Abstract
Objective:
To study the relationship of area- and volumetric-based visceral and subcutaneous adipose tissue (VAT and SAT) by MRI and their ratio in subjects with impaired glucose metabolism from the general population.
Methods:
Subjects from a population-based cohort with established prediabetes, diabetes and healthy controls without prior cardiovascular diseases underwent 3 T MRI. VAT and SAT were assessed as total volume and area on a single slice, and their ratio (VAT/SAT) was calculated. Clinical covariates and cardiovascular risk factors, such as hypertension and glycemic state were assessed in standardized fashion. Univariate and adjusted analyses were conducted.
Results:
Among 384 subjects (age: 56.2 ± 9.2 years, 58.1% male) with complete MRI data available, volumetric and single-slice VAT, SAT and VAT/SAT ratio were strongly correlated (all >r = 0.89). Similarly, VAT/SATvolume ratio was strongly correlated with VATvolume but not with SAT (r = 0.72 and r = −0.21, respectively). Significant higher levels of VAT, SAT and VAT/SAT ratio were found in subjects with impaired glucose metabolism (all p ≤ 0.01). After adjustment for potential cardiovascular confounders, VATvolume and VAT/SATvolume ratio remained significantly higher in subjects with impaired glucose metabolism (VATvolume = 6.9 ± 2.5 l and 3.4 ± 2.3 l; VAT/SATvolume ratio = 0.82 ± 0.34 l and 0.49 ± 0.29 l in patients with diabetes and controls, respectively, all p < 0.02), whereas the association for SATvolume attenuated. Additionally, there was a decreasing effect of glycemic status on VAT/SATvolume ratio with increasing body mass index and waist circumference (p < 0.05).
Conclusions:
VATvolume and VAT/SATvolume ratio are associated with impaired glucose metabolism, independent of cardiovascular risk factors or MRI-based quantification technique, with a decreasing effect of VAT/SATvolume ratio in obese subjects.
Advances in knowledge:
Quantification of VATvolume and VAT/SATvolume ratio by MRI represents a reproducable biomarker associated with cardiometabolic risk factors in subjects with impaired glucose metabolism, while the association of VAT/SATvolume ratio with glycemic state is attenuated in obese subjects.
Introduction
Diabetes is a common widespread disease with a steadily increasing prevalence worldwide. Age-standardized global prevalence of diabetes has almost doubled since 1980, rising from 4.7 to 8.5%, identifying diabetes as one of the leading growing health challenges.1 Patients with diabetes were previously shown to have a two- to threefold higher risk for the development of cardiovascular diseases.2 Furthermore, obesity, defined by a body mass index (BMI) of at least 30 kg m–2, is a strong predictive factor in the development of Type 2 diabetes, and obesity, in turn, represents a major risk factor for cardiovascular diseases such as coronary heart disease.3, 4
There is early evidence that BMI seems to be a valid indicator for the overall classification of obesity, however, the BMI does not reflect the individual distribution and functional differences of several fat compartments.5–7 Furthermore, early studies have determined an association of different fat compartments with different metabolic risk, especially insulin resistance.5–9 As a ratio of the body mass divided by the square of the body height, the BMI does not factor in the distribution of muscle and adipose tissue in individuals. Moreover, ethnical differences make BMI a rather inconsistent tool for estimating body composition.10
Specifically, visceral adipose tissue (VAT) seems to be more strongly associated with metabolic risk and is often considered to be a unique pathogenic adipose tissue depot, associated with adverse outcome and higher metabolic risk.5, 11,12 Besides dyslipidemia, for instance, it is well established that impaired glucose metabolism is associated with VAT.13 Furthermore, other ectopic fat depots such as epicardial fat are associated with VAT14 and it has become clear that adipocytes in VAT display a broader spectrum of inflammatory mediators than other fat depots.15 Notably, there is also early evidence that VAT is associated with specific genetic predispositions in females.16
The contributing role of subcutaneous adipose tissue (SAT) in the development of metabolic syndrome is still controversial. Moreover, several studies indicated that SAT may have beneficial effects on metabolism, emphasizing the intrinsic difference in adipose depots independent of the anatomic location.9, 17,18 In contrary, excess SAT has also been suggested to contribute to metabolic syndrome.19 Molecular studies previously showed that VAT is associated with a higher production of inflammatory cytokines leading to an increased metabolic activity, as it secrets more humoral mediators such as adiponectin and leptin, and therefore carries a greater predicition for mortality than SAT.20 However, the complexity of anatomic and functional fat depots such as VAT and SAT remains poorly understood.
The various fat compartments can be quantified non-invasively by MRI.21 Compared to other imaging modalities, such as ultrasound, CT, or dual X-ray absorptiometry,22–26 MRI represents a non-invasive tool in the prevention setting, without the need of ionizing radiation.27 However, there are a number of different parameters available, including volumetric and area-based estimates of fat depots at different transverse levels of the torso.28, 29 Earlier research has focused on the ratio between VAT and SAT (VAT/SAT ratio) as a metric of individual body fat, which has been shown to represent a predictor of cardiac events and adverse outcome, independent of the absolute fat volume.30, 31
Therefore, the aim of this study was to systematically study the association between the different parameters of fat depots obtainable by MRI and impaired glucose metabolism in subjects from the general population without cardiovascular disease. Our hypothesis was that there are parameters that are more strongly associated with diabetes status than others.
Methods and materials
Study population
The study was designed as a case control study nested in a prospective cohort from the “Cooperative Health Research in the Region of Augsburg” (KORA) between June 2013 and September 2014 and previously described elsewhere.32, 33 An oral glucose tolerance test was administered to all participants who had not been diagnosed for Type 2 diabetes, and established definitions of diabetes and prediabetes were applied.34, 35 Other established risk factors were collected in standardized fashion as part of the KORA study design, as previously described.32, 33
Subjects were eligible, if they met the following inclusion criteria: (a) willingness to undergo whole-body MRI and (b) qualification in either the prediabetes, diabetes, or control group, according to the definition of the World Health Organisation.34 Subjects, who met the following criteria, were excluded: (a) age above 72 years, (b) subjects with prior cardiovascular diseases, (c) contraindications against standard MRI examination such as cardiac pacemaker, surgical clip material, pregnancy or breastfeeding subjects, or subjects with claustrophobia, known allergy against gadolinium compounds, or an impaired renal function with a serum creatinine ≥1.3 mg dl−1.
Systolic and diastolic blood pressure measurements were obtained three times at the right arm of seated subjects after a 5-min resting period; the mean of the second and third measurements was used for analyzes. Hypertension was defined as increased systolic blood pressure ≥140 mmHg, increased diastolic blood pressure ≥90 mmHg or intake of antihypertensive medication under awareness of having hypertension. Subjects who reported current regular or sporadic cigarette smoking were defined as smokers, those who reported only previous regular or sporadic cigarette smoking were defined as ex-smokers; all others were defined as never smokers.
The study was approved by the institutional review board of the medical faculty of the Ludwig-Maximilian University Munich and all participants provided written informed consent prior to the commencement of the study.
MRI for assessment of body adipose tissue compartments
The body adipose protocol was embedded in a comprehensive, whole-body exam using a 3 T Magnetom Skyra (Siemens AG, Healthcare Sector, Erlangen, Germany) as detailed described elsewhere.32 This protocol comprised a three-dimensional in/opposed-phase VIBE-Dixon sequence using the following parameters: Slice Thickness 1.7 mm, spatial resolution: 1.7 × 1.7 mm2, field of view: 488 × 717 mm using a 256 × 256 mm matrix, repetition time: 4.06 ms echo time: 1.26; 2.49 ms, with a 9° flip angle.
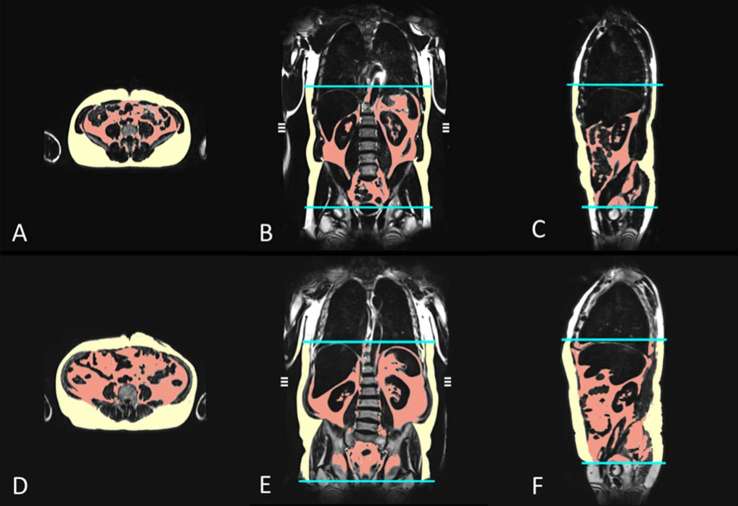
Based on the volume-interpolated three-dimensional in/opposed-phase VIBE-Dixon sequence, a fat selective tomogram was reconstructed (slice thickness 5 mm at 5 mm increment). For quantification of the adipose tissue compartments, an in-house algorithm based on Matlab R2013a was used.21 This algorithm automatically segments VAT and SAT based on fuzzy clustering and orthonormal snakes in about 2 min per data set. Cut-off values were set to 50% of the maximum fat signal, which was automatically derived in each slice. Slight imperfections at the transition between VAT and SAT—if present—were manually corrected in a second step. The volumetric VATvolume was measured from the femoral head to the cardiac apex, the volumetric SATvolume was calculated from the femoral head to the diaphragm, indicated in liter (l). The volumetric total adipose tissue (TATvolume) is defined as the summary of VATvolume and SATvolume, calculated from the femoral head to the diaphragm and cardiac apex, respectively, indicated in liter (l). In addition, both VAT and SAT compartments were measured at a single slice at the level of the umbilicus based on a VIBE-Dixon sequence (VATarea and SATarea, respectively), indicated in square centimeter (cm2), as previous studies showed, that axial MRI measurements at the umbilical level allow for a reliable estimation of the fat compartments with highest correlations regarding VAT and SAT and can easily be identified in axial slices.28 An example of the VAT and SAT compartments as total volume and area on a single slice, in a control and a subject with prediabetes is depicted in Figure 1.
Figure 1.
MRI-based assessment of adipose tissue depots in a 42-year-old male control (a–c); VATvolume 2.8 l, SATvolume 5.8 l, VATarea 89.8 cm2, SATarea 259.4 cm2) and an obese, 57-year-old male with prediabetes (d–f); VATvolume 9.1 l, SATvolume 10.8 l, VATarea 302.3 cm2, SATarea 332.2 cm2). The volumes of the different adipose tissue depots were measured automatically from the diaphragm to the femoral head by employing an in-house algorithm (b–c and e–f). VATarea and SATarea are derived from a single slice on the level of the umbilicus (a, d). (red area = VAT; yellow area = SAT). SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
All analyzes were performed in a blinded fashion by independent readers unaware of the glycemic status and clinical covariates.
Statistical analysis
Demographic characteristics, risk factors and adipose tissue parameters of participants are presented as arithmetic means and standard deviations for continuous variables and counts and percentages for categorical variables. A two-sample t-test with pooled variance was used to analyze differences in mean adipose tissue variables. The correlation between the respective adipose tissue parameters with the corresponding confidence interval was calculated by Pearson’s correlation coefficient and correlation was interpreted as very weak (r = 0–0.19), weak (r = 0.20–0.39), moderate (r = 0.40–0.59), strong (r = 0.60–0.79) and very strong (r = 0.80–1.00).36
The association of body adipose tissue on glycemic status was evaluated by an ordered logistic regression model adjusted for age and sex. The association of glycemic status to body adipose tissue was assessed by linear regression models adjusted for age, sex, smoking, BMI, hypertension, high density lipoprotein, low density lipoprotein and triglycerides. Interactions of glycemic status and BMI/waist circumference were evaluated by calculating marginal effects based on linear regression models including multiplicative interaction terms. p-values < 0.05 were considered to denote statistical significance. All calculations were performed with R v3.4.1.
Results
Among 400 subjects enrolled, a total of 384 subjects with complete MR data sets were included in the final analysis (96.0%). Of them, 235 were healthy controls, 97 were classified as prediabetes and 52 with diabetes (61.2, 25.3 and 13.5%, respectively). The mean age was 56.2 ± 9.2 years and 58.1% of the subjects were male (Table 1).
Table 1.
Demographic characteristics and cardiovascular risk factors of our study population
| Variable | All | Control | Prediabetes | Diabetes |
| N = 384 | N = 235 (61.2%) | N = 97 (25.3%) | N = 52 (13.5%) | |
| Age, years | 56.2 ± 9.2 | 54.0 ± 8.7 | 58.5 ± 8.9 | 62.1 ± 8.3 |
| Male gender | 223 (58.1%) | 121 (51.5%) | 63 (64.9%) | 39 (75.0%) |
| Weight, kg | 82.5 ± 15.9 | 78.6 ± 15.4 | 88.8 ± 13.4 | 88.1 ± 17.3 |
| Height, cm | 171.7 ± 9.7 | 171.6 ± 10.3 | 172.2 ± 9.4 | 171.5 ± 7.8 |
| BMI, kg m–2 | 27.9 ± 4.7 | 26.6 ± 4.2 | 30.0 ± 4.5 | 29.9 ± 4.9 |
| Waist circumference, cm | 98.0 ± 13.8 | 93.4 ± 12.5 | 104.4 ± 11.7 | 106.9 ± 14.1 |
| Waist-to-hip-ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 |
| Hypertension | 128 (33.3%) | 49 (20.9%) | 43 (44.3%) | 36 (69.2%) |
| HbA1c, % | 5.6 ± 0.7 | 5.3 ± 0.3 | 5.6 ± 0.3 | 6.7 ± 1.3 |
| HDL, mg dl–1 | 61.9 ± 17.7 | 65.1 ± 17.9 | 58.7 ± 14.3 | 53.8 ± 18.9 |
| LDL, mg dl–1 | 139.4 ± 32.6 | 138.2 ± 31.5 | 146.1 ± 30.3 | 132.8 ± 39.4 |
| Triglycerides, mg dl–1 | 131.5 ± 85.8 | 107.5 ± 64.3 | 152.0 ± 82.8 | 201.3 ± 122.3 |
| Total cholesterol, mg dl–1 | 217.7 ± 36.2 | 215.7 ± 35.6 | 225.5 ± 31.5 | 212.6 ± 44.7 |
| Smoking | ||||
| Never smoker | 141 (36.7%) | 92 (39.1%) | 34 (35.1%) | 15 (28.8%) |
| Ex-smoker | 165 (43.0%) | 91 (38.7%) | 45 (46.4%) | 29 (55.8%) |
| Smoker | 78 (20.3%) | 52 (22.1%) | 18 (18.6%) | 8 (15.4%) |
BMI, body mass index; LDL, low density lipoprotein; HDL, high density lipoprotein.
Data are presented as arithmetic means ± standard deviations (continuous variables) or counts and percentages (categorical variables).
Correlation between different MR- parameters of fat depots
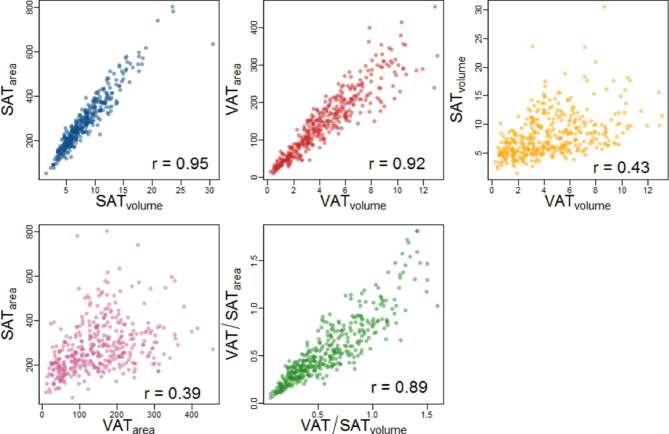
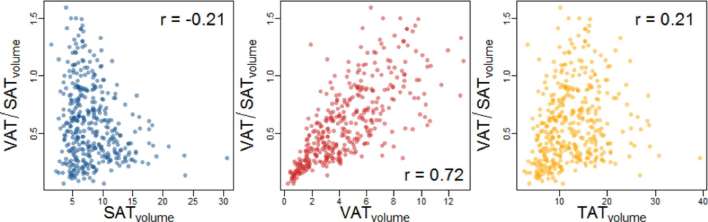
Independent of area-based or volumetric measurement technique, volumetric and single-sliced VAT and SAT strongly correlated (Figure 2, r = 0.92 and r = 0.95, respectively). Area-based and volumetric SAT and VAT were moderately correlated (r = 0.43 for and = 0.39 for volumetric and single-sliced measurements, respectively). However, the correlations between volumetric and single-sliced VAT/SAT ratios were strong (r = 0.89). However, as we found a slightly higher association of VATvolume with cardiometabolic risk factors, all subsequent analysis was carried out using the volumetric measurement. Comparing VAT/SATvolume ratios with the respective VATvolume and SATvolume, we found a strong correlation between the VAT/SATvolume ratio and the respective VATvolume (Figure 3, r = 0.72). VAT/SATvolume ratio and SATvolume or TATvolume were weakly (r = 0.21 for VAT/SATvolume and TATvolume) or not correlated (r = −0.21 for VAT/SATvolume and SATvolume).
Figure 2.
Scatter plots demonstrating the correlation between single-sliced and volumetric assessment of VAT and SAT determined by MRI. SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Figure 3.
Scatter plots demonstrating the correlation between VATvolume, SATvolume and TATvolume with the respective VAT/SATvolume ratio determined by MRI. SAT, subcutaneous adipose tissue; TAT, total adipose tissue; VAT, visceral adipose tissue.
Association of MR parameters with glycemic status
There were significant differences in the several fat depots between the subgroups (Table 2). TATvolume, SATvolume and VATvolume were significantly higher in subjects with prediabetes and diabetes as compared to healthy controls (all p ≤ 0.001). Also, the VAT/SATvolume ratio was significantly higher in subjects with prediabetes and diabetes. The association between VATvolume and glycemic status [odds ratio (OR): 3.1] was stronger than for SATvolume (OR: 2.1), VAT/SATvolume ratio (OR: 2.0), BMI (OR:2.1) or waist circumference (OR:2.6).
Table 2.
Difference of visceral and subcutaneous adipose tissue between subjects with prediabetes, diabetes, and healthy controls
| All | Controls | Prediabetes | p-valuea | Diabetes | p-value b | |
| N = 384 | N = 235 | N = 97 | N = 52 | |||
| Body adipose tissue | ||||||
| TATvolume, l | 12.6 ± 5.5 | 10.7 ± 4.7 | 15.3 ± 5.3 | <0.001 | 16.1 ± 5.4 | <0.001 |
| VATvolume, l | 4.5 ± 2.7 | 3.4 ± 2.3 | 5.8 ± 2.4 | <0.001 | 6.9 ± 2.5 | <0.001 |
| SATvolume, l | 8.1 ± 3.7 | 7.3 ± 3.2 | 9.6 ± 4.2 | <0.001 | 9.2 ± 3.8 | 0.001 |
| Ratio VAT/SATvolume | 0.59 ± 0.33 | 0.49 ± 0.29 | 0.68 ± 0.34 | <0.001 | 0.82 ± 0.34 | <0.001 |
SAT, subcutaneous adipose tissue; TAT, total adipose tissue; VAT, visceral adipose tissue.
p-values are Bonferroni corrected for the repeated comparison to the control group.
aprediabetes vs. controls. b diabetes vs. controls.
After adjustment for potential confounders, including age, sex and hypertension, prediabetes and diabetes remained significantly associated with TATvolume, VATvolume and VAT/SATvolume ratio (all p ≤ 0.006, Table 3). These associations persisted after additionally adjusting for smoking, BMI, and dyslipidemia, (all <0.016), while the association of SATvolume with glycemic state remained non-significant (all p ≥ 0.17).
Table 3.
Association of glycemic status to body adipose tissue after adjustment for potential confounders
| Adjusted for age, sex and BMI | ||||||
| Prediabetes | Diabetes | |||||
| β-coefficient | 95% CI | p-value | β-coefficient | 95% CI | p-value | |
| TATvolume, l | 1.08 | [0.46, 1.69] | <0.001 | 1.80 | [1.01, 2.58] | <0.001 |
| VATvolume, l | 0.76 | [0.37, 1.16] | <0.001 | 1.50 | [0.99, 2.01] | <0.001 |
| SATvolume, l | 0.32 | [−0.10, 0.73] | 0.14 | 0.30 | [−0.23, 0.83] | 0.271 |
| Ratio VAT/SATvolume | 0.10 | [0.04, 0.15] | <0.001 | 0.15 | [0.08, 0.22] | <0.001 |
| Adjusted for age, sex, smoking, body mass index, hypertension, HDL, LDL and triglycerides | ||||||
| Prediabetes | Diabetes | |||||
| β-coefficient | 95% CI | p-value | β-coefficient | 95% CI | p-value | |
| TATvolume, l | 0.82 | [0.21, 1.44] | 0.009 | 1.19 | [0.34, 2.04] | 0.006 |
| VATvolume, l | 0.52 | [0.14, 0.91] | 0.008 | 0.87 | [0.34, 1.40] | 0.001 |
| SATvolume, l | 0.30x | [−0.12, 0.72] | 0.166 | 0.32 | [−0.26, 0.90] | 0.281 |
| Ratio VAT/SATvolume | 0.07 | [0.02, 0.13] | 0.008 | 0.09 | [0.02, 0.16] | 0.02 |
BMI, body mass index; CI, confidence interval; SAT, subcutaneous adipose tissue; TAT, total adipose tissue; VAT, visceral adipose tissue.
Results from linear regression model.
Association with BMI and waist circumference
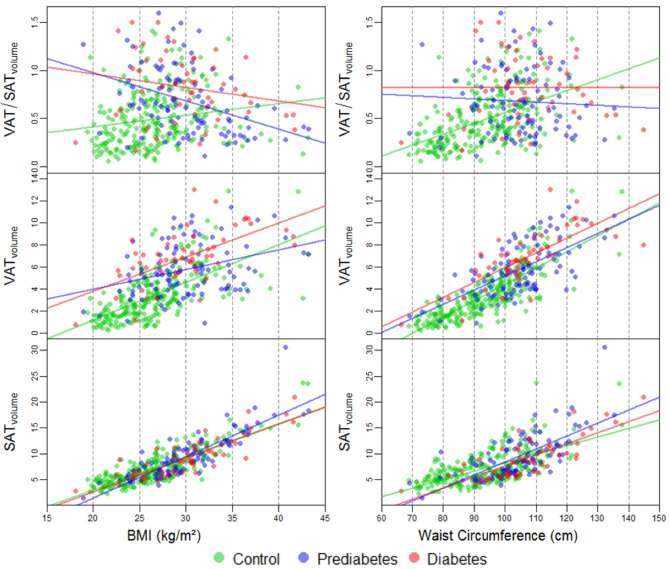
Figure 4 displays the correlation between the absolute fat depot volumes and the VAT/SATvolume ratio with rising BMI or waist circumference in controls as well as subjects with impaired glucose metabolism. With rising BMI and waist circumference, an increase of VATvolume and SATvolume was detected in all subgroups. The increase of SATvolume with rising BMI and waist circumference was similar in all subgroups, whereas there was a stronger increase of VATvolume in controls as compared to subjects with prediabetes and diabetes (r = 0.64 for controls vs r = 0.34 and 0.62 in subjects with prediabetes and diabetes, respectively).
Figure 4.
Association of adipose tissue depots SATvolume and VATvolume as well as the VAT/SATvolume ratio obtained with increasing BMI and waist circumference. BMI, body mass index; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
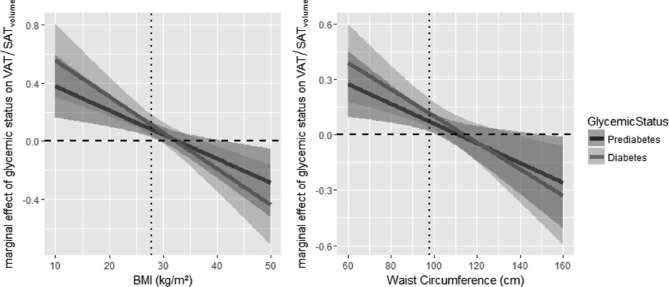
Figure 5 displays the marginal effect of glycemic status on the VAT/SATvolume ratio for multiplicative interactions with BMI and waist circumference. The marginal effect reached statistical significance for a BMI up to 29.5 and 31 kg m–2 in subjects with prediabetes and diabetes, respectively (p < 0.05). Similarly, the marginal effect of glycemic status on the VAT/SATvolume ratio reached statistical significance in the range of a waist circumference of 65–101 cm. The analysis of the absolute fat volumes VATvolume and SATvolume showed a decreasing marginal effect of diabetes on VATvolume in the range of a BMI of 19–34 kg m–2 and of prediabetes in the range of a BMI of 19.5–31 kg m–2. An increasing marginal effect of glycemic status on SATvolume was found, which did not reach statistical significance.
Figure 5.
Marginal effects of glycemic status on the ratio of VAT/SATvolume for multiplicative interactions with BMI (left) and waist circumference (right). Displayed are the marginal effects of prediabetes (solid line, dark gray) and diabetes (solid line, light gray) and the respective 95% confidence interval for a grid of possible values of BMI (range in data: 18.1–43.2 kg m–2) and waist circumference (range in data: 66.4–144.8 cm). The arithmetic mean is indicated by a dotted line. The dashed line indicates the line of no effect. BMI, body mass index; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Discussion
In this study, including adult individuals without known cardiovascular disease from the general population, we found a very strong correlation between volumetric and single-sliced measurements of VAT and SAT and its ratio. Increased MRI-based VATvolume and VAT/SATvolume ratios were associated with prediabetes and diabetes, independent of cardiometabolic confounders. Among measurements, VATvolume was stronger related to prediabetes or diabetes as compared to TATvolume, BMI, or waist circumference, while the association of SATvolume was not independent of potential confounders. Furthermore, we found an attenuated association of VAT/SATvolume ratio with glycemic state in obese subjects with high BMI or waist circumference, possibly dominated by the variation of VATvolume in obese subjects.
SAT and VAT were previously shown to be highly correlated with metabolic risk factors and seem to provide an individual metabolic risk profile associated with the variation in the several fat compartments, which cannot be reflected by general measurements such as BMI and waist circumference.5, 28 However, its real clinical value remains to be determined.
Similar to previous research, we found strong correlations between volumetric and single-sliced assessment of VAT and SAT as well as the VAT/SAT ratios. Schwenzer et al found similarly high correlations between single slices and volumetric measurements of the several adipose tissue departments.28 Furthermore, in a study with morbidly obese patients, Schaudinn et al found a strong correlation between volumetric VAT and sliced-based VAT, independent of the number of slices assessed.37 However, our data also indicate that volumetric measurements may provide a slightly higher discriminatory power, particularly in subjects with higher BMI. In contrast to these earlier efforts, our sample was drawn from a large European general population without prior cardiovascular disease and comprised subjects with impaired glycemic state as well as controls, thus, allows for higher generalizability. As such, while we confirm that a single-slice based quantification of adipose tissue depots represent a reliable alternative for risk stratification in larger cohorts, further more outcome-related research will be necessary.
Despite the strong association among these quantitative parameters, there is early evidence that VATvolume is a stronger predictor for metabolic disease and cardiovascular risk factors as compared to SAT.5, 12 Also, the VAT/SAT ratio seems to be a proxy for cardiometabolic risk, independent of VAT or absolute fat volumes.31, 38 Our results confirm these early findings, as we found a significant association of VAT, SAT and TAT as well as the VAT/SAT ratio with prediabetes and diabetes. Furthermore, our results indicate that VATvolume as well as the VAT/SATvolume ratio is strongly associated with diabetes and prediabetes state, independent of cardiometabolic risk factors, such as age, sex, hypertension, BMI, smoking and dyslipidemia. In contrast, the association of SAT and glycemic state attenuated after adjusting for these confounders. Furthermore, in contrast to VATvolume, the SATvolume as well as TATvolume did not exceed a weak correlation with the VAT/SATvolume ratio, potentially indicating a stronger influence of VATvolume on the composition of body fat depots. We also found a stronger association of VATvolume with increased risk of prediabetes and diabetes compared to SATvolume, VAT/SATvolume ratio, BMI and waist circumference. In a large sample drawn from the Framingham Heart Study, including 3001 participants without prior cardiovascular diseases, VAT was more strongly associated with adverse metabolic risk profile as compared to SAT; however, their measurements were performed on CT.5 Similar to our MR-based approach, in a large cohort of Chinese adults, Tang et al found a higher association of VAT with increased risk of prediabetes.12 However, the role of SAT in cardiometabolic risk is still controversial, as previous studies found an inverse association of SAT with insulin resistance in obese subjects.39 As such, our results confirm the strong role of VATvolume in predicting cardiovascular risk and potentially adverse outcome beyond SATvolume in an European cohort.
This finding is mirrored for the role of VAT/SAT ratio. Previously, Kaess et al found a significant correlation between VAT/SAT ratio and cardiometabolic risk factors, independent of BMI and absolute VAT.31 In a retrospective cohort including participants without known cardiovascular disease from Europe, Ladeiras-Lopes et al found that CT-based VAT/SAT ratio was, in contrary to the absolute fat volumes of VAT and SAT, an independent risk factor for cardiovascular events and death.38 Our results suggest a stronger predictive value for absolute VATvolume, while the VAT/SAT ratio remained an independently association of potential confounders. Further, outcome-based research is clearly needed to elucidate the most predictive parameter of VAT for risk stratification.
Interestingly, our results demonstrate an interaction effect between BMI and waist circumference and prediabetes and/or diabetes state, as the association between the VAT/SATvolume ratio attenuated with higher BMI or waist circumference. Specifically, the relationship between absolute fat volumes (VATvolume and SATvolume) and BMI or waist circumference was characterized by a stronger increase of VATvolume in controls as compared to subjects with impaired glucose metabolism, whereas SATvolume increased similarly between the subgroups. Thus, the VAT/SATvolume ratio decreased with increasing BMI or waist circumference in subjects with impaired glucose metabolism, which was the opposite in controls. Furthermore, in contrary to SATvolume, we found a strong correlation between VAT/SATvolume ratios with VATvolume measurement, indicating a stronger influence of VATvolume compared to SATvolume. These findings may suggest that the association of glycemic status with VAT/SATvolume ratio is less pronounced in subjects with higher BMI or waist circumference and consequently, limit the value of VAT/SATvolume ratios for the risk stratification in these obese subjects due to the varying VATvolume in obese patients. However, further confirmatory research also in other cohorts is clearly warranted.
Our study has several limitations. The small sample size as well as the inclusion of mainly middle-aged, Caucasian subjects limit the generalizability of our results and reported associations may differ according to ethnicity when comparing with other cohorts. Moreover, many studies are based on VAT and SAT measurements at the level of lumbar vertebra L3, however, previous research showed, that axial MRI measurements at the umbilical level also allow for a valid and reliable estimation of the fat compartments with high correlations regarding VAT and SAT, and are more easily depicted on axial slices.28 Focusing on the relation of the fat depots in obese patients, generalizability is limited due to the fact of the small number of subjects with high levels of BMI and waist circumference. However, our study population represent a representative sample from a western European population. Furthermore, the observational cross-sectional design of our study precludes definite causal interferences and more large-scale studies are warranted.
In conclusion, our results demonstrate that there is a strong correlation between the different parameters of fat deposition, including SAT, VAT and VAT/SAT ratios derived from area-based and volumetric MRI. Among them, elevated VATvolume and VAT/SATvolume ratio are highly associated with prediabetes and diabetes, above and beyond known cardiovascular risk factors and independent of single-sliced or volumetric quantification on MRI. However, VAT/SATvolume ratios appear to be more dependent on VATvolume as compared to SATvolume or TATvolume. In obese subjects with elevated BMI and/or waist circumference, the VAT/SATvolume ratio may be of limited value due to present interaction effects. Thus, quantification of VATvolume as well as VATarea represents a reproducable and reliable biomarker associated with cardiometabolic risk factors such as obesity and glycemic state. Further confirmatory research especially in large cohort studies is warranted.
Contributor Information
Corinna Storz, Email: corinna.storz@uni-tuebingen.de.
Sophia D Heber, Email: Sophia.heber@uni-tuebingen.de.
Susanne Rospleszcz, Email: susanne.rospleszcz@helmholtz-muenchen.de.
Jürgen Machann, Email: Juergen.Machann@med.uni-tuebingen.de.
Sabine Sellner, Email: Sabine.Sellner@med.uni-muenchen.de.
Konstantin Nikolaou, Email: Konstantin.Nikolaou@med.uni-tuebingen.de.
Roberto Lorbeer, Email: Roberto.Lorbeer@med.uni-muenchen.de.
Sergios Gatidis, Email: Sergios.Gatidis@uni-tuebingen.de.
Stefanie Elser, Email: Stefanie.Elser@med.uni-tuebingen.de.
Annette Peters, Email: peters@helmholtz-muenchen.de.
Christopher L Schlett, Email: Christopher.Schlett@med.uni-heidelberg.de.
Fabian Bamberg, Email: fabian.bamberg@uni-tuebingen.de.
REFERENCES
- 1. World Health Organization. Global report on diabetes. France: The British Institute of Radiology.; 2016. [Google Scholar]
- 2.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979; 241: 2035–8. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Krauss RM. American Heart Association call to action: obesity as a major risk factor for coronary heart disease. AHA Nutrition Committee. Circulation 1998; 97: 2099–100. doi: 10.1161/01.CIR.97.21.2099 [DOI] [PubMed] [Google Scholar]
- 4.Hjerkind KV, Stenehjem JS, Nilsen TI, Adiposity NTI. Adiposity, physical activity and risk of diabetes mellitus: prospective data from the population-based HUNT study, Norway. BMJ Open 2017; 7: e013142. doi: 10.1136/bmjopen-2016-013142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007; 116: 39–48. doi: 10.1161/CIRCULATIONAHA.106.675355 [DOI] [PubMed] [Google Scholar]
- 6.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000; 21: 697–738. doi: 10.1210/edrv.21.6.0415 [DOI] [PubMed] [Google Scholar]
- 7.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997; 46: 1579–85. doi: 10.2337/diacare.46.10.1579 [DOI] [PubMed] [Google Scholar]
- 8.Machann J, Thamer C, Stefan N, Schwenzer NF, Kantartzis K, Häring HU, et al. Follow-up whole-body assessment of adipose tissue compartments during a lifestyle intervention in a large cohort at increased risk for type 2 diabetes. Radiology 2010; 257: 353–63. doi: 10.1148/radiol.10092284 [DOI] [PubMed] [Google Scholar]
- 9.Stefan N, Schick F, Häring HU. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab 2017; 26: 292–300. doi: 10.1016/j.cmet.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 10.Rønn PF, Andersen GS, Lauritzen T, Christensen DL, Aadahl M, Carstensen B, et al. Ethnic differences in anthropometric measures and abdominal fat distribution: a cross-sectional pooled study in Inuit, Africans and Europeans. J Epidemiol Community Health 2017; 71: 536: 536–43. doi: 10.1136/jech-2016-207813 [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson heart study. J Clin Endocrinol Metab 2010; 95: 5419–26. doi: 10.1210/jc.2010-1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang L, Zhang F, Tong N. The association of visceral adipose tissue and subcutaneous adipose tissue with metabolic risk factors in a large population of Chinese adults. Clin Endocrinol 2016; 85: 46–53. doi: 10.1111/cen.13013 [DOI] [PubMed] [Google Scholar]
- 13.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA 2012; 308: 1150–9. doi: 10.1001/2012.jama.11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res 2003; 11: 304–10. doi: 10.1038/oby.2003.45 [DOI] [PubMed] [Google Scholar]
- 15.Cheng KH, Chu CS, Lee KT, Lin TH, Hsieh CC, Chiu CC, et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes 2008; 32: 268–74. doi: 10.1038/sj.ijo.0803726 [DOI] [PubMed] [Google Scholar]
- 16.Fox CS, Liu Y, White CC, Feitosa M, Smith AV, Heard-Costa N, et al. Genome-wide association for abdominal subcutaneous and visceral adipose reveals a novel locus for visceral fat in women. PLoS Genet 2012; 8: e1002695. doi: 10.1371/journal.pgen.1002695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care 2009; 32: 1068–75. doi: 10.2337/dc08-2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 2008; 7: 410–20. doi: 10.1016/j.cmet.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertoli S, Leone A, Vignati L, Spadafranca A, Bedogni G, Vanzulli A, et al. Metabolic correlates of subcutaneous and visceral abdominal fat measured by ultrasonography: a comparison with waist circumference. Nutr J 2016; 15: 2. doi: 10.1186/s12937-015-0120-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010; 11: 11–18. doi: 10.1111/j.1467-789X.2009.00623.x [DOI] [PubMed] [Google Scholar]
- 21.Würslin C, Machann J, Rempp H, Claussen C, Yang B, Schick F. Topography mapping of whole body adipose tissue using A fully automated and standardized procedure. J Magn Reson Imaging 2010; 31: 430–9. doi: 10.1002/jmri.22036 [DOI] [PubMed] [Google Scholar]
- 22.Armellini F, Zamboni M, Rigo L, Todesco T, Bergamo-Andreis IA, Procacci C, et al. The contribution of sonography to the measurement of intra-abdominal fat. J Clin Ultrasound 1990; 18: 563–7. doi: 10.1002/jcu.1870180707 [DOI] [PubMed] [Google Scholar]
- 23.Gong W, Ren H, Tong H, Shen X, Luo J, Chen S, et al. A comparison of ultrasound and magnetic resonance imaging to assess visceral fat in the metabolic syndrome. Asia Pac J Clin Nutr 2007; 16(Suppl 1): 339–45. [PubMed] [Google Scholar]
- 24.Val-Laillet D, Blat S, Louveau I, Malbert CH. A computed tomography scan application to evaluate adiposity in a minipig model of human obesity. Br J Nutr 2010; 104: 1719–28. doi: 10.1017/S0007114510002667 [DOI] [PubMed] [Google Scholar]
- 25.De Lucia Rolfe E, Norris SA, Sleigh A, Brage S, Dunger DB, Stolk RP, et al. Validation of ultrasound estimates of visceral fat in black South African adolescents. Obesity 2011; 19: 1892–7. doi: 10.1038/oby.2011.213 [DOI] [PubMed] [Google Scholar]
- 26.Neeland IJ, Grundy SM, Li X, Adams-Huet B, Vega GL. Comparison of visceral fat mass measurement by dual-X-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: the Dallas Heart Study. Nutr Diabetes 2016; 6: e221. doi: 10.1038/nutd.2016.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klopfenstein BJ, Kim MS, Krisky CM, Szumowski J, Rooney WD, Purnell JQ. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br J Radiol 2012; 85: e826–e830. doi: 10.1259/bjr/57987644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwenzer NF, Machann J, Schraml C, Springer F, Ludescher B, Stefan N, et al. Quantitative analysis of adipose tissue in single transverse slices for estimation of volumes of relevant fat tissue compartments: a study in a large cohort of subjects at risk for type 2 diabetes by MRI with comparison to anthropometric data. Invest Radiol 2010; 45: 788–94. doi: 10.1097/RLI.0b013e3181f10fe1 [DOI] [PubMed] [Google Scholar]
- 29.Machann J, Horstmann A, Born M, Hesse S, Hirsch FW. Diagnostic imaging in obesity. Best Pract Res Clin Endocrinol Metab 2013; 27: 261–77. doi: 10.1016/j.beem.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 30.Ladeiras-Lopes R, Sampaio F, Bettencourt N, Fontes-Carvalho R, Ferreira N, Leite-Moreira A, et al. The ratio between visceral and subcutaneous abdominal fat assessed by computed tomography is an independent predictor of mortality and cardiac events. Rev Esp Cardiol 2017; 70: 331–7. doi: 10.1016/j.rec.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 31.Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia 2012; 55: 2622–30. doi: 10.1007/s00125-012-2639-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bamberg F, Hetterich H, Rospleszcz S, Lorbeer R, Auweter SD, Schlett CL, et al. Subclinical disease burden as assessed by whole-body MRI in subjects with prediabetes, subjects with diabetes, and normal control subjects from the general population: the KORA-MRI study. Diabetes 2017; 66: 158–69. doi: 10.2337/db16-0630 [DOI] [PubMed] [Google Scholar]
- 33.Holle R, Happich M, Löwel H, Wichmann HE, MONICA/KORA Study Group. KORA--a research platform for population based health research. Gesundheitswesen 2005; 67(Suppl 1): 19–25. doi: 10.1055/s-2005-858235 [DOI] [PubMed] [Google Scholar]
- 34. World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Geneva: The British Institute of Radiology.; 2006. [Google Scholar]
- 35.Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force on diabetes, pre-diabetes, and cardiovascular diseases of the European society of cardiology (ESC) and developed in collaboration with the European association for the study of diabetes (EASD). Eur Heart J 2013; 34: 3035–87. doi: 10.1093/eurheartj/eht108 [DOI] [PubMed] [Google Scholar]
- 36.Evans JD. Straightforward statistics for the behavioral sciences. Pacific Grove: The British Institute of Radiology.; 1996. xxii, 600. [Google Scholar]
- 37.Schaudinn A, Linder N, Garnov N, Kerlikowsky F, Blüher M, Dietrich A, et al. Predictive accuracy of single- and multi-slice MRI for the estimation of total visceral adipose tissue in overweight to severely obese patients. NMR Biomed 2015; 28: 583–90. doi: 10.1002/nbm.3286 [DOI] [PubMed] [Google Scholar]
- 38.Ladeiras-Lopes R, Sampaio F, Bettencourt N, Fontes-Carvalho R, Ferreira N, Leite-Moreira A, et al. The ratio between visceral and subcutaneous abdominal fat assessed by computed tomography is an independent predictor of mortality and cardiac events. Rev Esp Cardiol 2017; 70: 331–7. doi: 10.1016/j.rec.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 39.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab 2011; 96: E1756–E1760. doi: 10.1210/jc.2011-0615 [DOI] [PMC free article] [PubMed] [Google Scholar]