Abstract
Morbid obesity is an increasing health problem, and bariatric surgery is a popular treatment option. Radiologists must be familiar with performing and interpreting studies in this patient population. The typical post-operative findings of the Roux-en-Y gastric bypass (RYGB), laparoscopic adjustable gastric banding (LAGB) and sleeve gastrectomy (SG) procedures on upper gastrointestinal (UGI) series and computerized tomography (CT) are presented. An overview of the potential complications is provided in addition to a description of potential pitfalls in interpreting these studies.
Introduction
Obesity presents an explicit health care concern particularly in wealthy countries where obesity rates have reached epidemic proportions, and is associated with significant health risks.1–3 Studies have demonstrated a strong relationship between increased body mass index (BMI) and the development of comorbidities including hypertension, Type 2 diabetes, atherosclerosis, sleep apnea, osteoarthritis, cancer and early death.4, 5 This public health threat continues to steadily increase, and worldwide trends show that more people are now obese (BMI ≥ 30) than underweight. In 2014, 18.4% of the world’s obese adults (118 million) lived in high-income English-speaking countries. For example, obesity in the US has more than doubled over the past three decades with an estimated 39.6% of adults in the US obese, and approximately 28% of the population in the UK is obese – the highest rate in Europe.1,6–8 Increasing rates of obesity lead not only to increased morbidity but also significant economic consequences, with a strong positive relationship between BMI and medical costs. It has been estimated that as much as 20.6% of US national health expenditure is spent treating obesity-related illnesses, in addition to indirect economic costs such as lost productivity and work absenteeism.9, 10
Bariatric surgery has demonstrated superiority over non-surgical treatments for obesity, producing greater sustained weight loss compared with conventional medical management and lifestyle changes, and has higher impact on obesity-related comorbidities.5 As a consequence, the number of bariatric surgeries performed worldwide has increased over the years, although type and frequency of preferred surgeries continues to evolve over time as more data regarding efficacy, complications and morbidity become available. The current most commonly performed bariatric procedures are the sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric band (LAGB) and these procedures are the focus of this manuscript. These procedures represent 45.9, 39.6 and 7.4% of all bariatric procedures performed worldwide.11 The most recent global data show that 5,79,517 bariatric surgeries were performed worldwide in 2014.11 RYGB remains the most common bariatric surgery in the UK followed by SG, although the latter has been gaining in popularity and is now the most common bariatric surgery in countries where the most bariatric surgeries are performed. Indeed, the UK performs a lower volume of bariatric surgeries compared with other European and North American countries; a total of 6,391 such surgeries were performed in the UK in 2014 compared with 1,91,920 in the USA and 46,960 in France.11, 12
Imaging studies are important tools to evaluate the post-operative patient, and radiologists must be aware of the expected post-operative findings and potential complications. This paper seeks to describe the radiological evaluation of the main bariatric surgeries, primarily done using fluoroscopic upper gastrointestinal (UGI) examination and computerized tomography (CT), focusing on expected post-operative appearance and the early and late post-operative complications.
Roux-en-Y Gastric Bypass
RYGB is a well-established surgery that results in the highest long-term success and most sustained weight loss among bariatric procedures.13–16 Although its frequency has slightly declined in recent years, RYGB remains popular, representing roughly a quarter of all bariatric surgeries performed in the USA12 and 40% of bariatric procedures worldwide.11
In RYGB a small 15–30 ml pouch is created from the proximal stomach, excluding the remainder of the stomach and the duodenum (i.e. the biliopancreatic limb) from the path of food (Figure 1). A Roux jejunal limb is brought up either antecolic or retrocolic (retrocolic through a defect created in the transverse mesocolon) and anastomosed to the gastric pouch, creating a gastrojejunal stoma. The stoma is usually 8–15 mm in diameter. The Roux limb typically has a short oversewn blind-ending component and an antegrade-flowing “alimentary” limb (typically 80–120 cm in length) leading to the downstream side-to-side jejunojejunal anastomosis. The jejunojejunal anastomosis is most commonly located in the left mid abdomen.17
Figure 1.
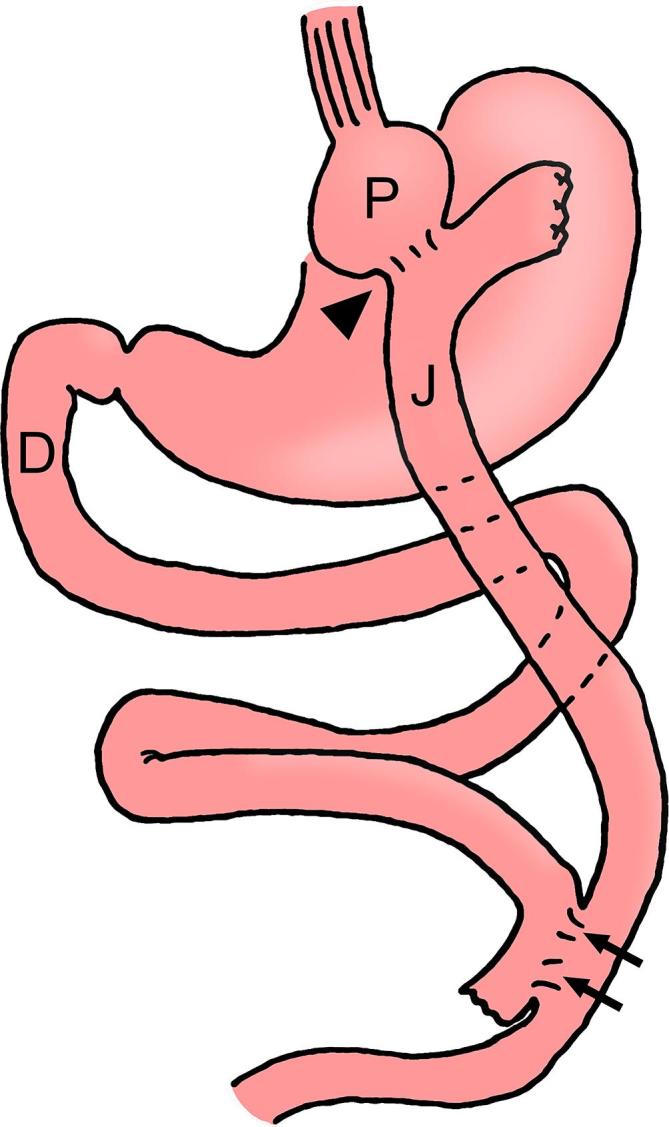
RYGB diagram. A small gastric pouch (P) is created to exclude the remainder of the stomach and the duodenum (D) (biliopancreatic limb) from the path of food. There is a gastrojejunostomy with a jejunal Roux limb (J) anastomosed to the pouch via a narrow stoma (arrowhead) and creating the alimentary limb. There is then a more downstream jejunojejunostomy (arrows). This creates an alimentary limb (i.e. pouch, Roux limb), a biliopancreatic limb (including the excluded stomach and duodenum) and a downstream common channel. RYGB, Roux-en-Y gastric bypass.
Weight loss occurs primarily by a restrictive mechanism, whereby the small gastric pouch and narrow gastrojejunal stoma create early and prolonged satiety. Malabsorption contributes to a lesser degree due to bypass of the duodenum and variable length of proximal jejunum.18
Expected imaging appearance following RYGB
Upper gastrointestinal examination
In the early post-operative period, UGI is often used following RYGB to assess for leak or obstruction. In the later post-operative period, UGI can be used to evaluate patients with abdominal pain, dysphagia, inadequate or excessive weight loss and/or signs of obstruction. A UGI in the early post-operative phase should first be performed using water-soluble contrast; if no leak is seen, barium can be used.19 In the later post-operative phase, barium can be utilized initially.
The examination should begin with assessment of the proximal post-surgical anatomy, including the gastric pouch stoma and proximal Roux limb. Fluoroscopic technique can vary depending on machine and local practice. However, the RYGB anatomy is best depicted with the patient in the left posterior oblique (LPO) position20 (Figure 2a). The patient is placed in the LPO position prior to administering oral contrast material, ideally with the fluoroscopy table horizontal in position. If the fluoroscopy equipment does not allow for supine positioning, the study may be performed with the patient in the upright position, however, this position may not depict optimal luminal distention. Additional alternatives with varying degrees of efficacy for superobese patients may include performing the study with overhead radiographs only or potentially with the use of a C-arm.21
Figure 2.
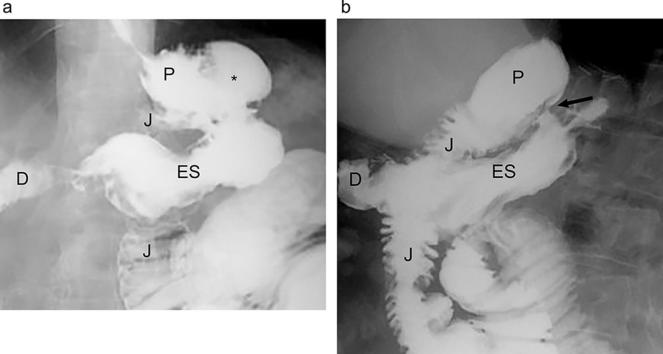
Expected anatomy following gastric bypass on UGI. (a). Fluoroscopic UGI spot image acquired with the patient in the supine LPO position shows the small gastric pouch (P), narrow gastrojejunal anastomosis (arrows) and adjacent Roux jejunal limb (J). (b). Supine overhead radiograph from UGI shows the gastric pouch (P), Roux jejunal limb (J) and expected location of the left mid-abdominal jejunojejunal anastomosis (arrow). LPO, left posterior oblique; UGI, upper gastrointestinal.
Rapid sequence imaging is helpful to achieve full distention views of the gastric pouch, stoma and roux limb; adequate distention of the pouch and stoma is essential to assess for a potential leak. Following assessment in the LPO position, additional fluoroscopic views can be obtained in additional obliquities. Overhead radiographs, or the largest no-magnification equivalent depending upon the available equipment, should then be obtained until contrast passes through the jejunojejunal anastomosis (Figure 2b) in the early post-operative phase, as rarely leak or obstruction can occur at this site.19 The study should be continued until contrast reaches the terminal ileum in the late post-operative phase, as an obstruction or internal hernia may not become conspicuous until the entire small bowel is opacified.22
CT
CT may be obtained in patients following RYGB who present with symptoms of abdominal pain, obstruction, or internal hernia. Additionally, evidence of prior bariatric surgery may be found incidentally on studies performed for other indications.
Patients are optimally imaged following the administration of positive oral contrast as well as i.v. contrast.23 Positive oral contrast can help to distinguish the alimentary limb and common channel from the excluded limb (Figure 3). Ideally the patient should drink positive oral contrast 30–60 min prior to the scan and they should drink additional oral contrast material immediately before the scan to opacify the gastric pouch and proximal Roux limb. As with UGI, water soluble contrast (diluted for CT) is preferable in the early post-operative course or with suspected leak. Barium may be administered in the late post-operative period. The gastric pouch, gastrojejunal anastomosis, roux limb, excluded stomach and biliopancreatic limb should be readily identified on CT. The jejunojejunal anastomosis should be identified as well and is usually located in the left mid abdomen.20
Figure 3.
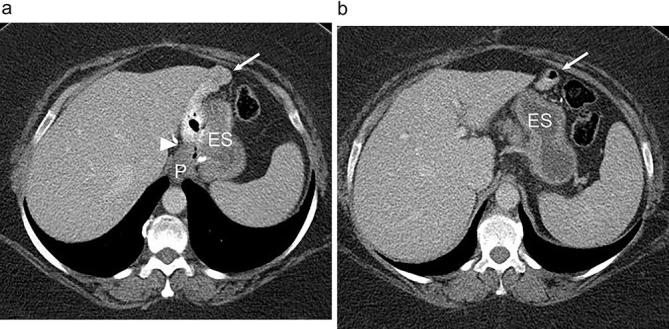
Expected anatomy following RYGB on CT. (a and b). Axial abdominal CT images acquired with both oral and i.v. contrast show a small gastric pouch (P), gastrojejunal anastomosis (arrowhead), Roux jejunal limb (arrow) and the excluded stomach (ES). Note the opacification of the alimentary jejunal limb (arrow) without opacification of the excluded stomach. RYGB, Roux-en-Y gastric bypass.
RYGB complications
Extraluminal leak
The most common serious complication in the early post-operative phase following RYGB is post-operative leak, occurring in up to 6% of patients.14, 16,19 Post-operative leak leads to high morbidity and increased mortality, requiring repeat surgery in as many as 80% of cases.19 Leak is most often diagnosed within 10 days after surgery, and early diagnosis and treatment are essential to reduce the associated morbidity.14, 16,19,24 UGI examination is the imaging study of choice to evaluate for possible leak.19, 20
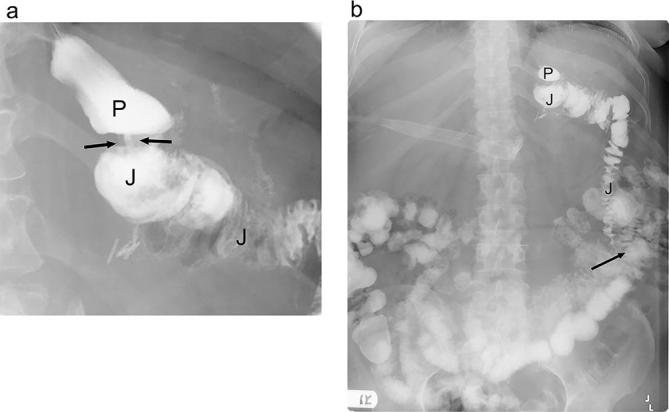
Most post-operative leaks after RYGB extend into the left upper quadrant, to the left of the gastrojejunal anastomosis19 (Figure 4). The vast majority of leaks arise from the gastrojejunal anastomosis19 (77%), but leak may also originate from the gastric pouch, jejunal stump, distal esophagus and even rarely from the jejunojejunal anastomosis.19, 25
Figure 4.
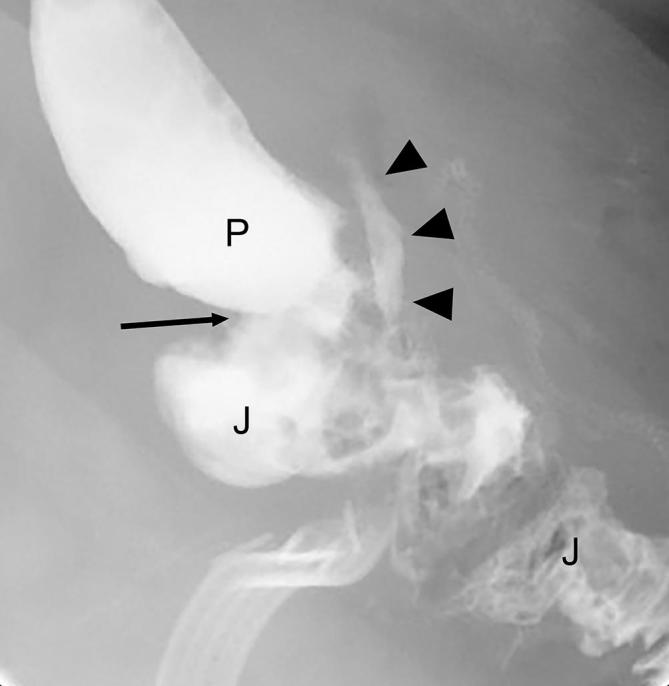
Small leak following RYGB. Fluoroscopic UGI spot image in the LPO position shows the gastric pouch (P), gastrojejunal anastomosis (arrow) and Roux jejunal limb (J). There is extravasated contrast in the left of the anastomosis (arrowheads), consistent with a small extraluminal leak. Also noted extraluminal gas in the vicinity and an indwelling surgical drainage catheter. LPO, left posterior oblique; RYGB, Roux-en-Y gastric bypass; UGI, upper gastrointestinal.
Care should be taken to closely assess for a leak at the time of initial fluoroscopy, as later on during the study contrast may reflux into the excluded stomach, particularly in the setting of ileus or obstruction. This may appear as a collection of contrast in the left upper quadrant to the left of the gastrojejunal anastomosis and can obscure or mimic a leak. Intragastric location can be confirmed by rotating the patient to the right and shifting the contrast into the distal stomach and duodenum.19 Occasionally, a leak will only be detected by contrast opacification of a surgical drain. Overhead radiographs following initial fluoroscopy are particularly helpful in this patient population.19, 25
Communication with the excluded stomach
Communication between the gastric pouch and the excluded stomach can occur by way of staple line dehiscence or disruption or via a gastrogastric fistula. Communication with the excluded stomach occurs in up to 4% of patients.26 This abnormal communication can allow ingested food to enter the excluded stomach and lead to failed weight loss, often necessitating non-emergent corrective surgery.26
In the past, the gastric pouch was predominantly created with a staple or suture line separating it from the remainder of the stomach. Dehiscence or disruption of the staple line in this setting can be the result of overdistention of the gastric pouch with food or inadequate division of the pouch from the excluded stomach at the time of surgery.26–28 More recently, it is more common for the gastric pouch to be transected from the remainder of the stomach. In this case, a gastro-gastric fistula may form and lead to communication between the pouch and excluded stomach. This may occur in the setting of post-operative leak.19, 26
Communication with the excluded stomach is most readily diagnosed with UGI. UGI examination will reveal contrast material entering the gastric pouch and jejunum, but also traveling into the excluded stomach either across the staple line or via a gastro-gastric fistula (Figure 5). Contrast may show preferential flow either into the excluded stomach or into the Roux limb. As with a free leak, it is important to assess for communication with the excluded stomach early during the examination to avoid opacification of the excluded stomach via retrograde flow later in the study.25, 26
Figure 5.
Staple line dehiscence following RYGB - communication with the excluded stomach. (a) UGI spot image in the supine position shows contrast opacifying the gastric pouch (P) and jejunal limb (J). There is also a collection of contrast to the left of the anastomosis (*). Contrast is seen more distally within the excluded stomach (ES) and duodenum (D). (b) With rotation of the patient into the RPO position, an opacified tract across the gastric staple line is noted (arrow) allowing for communication between the gastric pouch (P) and excluded stomach (ES). RYGB, Roux-en-Y gastric bypass; UGI, upper gastro intestinal; RPO, right posterior oblique.
This complication is more difficult to differentiate with CT, since contrast can be seen within the excluded stomach via retrograde flow. If contrast is seen in the excluded stomach on CT but not in the duodenum or elsewhere in the biliopancreatic limb, the diagnosis can be suggested, and the diagnosis can be confirmed with UGI.26, 29
Stomal edema or stenosis
Early in the post-operative period, obstruction can occur as the result of hematoma or edema at either the gastrojejunal (Figure 6) and/or jejunojejunal anastomosis. Gastrojejunal anastomotic narrowing can lead to pouch and esophageal dilatation resulting in an increased risk of aspiration. When this occurs, delayed initiation of diet is beneficial. Edema or stenosis at the jejunojejunal anastomosis can lead to three patterns of small bowel obstruction (see below) including obstruction of the excluded limb, a potentially life-threatening complication. This represents a closed-loop type obstruction, as there is no natural means for the excluded limb to decompress; gastric perforation or necrosis can result. Percutaneous decompression of the excluded stomach can be used to temporarily alleviate the obstruction until the anastomotic edema and/or hematoma resolve.
Figure 6.
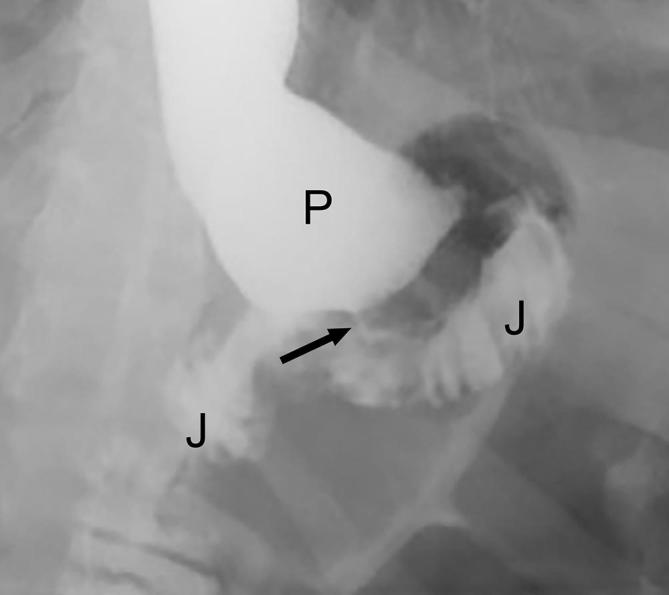
Gastrojejunal stomal narrowing following RYGB. Fluoroscopic UGI spot image acquired in the supine LPO position shows a dilated gastric pouch (P) with significant narrowing of the gastrojejunal anastomosis (arrow) due to post-operative edema. A small amount of contrast is noted opacifying the adjacent Roux jejunal limb (J). LPO, left posterior oblique; RYGB, Roux-en-Y gastric bypass; UGI, upper gastrointestinal.
Later in the post-operative period, stomal narrowing is usually a consequence of stenosis with anastomotic fibrosis. This occurs most frequently at the gastrojejunal anastomosis, and is seen in up to 10% of patients.25 Stenosis of the gastrojejunal stoma can lead to distention of the gastric pouch and esophagus, and delayed emptying of the pouch. This may be effectively treated with endoscopic dilatation. Stenosis of the jejunojejunal anastomosis on the other hand is much less common, occurring in <0.9% of patients, and can lead to small bowel obstruction. Jejunojejunal stenosis more commonly requires surgical revision.14, 15,30,31
Marginal ulcers are another cause of gastrojejunal anastomotic narrowing. Marginal ulcers develop due to increased exposure of the jejunal mucosa to gastric secretions, and are seen in up to 3% of RYGB patients. The incidence of marginal ulcers decreases with smaller pouch size, and marginal ulcers tend to respond well to medical treatment.15, 28,30,32 On UGI, marginal ulcers are characterized by small focal outpouchings with stasis of contrast and associated fold thickening along the anastomosis. Small marginal ulcers may be difficult to detect on UGI due to overlapping bowel segments, and may require diagnosis with endoscopy, which has a higher sensitivity for their detection.33
Small bowel obstruction (SBO)
Several entities can cause SBO in patients with RYGB, including adhesions, internal hernia, abdominal wall hernia, stomal stenosis and intussusception.34–36 Adhesions are the most common cause of SBO in patients following open RYGB, whereas internal hernia is the most common cause of SBO following laparoscopic RYGB, presumably due to decreased adhesions allowing for increased bowel motility as compared with open technique.15
Three main patterns of SBO following RYGB (types A, B and C), with different appearances on UGI and CT related to altered post-surgical anatomy and site of obstruction:37
Type A SBO consists of dilatation (Figure 7) of the alimentary (roux) limb, with decompressed biliopancreatic limb and distal common channel.
Type B SBO involves dilatation of the biliopancreatic limb only, with decompressed Roux limb and common channel. This represents a closed-loop obstruction and can lead to perforation of the excluded stomach if not recognized and treated in a timely fashion. On UGI, the obstructed biliopancreatic limb will not opacify with oral contrast, and the presence of the dilated excluded limb must be inferred by mass effect from dilated fluid-filled bowel on adjacent bowel loops. The diagnosis is more easily made with CT, by recognition of the dilated biliopancreatic limb and decompressed alimentary limb (Figure 8). The excluded stomach should normally be decompressed following RYGB.
Type C SBO involves obstruction of the common channel, resulting in dilatation of both the alimentary and biliopancreatic limbs.
Figure 7.
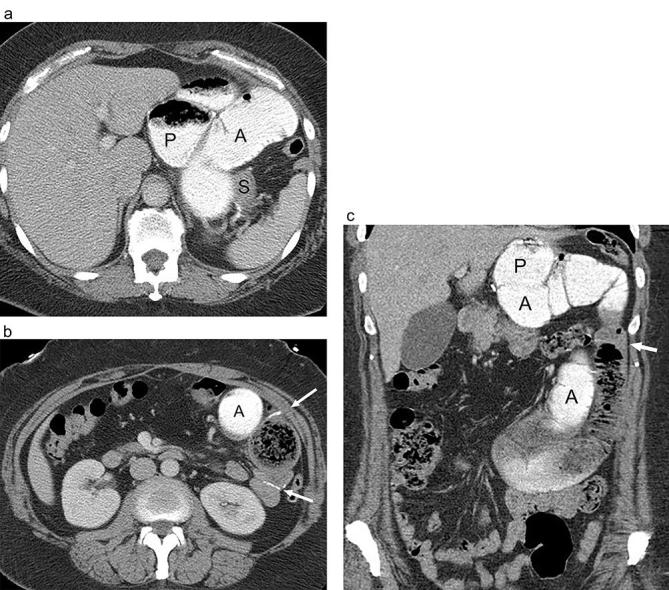
Small bowel obstruction of following RYGB with obstruction of the alimentary limb due to jejunojejunal stomal stenosis. (a and b) Axial and (c) coronal CT images following positive oral and i.v. contrast show marked dilatation of the gastric pouch (P) and Roux jejunal limb (A) (Alimentary limb) extending towards an abrupt transition at the jejunojejunal anastomosis (arrows) due to stomal stenosis and fibrosis. Distal small bowel is decompressed. The excluded stomach (S) is collapsed and is not opacified with luminal contrast. RYGB, Roux-en-Y gastric bypass.
Figure 8.
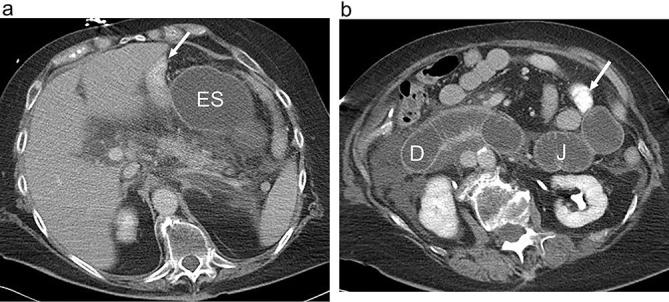
Small bowel obstruction following RYGB with obstruction of the excluded, biliopancreatic limb. (a and b). Axial CT images following positive oral and i.v. contrast show marked dilatation of the fluid-filled unopacified excluded stomach (ES), duodenum (D) and proximal jejunum (J) (biliopancreatic limb). The opacified alimentary Roux limb is not dilated (arrow). The RYGB anatomy and the jejunal limb must be recognized in order to make the appropriate diagnosis. Distal small bowel is also decompressed. Also note abdominal free fluid. RYGB, Roux-en-Y gastric bypass.
Internal hernia
Internal hernia occurs in up to 3% of patients following RYGB.38 It is a potentially fatal complication that can occur at any time and in multiple instances after surgery, but is generally considered a late complication.14, 15,22,31,38 In internal hernia, bowel herniates through a mesenteric defect which can lead to obstruction, bowel ischemia, infarction, or perforation.15, 30,31,38,39 There are characteristic mesenteric defects that form after RYGB and serve as sites for potential internal hernias, and include a defect created in the transverse mesocolon for a retrocolic Roux limb, a mesenteric defect near the jejunojejunal anastomosis and a defect posterior to the Roux limb (i.e. Petersen’s defect).22,30,31,38–40 However, internal hernia can occur through any potential defect.
Diagnosis of internal hernia is challenging, both clinically and radiologically and requires a high index of suspicion. Clinical symptoms are often intermittent and/or non-specific. Imaging findings can be difficult to identify, both on UGI and CT, and diagnosis requires knowledge of the expected post-surgical anatomy and detection of an abnormal or unexpected bowel configuration.36, 38,39 Internal hernia typically requires urgent surgical repair.
UGI with small bowel follow through (SBFT) and CT will both show a change in bowel configuration which often includes migration of an anastomotic suture line (Figure 9). Displaced small bowel loops in an internal hernia have a clustered appearance, and can displace other bowel loops. In up to 90% of cases of internal hernia, small bowel loops are displaced into the left abdomen, but can migrate anywhere in the abdomen or pelvis.22, 25 Most often after RYGB, the jejunojejunal anastomotic suture line is found in the left mid abdomen. With internal hernia, the suture line may migrate into various positions in the abdomen or pelvis, but is most often displaced into the left upper quadrant (Figure 9b).22 SBFT has the advantage of providing a dynamic picture of the bowel which may show bowel loops entering and exiting the clustered segment over the course of the examination. Stasis of contrast in the clustered loops may be seen.22, 30 Obstruction may or may not be present.22
Figure 9.
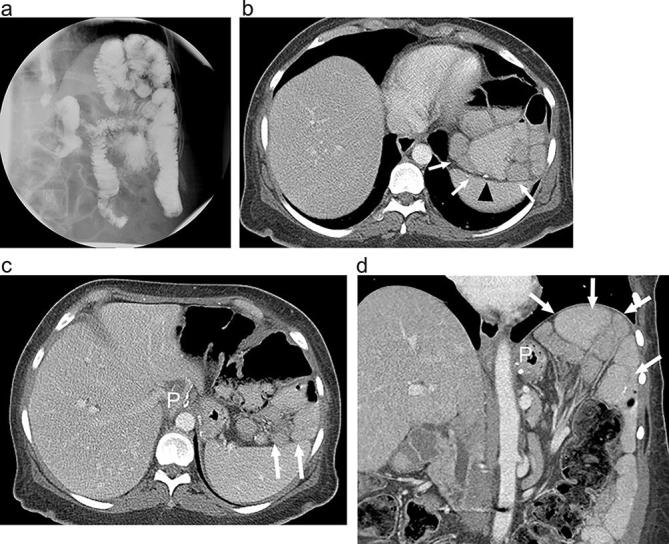
Internal hernia following RYGB on UGI and CT (a). Supine UGI imaging shows an atypical bowel configuration following RYGB with clustered, displaced small bowel loops (arrows), high in the left upper quadrant, above the gastric pouch (P) and abutting the diaphragm. Small bowel can be seen entering and exiting the clustered segment (arrowhead). (b and c) Axial and (d) coronal CT images with oral and i.v. contrast show RYGB anatomy with clustered displaced small bowel loops high in the left upper quadrant (arrows) above the gastric pouch (P). The jejunojejunal anastomosis is also displaced cephalad (arrowhead) due to internal hernia. Mesenteric vessels are tethered superiorly. At surgery the patient was found to have a large transverse mesocolic internal hernia. RYGB, Roux-en-Y gastric bypass; UGI, upper gastrointestinal.
In addition to detecting abnormal clustered positioning of the bowel, CT can reveal additional findings of internal hernia including swirling, stretching or displacement of mesenteric vessels and mesenteric engorgement and edema.25,40–42 Described findings of internal hernia on CT include displacement of small bowel into the left upper quadrant above the transverse mesocolon, high jejunojejunal anastomosis, clustered blood vessels in the left upper quadrant and the mesenteric swirl sign.41, 42
Laparoscopic Adjustable Gastric Banding
Gastric banding was first introduced in 1986, with a laparoscopic version available in the 1990s followed by an adjustable band made available in 2001. Gastric banding is a restrictive procedure that is technically simpler to perform than RYGB, and has the added benefit of being reversible. Early post-operative morbidity and weight loss are similar to other common bariatric procedures.43–46 More recent long-term studies, however, have shown low attrition of LAGB with a high proportion of patients requiring revision either due to complications or insufficient weight loss.47–49 As such, the popularity of this procedure has declined; LAGB represents only about 6% of all bariatric procedures performed in 2015 in the USA compared with 35% in 2011.12
The surgical technique consists of placing a silicone band around the proximal stomach, creating a small gastric pouch with a narrow stoma through the band, communicating with the remainder of the stomach (Figure 10).44, 50,51 The band is sutured to the adjacent stomach to prevent slipping. The inner portion of the band has an inflatable balloon cuff, connected with tubing to a subcutaneous port placed in the anterior abdominal wall. The port can be accessed percutaneously to inflate or deflate the balloon cuff, adjusting the size of the stoma. Adjustments can be made based on the patient’s weight loss curve or symptoms. Fluid can be added to narrow the stoma, or can be removed if the patient is experiencing obstructive symptoms. LAGB procedure involves no cutting, stapling or bypassing of the gastrointestinal tract and produces weight loss via a restrictive mechanism due to the small gastric pouch and narrow stoma.
Figure 10.
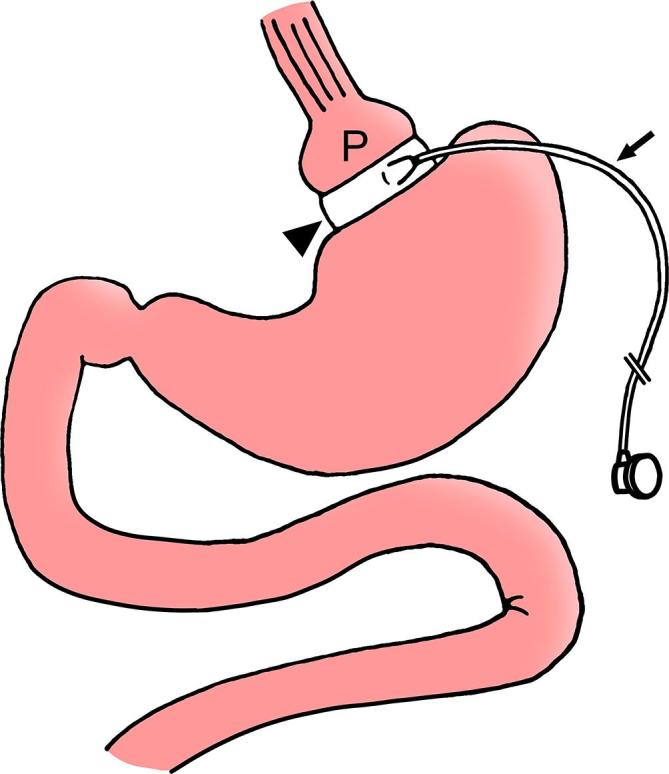
LAGB diagram. Diagram depicts a silicone band (arrowhead) placed around the upper stomach to create a small gastric pouch (P) and a narrow stoma through the band to communicate with the remainder of the stomach. Tubing (arrow) connects the band to a reservoir along the abdominal wall (not shown). The band has an inner inflatable balloon cuff. LAGB, laparoscopic adjustable gastric banding.
Expected imaging appearance following LAGB
Radiography
The band is radiopaque, and should be seen in the left epigastric region (Figure 11a). The angle of the long axis of the band to vertical with the patient in the supine position is known as the phi angle and should measure between 4 and 58 degrees.52 The tubing, if radiopaque, should be assessed for any discontinuity or kinking. The location and position of the injectable reservoir should also be evaluated on abdominal X-rays in this patient population.
Figure 11.
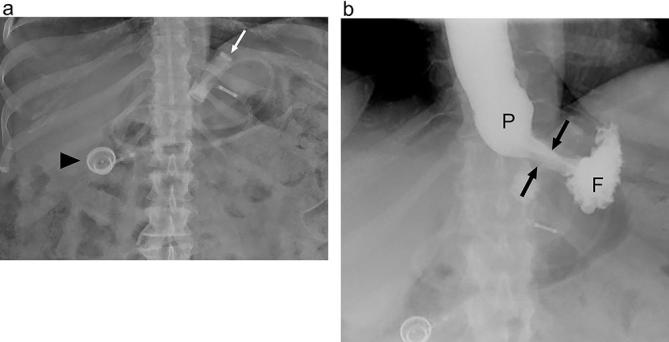
Expected appearance following LAGB on UGI (a) Supine radiograph shows the expected appearance following LAGB with a band in the left epigastric region (white arrow). Radio-opaque connecting tubing can be assessed as it extends to the injectable port (arrowhead). (b) Supine UGI image acquired while the patient is drinking shows a small pouch (P) with a narrow stoma (arrows) through the band and communicating with the gastric fundus (F). Note that in order to optimally asses the stoma the band must appear linear rather than as a ring shape. LAGB, laparoscopic adjustable gastric banding; UGI, upper gastrointestinal.
UGI examination
UGI examination is useful in the early post-operative period to evaluate band position and for signs of leak or obstruction. The band and reservoir should be assessed on the scout radiograph, evaluating device position and integrity. Before giving oral contrast, the patient should be positioned so that the band is seen in profile (appearing as a straight line rather than as a ring or O shape), most often achieved with the patient in straight anteroposterior or slight right posterior oblique position. This will allow for optimal visualization of the stoma (Figure 11b). If the patient is not properly positioned prior to administering contrast, rapid opacification of the fundus will obscure the stoma through the band. Supine positioning may allow for more optimal distension of the stoma. However, upright positioning may be necessary or beneficial, especially if there is any degree of obstruction due to the band. Early post-operative imaging should be performed using water soluble contrast, and if no leak is seen this can be followed by barium. In the late post-operative phase, barium can be used initially.50, 53
Initially, a focused examination of the post-operative anatomy is performed by following contrast through the distal esophagus, gastric pouch, stoma and remainder of the stomach. Assessment with the patient in the upright position may provide beneficial information regarding motility issues and pouch emptying through the stoma. Rapid sequence fluoroscopic imaging is helpful to acquire images of the maximally distended pouch and stoma. The ideal stoma size has been reported to be between 3 and 5 mm.53 Esophageal caliber and motility, gastric pouch size and distension can be assessed fluoroscopically.
Computerized tomography
CT may be used after LAGB to evaluate for a source of infection and to delineate soft tissue changes associated with the device. Care should be taken to include the entire overlying soft tissues in the field of view, to avoid excluding a portion of the tubing or reservoir. Proper positioning of the band around the proximal stomach (Figure 12) and the reservoir in the abdominal wall can be identified, and the tubing should be followed from the band to the reservoir.
Figure 12.
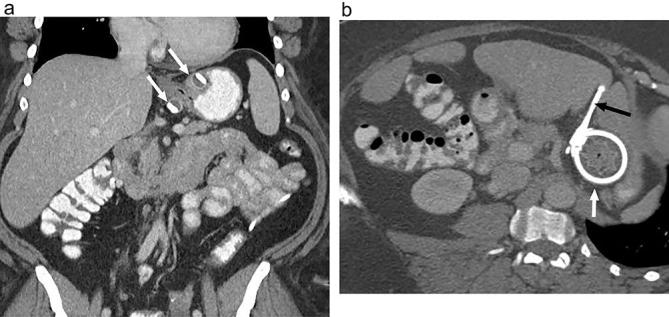
Expected appearance following LAGB on CT (a) Coronal and (b) axial CT images with oral and i.v. contrast shows the inflatable balloon cuff of the band (white arrows) positioned around the proximal stomach. The connecting tubing is partially imaged (black arrow). LAGB, laparoscopic adjustable gastric banding.
Band adjustment
Periodic band adjustments are necessary following LAGB to achieve optimal weight loss and avoid symptoms of obstruction, with an average of three adjustments done per patient.52 Band adjustment can be performed with fluoroscopy, where the oral administration of contrast before and after adjustment can be used to ensure adequate change in stomal caliber and avoid excessive stomal narrowing and obstruction.50, 51,54
The subcutaneous port can be localized fluoroscopically, and is accessed with a 20- to 22-gauge non-coring needle. With a saline-filled syringe attached, the needle is advanced until it hits the back of the reservoir. Saline should be easily injected and withdrawn to confirm appropriate placement. The full volume of saline can be withdrawn into the syringe to determine the total volume, followed by refilling and adjusting the system. The volume of saline instilled or removed should be documented.52, 54 Oral contrast is administered after band adjustment to confirm adequate stomal narrowing without obstruction. The amount of adjustment should be based on the patient’s weight loss curve and symptomatology, and can be decided in conjunction with the surgeon depending on local practice.
LAGB complications
LAGB has minimal perioperative mortality, and early post-operative complications are rare. These include gastric perforation (<0.5% of patients), improper band positioning at initial surgery, early band slippage (<0.1% of patients) and acute stomal obstruction (<1.4% of patients).43–45,52,53 Dysphagia and esophageal reflux are common until the patient’s dietary habits change.
Most complications after LAGB occur in the late post-operative phase. Up to 53–71% of patients ultimately will lose their band, either by removal or conversion to RYGB.48, 49 The most common late complications other than inadequate weight loss include pouch dilatation and band slippage, port or tube leak and band migration.48, 49,51,52 Gastric necrosis is a rare late complication that can be caused by band slippage and strangulation.43–45
Pouch dilatation
Pouch dilatation is a common complication that occurs in up to 25% of patients after LAGB, although incidence has decreased with newer modifications to surgical technique.55 Pouch dilatation can lead to failed weight loss due to insufficient restriction. Although pouch dilatation can be multifactorial, it is useful to categorize pouch dilatation as occurring with a normal or widened stoma, a narrow stoma, or with band slippage.
When pouch dilatation occurs with a normal or widened stoma, it is typically the result of dietary non-compliance and chronic overfilling of the pouch. Imaging will show a dilated pouch with concentric appearance and widely patent stoma. Treatment may require nutritional counseling.50, 53,56
In the case of pouch dilatation with a narrowed stoma, the pouch should be examined for concentric vs eccentric morphology. Concentric pouch dilatation with a narrow stoma (Figure 13) is usually caused by overinflation of the balloon cuff at adjustment, or rarely by focal weakness in the balloon cuff (with eccentric stomal narrowing). Acute symptoms can occur including dysphagia, esophageal dysmotility, vomiting and obstruction. The balloon cuff should be immediately deflated to alleviate symptoms and prevent further complications, including band slippage.50, 52 Eccentric pouch dilatation with a narrow stoma is indicative of band slippage (Figure 14), discussed below.
Figure 13.
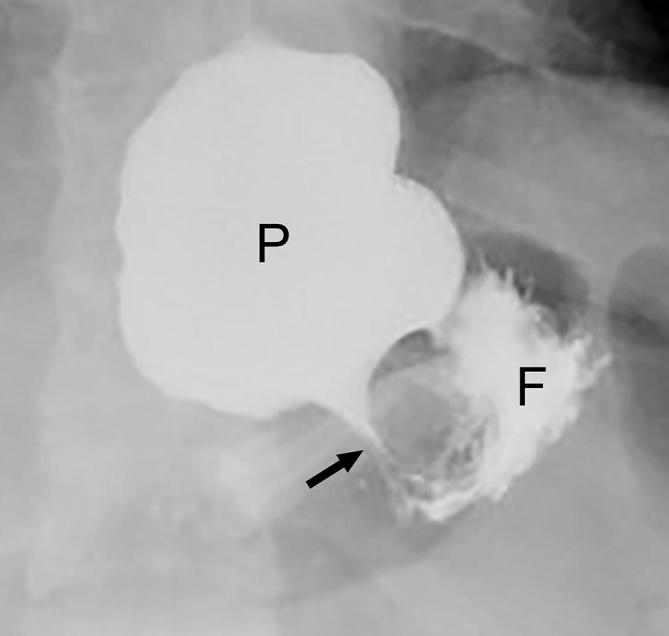
Concentric pouch dilatation following LAGB with a narrow stoma. UGI image acquired during drinking shows a concentrically dilated pouch (P) with a tight stoma (arrow) through the band. A small amount of contrast is seen in the gastric fundus (F). LAGB, laparoscopic adjustable gastric banding; UGI, upper gastrointestinal.
Figure 14.
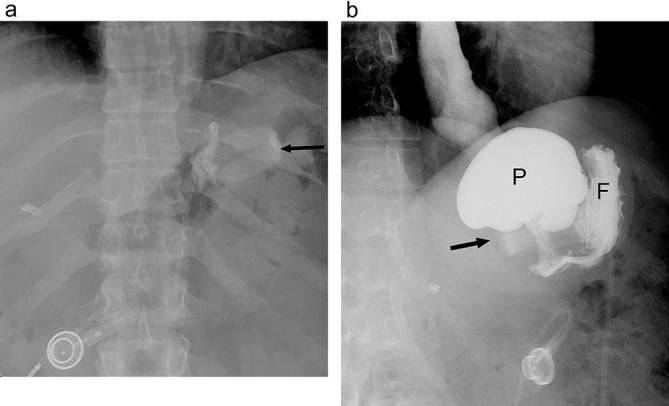
Band slippage with fundic herniation following LAGB. (a) A supine radiograph shows a change in the configuration of the gastric band (arrow) as compared with a prior post-operative study (not shown). It is now inferiorly located and horizontal in configuration. (b) UGI image shows an eccentrically dilated gastric pouch (P) above the inferior, horizontal band (arrow) due to band slippage. A small amount of contrast is seen in the gastric fundus (F). LAGB, laparoscopic adjustable gastric banding; UGI, upper gastrointestinal.
Pouch dilatation with band slippage
Pouch dilatation can occur because of band slippage, when the band becomes dislodged from its original position and a portion of stomach herniates above the band. This leads to an eccentrically dilated pouch with a narrow stoma. Band slippage has been reported in as many as 24% of patients, although incidence varies depending on surgical technique.44,53,55–57 It is considered a late complication and risk factors include overeating with overdistension of the pouch, excessive vomiting and overinflation of the band.
Three types of band slippage have been described – anterior, posterior and concentric slippage with complete displacement of the band distally. All types of slippage lead to similar consequences; if untreated, band slippage can progress with increased pouch dilatation and gastric herniation above the band. Potential sequelae include acute gastric obstruction, gastric volvulus, ischemia, infarction, perforation and hemorrhage. The most severe consequence is necrosis of the gastric pouch. Early detection of band slippage is essential to avoid severe complications. After diagnosis, the band should be immediately deflated.58
Radiography of band slippage will show downward displacement of the band with increased space between the band and the left hemidiaphragm (Figure 14a). With progressive slippage the band may rotate along its horizontal axis, and in the AP projection the anterior and posterior portions of the band will no longer overlap resulting in an O shape configuration, termed the “O-sign”.59 Tilting of the band in the sagittal plane will cause an abnormal phi angle. An air-fluid level may become visible in the dilated pouch. Ingestion of contrast will demonstrate eccentric dilatation of the pouch (Figure 14b) and tight stoma through the band. In posterior slippage, the posterior gastric wall herniated up through the band, and in anterior slippage, the anterior portion of the band displaced downward over the anterior wall of the stomach.50, 52
Intragastric erosion and band migration
The band itself can erode through the gastric wall partially or completely into the lumen, and can even migrate distally and cause downstream obstruction. Band erosion occurs in approximately 1% of patients, with incidence increasing after longer follow-up.50, 60,61 Risk factors include NSAID use, excessive vomiting, or overinflation of the band causing excessive pressure on the stomach.50 In the setting of erosion, the band should be removed and the stomach repaired to avoid hemorrhage and infection.47
On UGI, the band will appear as an intraluminal filling defect, with contrast surrounding the intragastric portion of the band (Figure 15).47, 50 Contrast may or may not pass through the stoma, depending on the degree of erosion. Erosion can be visible on CT if the band is completely intraluminal or if contrast can be seen surrounding the band. Associated abscess, surrounding inflammatory change, or peritonitis may be seen.50
Figure 15.
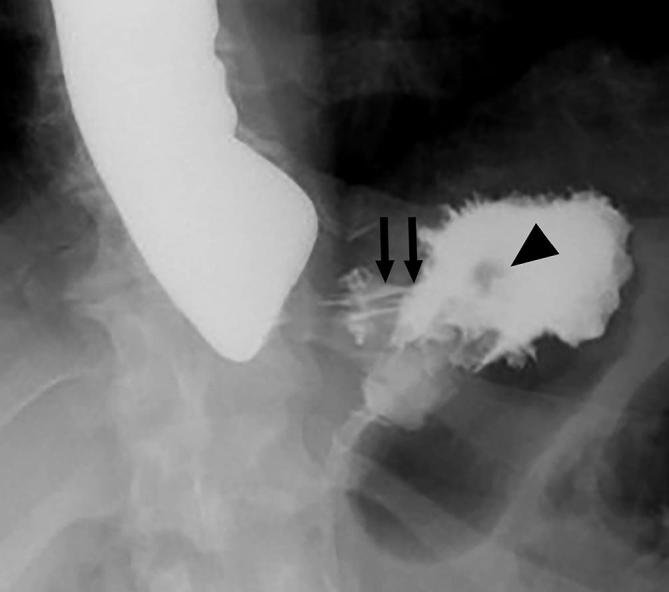
Band erosion following LAGB. UGI image following LAGB acquired with the patient drinking shows contrast extending superiorly and along the left aspect of the band (arrows), partially surrounding the band rather than opacifying a stoma through the band. The band is seen as a filling defect (arrowhead). This is due to intragastric erosion of the band. LAGB, laparoscopic adjustable gastric banding; UGI, upper gastrointestinal.
Device-related complications
Malfunction of the indwelling devices including the band, tubing and reservoir have been reported in 1.4 to 26% of patients, in part depending on length of follow-up, and these complications tend to require surgical repair.50 The most common device-related complication is infection of the port, tubing or band, occurring in up to 6% of patients.48, 50 The reservoir may migrate through the abdominal wall soft tissues or become inverted in up to 3% of patients, preventing adjustment of the band.48, 50
A defect in the reservoir, tubing, or band can cause fluid loss from the system and band deflation. Fluid loss can also be caused by accessing the reservoir with the incorrect needle. This will lead to stomal widening, with change in the patient’s dietary habits and poor weight loss. To detect a leak from the band system, a designated volume of saline can be injected into the reservoir and measuring the volume of saline returned for discrepancy. Water soluble contrast can also be injected into the system to localize a leak, which can help direct surgical intervention.51, 52,55
Sleeve Gastrectomy
SG, or gastric sleeve, is a restrictive bariatric procedure. This procedure was originally performed as a first step of a stage procedure, prior to RGB or duodenal switch, particularly in high-risk obese patients. It was found that SG alone achieved high rates of weight loss,62, 63 and has since been performed as a standalone procedure. SG has rapidly increased in popularity in recent years and is now the most commonly performed bariatric surgery in the USA and worldwide, representing 54% of all bariatric surgeries performed in the USA in 2015 compared with 18% in 2011.11, 12 It is usually performed laparoscopically and is relatively technically simple, not requiring placement of a prosthesis, creation of an anastomosis or interventional adjustments. The amount of excess weight loss is comparable to other bariatric procedures.63–65
In SG, the stomach is divided longitudinally with removal of 70–80% of the stomach including the greater curvature of the gastric fundus, body, and proximal antrum, leaving the pylorus intact (Figure 16). This results in a long, narrow stomach and the procedure is irreversible. The size of the gastric remnant is calibrated around a bougie tube placed in the stomach at the time of resection, leaving behind a narrow tube-shaped stomach.62, 66,67
Figure 16.
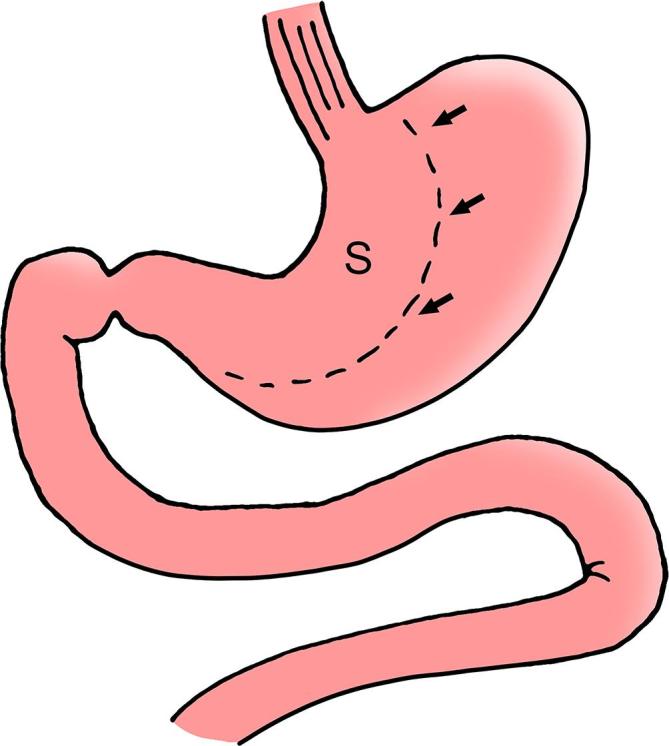
SG diagram depicts the gastric sleeve (S) with approximately 70% of the stomach resected along the greater curvature of the stomach and with relative sparing of the antrum. Note the resection margin (arrows). The pylorus and duodenum are left intact. SG, sleeve gastrectomy.
Expected imaging appearance following SG
UGI examinationSG
Imaging is not routinely performed after SG surgery. UGI with water-soluble contrast may be used when there is concern for complication in the early post-operative period, including suspected leak or obstruction.68 In the later post-operative period, barium can be used. On UGI, the post-operative stomach has a narrow elongated tubular appearance, with relative preservation of the antrum (Figure 17). Gastric peristalsis is usually diminished or absent. Leak will almost always occur in the proximal stomach involving the proximal third of the staple line.69
Figure 17.
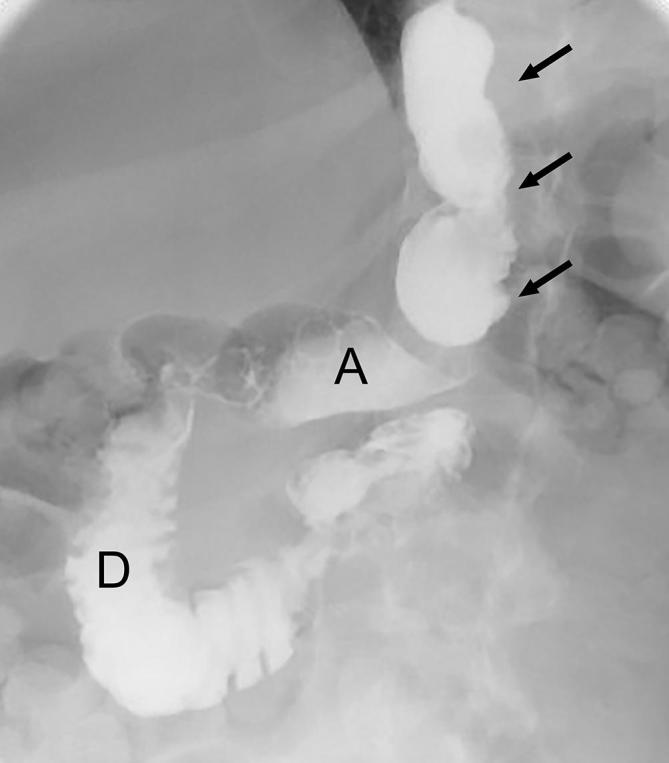
Expected appearance following SG on UGI. Supine UGI image show the narrowed, tubular configuration of the gastric sleeve (arrows) with an intact distal antrum (A), pylorus and duodenum (D). SG, sleeve gastrectomy; UGI, upper gastrointestinal.
Computerized tomography
CT is useful in cases of suspected post-operative abscess, splenic infarction, obstruction, fistula or leak. With CT, a staple line is visible along the length of the greater curvature of the stomach, and the stomach is small in caliber along its long axis with a tubular configuration (Figure 18). The duodenum and small bowel have a normal appearance, and there is no left upper quadrant roux limb or other gastroenteric anastomosis.
Figure 18.
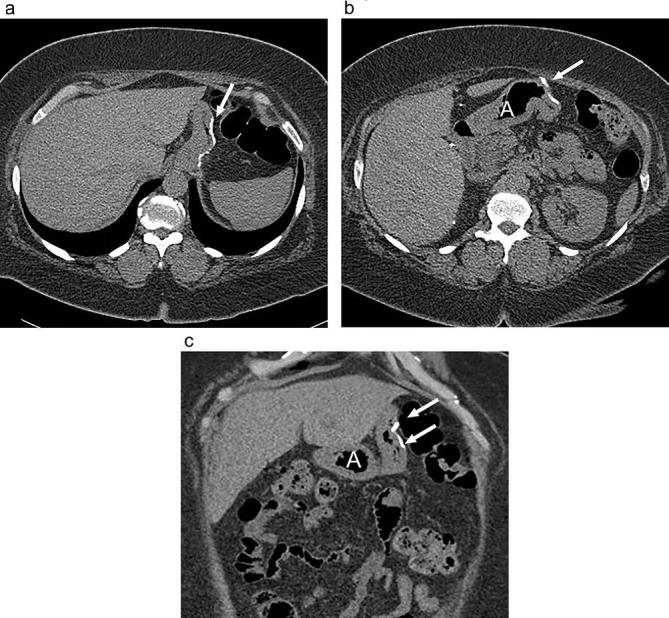
Expected appearance following sleeve gastrectomy on CT (a and b) axial and (c) coronal non-contrast CT images shows a narrowed stomach with suture along the resected greater curvature (arrows) and prominence of mesenteric fat in the expected location of the remainder of the stomach. Note the intact antrum (A).
SG complications
Leak
The primary early post-operative complication in SG is leak, occurring in 1 to 8% of cases, leading to significant morbidity.69–71 The leak most commonly arises from the proximal staple line near the gastroesophageal junction (Figure 19), and can result from mechanical failure or ischemia. Ischemia is hypothesized to be related to relatively lower blood perfusion in this location, a condition found more frequently in obese patients making them particularly susceptible.69, 72 Clinical signs of leak may include tachycardia, fever and abdominal pain, but are non-specific.73, 74
Figure 19.
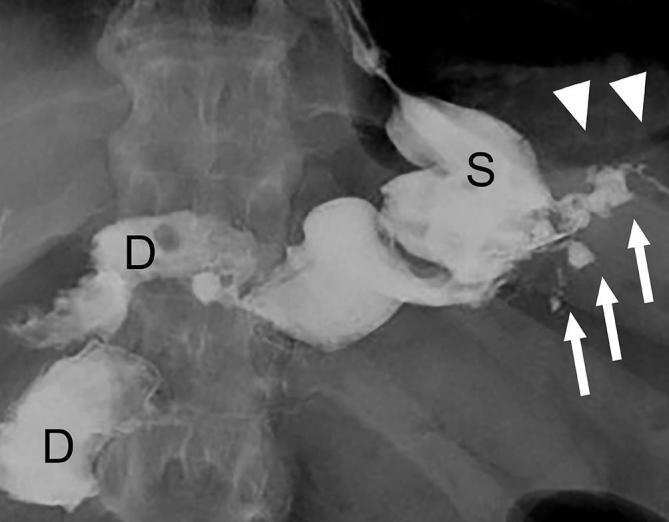
Small leak on UGI following SG. UGI image following SG shows a small amount of extravasated, extraluminal contrast (arrows) extending left laterally from the proximal gastric sleeve (S) in this recently post-operative patient. Also note extraluminal gas in the left upper quadrant (arrowheads). D, duodenum; SG, sleeve gastrectomy; UGI, upper gastrointestinal.
Radiological evaluation for leak can be performed with UGI or CT. The difference in sensitivity between these modalities is not well studied in SG; however, CT can provide additional information such as the presence of a hematoma or abscess (Figure 20). UGI allows for more optimal distension of the post-operative anatomy. With a leak, imaging will reveal extension of ingested contrast beyond the gastric lumen, and will most often occur at the proximal staple line near the incisura. The presence of a small proximal gastric pouch created by preservation of a part of the fundus at surgery can mimic the appearance of a leak and lead to a false positive leak.75 If a surgical drain is in place, a leak can be confirmed if contrast is seen to opacify the drain. Conservative treatment of leaks can be performed by endoscopic placement of a covered stent across the leak, and percutaneous drainage of the leak or any undrained collection (Figure 21).70,73,75–77
Figure 20.
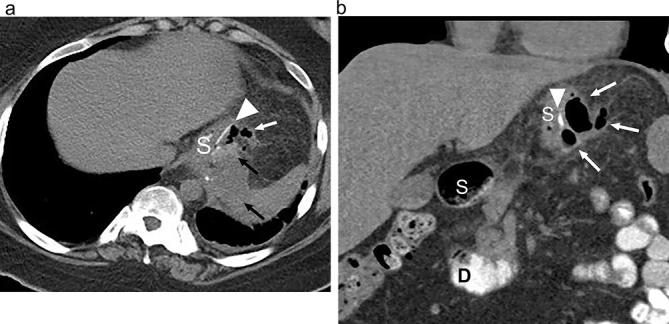
Leak on CT following SG. (a) Axial and (b) coronal CT images following positive oral contrast administration show an ill-defined fluid collection (black arrows) and extraluminal gas (white arrows) in the left upper quadrant adjacent to the suture line (arrowhead) of the proximal gastric sleeve (S). The fluid collection is of subtle increased density anteriorly due to a small amount of extravasation administered oral contrast. D, duodenum; SG, sleeve gastrectomy.
Figure 21.
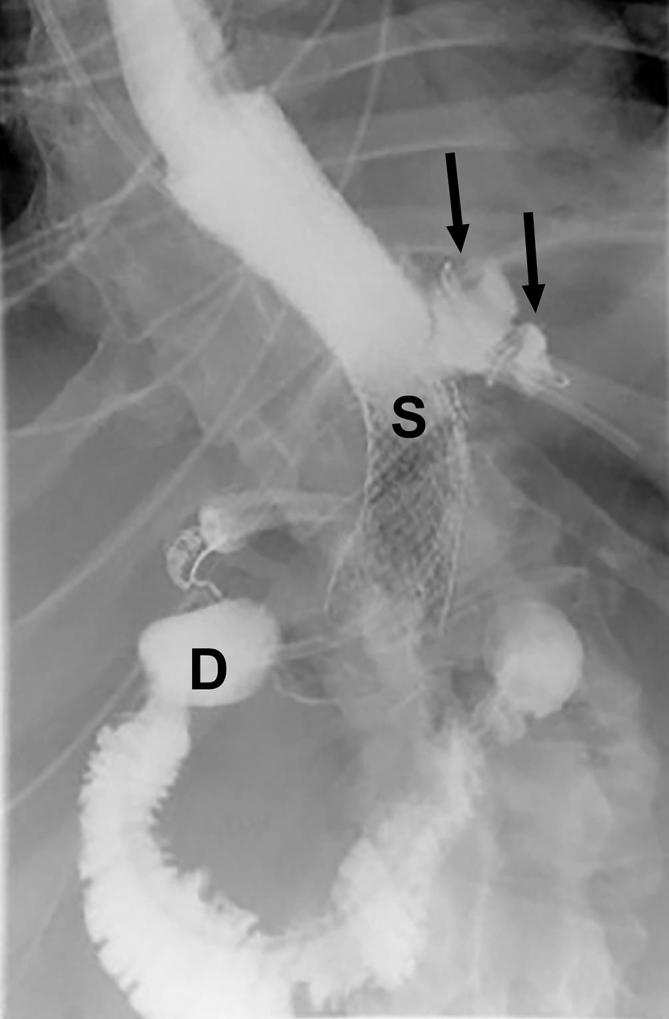
Leak on UGI following SG with endoscopic stent placement. UGI image following sleeve gastrectomy shows a stent placed in the distal esophagus and across the gastric sleeve (S). There is a persistent leak (arrows) from the proximal sleeve extending into the left upper quadrant despite stent placemen. A drainage catheter is coiled in the extraluminal collection (arrows). D, duodenum; SG, sleeve gastrectomy; UGI, upper gastrointestinal.
Strictures
Stricture of the sleeve is a rare complication, reported to occur in up to 3.9% of patients.78 Focal sleeve narrowing can occur early post-operatively due to edema or ischemia, but fixed narrowing is more commonly seen in the late post-operative course due to scarring and fibrosis along the staple line. Stricture can lead to pouch dilatation and obstruction. Clinical symptoms include nausea, vomiting, dysphagia and epigastric pain. As with other restrictive procedures, failed weight loss may also be a consequence of pouch dilatation.78–81
UGI is more effective than CT to detect stricture, and will reveal significant narrowing of the sleeve lumen with delayed or no passage of contrast. In addition, the proximal gastric pouch may be dilated (Figure 22).68, 81 The primary treatment for a stricture is serial endoscopic balloon dilatation. If a stenosis is discovered very early in the post-operative period when dilatation may disrupt the staple line, a covered stent can be placed to facilitate nutrition. When conservative measures fail, definitive treatment often requires surgical conversion to RYGB.78–80,82–84
Figure 22.
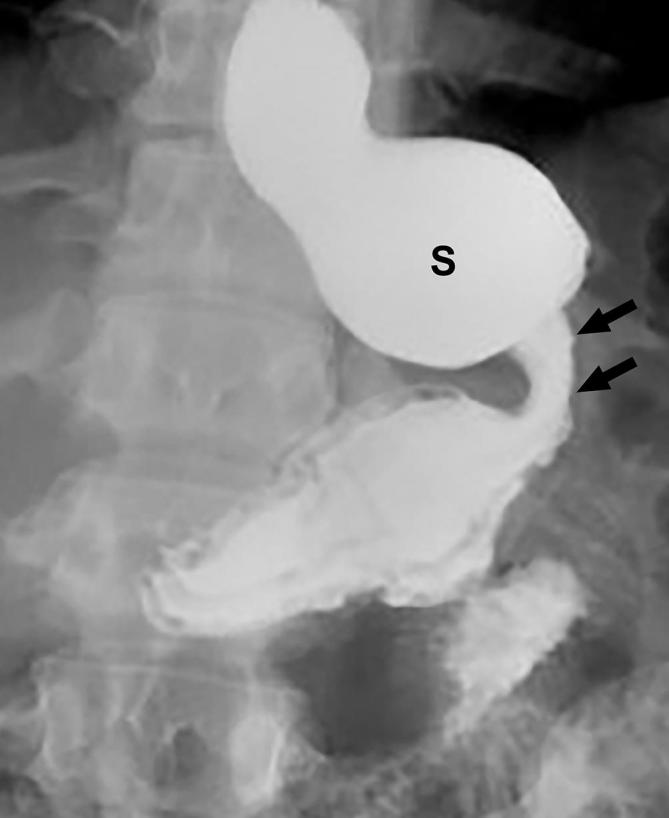
Stricture and proximal pouch dilatation following SG. Supine UGI image following SG shows a focal stricture in the mid sleeve (arrow) with proximal dilatation of the sleeve (S). SG, sleeve gastrectomy; UGI, upper gastrointestinal.
Additional reported complications following SG include gastric motility problems, bleeding, infection and splenic infarction/ischemia.62, 82,85 Splenic infarction occurs due to compromise of vascular supply to the spleen after ligation of short gastric arteries.
Conclusion
As obesity rates continue to increase across the globe, bariatric surgery continues to become more commonplace. The three most common bariatric procedures currently performed are the RYGB, LAGB and SG. The radiologist must be familiar with these surgeries including the expected post-operative anatomy, important complications and potential imaging pitfalls. Patients with complications after bariatric surgery can have non-specific clinical presentations, and an understanding of the expected post-operative anatomy and radiological examination techniques is essential to arrive at an accurate diagnosis.
Contributor Information
Ryan D Clayton, Email: rdclayto@gmail.com; ryan.clayton@vcuhealth.org.
Laura R Carucci, Email: laura.carucci@vcuhealth.org.
REFERENCES
- 1.NCD Risk Factor Collaboration Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017; 6736: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spieker EA, Pyzocha N. Economic Impact of Obesity. Prim Care 2016; 43: 83–95. doi: 10.1016/j.pop.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 3.Di Cesare M, Bentham J, Stevens GA, Zhou B, Danaei G, Lu Y, et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet . 2016; 387: 1377–96. doi: 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel, 2013 Expert Panel Report: Guidelines (2013) for the management of overweight and obesity in adults. Obesity 2014; 22 Suppl 2(Suppl 2): S41–10. doi: 10.1002/oby.20660 [DOI] [PubMed] [Google Scholar]
- 5.Ravussin E, Ryan DH. . Expert panel report: guidelines (2013) for the management of overweight and obesity in adults. Obesity 2014; 22(Suppl 2): S41–10. doi: 10.1002/oby.20660 [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord 1998; 22: 39–47. doi: 10.1038/sj.ijo.0800541 [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 2015; 219: 1–8. [PubMed] [Google Scholar]
- 8.World Health Organization. Obesity and overweight [Internet]. 2017. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/ [cited 2017 Dec 12].
- 9.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ 2012; 31: 219–30. doi: 10.1016/j.jhealeco.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Lehnert T, Sonntag D, Konnopka A, Riedel-Heller S, König HH. Economic costs of overweight and obesity. Best Pract Res Clin Endocrinol Metab 2013; 27: 105–15. doi: 10.1016/j.beem.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 11.Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, et al. Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes Surg 2017; 27: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponce J, DeMaria EJ, Nguyen NT, Hutter M, Sudan R, Morton JM. American society for metabolic and bariatric surgery estimation of bariatric surgery procedures in 2015 and surgeon workforce in the United States. Surg Obes Relat Dis . 2016; 12: 1637–9. doi: 10.1016/j.soard.2016.08.488 [DOI] [PubMed] [Google Scholar]
- 13.Fisher BL, Schauer P. Medical and surgical options in the treatment of severe obesity. Am J Surg 2002; 184:S9–S16. doi: 10.1016/S0002-9610(02)01173-X [DOI] [PubMed] [Google Scholar]
- 14.DeMaria EJ, Sugerman HJ, Kellum JM, Meador JG, Wolfe LG. Results of 281 consecutive total laparoscopic roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg 2002; 235: 640–7. doi: 10.1097/00000658-200205000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higa KD, Boone KB, Ho T. Complications of the laparoscopic Roux-en-Y gastric bypass: 1,040 patients-what have we learned? Obes Surg 2000; 10: 509–13. doi: 10.1381/096089200321593706 [DOI] [PubMed] [Google Scholar]
- 16.Schauer PR, Ikramuddin S, Gourash W, Ramanathan R, Luketich J. Outcomes after laparoscopic roux-en-Y gastric bypass for morbid obesity. Ann Surg . 2000; 232: 515–29. doi: 10.1097/00000658-200010000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schauer PR, Ikramuddin S, Hamad G, Eid GM, Mattar S, Cottam D, et al. Laparoscopic gastric bypass surgery: current technique. J Laparoendosc Adv Surg Tech A 2003; 13: 229–39. doi: 10.1089/109264203322333557 [DOI] [PubMed] [Google Scholar]
- 18.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab . 2004; 89: 2608–15[Internet]. doi: 10.1210/jc.2004-0433 [DOI] [PubMed] [Google Scholar]
- 19.Carucci LR, Turner MA, Conklin RC, DeMaria EJ, Kellum JM, Sugerman HJ. Roux-en-Y gastric bypass surgery for morbid obesity: evaluation of postoperative extraluminal leaks with upper gastrointestinal series. Radiology . 2006; 238: 119–27. doi: 10.1148/radiol.2381041557 [DOI] [PubMed] [Google Scholar]
- 20.Carucci LR, Turner MA, Yu J. Imaging evaluation following roux-en-Y gastric bypass surgery for morbid obesity. Radiographics . 2008; 45: 247–60. [DOI] [PubMed] [Google Scholar]
- 21.Carucci LR. Imaging obese patients: problems and solutions. Abdom Imaging 2013; 38: 630–46. doi: 10.1007/s00261-012-9959-2 [DOI] [PubMed] [Google Scholar]
- 22.Carucci LR, Turner MA, Shaylor SD, Carucci TMA, Shaylor SD. Internal hernia following roux-en-Y gastric bypass surgery for morbid obesity: evaluation of radiographic findings at small-bowel examination. Radiology . 2009; 251: 762–70. doi: 10.1148/radiol.2513081544 [DOI] [PubMed] [Google Scholar]
- 23.Levine MS, Carucci LR. Imaging of bariatric surgery: normal anatomy and postoperative complications. Radiology . 2014; 270: 327–41. doi: 10.1148/radiol.13122520 [DOI] [PubMed] [Google Scholar]
- 24.Buckwalter JA, Herbst CA. Leaks occurring after gastric bariatric operations. Surgery 1988; 103: 156–60. [PubMed] [Google Scholar]
- 25.Carucci LR, Turner MA, Yu J. Imaging evaluation following roux-en-Y gastric bypass surgery for morbid obesity. Radiol Clin North Am 2007; 45: 247–60. doi: 10.1016/j.rcl.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 26.Carucci LR, Conklin RC, Turner MA. Roux-en-Y gastric bypass surgery for morbid obesity: evaluation of leak into excluded stomach with upper gastrointestinal examination. Radiology . 2008; 248: 504–10. doi: 10.1148/radiol.2482070926 [DOI] [PubMed] [Google Scholar]
- 27.Buckwalter JA, Herbst CA. Complications of gastric bypass for morbid obesity. Am J Surg 1980; 139: 55–60. doi: 10.1016/0002-9610(80)90230-5 [DOI] [PubMed] [Google Scholar]
- 28.Goodman P, Halpert RD. Radiological evaluation of gastric stapling procedures for morbid obesity. Crit Rev Diagn Imaging 1991; 32: 37–67. [PubMed] [Google Scholar]
- 29.Carucci LR, Turner MA. Imaging following bariatric procedures: roux-en-Y gastric bypass, gastric sleeve, and biliopancreatic diversion. Abdom Imaging 2012; 37: 697–711. doi: 10.1007/s00261-012-9860-z [DOI] [PubMed] [Google Scholar]
- 30.Blachar A, Federle MP. Gastrointestinal complications of laparoscopic roux-en-Y gastric bypass surgery in patients who are morbidly obese: findings on radiography and CT. Am J Roentgenol 2002; 179:1437–42. doi: 10.2214/ajr.179.6.1791437 [DOI] [PubMed] [Google Scholar]
- 31.Blachar A, Federle MP, Pealer KM, Ikramuddin S, Schauer PR. Gastrointestinal complications of laparoscopic roux-en-Y gastric bypass surgery: clinical and imaging findings. Radiology . 2002; 223: 625–32. doi: 10.1148/radiol.2233011323 [DOI] [PubMed] [Google Scholar]
- 32.Fobi MA, Lee H, Holness R, Cabinda D. Gastric bypass operation for obesity. World J Surg 1998; 22: 925–35. doi: 10.1007/s002689900496 [DOI] [PubMed] [Google Scholar]
- 33.Schirmer BD, Meyers WC, Hanks JB, Kortz WJ, Jones RS, Postlethwait RW, et al. A difficult surgical problem. Ann Surg 1982; 195: 653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Champion JK, Williams M. Small bowel obstruction and internal hernias after laparoscopic Roux-en-Y gastric bypass. Obes Surg 2003; 13: 596–600. doi: 10.1381/096089203322190808 [DOI] [PubMed] [Google Scholar]
- 35.Chandler RC, Srinivas G, Chintapalli KN, Schwesinger WH, Prasad SR. Imaging in bariatric surgery: a guide to postsurgical anatomy and common complications. AJR Am J Roentgenol 2008; 190: 122–35. doi: 10.2214/AJR.07.2134 [DOI] [PubMed] [Google Scholar]
- 36.Filip JE, Mattar SG, Bowers SP, Smith CD. Internal hernia formation after laparoscopic roux-en-Y gastric bypass for morbid obesity. Am Surg 2002; 68: 640–3. [PubMed] [Google Scholar]
- 37.Tucker ON, Escalante-Tattersfield T, Szomstein S, Rosenthal RJ. The ABC System: a simplified classification system for small bowel obstruction after laparoscopic roux-en-Y gastric bypass. Obes Surg 2007; 17: 1549–54. doi: 10.1007/s11695-007-9273-1 [DOI] [PubMed] [Google Scholar]
- 38.Higa KD, Ho T, Boone KB. Internal hernias after laparoscopic roux-en-Y gastric bypass: incidence, treatment and prevention. Obes Surg . 2003; 13: 350–4. doi: 10.1381/096089203765887642 [DOI] [PubMed] [Google Scholar]
- 39.Iannelli A, Buratti MS, Novellas S, Dahman M, Amor IB, Sejor E, et al. Internal hernia as a complication of laparoscopic roux-en-Y gastric bypass. Obes Surg 2007; 17: 1283–6. doi: 10.1007/s11695-007-9229-5 [DOI] [PubMed] [Google Scholar]
- 40.Blachar A, Federle MP, Dodson SF. Internal hernia: clinical and imaging findings in 17 patients with emphasis on CT criteria. Radiology . 2001; 218: 68–74. doi: 10.1148/radiology.218.1.r01ja5368 [DOI] [PubMed] [Google Scholar]
- 41.Lockhart ME, Tessler FN, Canon CL, Smith JK, Larrison MC, Fineberg NS, et al. Internal hernia after gastric bypass: sensitivity and specificity of seven CT signs with surgical correlation and controls. AJR Am J Roentgenol 2007; 188: 745–50. doi: 10.2214/AJR.06.0541 [DOI] [PubMed] [Google Scholar]
- 42.Reddy SA, Yang C, McGinnis LA, Seggerman RE, Garza E, Ford KL. Diagnosis of transmesocolic internal hernia as a complication of retrocolic gastric bypass: CT imaging criteria. AJR Am J Roentgenol 2007; 189: 52–5. doi: 10.2214/AJR.06.0898 [DOI] [PubMed] [Google Scholar]
- 43.Zinzindohoue F, Chevallier JM, Douard R, Elian N, Ferraz JM, Blanche JP, et al. Laparoscopic gastric banding: a minimally invasive surgical treatment for morbid obesity: prospective study of 500 consecutive patients. Ann Surg . 2003; 237: 1–9. doi: 10.1097/01.SLA.0000041162.55494.4C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chevallier JM, Zinzindohoué F, Douard R, Blanche JP, Berta JL, Altman JJ, et al. Complications after laparoscopic adjustable gastric banding for morbid obesity: experience with 1,000 patients over 7 years. Obes Surg . 2004; 14: 407–14. doi: 10.1381/096089204322917954 [DOI] [PubMed] [Google Scholar]
- 45.DeMaria EJ, Jamal MK. Laparoscopic adjustable gastric banding: evolving clinical experience. Surg Clin North Am 2005; 85: 773–87. doi: 10.1016/j.suc.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 46.Mognol P, Chosidow D, Marmuse JP. Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding in the super-obese: a comparative study of 290 patients. Obes Surg 2005; 15: 76–81. doi: 10.1381/0960892052993486 [DOI] [PubMed] [Google Scholar]
- 47.Hainaux B, Agneessens E, Rubesova E, Muls V, Gaudissart Q, Moschopoulos C, et al. Intragastric band erosion after laparoscopic adjustable gastric banding for morbid obesity: imaging characteristics of an underreported complication. AJR Am J Roentgenol 2005; 184: 109–12. doi: 10.2214/ajr.184.1.01840109 [DOI] [PubMed] [Google Scholar]
- 48.Vinzens F, Kilchenmann A, Zumstein V, Slawik M, Gebhart M, Peterli R. Long-term outcome of laparoscopic adjustable gastric banding (LAGB): results of a Swiss single-center study of 405 patients with up to 18 years' follow-up. Surg Obes Relat Dis . 2017; 13: 1313–9. doi: 10.1016/j.soard.2017.04.030 [DOI] [PubMed] [Google Scholar]
- 49.Aarts EO, Dogan K, Koehestanie P, Aufenacker TJ, Janssen IM, Berends FJ. Long-term results after laparoscopic adjustable gastric banding: a mean fourteen year follow-up study. Surg Obes Relat Dis . 2014; 10: 633–40. doi: 10.1016/j.soard.2014.03.019 [DOI] [PubMed] [Google Scholar]
- 50.Carucci LR, Turner MA, Szucs RA. Adjustable laparoscopic gastric banding for morbid obesity: imaging assessment and complications. Radiol Clin North Am 2007; 45: 261 ––74p. . doi: 10.1016/j.rcl.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 51.Favretti F, Cadière GB, Segato G, Himpens J, De Luca M, Busetto L, et al. Laparoscopic banding: selection and technique in 830 patients. Obes Surg 2002; 12: 385–90. doi: 10.1381/096089202321087922 [DOI] [PubMed] [Google Scholar]
- 52.Carucci LR, Turner MA. Imaging after bariatric surgery for morbid obesity: Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding. Semin Roentgenol 2009; 44: 283–96. doi: 10.1053/j.ro.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 53.Wiesner W, Schöb O, Hauser RS, Hauser M. Adjustable laparoscopic gastric banding in patients with morbid obesity: radiographic management, results, and postoperative complications. Radiology . 2000; 216: 389–94. doi: 10.1148/radiology.216.2.r00au28389 [DOI] [PubMed] [Google Scholar]
- 54.Frigg A, Peterli R, Zynamon A, Lang C, Tondelli P. Radiologic and endoscopic evaluation for laparoscopic adjustable gastric banding: preoperative and follow-up. Obes Surg 2001; 11: 594–9. doi: 10.1381/09608920160557075 [DOI] [PubMed] [Google Scholar]
- 55.Zappa MA, Micheletto G, Lattuada E, Mozzi E, Spinola A, Meco M, et al. Prevention of pouch dilatation after laparoscopic adjustable gastric banding. Obes Surg 2006; 16: 132–6. doi: 10.1381/096089206775565140 [DOI] [PubMed] [Google Scholar]
- 56.Zacharoulis D, Roy-Chadhury SH, Dobbins B, Kumar H, Goutzamani E, Boyle CJ, et al. Laparoscopic adjustable gastric banding: surgical and radiological approach. Obes Surg 2002; 12: 280–4. doi: 10.1381/096089202762552511 [DOI] [PubMed] [Google Scholar]
- 57.Weiner R, Blanco-Engert R, Weiner S, Matkowitz R, Schaefer L, Pomhoff I. Outcome after laparoscopic adjustable gastric banding - 8 years experience. Obes Surg . 2003; 13: 427–34. doi: 10.1381/096089203765887787 [DOI] [PubMed] [Google Scholar]
- 58.Kriwanek S, Schermann M, Ali Abdullah S, Roka R. Band slippage--a potentially life-threatening complication after laparoscopic adjustable gastric banding. Obes Surg 2005; 15: 133–6. doi: 10.1381/0960892052993503 [DOI] [PubMed] [Google Scholar]
- 59.Pieroni S, Sommer EA, Hito R, Burch M, Tkacz JN. The “O” sign, a simple and helpful tool in the diagnosis of laparoscopic adjustable gastric band slippage. Am J Roentgenol 2010; 195: 137–41. doi: 10.2214/AJR.09.3933 [DOI] [PubMed] [Google Scholar]
- 60.Singhal R, Bryant C, Kitchen M, Khan KS, Deeks J, Guo B, et al. Band slippage and erosion after laparoscopic gastric banding: a meta-analysis. Surg Endosc 2010; 24: 2980–6. doi: 10.1007/s00464-010-1250-4 [DOI] [PubMed] [Google Scholar]
- 61.Favretti F, Segato G, Ashton D, Busetto L, De Luca M, Mazza M, et al. Laparoscopic adjustable gastric banding in 1,791 consecutive obese patients: 12-year results. Obes Surg 2007; 17: 168–75. doi: 10.1007/s11695-007-9043-0 [DOI] [PubMed] [Google Scholar]
- 62.Basso N, Casella G, Rizzello M, Abbatini F, Soricelli E, Alessandri G, et al. Laparoscopic sleeve gastrectomy as first stage or definitive intent in 300 consecutive cases. Surg Endosc 2011; 25: 444–9. doi: 10.1007/s00464-010-1187-7 [DOI] [PubMed] [Google Scholar]
- 63.Gluck B, Movitz B, Jansma S, Gluck J, Laskowski K. Laparoscopic sleeve gastrectomy is a safe and effective bariatric procedure for the lower BMI (35.0-43.0 kg/m2) population. Obes Surg 2011; 21: 1168–71. doi: 10.1007/s11695-010-0332-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirth DA, Jones EL, Rothchild KB, Mitchell BC, Schoen JA. Laparoscopic sleeve gastrectomy: long-term weight loss outcomes. Surg Obes Relat Dis . 2015; 11: 1004–7. doi: 10.1016/j.soard.2015.02.016 [DOI] [PubMed] [Google Scholar]
- 65.Sepúlveda M, Alamo M, Saba J, Astorga C, Lynch R, Guzmán H. Long-term weight loss in laparoscopic sleeve gastrectomy. Surg Obes Relat Dis 2017; 13: 1676–81. doi: 10.1016/j.soard.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 66.Abu-Jaish W, Rosenthal RJ. Sleeve gastrectomy: a new surgical approach for morbid obesity. Expert Rev Gastroenterol Hepatol . 2010; 4: 101–19. doi: 10.1586/egh.09.68 [DOI] [PubMed] [Google Scholar]
- 67.Balla A, Quaresima S, Leonetti F, Paone E, Brunori M, Messina T, et al. Laparoscopic sleeve gastrectomy changes in the last decade: differences in morbidity and weight loss. J Laparoendosc Adv Surg Tech A 2017; 27: 1165–71. doi: 10.1089/lap.2017.0059 [DOI] [PubMed] [Google Scholar]
- 68.Chivot C, Rebibo L, Robert B, Dhahri A, Regimbeau JM, Yzet T. Value of routine upper gastrointestinal swallow study after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis . 2017; 13: 758–65. doi: 10.1016/j.soard.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 69.Cesana G, Cioffi S, Giorgi R, Villa R, Uccelli M, Ciccarese F. Proximal leakage after laparoscopic sleeve gastrectomy: an analysis of preoperative and operative predictors on 1738 consecutive procedures. Obes Surg 2017; 28: 1–9. [DOI] [PubMed] [Google Scholar]
- 70.Jurowich C, Thalheimer A, Seyfried F, Fein M, Bender G, Germer CT, et al. Gastric leakage after sleeve gastrectomy-clinical presentation and therapeutic options. Langenbecks Arch Surg 2011; 396: 981–7. doi: 10.1007/s00423-011-0800-0 [DOI] [PubMed] [Google Scholar]
- 71.Hutter MM, Schirmer BD, Jones DB, CY K, Cohen ME, Merkow RP. First report from the American college of surgeons bariatric surgery center network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. The British Institute of Radiology.; 2011. 410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saber AA, Azar N, Dekal M, Abdelbaki TN. Computed tomographic scan mapping of gastric wall perfusion and clinical implications. Am J Surg . 2015; 209: 999–1006. doi: 10.1016/j.amjsurg.2014.05.023 [DOI] [PubMed] [Google Scholar]
- 73.de Aretxabala X, Leon J, Wiedmaier G, Turu I, Ovalle C, Maluenda F, et al. Gastric leak after sleeve gastrectomy: analysis of its management. Obes Surg 2011; 21: 1232–7. doi: 10.1007/s11695-011-0382-5 [DOI] [PubMed] [Google Scholar]
- 74.Goitein D, Goitein O, Feigin A, Zippel D, Papa M. Sleeve gastrectomy: radiologic patterns after surgery. Surg Endosc 2009; 23: 1559–63. doi: 10.1007/s00464-009-0337-2 [DOI] [PubMed] [Google Scholar]
- 75.Triantafyllidis G, Lazoura O, Sioka E, Tzovaras G, Antoniou A, Vassiou K, et al. Anatomy and complications following laparoscopic sleeve gastrectomy: radiological evaluation and imaging pitfalls. Obes Surg 2011; 21: 473–8. doi: 10.1007/s11695-010-0236-6 [DOI] [PubMed] [Google Scholar]
- 76.Kim J, Azagury D, Eisenberg D, DeMaria E, Campos GM. American Society for Metabolic and Bariatric Surgery Clinical Issues Committee ASMBS position statement on prevention, detection, and treatment of gastrointestinal leak after gastric bypass and sleeve gastrectomy, including the roles of imaging, surgical exploration, and nonoperative management. Surg Obes Relat Dis 2015; 11: 739–48. doi: 10.1016/j.soard.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 77.Tan JT, Kariyawasam S, Wijeratne T, Chandraratna HS. Diagnosis and management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg 2010; 20: 403–9. doi: 10.1007/s11695-009-0020-7 [DOI] [PubMed] [Google Scholar]
- 78.Binda A, Jaworski P, Tarnowski W. Stenosis after sleeve gastrectomy-cause, diagnosis and management strategy. Pol Przegl Chir 2013; 85: 730–6. doi: 10.2478/pjs-2013-0112 [DOI] [PubMed] [Google Scholar]
- 79.Burgos AM, Csendes A, Braghetto I. Gastric stenosis after laparoscopic sleeve gastrectomy in morbidly obese patients. Obes Surg 2013; 23: 1481–6. doi: 10.1007/s11695-013-0963-6 [DOI] [PubMed] [Google Scholar]
- 80.Rebibo L, Hakim S, Dhahri A, Yzet T, Delcenserie R, Regimbeau JM. Gastric stenosis after laparoscopic sleeve gastrectomy: diagnosis and management. Obes Surg 2016; 26: 995–1001. doi: 10.1007/s11695-015-1883-4 [DOI] [PubMed] [Google Scholar]
- 81.Chivot C, Robert B, Lafaye N, Fuks D, Dhahri A, Verhaeghe P, et al. Laparoscopic sleeve gastrectomy: imaging of normal anatomic features and postoperative gastrointestinal complications. Diagn Interv Imaging 2013; 94: 823–34. doi: 10.1016/j.diii.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 82.Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis 2009; 5: 469–75. doi: 10.1016/j.soard.2009.05.011 [DOI] [PubMed] [Google Scholar]
- 83.Parikh A, Alley JB, Peterson RM, Harnisch MC, Pfluke JM, Tapper DM, et al. Management options for symptomatic stenosis after laparoscopic vertical sleeve gastrectomy in the morbidly obese. Surg Endosc 2012; 26: 738–46. doi: 10.1007/s00464-011-1945-1 [DOI] [PubMed] [Google Scholar]
- 84.El-Sayes IA, Frenken M, Weiner RA. Management of leakage and stenosis after sleeve gastrectomy. Surgery 2017; 162: 652–61. doi: 10.1016/j.surg.2017.04.015 [DOI] [PubMed] [Google Scholar]
- 85.Stamou KM, Menenakos E, Gomatos IP, Panousopoulos SG, Smparounis S, Leandros E, et al. Clinical implications of sleeve gastrectomy as a source of spleen infarction or ischemia. Obes Surg 2011; 21: 1490–3. doi: 10.1007/s11695-010-0302-0 [DOI] [PubMed] [Google Scholar]