Abstract
Hepatic steatosis is a frequently encountered imaging finding that may indicate chronic liver disease, the most common of which is non-alcoholic fatty liver disease. Non-alcoholic fatty liver disease is implicated in the development of systemic diseases and its progressive phenotype, non-alcoholic steatohepatitis, leads to increased liver-specific morbidity and mortality. With the rising obesity epidemic and advent of novel therapeutics aimed at altering metabolism, there is a growing need to quantify and monitor liver steatosis. Imaging methods for assessing steatosis range from simple and qualitative to complex and highly accurate metrics. Ultrasound may be appropriate in some clinical instances as a screening modality to identify the presence of abnormal liver morphology. However, it lacks sufficient specificity and sensitivity to constitute a diagnostic modality for instigating and monitoring therapy. Newer ultrasound techniques such as quantitative ultrasound show promise in turning qualitative assessment of steatosis on conventional ultrasound into quantitative measurements. Conventional unenhanced CT is capable of detecting and quantifying moderate to severe steatosis but is inaccurate at diagnosing mild steatosis and involves the use of radiation. Newer CT techniques, like dual energy CT, show potential in expanding the role of CT in quantifying steatosis. MRI proton-density fat fraction is currently the most accurate and precise imaging biomarker to quantify liver steatosis. As such, proton-density fat fraction is the most appropriate noninvasive end point for steatosis reduction in clinical trials and therapy response assessment.
Introduction
Fatty liver, or hepatic steatosis, refers to the abnormal accumulation of triglycerides (TG) within hepatocytes.1 While potentially an inconsequential or self-limited finding, hepatic steatosis is also associated with chronic liver disease, the most common of which is non-alcoholic fatty liver disease (NAFLD). NAFLD comprises two main phenotypes: nonalcoholic fatty liver (NAFL), which is thought to have little histological progression over time, and non-alcoholic steatohepatitis (NASH), which is thought to be the more progressive form with higher risk of evolution to cirrhosis and its complications.2 An estimated 6–26% of all NAFLD patients have NASH.3–5
Patients with NAFLD have increased overall mortality than the general population, with cardiovascular complications being the leading cause of death followed by metabolic and liver-related causes.6–10 The increased cardiovascular risk amongst NAFLD patients correlates with steatosis severity.11, 12 A smaller subset of NAFLD patients with advanced stage fibrosis or NASH also have increased liver-related morbidity and mortality consequent to the higher risk of progression to cirrhosis and cirrhosis-related liver transplantation.2,6,13–16 In addition to histological fibrosis and NASH, changes in liver steatosis may also impact NAFLD progression. Recent studies found that steatosis severity correlates with the risk of fibrotic progression in NAFLD and regression in NASH, and that a reduction in steatosis severity is associated with improvement in NASH.17, 18
The systemic and hepatic diseases associated with NASH and progressive NAFLD warrant accurate detection and staging of these conditions. In particular, distinguishing between physiological vs pathological fat accumulation and longitudinal monitoring for treatment response are desired. Liver biopsy is the clinical reference standard for the assessment of NAFLD and histological evaluation includes features not currently detectable on imaging, such as specific patterns of inflammation and hepatocyte injury seen in NASH.2, 19 Histological steatosis is graded on a semi-quantitative scale based on the number of hepatocytes containing microscopically discernible cytoplasmic fat droplets: 0 (<5% hepatocytes), 1 (5–33% hepatocytes), 2 (33–66% hepatocytes), and 3 (>66% hepatocytes).19, 20 However, liver biopsy is observer dependent and invasive, conveying non-negligible risk of significant morbidity and mortality.21, 22 The relatively small core size of biopsy also introduces sampling errors, especially as steatosis is known to be heterogeneous.23, 24 These shortcomings make liver biopsy a suboptimal tool for screening, monitoring, and research.
As a non-invasive alternative to liver biopsy, imaging is increasingly utilised in the diagnosis and management of NAFLD. Imaging and related non-imaging techniques can accurately assess the important disease markers of liver steatosis and advanced liver fibrosis. Non-invasive techniques for staging liver fibrosis is beyond the scope of this review. Conventional techniques for evaluating steatosis include ultrasound, CT and MR spectroscopy and MRI. This article outlines the performance and clinical utility of each modality. Future directions including quantitative approaches will also be discussed.
Ultrasound
Normal liver parenchyma is the same as or slightly more echogenic (“brighter”) than the adjacent kidney and spleen.25 Ultrasound beam scattering by lipid droplets in steatosis causes more echo signals to return to the transducer, creating the appearance of a “bright” or hyperechoic liver.26 Fat also attenuates the beam which decreases beam penetration into tissue. This attenuation leads to poor visualisation of structures within the steatotic liver parenchyma—such as intrahepatic vessels, bile ducts and in some cases liver lesions26—and of structures deep to the liver, such as the diaphragm. Thus, the presence of steatosis can be inferred if the liver is too bright and/or if liver structures are blurry or poorly visualised.
In addition to diagnosing steatosis, ultrasound can be used to grade the severity of steatosis by scoring the degree of liver brightening and/or blurring of vessels and diaphragm. See Figure 1 for examples. To avoid inaccurate assessment due to parameters of acquisition (frequency, gain, etc.), liver brightness is assessed by comparing to an internal standard such as the kidney or the spleen.26 Ultrasound performs best at qualifying liver steatosis when there is no other background liver disease; however, it remains relatively insensitive to detection of mild steatosis. See Table 1 for a summary of studies on ultrasound diagnostic accuracy. The sensitivity and specificity of ultrasound at detecting moderate to severe steatosis, using histology as reference standard, are 80–89 and 87–90%, respectively.27–31 The sensitivity and specificity drops to 65 and 81%, respectively, when all grades of steatosis are considered.27
Figure 1.
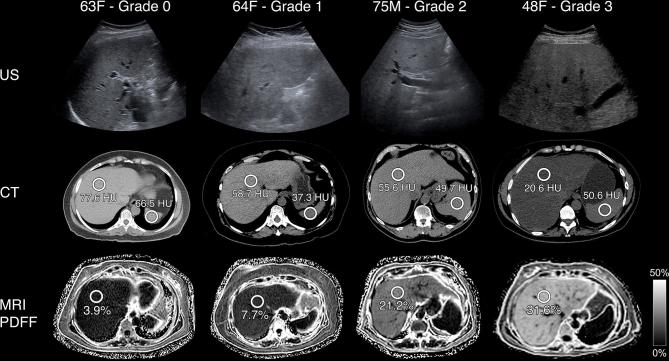
Ultrasound, CT and MR at steatosis—examples. B-mode ultrasound transverse images of the liver (first row), axial unenhanced CT images of the liver at the level of the spleen (second row), and axial MRI PDFF images of the liver (third row) are shown for four patients. Steatosis grade was determined at liver biopsy with direct histological visualisation for the number of cells with intracellular fat vacuoles: none (0% hepatocytes), mild (0–33% hepatocytes), moderate (33–66% hepatocytes) and severe (>66% hepatocytes). As steatosis grade increases from left to right in each row, the following patterns are seen: on ultrasound, increased liver parenchyma echogenicity and decreased definition of intrahepatic structures such as vessel walls; on unenhanced CT, liver density on CT in HU decreases though spleen density in HU is variable; on MR, PDFF values increase. HU, Hounsfield unit; PDFF, proton-density fat fraction.
Table 1.
Studies on diagnostic accuracy of ultrasound on steatosis
| Author, year (reference) | Design | N | Indication | Reference standard | Sensitivity | Specificity |
| Palmentieri et al27 | Prospective | 235 | Suspected liver disease | Liver biopsy | Steatosis ≥5%: 0.64 Steatosis ≥30%: 0.93 |
Steatosis ≥5%: 0.97 Steatosis ≥30%: 0.93 |
| Lee et al28 | Prospective | 161 | Potential liver donors | Liver biopsy | Steatosis ≥5%: 0.62 Steatosis ≥30%: 0.82 |
Steatosis ≥5%: 0.81 Steatosis ≥30%: 0.98 |
| van Werven et al29 | Prospective | 46 | Liver resection | Liver biopsy | Steatosis >5%: 0.65 | Steatosis >5%: 0.77 |
| Hernaez et al30 | Meta-analysis: 49 studies from 1967 to 2010 | 4720 | Suspected/known liver disease | Liver biopsy | Steatosis >5%: 0.65 Steatosis ≥20–30%: 0.91 |
Steatosis >5%: 0.81 Steatosis ≥20–30%: 0.99 |
| Bril et al31 | Prospective | 146 | High BMI with suspected NAFLD | Liver biopsy and MRS | Steatosis >12.5%: 0.85 | Steatosis >12.5%: 0.70 |
BMI, body mass index; MRS, magnetic resonance spectroscopy; N, sample size; NAFLD, non-alcoholic fatty liver disease.
Advantages and limitations
Advantages of ultrasound include safety, wide availability, and little associated patient discomfort.32, 33 The relative cost of abdominal ultrasound is low compared to CT or MR. Unlike CT and MRI, liver iron has little effect on the ultrasound beam.34
Ultrasound has several disadvantages for steatosis detection and grading. In patients with large body habitus, steatosis may be overestimated due to beam attenuation by overlying fat rather than liver fat. Echogenicity of the liver may be confounded by fibrosis, inflammation, and other features of chronic liver disease.27 Fibrosis and fat can superficially resemble each other by causing coarsening of the echotexture and increased echogenicity of the liver.34 In principle, fat causes more vessel wall blurring and beam attenuation than fibrosis, but qualitative assessment of such differences is prone to misclassification. Thus, in the setting of chronic liver disease, it may be difficult to ascertain the extent that hyperechogenicity is attributable to steatosis, fibrosis, or both.35
Ultrasound yields relatively imprecise qualitative classifications of mild, moderate, and severe steatosis. Additionally, conventional ultrasound is operator- and reader-dependent, resulting in variable results and reproducibility. Among patients with known or suspected steatosis, the intra- and inter-reader correlation ranged from 0.5 to 0.6 and 0.4–0.5, respectively.34, 36 In addition, liver steatosis can be diffuse, focal or mixed; but ultrasound may not visualise the entire liver due to shadowing from ribs, gas, and other patient factors.34, 37 Finally, ultrasound measurements are indirect indices of fat and hence, the values depend on calibration and acquisition parameters. This can lead to variations between manufacturers, machines and operators that confound interpretation of results.
Recommendations for clinical care and clinical trials
Despite its many limitations, it may be reasonable to use ultrasound in the appropriate clinical setting as an initial screen for steatosis. However, providers and patients should recognise that a grossly abnormal ultrasound indicates the presence of disease including steatosis, but a diagnostic test such as CT, MRI or biopsy may be considered as a next step to differentiate between disease states (fibrosis and fat) and to accurately quantify severity. For clinical trials, ultrasound is not considered a reliable tool to accurately assess liver steatosis. Ultrasound lacks sufficient precision for longitudinal measurements. As quantitative ultrasound techniques emerge, they may play a more central role in clinical and research usage. However, there is insufficient evidence to make a recommendation regarding their use at this time.
Future directions
Quantitative ultrasound is a technique designed to address the subjectivity, operator- and machine-dependency, and diagnostic and grading inaccuracy of conventional ultrasound on steatosis. With quantitative ultrasound, two quantitative parameters—attenuation coefficient, which is analogous to obscuration of liver structures, and backscatter coefficient, which is analogous to echogenicity—are estimated. Calibrated tissue phantoms are used to address machine and operator variability. Importantly, quantitative ultrasound can be implemented on any clinical ultrasound system and is acquired during conventional ultrasound, adding negligibly to examination time. Preliminary studies have shown that quantitative ultrasound can diagnose and grade hepatic steatosis more accurately than conventional ultrasound38, 39 and has superior intra- and inter-observer agreement.39, 40
Other possibilities for improvement include examining the effect of variable operator expertise and acquisition parameters on the accuracy of ultrasound evaluation of steatosis. Development of methods to improve agreement between readers, such as a training atlas, is another area under investigation. Automated tools for detecting steatosis and improved technology allowing better penetration for patients with higher body mass index would also potentially improve results.
Computed Tomography
Similar to ultrasound, attenuation is a relevant factor in determining final image brightness on CT (Figure 1). CT images are generated by X-ray photons traversing tissues and exposing a detector opposite the beam. The denser the tissue, the more attenuated the X-ray is and the brighter the corresponding image pixel. CT scanners are calibrated to yield pixel value measurements relative to water using a unit of measurement known as the Hounsfield Unit (HU). Water, by definition, is 0 HU, and air is defined as −1000 HU. At unenhanced CT, normal liver parenchyma is about 60 HU and hyperattenuates (appears brighter) relative to the spleen.41, 42 With increased steatosis, the liver tissue becomes hypoattenuating (darker) relative to the adjacent fat-free spleen.43, 44 In cases of severe steatosis, the normally hypoattenuating intrahepatic vessels may appear bright relative to the steatotic liver and mimic the effect of contrast enhancement.45, 46
Over the years, various criteria for diagnosing steatosis at unenhanced CT have been proposed. Because materials other than fat, such as iron, can also affect the attenuation of the X-ray beam,34 and because the calibration of HU varies by scanner and manufacturer, some investigators have advocated comparing the liver to the spleen which serves as an internal standard.41 Absolute liver HU less than 40 or liver-minus-spleen difference of less than −10 HU have been used to diagnose steatosis with reported sensitivity and specificity ranging 46–72% and 88–95%.29, 47 Retrospective evaluations of steatosis at unenhanced CT have established absolute liver HU less than 48 as highly specific for moderate to severe steatosis.48 See Table 2 for a summary of studies on the diagnostic accuracy of CT. Like ultrasound, the diagnostic performance of CT decreases with lesser severity of steatosis. In mild steatosis with fat fraction of 10–20%, CT diagnostic sensitivity ranges from 52 to 62%.47 As a quantitative method, liver HU at unenhanced CT has demonstrated an inverse linear relationship with MR spectroscopic proton-density fat fraction (PDFF), an MR-related biomarker currently accepted as the noninvasive reference standard for steatosis quantification (see more under section “Magnetic Resonance”).50 Contrast-enhanced CT utilising a liver-minus-spleen difference of less than or equal to 19 HU has been found to diagnose moderate to severe steatosis with modest sensitivity and high specificity on portal venous phase post-contrast.51 However, contrast-enhanced CT is generally not used for clinical assessment of steatosis due to the overlap in HU between normal and abnormal liver tissues and to the HU dependence on scan delay and contrast protocol.52, 53
Table 2.
Studies on diagnostic performance of non-contrast conventional CT
| Author, year (reference) | Design | N | Indication | Reference standard | Sensitivity | Specificity |
| Lee et al28 | Prospective | 161 | Potential liver donors | Liver biopsy | Steatosis ≥5%: 0.50 Steatosis ≥30%: 0.73 |
Steatosis ≥5%: 0.77 Steatosis ≥30%: 0.91 |
| van Werven et al29 | Prospective | 46 | Liver resection | Liver biopsy | Steatosis >5%: 0.74 | Steatosis >5%: 0.70 |
| Park et al49 | Prospective | 154 | Potential liver donors | Liver biopsy | Steatosis ≥30%: 0.91 | Steatosis ≥30%: 0.97 |
| Bohte et al47 | Meta-analysis: 12 studies from 2001 to 2009 | 1721 | Potential liver donors/NAFLD/liver resection | Liver biopsy | Steatosis >0%: 0.46 Steatosis >10%: 0.57 Steatosis >25%: 0.72 |
Steatosis >0%: 0.94 Steatosis >10%: 0.88 Steatosis >25%: 0.72 |
| Saadeh et al34 | Prospective | 25 | Biopsy-proven NAFLD | Liver biopsy | Steatosis >33%: 0.93 | N/A |
N, sample size; N/A, not applicable; NAFLD, non-alcoholic fatty liver disease.
Advantages and limitations
The main advantages of CT for assessing steatosis are relatively fast acquisition, ease of performance, straightforward analysis, and quantitative results.
Like ultrasound, however, CT cannot accurately diagnose mild steatosis. CT uses tissue density as an indirect index of steatosis and thus relies on calibration which is known to vary between scanners, manufacturers, and reconstruction algorithms.48, 51,52 Like hyperechogenicity of the liver on ultrasound, X-ray beam attenuation is not specific for steatosis. Liver density is influenced by the presence of materials such as iron, glycogen and less well-understood factors including haematocrit, copper and other metallic ions; all of these can alter X-ray beam attenuation.45, 53 Likewise, the spleen is an imperfect reference standard as it can be affected by haemosiderosis and haemochromatosis in a small minority of patients.45 The use of ionising radiation is also a drawback. Finally, the vast majority of CT examinations performed for clinical care are performed following intravenous contrast injection.45 Quantification of steatosis on conventional post-contrast images involves specific contrast protocols and imaging delay which limits its utility as a standard metric. While available as research protocols, methods designed to subtract iodine from contrast-enhanced studies have not been fully validated or widely utilised in clinical settings.54, 55
Recommendations for clinical care and clinical trials
Due to exposure to ionising radiation and low sensitivity for mild steatosis, we would not recommend CT as a primary modality for measuring liver steatosis. Ultrasound and MRI are better alternatives. If CT is done for other purposes, then we recommend that radiologists assess for steatosis using conservative thresholds. In addition, the widespread availability and quantitative metric of CT make it potentially useful for identifying patients with steatosis in retrospective studies.
To diagnose steatosis at unenhanced CT, no one method is clearly superior. Measuring absolute liver attenuation with a threshold of equal to or less than 40 HU raises specificity but lowers sensitivity. On the other hand, measuring the liver to spleen attenuation ratio may increase the number of false positive cases. Fat sparing on CT would be the one unequivocal finding for the presence of steatosis.37 If there is fat-sparing around the gallbladder fossa or other such characteristic fat-sparing patterns, then there is at least some degree of steatosis present in at least some parts of the liver.37
Future directions
Recent advances in CT technology, such as dual energy CT (DECT), show promise in separating the fat component from water and in reconstructing virtual unenhanced CT images. This technique exploits the observation that different tissues have characteristic attenuation profiles across a range of photon energies.50 Most tissues exhibit decreased attenuation as the incident photon energies increase.8 In contrast, fat preferentially attenuates high-energy photon over low-energy photons for the photon range used in conventional CT imaging.8 This is thought to be due to the different mechanisms of X-ray beam interference at different energy levels. At lower photon energies, the photoelectric effect predominates. Since fat has more hydrogen atoms (lower effective atomic number) than other soft tissues and the magnitude of the photoelectric effect correlates with the effective atomic number, fat causes less attenuation at lower photon energies.53 At higher photon energies, the Compton effect, which scatters the incident beam, predominates. Since the Compton effect correlates with electron density and fat is more electron-dense relative to other tissue types, fat preferentially attenuates high-energy photons.53 This results in the observation that as the tube voltage used to acquire the projection data increases, fat becomes denser on CT images even as the surrounding soft tissue becomes less dense.50, 53,55 DECT uses the characteristic attenuation profiles of different tissues, including fat, to decompose images specific for different material composition (Figure 2). Thus, “fat maps” may be recreated from a study done at different energy levels.56 Likewise, the characteristic attenuation profile of iodinated contrast may be utilised to “subtract” contrast enhancement from studies and create virtual unenhanced images.50
Figure 2.
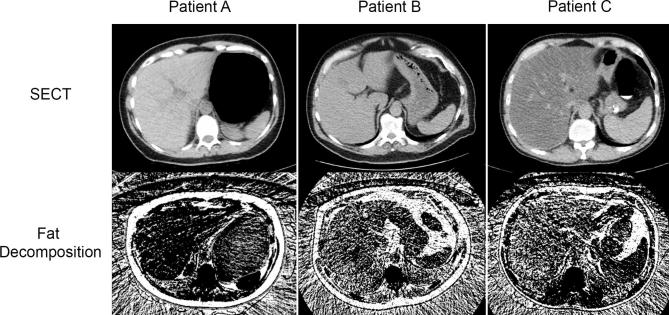
Conventional unenhanced CT and DECT at steatosis– adapted from Kramer et al.50 Conventional unenhanced CT acquired at 120 kVp (first row) and DECT acquired by rapidly switching tube voltages between 80 and 140 kVp, then post-processed into fat-density images (second row) are shown for three patients with varying degrees of steatosis. Patients A, B, and C have 0, 10, and 40% liver fat fraction, respectively, as determined by MRS PDFF (not shown). As liver fat fraction increases across the rows, liver attenuation at conventional unenhanced CT visibly decreases and liver fat density on DECT visibly increases. 50 Reprinted with permission from the American Journal of Roentgenology. DECT, dual energy CT; PDFF, proton-density fat fraction; MRS, MR spectroscopy.
Although it remains an investigational technique, DECT has shown promising results in some studies. Hyodo et al demonstrated that using a method of decomposition CT, it was possible to quantify fat fraction by volume.56 This is potentially useful for clinical care in which patients may have images acquired only after contrast.56 DECT-based algorithm can be applied to both contrast-enhanced and non-contrast-enhanced images, though DECT has yet to demonstrate clinical utility in the analysis of single-energy unenhanced CT. Further validation of DECT is required prior to routine use in the clinical setting at this time.50, 56
Magnetic Resonance Imaging
MRI is considered the most sensitive and specific technique for assessing steatosis. Unlike ultrasound and CT, which measure steatosis by proxy, MRI measures the signal intensity (brightness) of protons at different resonance frequencies.57 Water resonates at a single frequency, while TG in steatosis exhibits more complex behaviour (Figure 3).57, 58 MRI exploits the difference in proton resonance frequencies of water and TG by acquiring images at echo times at which water and TG are nominally in and out of phase.57, 59
Figure 3.
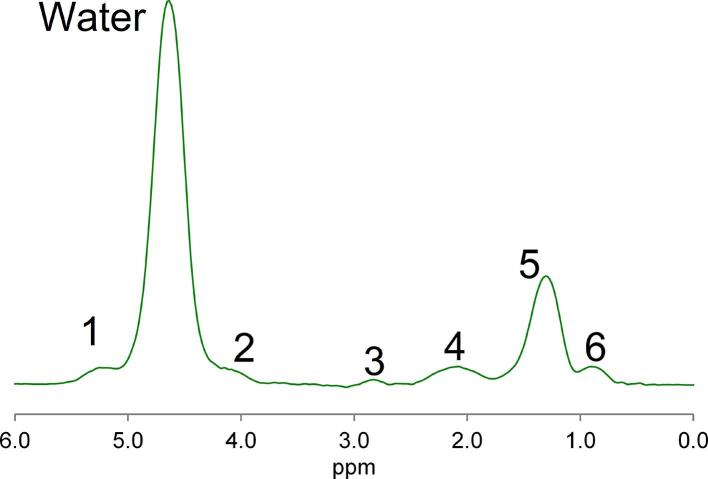
Typical liver MR spectrum showing water peak at 4.7 ppm (chemical shift measured in ppm) and multiple fat peaks (Peaks 1–6). There is one main fat peak (Peak 5). There are also Peak 4 and Peak 6, which partially overlap with the main fat peak. Peak 1 and Peak 2 overlap with the single water peak. Different correction techniques exist in advanced MR to address the problem of teasing apart contributions from individual peaks. ppm, parts per million.
Hepatic steatosis assessment on MRI has evolved from early methods that only gave qualitative estimates (i.e. dual echo chemical shift imaging) to more advanced and fully quantitative methods, meeting the ultimate goal of accurate and precise steatosis measurement. Identification and incorporation of the main confounders were essential steps. These confounders on MRI are R2* decay, the complexity of the fat spectrum and T1 weighting due to the differing T1 values of fat and water.57, 60 Confounder corrected chemical shift-encoded MRI corrects for these three confounders by collecting images at multiple echo times with low flip angle to minimise T1 weighting and by incorporating the multi-peak structure of fat in the analysis algorithm.57, 61,62 The end result is the PDFF, which is the (liver fat signal)/(total signal).
The liver fat signal generated through PDFF imaging is due almost entirely to protons in TG, which make up virtually all of the pathological fat accumulation in hepatic steatosis.63 While liver fat may contain trace contributions from other lipids, these are not detected on MR as they have ultrashort T2s due to being bound in components of normal tissue, i.e. cell walls. Water also has an invisible ultrashort T2 fraction, as it is bound to protein such as collagen. Thus, PDFF signal can be defined as the fraction of proton signal from mobile fat normalised by the total proton signal from all mobile proton species.
Over the years, three classes of advanced technique have emerged: magnitude data-based MRI, complex data-based MRI, and MRS. Performed properly, they agree so closely with each other they can essentially be assumed to be equivalent (Figure 4).64–69 This review will not address MRS, as it is often used as a reference standard and is a biochemical rather than imaging-based technique. For details on the diagnostic performance of MRS, Yokoo et al recently performed a meta-analysis with histology as the reference standard. MRS demonstrated superior diagnostic accuracy compared to other noninvasive methods for detecting mild steatosis (histological grade <5–<10%) with sensitivity and specificity ranging 77–95 and 81–97%, respectively.62
Figure 4.
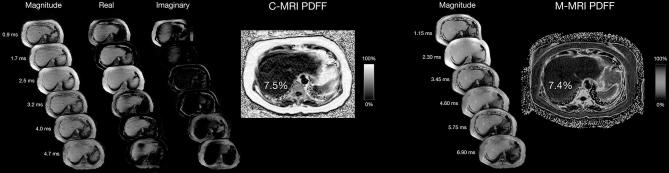
Complex data-based (c-MRI) and magnitude data-based (m-MRI) MR, acquisitions from 64-year-old female patient with mild histological grade steatosis in Figure 1. Startinginary source images with the output PDFF map to the right. The echo times at which the c-MRIs are acquired are listed on the far left. Further to the right are the magnitude source echoes for the m-MRI with the corresponding PDFF map on the far right. The echo times at which the m-MRIs are acquired are adjacent to the magnitude source echoes. The average PDFF measurement derived from c-MRI is 7.5% while that derived from m-MRI on the same patient is 7.4%. PDFF, proton-density fat fraction.
Prior studies in children and adults with known or suspected NAFLD have shown that MRI-PDFF have high intra- and interexam repeatability across scanners and magnets.70–73 MRI-PDFF correlates strongly with both biochemically determined TG concentration and MR spectroscopy.34, 72,74 See Table 3 for a summary of studies on the diagnostic accuracy of MRI-PDFF. Using contemporaneous histology as the reference standard, MRI-PDFF accurately classifies dichotomised steatosis grades cross-sectionally, and change in PDFF accurately classifies change in steatosis longitudinally.18,39,47,64,75–80 MRI-PDFF demonstrates high interexam precision at all anatomic levels of the liver and accuracy in detecting as low as 1.6% change in fat fraction over time.69, 72,81,82 Most MRI vendors offer FDA approved packages that can generate PDFF maps making it relatively accessible clinically.
Table 3.
Studies on diagnostic accuracy of MR with liver biopsy as reference standard
| Author, year (reference) | Design | N | Indication | PDFF threshold (%) | Sensitivity | Specificity |
| Bohte et al47 | Meta-analysis: 11 studies from 2001 to 2009 | 569 | Potential liver donor/NAFLD/liver resection | N/A | Grade > 0%: 0.82 Grade > 10%: 0.90 Grade > 25%: 0.97 |
Grade > 0%: 0.90 Grade > 10%: 0.95 Grade > 25%: 0.76 |
| Idilman et al | Retrospective | 70 | Biopsy-proven NAFLD | Grade ≥ 2: 15% | Grade ≥ 2: 0.93 | Grade ≥ 2: 0.85 |
| Tang et al76 | Prospective | 89 | NAFLD | Grade ≥ 1: 6.4 Grade ≥ 2: 17.4 Grade = 3: 22.1 |
Grade ≥ 1: 0.86 Grade ≥ 2: 0.64 Grade = 3: 0.71 |
Grade ≥ 1: 0.83 Grade ≥ 2: 0.96 Grade = 3: 0.92 |
| Paige et al39 | Prospective | 61 | Biopsy-proven NAFLD | Grade ≥ 2: 13.45 Grade = 3: 16.83 |
Grade >= 2: 0.85 Grade = 3: 1.00 |
Grade >= 2: 0.96 Grade = 3: 0.81 |
| Middleton et al., 201777 | Multicenter RCT | 110 | Paediatric NAFLD clinical trial | Grade ≥ 2: 17.5 Grade = 3: 23.3 |
Grade ≥ 2: 0.74 Grade = 3: 0.60 |
0.90a |
| Middleton et al., 201777 | Multicenter RCT | 113 | NASH clinical trial | Grade ≥ 2: 16.3 Grade = 3: 21.7 |
Grade ≥ 2: 0.83 Grade = 3: 0.84 |
0.90a |
N, sample size; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PDFF, proton-density fat fraction; RCT, randomised controlled trial.
aPDFF threshold was chosen for a target specificity of 0.90.
Advantages and limitations
These advanced MRI techniques have many advantages over ultrasound or CT. Primarily, MRI measures the PDFF which is a fundamental property of tissue and requires no internal calibration or reference standard. Advanced sequences can address biological confounders such as iron overload by simultaneously measuring and correcting for R2*. The images needed for PDFF measurements can be acquired very quickly (the entire liver may be imaged in a single or two breath-holds). As such, volumetric assessment of steatosis is possible. This is not possible with ultrasound or MRS.
Despite its superior diagnostic performance to ultrasound and CT, MRI does have several drawbacks. Areas of motion and parallel image artefact negatively impact measurement accuracy, and so these regions need to be identified and avoided when placing ROIs. Magnitude data-based MRI does not readily differentiate fat fraction greater than 50% from fat fraction less than 50%.59 Though rare, human liver fat fraction does occasionally exceed 50%. Another issue is the limited ability of current MRI techniques for R2* correction. In cases of extreme iron overload, the signal loss may be so fast that it is not feasible to measure the oscillation.
Finally, we have limited knowledge about proton pool variability between patients and within patients over time. One of the implicit assumptions in PDFF is that the pool of protons invisible on MR—e.g. those bound to cholesterol crystals and to water in collagen—remains the same across and within patients. To the extent that the invisible pool changes between or within the same patient in the course of steatosis or other liver diseases, PDFF may not accurately reflect the actual degree of steatosis in the patient.
Besides limitations related to fat fraction analysis, MRI can also be limited by patient factors, operators and institutions. While commercially available on new MR platforms, the software packages capable of processing PDFF maps may not be readily available due to budgetary and hardware constraints in some imaging centres. MRI suitability may be limited by patient factors including claustrophobia, implanted devices, and discomfort. There is also a higher relative charge for MRI compared to ultrasound and CT. This is an area for future research and development to help offset the cost.
Recommendations for clinical care and clinical trials
Clinical care recommendations depend largely on availability and patient tolerance. If a PDFF technique is available, it should be the method of choice in all patients for whom assessment of liver steatosis is clinically requested. The currently available software packages can generate parametric maps in less than 30 s, making them easy to incorporate into routine clinical practice. Importantly, the PDFF technique can be done after gadolinium injection because these techniques are robust in the setting of the T1 and T2* shortening effects of gadolinium.
For clinical trials, PDFF is the most accurate and precise imaging measure of liver steatosis. Hence, PDFF should be used as an end point for clinical trials for inclusion criteria and in settings where quantifying steatosis is relevant. Since commercial variants of PDFF technique may not be available at all sites, trials may be done in partnership with radiology coordinating centres that can standardise the appropriate PDFF technique across all sites participating in a trial.
A factor to consider when assessing longitudinal data for either clinical care or clinical trials is the fine margin of error in diagnosing mild steatosis. While more accurate than US and CT in quantifying steatosis, MRI does have error rates of ± 1.5% as an order of magnitude.62,67,82–84 This margin of error is largely independent of the actual fat fraction, which poses a problem for screening steatosis in children and healthy adults since a 1% margin of error could be consequential for a diagnosis threshold of 5%. Our limited knowledge of steatosis further confounds margin of error analysis. Using metabolic indices as a reference, a PDFF cutoff of 3% fat fraction has been suggested for diagnosing steatosis; using histology as the standard, however, a cutoff of 6% fat fraction has been suggested.2, 85 Further research is needed to refine our understanding of what constitutes a normal amount of fat in the liver.
Future directions
In the last decade, PDFF has grown from an experimental method being tested at a few research centres to a validated clinical standard for steatosis assessment. The availability and adoption of PDFF technique has become widespread in the last few years, and will continue to increase in response to the worldwide steatosis epidemic. Future directions would involve addressing the various known limitations of PDFF. Techniques are being investigated that can reliably measure PDFF in the setting of extreme iron overload. Improvement in our knowledge of the biological and clinical significance of PDFF values and their longitudinal change would also make PDFF a better diagnostic tool. For instance, there is yet to be consensus on the cutoff value(s) that differentiate normal from abnormal and the clinical significance of the range of PDFF values. Investigators are also trying to automate PDFF analysis and to improve the accuracy and precision of PDFF in the low-fat fraction range.
Summary
Hepatic steatosis can be seen in a wide variety of chronic liver diseases, the most common of which is NAFLD. Studies suggest that steatosis severity as well as steatosis change over time influence disease progression in NAFLD and its high-risk subtype, NASH. With the increasing global prevalence of NAFLD and the recent surge of clinical trials aimed at disease-altering therapy, there is an ever more important need for safe and accurate quantification of steatosis. Liver biopsy is currently the reference standard for disease assessment in NAFLD and NASH. However, it is observer dependent and invasive, and it carries non-negligible risks. Imaging techniques for assessing steatosis range from qualitative tools available at the bedside to highly accurate and precise metrics. Table 4 lists the strengths, weaknesses, and clinical care recommendations for these techniques. Ultrasound is a safe and widely available technique that may serve in certain clinical scenarios as an initial screen. Its main drawbacks are machine and operator dependencies, qualitative assessment, and inaccuracy at detecting mild steatosis. Recent innovations in quantitative ultrasound promise to address some of these deficiencies. Like ultrasound, conventional unenhanced CT is accessible, easy to perform, and can be highly specific for moderate to severe steatosis. Quantitative evaluation of steatosis is an additional benefit of CT. However, CT is inaccurate in the mild steatosis range and involves the use of radiation. Newer CT techniques such as DECT could potentially expand the utility of this modality at steatosis quantification. MRI PDFF is currently the most accurate quantitative imaging biomarker of steatosis. The availability and utilization of PDFF have grown rapidly in recent years, with continued progress being made in technical refinement and validation. Where available, PDFF may serve as the noninvasive endpoint for steatosis reduction in clinical trials and therapy response assessment.
Table 4.
Comparison of ultrasound, CT and MRI-PDFF for clinical care and clinical trials in hepatic steatosis
| Advantages | Disadvantages | Recommendations | |
| Ultrasound | Safe Widely available Low cost |
Indirect measurement Qualitative Operator and calibration dependent Inaccurate for mild steatosis Inaccurate steatosis grading Confounders: obesity, fibrosis Imprecise localization |
Clinical care: initial screen Clinical trials: do not recommend |
| CT | Fast acquisition Easy to perform Straightforward analysis Quantitative |
Indirect measurement Variable calibration Inaccurate for mild steatosis Confounders: iron, glycogen Ionising radiation Requires standard acquisition if contrast-enhanced |
Clinical care: retrospective with conservative thresholds Clinical trials: do not recommend |
| MRI-PDFF | Direct measurement Precise fat quantification Highly sensitive and specific Corrects for confounders Fast acquisition |
Relatively limited access Claustrophobia Implantable devices |
Clinical care: study of choice (if available) Clinical trials: study of choice (if available) |
PDFF, proton-density fat fraction.
Contributor Information
Yingzhen N Zhang, Email: y8zhang@ucsd.edu.
Kathryn J Fowler, Email: fowlerk@wustl.edu.
Gavin Hamilton, Email: ghamilton@ucsd.edu.
Jennifer Y Cui, Email: jenncui@ucsd.edu.
Ethan Z Sy, Email: ezsy@ucsd.edu.
Michelle Balanay, Email: mbalanay@ucsd.edu.
Jonathan C Hooker, Email: jhooker@ucsd.edu.
Nikolaus Szeverenyi, Email: nszeverenyi@mail.ucsd.edu.
Claude B Sirlin, Email: csirlin@ucsd.edu.
REFERENCES
- 1.Fabbrini E, Sullivan S, Klein S, Ekstedt M, Hagström H, Nasr P. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 2010; 51: 679–89. doi: 10.1002/hep.23280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol 2012; 107: 811–26. doi: 10.1038/ajg.2012.128 [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73–84. doi: 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 4.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011; 140: 124–31. doi: 10.1053/j.gastro.2010.09.038 [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Lee K, Lee KW, Yi NJ, Lee HW, Hong G, et al. Histologically proven non-alcoholic fatty liver disease and clinically related factors in recipients after liver transplantation. Clin Transplant 2014; 28: 521–9. doi: 10.1111/ctr.12343 [DOI] [PubMed] [Google Scholar]
- 6.Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015; 61: 1547–54. doi: 10.1002/hep.27368 [DOI] [PubMed] [Google Scholar]
- 7.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006; 44: 865–73. doi: 10.1002/hep.21327 [DOI] [PubMed] [Google Scholar]
- 8.Graffy PM, Pickhardt PJ. Quantification of hepatic and visceral fat by CT and MR imaging: relevance to the obesity epidemic, metabolic syndrome and NAFLD. Br J Radiol 2016; 89: 20151024. doi: 10.1259/bjr.20151024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickhardt PJ, Hahn L, Muñoz del Rio A, Park SH, Reeder SB, Said A. Natural history of hepatic steatosis: observed outcomes for subsequent liver and cardiovascular complications. AJR Am J Roentgenol 2014; 202: 752–8. doi: 10.2214/AJR.13.11367 [DOI] [PubMed] [Google Scholar]
- 10.Hahn L, Reeder SB, Muñoz del Rio A, Pickhardt PJ. Longitudinal changes in liver fat content in asymptomatic adults: hepatic attenuation on unenhanced CT as an imaging biomarker for steatosis. AJR Am J Roentgenol 2015; 205: 1167–72. doi: 10.2214/AJR.15.14724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brouha SS, Nguyen P, Bettencourt R, Sirlin CB, Loomba R. Increased severity of liver fat content and liver fibrosis in non-alcoholic fatty liver disease correlate with epicardial fat volume in type 2 diabetes: a prospective study. Eur Radiol 2018; 28: 1345–55. doi: 10.1007/s00330-017-5075-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granér M, Nyman K, Siren R, Pentikäinen MO, Lundbom J, Hakkarainen A, et al. Ectopic fat depots and left ventricular function in nondiabetic men with nonalcoholic fatty liver disease. Circ Cardiovasc Imaging 2015; 8: e001979. doi: 10.1161/CIRCIMAGING.114.001979 [DOI] [PubMed] [Google Scholar]
- 13.Gramlich T, Kleiner DE, McCullough AJ, Matteoni CA, Boparai N, Younossi ZM. Pathologic features associated with fibrosis in nonalcoholic fatty liver disease. Hum Pathol 2004; 35: 196–9. doi: 10.1016/j.humpath.2003.09.018 [DOI] [PubMed] [Google Scholar]
- 14.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149: 389–97. doi: 10.1053/j.gastro.2015.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fassio E, Alvarez E, Domínguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology 2004; 40: 820–6. doi: 10.1002/hep.20410 [DOI] [PubMed] [Google Scholar]
- 16.Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut 2010; 59: 969–74. doi: 10.1136/gut.2009.205088 [DOI] [PubMed] [Google Scholar]
- 17.Ajmera V, Park CC, Caussy C, Singh S, Hernandez C, Bettencourt R, et al. Magnetic resonance imaging proton density fat fraction associates with progression of fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2018; In Press. doi: 10.1053/j.gastro.2018.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel J, Bettencourt R, Cui J, Salotti J, Hooker J, Bhatt A, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol 2016; 9: 692–701. doi: 10.1177/1756283X16656735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313–21. doi: 10.1002/hep.20701 [DOI] [PubMed] [Google Scholar]
- 20.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999; 94: 2467–74. doi: 10.1111/j.1572-0241.1999.01377.x [DOI] [PubMed] [Google Scholar]
- 21.El-Badry AM, Breitenstein S, Jochum W, Washington K, Paradis V, Rubbia-Brandt L, et al. Assessment of hepatic steatosis by expert pathologists. Ann Surg 2009; 250: 691–7. doi: 10.1097/SLA.0b013e3181bcd6dd [DOI] [PubMed] [Google Scholar]
- 22.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005; 128: 1898–906. doi: 10.1053/j.gastro.2005.03.084 [DOI] [PubMed] [Google Scholar]
- 23.Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, et al. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet 1986; 1: 523–5. doi: 10.1016/S0140-6736(86)90883-4 [DOI] [PubMed] [Google Scholar]
- 24.Arun J, Jhala N, Lazenby AJ, Clements R, Abrams GA. Influence of liver biopsy heterogeneity and diagnosis of nonalcoholic steatohepatitis in subjects undergoing gastric bypass. Obes Surg 2007; 17: 155–61. doi: 10.1007/s11695-007-9041-2 [DOI] [PubMed] [Google Scholar]
- 25.Zwiebel WJ. Sonographic diagnosis of diffuse liver disease. Semin Ultrasound CT MR 1995; 16: 8–15. doi: 10.1016/0887-2171(95)90011-X [DOI] [PubMed] [Google Scholar]
- 26.Charatcharoenwitthaya P, Lindor KD. Role of radiologic modalities in the management of non-alcoholic steatohepatitis. Clin Liver Dis 2007; 11: 37–54. doi: 10.1016/j.cld.2007.02.014 [DOI] [PubMed] [Google Scholar]
- 27.Palmentieri B, de Sio I, La Mura V, Masarone M, Vecchione R, Bruno S, et al. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig Liver Dis 2006; 38: 485–9. doi: 10.1016/j.dld.2006.03.021 [DOI] [PubMed] [Google Scholar]
- 28.Lee SS, Park SH, Kim HJ, Kim SY, Kim MY, Kim DY, et al. Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol 2010; 52: 579–85. doi: 10.1016/j.jhep.2010.01.008 [DOI] [PubMed] [Google Scholar]
- 29.van Werven JR, Marsman HA, Nederveen AJ, Smits NJ, ten Kate FJ, van Gulik TM, et al. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology . 2010; 256: 159–68. doi: 10.1148/radiol.10091790 [DOI] [PubMed] [Google Scholar]
- 30.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011; 54: 1082–90. doi: 10.1002/hep.24452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bril F, Ortiz-Lopez C, Lomonaco R, Orsak B, Freckleton M, Chintapalli K, et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int 2015; 35: 2139–46. doi: 10.1111/liv.12840 [DOI] [PubMed] [Google Scholar]
- 32.Debongnie JC, Pauls C, Fievez M, Wibin E. Prospective evaluation of the diagnostic accuracy of liver ultrasonography. Gut 1981; 22: 130–5. doi: 10.1136/gut.22.2.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathiesen UL, Franzén LE, Aselius H, Resjö M, Jacobsson L, Foberg U, et al. Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig Liver Dis 2002; 34: 516–22. doi: 10.1016/S1590-8658(02)80111-6 [DOI] [PubMed] [Google Scholar]
- 34.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123: 745–50. doi: 10.1053/gast.2002.35354 [DOI] [PubMed] [Google Scholar]
- 35.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J 1986; 292: 13–15. doi: 10.1136/bmj.292.6512.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol 2007; 189: W320–W323. doi: 10.2214/AJR.07.2123 [DOI] [PubMed] [Google Scholar]
- 37.Hamer OW, Aguirre DA, Casola G, Lavine JE, Woenckhaus M, Sirlin CB. Fatty liver: imaging patterns and pitfalls. Radiographics 2006; 26: 1637–53. doi: 10.1148/rg.266065004 [DOI] [PubMed] [Google Scholar]
- 38.Xu L, Lu W, Li P, Shen F, Mi YQ, Fan JG. A comparison of hepatic steatosis index, controlled attenuation parameter and ultrasound as noninvasive diagnostic tools for steatosis in chronic hepatitis B. Dig Liver Dis 2017; 49: 910–7. doi: 10.1016/j.dld.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 39.Paige JS, Bernstein GS, Heba E, Costa EAC, Fereirra M, Wolfson T, et al. A pilot comparative study of quantitative ultrasound, conventional ultrasound, and MRI for predicting histology-determined steatosis grade in adult nonalcoholic fatty liver disease. AJR Am J Roentgenol 2017; 208: W168–W177. doi: 10.2214/AJR.16.16726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han A, Andre MP, Erdman JW, Loomba R, Sirlin CB, O'Brien WD. Repeatability and reproducibility of a clinically based QUS phantom study and methodologies. IEEE Trans Ultrason Ferroelectr Freq Control 2017; 64: 218–31. doi: 10.1109/TUFFC.2016.2588979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piekarski J, Goldberg HI, Royal SA, Axel L, Moss AA. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology 1980; 137: 727–9. doi: 10.1148/radiology.137.3.6934563 [DOI] [PubMed] [Google Scholar]
- 42.Boyce CJ, Pickhardt PJ, Kim DH, Taylor AJ, Winter TC, Bruce RJ, et al. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR Am J Roentgenol 2010; 194: 623–8. doi: 10.2214/AJR.09.2590 [DOI] [PubMed] [Google Scholar]
- 43.Wells MM, Li Z, Addeman B, McKenzie CA, Mujoomdar A, Beaton M, et al. Computed tomography measurement of hepatic steatosis: prevalence of hepatic steatosis in a canadian population. Can J Gastroenterol Hepatol 2016; 2016: 1–7. doi: 10.1155/2016/4930987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park YS, Park SH, Lee SS, Kim DY, Shin YM, Lee W, et al. Biopsy-proven nonsteatotic liver in adults: estimation of reference range for difference in attenuation between the liver and the spleen at nonenhanced CT. Radiology 2011; 258: 760–6. doi: 10.1148/radiol.10101233 [DOI] [PubMed] [Google Scholar]
- 45.Johnston RJ, Stamm ER, Lewin JM, Hendrick RE, Archer PG. Diagnosis of fatty infiltration of the liver on contrast enhanced CT: limitations of liver-minus-spleen attenuation difference measurements. Abdom Imaging 1998; 23: 409–15. doi: 10.1007/s002619900370 [DOI] [PubMed] [Google Scholar]
- 46.Jacobs JE, Birnbaum BA, Shapiro MA, Langlotz CP, Slosman F, Rubesin SE, et al. Diagnostic criteria for fatty infiltration of the liver on contrast-enhanced helical CT. AJR Am J Roentgenol 1998; 171: 659–64. doi: 10.2214/ajr.171.3.9725292 [DOI] [PubMed] [Google Scholar]
- 47.Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol 2011; 21: 87–97. doi: 10.1007/s00330-010-1905-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pickhardt PJ, Park SH, Hahn L, Lee SG, Bae KT, Yu ES. Specificity of unenhanced CT for non-invasive diagnosis of hepatic steatosis: implications for the investigation of the natural history of incidental steatosis. Eur Radiol 2012; 22: 1075–82. doi: 10.1007/s00330-011-2349-2 [DOI] [PubMed] [Google Scholar]
- 49.Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology 2006; 239: 105–12. doi: 10.1148/radiol.2391050361 [DOI] [PubMed] [Google Scholar]
- 50.Kramer H, Pickhardt PJ, Kliewer MA, Hernando D, Chen GH, Zagzebski JA, et al. Accuracy of liver fat quantification with advanced CT, MRI, and ultrasound techniques: prospective comparison with MR spectroscopy. AJR Am J Roentgenol 2017; 208: 92–100. doi: 10.2214/AJR.16.16565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim DY, Park SH, Lee SS, Kim HJ, Kim SY, Kim MY, et al. Contrast-enhanced computed tomography for the diagnosis of fatty liver: prospective study with same-day biopsy used as the reference standard. Eur Radiol 2010; 20: 359–66. doi: 10.1007/s00330-009-1560-x [DOI] [PubMed] [Google Scholar]
- 52.Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol 2007; 188: 1307–12. doi: 10.2214/AJR.06.0992 [DOI] [PubMed] [Google Scholar]
- 53.Fischer MA, Gnannt R, Raptis D, Reiner CS, Clavien P-A, Schmidt B, et al. Quantification of liver fat in the presence of iron and iodine. Invest Radiol 2011; 46: 351–8. doi: 10.1097/RLI.0b013e31820e1486 [DOI] [PubMed] [Google Scholar]
- 54.Zheng D, Tian W, Zheng Z, Gu J, Guo Z, He X. Accuracy of computed tomography for detecting hepatic steatosis in donors for liver transplantation: a meta-analysis. Clin Transplant 2017; 31: e13013. doi: 10.1111/ctr.13013 [DOI] [PubMed] [Google Scholar]
- 55.Artz NS, Hines CD, Brunner ST, Agni RM, Kühn JP, Roldan-Alzate A, et al. Quantification of hepatic steatosis with dual-energy computed tomography: comparison with tissue reference standards and quantitative magnetic resonance imaging in the ob/ob mouse. Invest Radiol 2012; 47: 603–10. doi: 10.1097/RLI.0b013e318261fad0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hyodo T, Hori M, Lamb P, Sasaki K, Wakayama T, Chiba Y, et al. Multimaterial decomposition algorithm for the quantification of liver fat content by using fast-kilovolt-peak switching dual-energy CT: experimental validation. Radiology 2017; 282: 381–9. doi: 10.1148/radiol.2016160129 [DOI] [PubMed] [Google Scholar]
- 57.Hamilton G, Middleton MS, Heba ER, Sirlin CB. Imaging techniques for the assessment of ectopic fat in liver and skeletal muscle : Translational research methods for diabetes, obesity and cardiometabolic drug development [Internet]. London: The British Institute of Radiology.; 2015. 99–119. http://link.springer.com/10.1007/978-1-4471-4920-0_4. [Google Scholar]
- 58.Hamilton G, Yokoo T, Bydder M, Cruite I, Schroeder ME, Sirlin CB, et al. In vivo characterization of the liver fat ¹H MR spectrum. NMR Biomed 2011; 24: 784–90. doi: 10.1002/nbm.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging 2011; 34: 729–49. doi: 10.1002/jmri.22580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong CW, Fazeli Dehkordy S, Hooker JC, Hamilton G, Sirlin CB. Fat quantification in the abdomen. Top Magn Reson Imaging 2017; 26: 221–7. doi: 10.1097/RMR.0000000000000141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology 2011; 258: 767–75. doi: 10.1148/radiol.10100708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yokoo T, Bydder M, Hamilton G, Middleton MS, Gamst AC, Wolfson T, et al. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology 2009; 251: 67–76. doi: 10.1148/radiol.2511080666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi SS, Diehl AM. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr Opin Lipidol 2008; 19: 295–300. doi: 10.1097/MOL.0b013e3282ff5e55 [DOI] [PubMed] [Google Scholar]
- 64.Zand KA, Shah A, Heba E, Wolfson T, Hamilton G, Lam J, et al. Accuracy of multiecho magnitude-based MRI (M-MRI) for estimation of hepatic proton density fat fraction (PDFF) in children. J Magn Reson Imaging 2015; 42: 1223–32. doi: 10.1002/jmri.24888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yokoo T, Serai SD, Pirasteh A, Bashir MR, Hamilton G, Hernando D, et al. Linearity, bias, and precision of hepatic proton density fat fraction measurements by using MR imaging: a meta-analysis. Radiology 2018; 286: 486–98. doi: 10.1148/radiol.2017170550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Achmad E, Yokoo T, Hamilton G, Heba ER, Hooker JC, Changchien C, et al. Feasibility of and agreement between MR imaging and spectroscopic estimation of hepatic proton density fat fraction in children with known or suspected nonalcoholic fatty liver disease. Abdom Imaging 2015; 40: 3084–90. doi: 10.1007/s00261-015-0506-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rehm JL, Wolfgram PM, Hernando D, Eickhoff JC, Allen DB, Reeder SB. Proton density fat-fraction is an accurate biomarker of hepatic steatosis in adolescent girls and young women. Eur Radiol 2015; 25: 2921–30. doi: 10.1007/s00330-015-3724-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haufe WM, Wolfson T, Hooker CA, Hooker JC, Covarrubias Y, Schlein AN, et al. Accuracy of PDFF estimation by magnitude-based and complex-based MRI in children with MR spectroscopy as a reference. J Magn Reson Imaging 2017; 46: 1641–7. doi: 10.1002/jmri.25699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Satkunasingham J, Nik HH, Fischer S, Menezes R, Selzner N, Cattral M, et al. Can negligible hepatic steatosis determined by magnetic resonance imaging-proton density fat fraction obviate the need for liver biopsy in potential liver donors? Liver Transplantation 2018; 24: 470–7. doi: 10.1002/lt.24965 [DOI] [PubMed] [Google Scholar]
- 70.Artz NS, Haufe WM, Hooker CA, Hamilton G, Wolfson T, Campos GM, et al. Reproducibility of MR-based liver fat quantification across field strength: same-day comparison between 1.5T and 3T in obese subjects. J Magn Reson Imaging 2015; 42: 811–7. doi: 10.1002/jmri.24842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang GH, Cruite I, Shiehmorteza M, Wolfson T, Gamst AC, Hamilton G, et al. Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging 2011; 34: 928–34. doi: 10.1002/jmri.22701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bannas P, Kramer H, Hernando D, Agni R, Cunningham AM, Mandal R, et al. Quantitative magnetic resonance imaging of hepatic steatosis: validation in ex vivo human livers. Hepatology 2015; 62: 1444–55. doi: 10.1002/hep.28012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tyagi A, Yeganeh O, Levin Y, Hooker JC, Hamilton GC, Wolfson T, et al. Intra- and inter-examination repeatability of magnetic resonance spectroscopy, magnitude-based MRI, and complex-based MRI for estimation of hepatic proton density fat fraction in overweight and obese children and adults. Abdom Imaging 2015; 40: 3070–7. doi: 10.1007/s00261-015-0542-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee SW, Park SH, Kim KW, Choi EK, Shin YM, Kim PN, et al. Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: comparison of visual grading with liver attenuation index. Radiology 2007; 244: 479–85. doi: 10.1148/radiol.2442061177 [DOI] [PubMed] [Google Scholar]
- 75.Idilman IS, Aniktar H, Idilman R, Kabacam G, Savas B, Elhan A, et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology 2013; 267: 767–75. doi: 10.1148/radiol.13121360 [DOI] [PubMed] [Google Scholar]
- 76.Tang A, Desai A, Hamilton G, Wolfson T, Gamst A, Lam J, et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 2015; 274: 416–25. doi: 10.1148/radiol.14140754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Middleton MS, Heba ER, Hooker CA, Bashir MR, Fowler KJ, Sandrasegaran K, et al. Agreement between magnetic resonance imaging proton density fat fraction measurements and pathologist-assigned steatosis grades of liver biopsies from adults with nonalcoholic steatohepatitis. Gastroenterology 2017; 153: 753–61. doi: 10.1053/j.gastro.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013; 58: 1930–40. doi: 10.1002/hep.26455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015; 61: 1239–50. doi: 10.1002/hep.27647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, et al. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol 2016; 65: 369–76. doi: 10.1016/j.jhep.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin SC, Heba E, Bettencourt R, Lin GY, Valasek MA, Lunde O, et al. Assessment of treatment response in non-alcoholic steatohepatitis using advanced magnetic resonance imaging. Aliment Pharmacol Ther 2017; 45: 844–54. doi: 10.1111/apt.13951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Negrete LM, Middleton MS, Clark L, Wolfson T, Gamst AC, Lam J, et al. Inter-examination precision of magnitude-based MRI for estimation of segmental hepatic proton density fat fraction in obese subjects. J Magn Reson Imaging 2014; 39: 1265–71. doi: 10.1002/jmri.24284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hong CW, Mamidipalli A, Hooker JC, Hamilton G, Wolfson T, Chen DH, et al. MRI proton density fat fraction is robust across the biologically plausible range of triglyceride spectra in adults with nonalcoholic steatohepatitis. J Magn Reson Imaging 2018; 47: 995–1002. doi: 10.1002/jmri.25845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heba ER, Desai A, Zand KA, Hamilton G, Wolfson T, Schlein AN, et al. Accuracy and the effect of possible subject-based confounders of magnitude-based MRI for estimating hepatic proton density fat fraction in adults, using MR spectroscopy as reference. J Magn Reson Imaging 2016; 43: 398–406. doi: 10.1002/jmri.25006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nasr P, Forsgren MF, Ignatova S, Dahlström N, Cedersund G, Leinhard OD, et al. Using a 3% proton density fat fraction as a cut-off value increases sensitivity of detection of hepatic steatosis, based on results from histopathology analysis. Gastroenterology 2017; 153: 53–5. doi: 10.1053/j.gastro.2017.03.005 [DOI] [PubMed] [Google Scholar]