figure 2.
Color wash dose distribution of the DIL in axial plane. DIL, dominant intraprostatic lesion.
The following structures were contoured as organs at risk (OARs): urinary bladder, rectum, posterior rectal wall, anal canal, urethra, peritoneal cavity/bowel bag, penile bulb, penis, testicles, femoral heads and necks, and cauda equina.
Dose-volume histograms were calculated for GTV/CTVs, PTVs and OARs, in order to evaluate the feasibility of the treatment plan, according to dose constraints.1, 5
Treatment delivery
The treatment was delivered by TrilogyTM with RapidArc® technology (Varian Medical Systems, Palo Alto, CA). Before each treatment delivery, a cone beam CT (CBCT) was acquired in order to ensure treatment precision despite possible intra-fraction errors. Patients were trained to have empty rectum and full bladder at each treatment fraction. The use of α−1 blockers and low doses of steroids was recommended to lower the risk of urinary obstruction and minimize inflammatory effects.
Statistical methods
Being early toxicity the primary endpoint of the trial, a rate of success (patients with no cumulative early toxicity grade ≥ 3) of 95% has been considered sufficient to warrant further investigation, whereas a rate of success of 85% or less (or even one event of rectal/urinary bladder necrosis) would have been considered unacceptable. To test this hypothesis, the initial stage of the study required 13 patients: the treatment schedule would have been rejected and the study prematurely closed if 2 or more Grade 3 or 4 early events (or one necrosis) occurred in this first stage, otherwise the trial would have proceeded with the recruitment to a total of 65 patients.
RESULTS
Between June 2015 and January 2016, 13 consecutive patients who fulfilled the inclusion criteria were treated in our Institution; 11 further patients were initially enrolled but then excluded mainly due to mp-MRI findings (T3 tumors), unsatisfactory urofluximetry or unfeasible CT-MRI fusion. The patients’ characteristics are listed in Table 2. Median clinical follow-up is 17 months (range 11–26).
Table 2.
Patients’ and disease’s characteristics
| Characteristic | Value |
| Median age (range) [years] | 75.4 (62.7–79.9) |
| Median initial PSA (range) [ng ml–1] | 5.8 (4.3–17) |
| Gleason Score | |
| 3 + 3 | 8 |
| 3 + 4 | 4 |
| 4 + 3 | 1 |
| Clinical stage (according to mp-MRI) | |
| T2a N0 | 5 |
| T2b N0 | 2 |
| T2c N0 | 6 |
| Risk (according to NCCN): | |
| Low | 3 |
| Intermediate | 10 |
| Median prostate volume (range) [cm3] | 41.50 (24.70–61.90) |
| Number of DIL(s) | |
| 1 lesion | 11 |
| 2 lesions | 2 |
| Median DIL(s) volume (range) [cm3] | 1.18 (0.20–5.40) |
| Median distance of DIL from urethra (range) [mm] | 5.9 (0.2–10.7) |
| DIL locationa | |
| Peripheral zone - intermediate/cranial + apex | 7 |
| Peripheral zone - intermediate/cranial | 3 |
| Peripheral zone - apex | 3 |
| Transition zone | 2 |
| Androgen deprivation therapy | 1 |
DIL, dominant intraprostatic lesion; mp-MRI, multiparametric MRI; NCCN, National Comprehensive Cancer Network; PSA, prostatic specific antigen;
Data from 15 DILs.
Dosimetric aspects
The planning goals were achieved, and dose-constraints for OARs were respected: Table 3 shows dosimetrical data obtained in our treatment plans. Regarding dose distribution and target coverage, median D95% of the PTV-P, D95% of the PTV-DIL and D98% of the GTV were 97.7%, 99.2 and 101.7%, respectively.
Table 3.
Dose-constraints established for the current study and dosimetrical data of our treatment plans (median and range)
| OAR | Dose/volume limits | Median | Range | |
| Rectum | V18 Gy < 50% | % | 17.90 | 7.0–28.2 |
| V29 Gy < 20% | % | 4.80 | 0.7–10.5 | |
| V33 Gy < 10% | % | 1.40 | 0.02–23.0 | |
| V36 Gy < 5% | % | 0 | 0–1.6 | |
| Posterior rectal wall | D1 cc < 16 Gy | Gy | 15.20 | 10.30–15.80 |
| Anal canal | Dmean <15 Gy | Gy | 9.45 | 1.6–14.90 |
| Posterior anal canal wall | Dmax < 16 Gy | Gy | 12.10 | 8.40–14.90 |
| Urinary bladder | V36 Gy< 10% | % | 0.50 | 0–1.50 |
| V36 Gy < 5 cm3 | cm3 | 1.20 | 0.01–4.50 | |
| V18 Gy < 40% | % | 12.80 | 3.90–36.00 | |
| Urethra | V36 Gy < 50% | % | 20.15 | 0.001–4.50 |
| Dmax < 40 Gy | Gy | 36.85 | 36.00–38.50 | |
| Hip joint (left) | V15 Gy < 5% | % | 0 | 0–1.70 |
| Hip joint (right) | V15 Gy < 5% | % | 0 | 0 |
| Peritoneal cavity | V30 Gy < 1 cm3 | cm3 | 0 | 0 |
| Dmean <5.4 Gy | % | 0.30 | 0.10–1.70 | |
| V17 Gy < 195 cm3 | cm3 | 0 | 0–0.1 | |
| Penile bulb | V29 Gy < 50% | % | 0 | 0–6.60 |
| Penis | V13 Gy <1 cm3 | cm3 | 0.01 | 0–0.60 |
| Testicles | D20% < 2 Gy | Gy | 0.30 | 0.20–0.50 |
| Cauda equina | Dmax <19 Gy | Gy | 0.50 | 0.40–0.60 |
Toxicity
Early toxicity, the primary endpoint of the study, was assessed at the end of treatment and 1 month later for all 13 patients: no Grade 3 or 4 events occurred. Toxicity included increased urinary frequency, mild incontinence, urgency, nocturia, dysuria, mild proctitis and occasional rectal bleeding. Furthermore, no ≥Grade 2 events were reported within 6 months.
Early toxicity profile of the first 13 patients is reported in Table 4.
Table 4.
Early toxicity (median follow-up of 12 months)
| Toxicity and follow-up time | Type of toxicity | Grade | Occurrences |
| Early—end of treatment | Gastrointestinal | 0 | 12 |
| 1 | 0 | ||
| 2 | 1 | ||
| Genitourinary | 0 | 9 | |
| 1 | 4 | ||
| 2 | 0 | ||
| Early—at 1 month | Gastrointestinal | 0 | 11 |
| 1 | 2 | ||
| 2 | 0 | ||
| Genitourinary | 0 | 9 | |
| 1 | 4 | ||
| 2 | 0 |
Following the results of our preliminary analysis of the first 13 patients, the study has continued. To date (December 2017), the enrollment of the 65 patients have been completed: they all have been evaluated for early toxicity, and none experienced grade >3 events.
DISCUSSION
Our prospective study, including patients affected by early stage PCa undergoing extreme hypofractionated radiotherapy with simultaneous boost on the DIL, showed a good toxicity profile.
Since PCa is a common disease with a generally good outcome, the pursuit of new effective, safe and affordable treatment options should be mandatory. This is the reason why in the last decade hypofractionated radiotherapy for localized PCa has been a central topic and a widely investigated strategy. The possibility to decrease treatment duration from 7 to 8 weeks to 10 days is appealing and convenient both for patients and radiotherapy facilities.
From a radiobiological point of view, the low α/β ratio of PCa, as well as its resulting sensitivity to higher fraction doses compared to surrounding normal tissues, should allow a more hypofractionated treatment, as long as OAR constraints are respected, in order to increase therapeutic ratio.6 Furthermore, the delivery of the dose in a short time (<2 weeks) should favorably impact on the treatment efficacy.
The dose escalation to the DIL represents the most innovative aspect of our trial. Conventionally, the target of our treatments has always been the whole prostate, indiscriminately. The fact that we are now identifying and irradiating to higher dose the actual disease could further improve the already good outcomes of patients with early stage PCa. Few similar experiences have been conducted using IMRT or a combination of external beam radiation therapy and brachytherapy.7, 8 A recent systematic review found that a boost to the DIL is a feasible option for increasing the radiation dose: with an EQD2 to the DIL > 80 Gy, as in our study, a 10-year disease-free survival rate of 98% can be reached.8
The use of mp-MRI is essential when performing this kind of high-precision treatment, both in the diagnostic and in the treatment planning setting. This tool, with the partnership of a dedicated radiologist, allows the identification of the DIL, which can be outlined as GTV, and a more accurate definition of the whole prostate, since CT can overestimate prostate volume up to 30%.9 Unfortunately, it is not always possible to perform an acceptable CT-MRI rigid fusion: even if patients undergo the two examinations in a short period of time, at least 30–40 min usually pass between planning CT scan and planning MRI, due to logistic reasons. In this amount of time, bladder filling may change, and so the rectum. Since the status of these two organs has a strong impact on prostate imaging, in some cases the changes prevented us from performing a suitable co-registration. A correct CT-MRI fusion is mandatory to identify and correctly delineate the DIL to safely deliver the higher dose to it, so patients whose imaging fusion was not acceptable were excluded from the study. The imaging fusion issue and its clinical implications have already been studied in literature.10 Other patients had to be excluded due to mp-MRI findings not matching the inclusion criteria (e.g. cT3 tumors) or to an unsatisfactory urinary flow.
Most experiences with prostate stereotactic radiotherapy have been conducted using Cyberknife, intensity modulated radiotherapy or Tomotherapy, usually with the implant of fiducials.11, 12 A recent study used a volumetric modulated arc therapy system and a CBCT for image guidance, instead of fiducials.13 This less invasive approach is endorsed by our Institution, in order to avoid the implant of markers. Performing a suitable image guidance without the need for implantation might be more comfortable for the patients, less time consuming and may prevent any procedure-related complication.
When a high dose per fraction is delivered, an important issue is to control intra-fraction motion, since geographical miss can lead both to an increase of toxicity and a decrease of tumor control. Although some tracking systems have been developed and used to minimize this limitation, the short beam-on time allowed by RapidArc® technology (usually less than 4 min in our experience) should not require to check intra-fraction errors or organ motion.
In recent literature, toxicity of extreme hypofractionated radiotherapy is reported to be quite low, with no Grade 3 or 4 early events.14–16 Hannan et al observed that doses up to 47.5 Gy in 5 fractions resulted in high control with acceptable toxicity, while 50 Gy in 5 fractions were associated with an increase in late high grade toxicity.17 In our experience no Grade 3 or 4 events were recorded within 1 month after treatment completion or at 6 months. We observed few mild urinary events at the end of treatment, such as increased urinary frequency, mild incontinence, urgency, nocturia, and dysuria, not different from usual radiotherapy-related early toxicity. Similarly, only mild proctitis and occasional rectal bleeding were reported regarding gastrointestinal early events.
These are preliminary but encouraging results, and allowed us to complete the enrollment: to date, all 65 patients have been safely treated.
CONCLUSION
To the best of our knowledge this is the first prospective study on the DIL-dose escalation using extreme hypopfractionation. Preliminary data of our pilot study including 13 patients with organ confined PCa undergoing extreme hypofractionated dose escalation using concomitant boost to mp-MRI-identified DIL showed no limiting toxicity, so the study have been completed to the enrollment of 65 patients. Furthermore, we started a biological substudy, performing mp-MRI-guided biopsies of the DIL in order to establish the potential role of some molecular markers (PTEN, Ki-67) as prognostic factors.
We believe that the actual goal for specialists dealing with PCa should be to identify a treatment which is equally or more effective than current treatments, better tolerated, shorter in order to improve the patients’ quality of life, and also convenient for both the patients and the facilities. If the results in terms of efficacy and toxicity of our trial, like other ongoing studies, will be confirmed after the evaluation of all the patients enrolled and a longer follow-up, extreme hypofractionated radiotherapy could be considered as the treatment fulfilling our expectations.
Footnotes
Acknowledgements: This study was partially supported by the research grants from Associazione Italiana per la Ricerca sul Cancro (AIRC): IG-13218 “Short-term high precision RT for early prostate cancer with concomitant boost to the dominant lesion”, and IG-14300 “Carbon ions boost followed by pelvic photon radiotherapy for high risk prostate cancer”. ClinicalTrials.gov registration: NCT01913717
Contributor Information
Giorgia Timon, Email: giorgiatimon@gmail.com.
Delia Ciardo, Email: delia.ciardo@ieo.it.
Alessia Bazani, Email: alessia.bazani@ieo.it.
Giulia Marvaso, Email: giulia.marvaso@ieo.it.
Giulia Riva, Email: giulia.riva@ieo.it.
Stefania Volpe, Email: stefania.volpe@ieo.it.
Damaris P Rojas, Email: damarispatricia.rojas@ieo.it.
Giuseppe Renne, Email: giuseppe.renne@ieo.it.
Giuseppe Petralia, Email: giuseppe.petralia@ieo.it.
Dario Zerini, Email: dario.zerini@ieo.it.
Cristiana Fodor, Email: cristiana.fodor@ieo.it.
Samantha Dicuonzo, Email: samantha.dicuonzo@ieo.it.
Davide Maestri, Email: davide.maestri@ieo.it.
Floriana Pansini, Email: floriana.pansini@ieo.it.
Raffaella Cambria, Email: raffaella.cambria@ieo.it.
Federica Cattani, Email: federica.cattani@ieo.it.
Federica Golino, Email: federica.golino@ieo.it.
Valerio Scroffi, Email: valerio.scroffi@ieo.it.
Daniela De Lorenzo, Email: daniela.delorenzo@ieo.it.
Ottavio De Cobelli, Email: ottavio.decobelli@ieo.it.
Roberto Orecchia, Email: roberto.orecchia@ieo.it.
Barbara Alicja Jereczek-Fossa, Email: barbara.jereczek@ieo.it.
REFERENCES
- 1.Henderson DR, Tree AC, van As NJ. Stereotactic body radiotherapy for prostate cancer. Clin Oncol 2015; 27: 270–9. doi: 10.1016/j.clon.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 2.Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys 2009; 74: 1405–18. doi: 10.1016/j.ijrobp.2008.10.091 [DOI] [PubMed] [Google Scholar]
- 3.Lips IM, van der Heide UA, Haustermans K, van Lin EN, Pos F, Franken SP, et al. Single blind randomized phase III trial to investigate the benefit of a focal lesion ablative microboost in prostate cancer (FLAME-trial): study protocol for a randomized controlled trial. Trials 2011; 12: 255. doi: 10.1186/1745-6215-12-255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timon G, Ciardo D, Bazani A, Garioni M, Maestri D, De Lorenzo D, et al. Rationale and protocol of AIRC IG-13218, short-term radiotherapy for early prostate cancer with concomitant boost to the dominant lesion. Tumori 2016; 102: 536–40. doi: 10.5301/tj.5000547 [DOI] [PubMed] [Google Scholar]
- 5.Chen LN, Suy S, Uhm S, Oermann EK, Ju AW, Chen V, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol 2013; 8: 58. doi: 10.1186/1748-717X-8-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys 2012; 82: e17–e24. doi: 10.1016/j.ijrobp.2010.10.075 [DOI] [PubMed] [Google Scholar]
- 7.Bauman G, Haider M, Van der Heide UA, Ménard C. Boosting imaging defined dominant prostatic tumors: a systematic review. Radiother Oncol 2013; 107: 274–81. doi: 10.1016/j.radonc.2013.04.027 [DOI] [PubMed] [Google Scholar]
- 8.von Eyben FE, Kiljunen T, Kangasmaki A, Kairemo K, von Eyben R, Joensuu T. Radiotherapy boost for the dominant intraprostatic cancer lesion-a systematic review and meta-analysis. Clin Genitourin Cancer 2016; 14: 189–97. doi: 10.1016/j.clgc.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 9.Rasch C, Barillot I, Remeijer P, Touw A, van Herk M, Lebesque JV. Definition of the prostate in CT and MRI: a multi-observer study. Int J Radiat Oncol Biol Phys 1999; 43: 57–66. doi: 10.1016/S0360-3016(98)00351-4 [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, Welsh D, McDonald K, Carruthers L, Cheng K, Montgomery D, et al. Identifying the dominant prostate cancer focal lesion using image analysis and planning of a simultaneous integrated stereotactic boost. Acta Oncol 2015; 54: 1543–50. doi: 10.3109/0284186X.2015.1063782 [DOI] [PubMed] [Google Scholar]
- 11.King CR, Freeman D, Kaplan I, Fuller D, Bolzicco G, Collins S, et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol 2013; 109: 217–21. doi: 10.1016/j.radonc.2013.08.030 [DOI] [PubMed] [Google Scholar]
- 12.Macdougall ND, Dean C, Muirhead R. Stereotactic body radiotherapy in prostate cancer: is rapidarc a better solution than cyberknife? Clin Oncol 2014; 26: 4–9. doi: 10.1016/j.clon.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 13.Alongi F, Cozzi L, Arcangeli S, Iftode C, Comito T, Villa E, et al. Linac based SBRT for prostate cancer in 5 fractions with VMAT and flattening filter free beams: preliminary report of a phase II study. Radiat Oncol 2013; 8: 171. doi: 10.1186/1748-717X-8-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tree AC, Ostler P, Hoskin P, Dankulchai P, Nariyangadu P, Hughes RJ, et al. Prostate stereotactic body radiotherapy—first UK experience. Clin Oncol 2014; 26: 757–61. doi: 10.1016/j.clon.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 15.Oliai C, Lanciano R, Sprandio B, Yang J, Lamond J, Arrigo S, et al. Stereotactic body radiation therapy for the primary treatment of localized prostate cancer. J Radiat Oncol 2013; 2: 63–70. doi: 10.1007/s13566-012-0067-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King CR, Brooks JD, Gill H, Presti JC. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012; 82: 877–82. doi: 10.1016/j.ijrobp.2010.11.054 [DOI] [PubMed] [Google Scholar]
- 17.Hannan R, Tumati V, Xie XJ, Cho LC, Kavanagh BD, Brindle J, et al. Stereotactic body radiation therapy for low and intermediate risk prostate cancer-results from a multi-institutional clinical trial. Eur J Cancer 2016; 59: 142–51. doi: 10.1016/j.ejca.2016.02.014 [DOI] [PubMed] [Google Scholar]