Abstract
Altered by ionising radiation, the vascular network is considered as a prime target to limit normal tissue damage and improve tumour control in radiotherapy (RT). Irradiation damages and/or activates endothelial cells, which then participate in the recruitment of circulating cells, especially by overexpressing cell adhesion molecules, but also by other as yet unknown mechanisms. Radiation-induced lesions are associated with infiltration of immune-inflammatory cells from the blood and/or the lymph circulation. Damaged cells from the tissues and immune-inflammatory resident cells release factors that attract cells from the circulation, leading to the restoration of tissue balance by fighting against infection, elimination of damaged cells and healing of the injured area. In normal tissues that surround the tumours, the development of an immune-inflammatory reaction in response to radiation-induced tissue injury can turn out to be chronic and deleterious for the organ concerned, potentially leading to fibrosis and/or necrosis of the irradiated area. Similarly, tumours can elicit an immune-inflammation reaction, which can be initialised and amplified by cancer therapy such as radiotherapy, although immune checkpoints often allow many cancers to be protected by inhibiting the T-cell signal. Herein, we have explored the involvement of vascular endothelium in the fate of healthy tissues and tumours undergoing radiotherapy. This review also covers current investigations that take advantage of the radiation-induced response of the vasculature to spare healthy tissue and/or target tumours better.
INTRODUCTION
All tissues can be damaged by ionising radiation beyond a dose-volume threshold.1 The damage results from the initial deposition of energy within the tissue. The processes of radiation injury begin at once after the irradiation, but some of the clinical and histological signs, such as necrosis and fibrosis, become visible weeks, months, or even years after a therapeutic treatment.1, 2 Damage is characterised by cell death, phenotypic changes, immune-inflammatory cell infiltration and vascular and tissue remodelling, which can lead to chronic injury and ultimately necrosis.
Radiotherapy (RT) is used to treat a variety of cancers, as well as benign tumours, in more than half of patients with tumours.3 Despite great advances in radiation dose delivery techniques, the therapeutic index of RT is still limited by normal tissue injury in organs at risk and by the radiation resistance of some tumours.4 New approaches to optimise the response of normal tissue and tumours thus remain essential for improving the outcome of RT, by increasing the likelihood of cancer cure or by decreasing normal tissue toxicity, or both.5, 6
In the vessels, the endothelium is a key cell compartment for the response to ionising radiation of healthy tissue and tumours, and represents a promising target to improve the differential effect of RT in the future.6, 7 Following radiation exposure, the global endothelial cell response covers a wide range of molecular changes with a global expression pattern of modifications.8, 9 Changes occur at the transcriptional, translational and post-translational levels and impact cell phenotype as well as the microenvironment by production and secretion of soluble factors such as reactive oxygen species (ROS), reactive nitrogen species, chemokines, cytokines and growth factors. These radiation-induced dynamic modifications of molecular networks may control the endothelial cell phenotype and govern recruitment of immune cells.
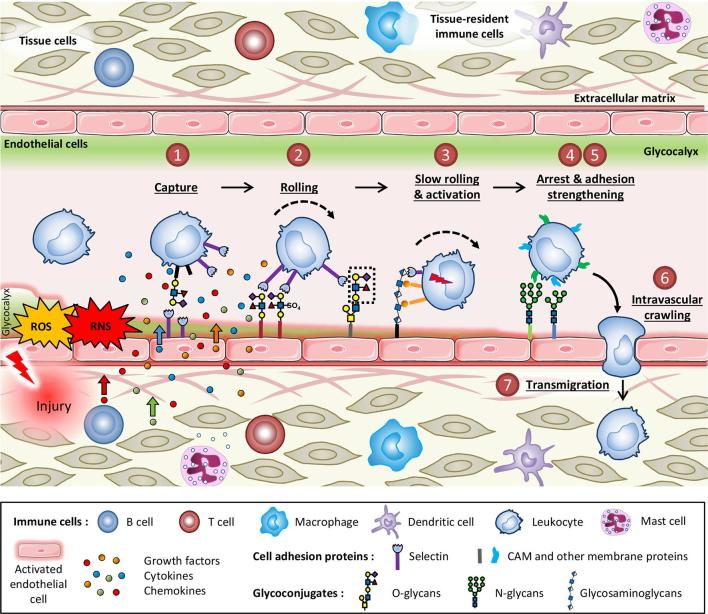
Ionising radiation induces an inflammatory response in organs10 and tumours11 characterised by immune-inflammatory cell infiltration. Vascular endothelium plays an integrative role in the tissue response following stress and controls the initiation and resolution of inflammatory responses through the regulation of chemotaxis and activation of leukocytes in the periphery.11, 12 The development of this inflammatory response is regulated by a complex process that involves leukocyte-endothelium interactions composed of activation, rolling, adhesion and transmigration in the surrounding tissue13 (Figure 1). On the other hand, engaging the immune system for optimal anti-cancer therapy is an attractive contemporary concept.14 Promising current strategies generate an effective immune response to destroy the tumour in combination with RT.15 In this context, control of the adaptive immune response by the tumour endothelium is a crucial process.
Figure 1.
The leukocyte adhesion cascade is triggered by a pro-inflammatory stimulus. Injury activates endothelial cells, allows production of free radicals and damages tissue and tissue-resident immune cells leading to the release of cytokines, chemokines and growth factors, which then attract leukocytes from the circulation. Circulating leukocytes undertake a seven-step process of capture, rolling, slow rolling and activation, arrest and adhesion strengthening, intravascular crawling and finally transmigration to reach sites of inflammation. Each step of this process is controlled by various adhesion molecules at the surface of the endothelium. All of these proteins are glycosylated, a post-translational modification process that may be regulated during inflammation. During this cascade of events, leukocytes are also activated by interactions with cytokines, chemokines and growth factors sequestrated by the glycosaminoglycans of the endothelial glycocalyx. RNS, reactive nitrogen species; ROS, reactive oxygen species.
THE VASCULAR ENDOTHELIUM
A healthy endothelium is in a well-balanced state between pro- and anti-oxidants, vasodilators and vasoconstrictors, pro- and anti-inflammatory molecules and pro- and anti-thrombotic signals. It provides key functions in angiogenic and inflammatory processes, which are activated and finely regulated when required. As can happen in the case of inflammation, breakdown of this complex balance leads to a diseased or pathological endothelium displaying pro-oxidant, vasoconstrictor, pro-inflammatory and pro-thrombotic properties.
Role in immune-inflammatory cell recruitment
The endothelium is able to activate a global molecular program in physiological conditions or in response to stress and serves as a key checkpoint to control the immune response. The adhesion of leukocytes to the vascular endothelium is one of the major characteristics of inflammation. This process involves glycoproteins which allow the adhesion of circulating leukocytes in the blood stream to endothelial cells. The objective of this adhesion is the passage of leukocytes from the bloodstream to the injured site. The endothelial cell-leukocyte interaction is the result of a large number of physical (shear forces), chemical (weak interactions and impairment of nitric oxide [NO] production) and biological (proteins and specific glycoproteins, substrates and cytokines) factors. Cell recruitment takes place through a cascade of seven events consisting of capture (or tethering), rolling, slow rolling, arrest, adhesion strengthening and spreading, intravascular crawling and paracellular and transcellular transmigration13 (Figure 1). The multiplicity of molecular choices for each of the stages provides a great diversity of very specific combinations of leukocyte recruitment that makes it possible to adapt recruitment to the type of tissue and injury.13, 16
Cell adhesion molecules (CAMs) and activation of integrins are at the heart of the adhesion process following selectin ligand recognition. Modulation of adhesion occurs through the action of chemical and biological factors released by injured tissues, endothelial cells, leukocytes and innate lymphoid cells. For instance, the degranulation of activated mast cells releases histamine, leukotrienes and platelet activating factor. Histamine then activates selectins, which allows the capture of leukocytes and the initiation of rolling. Subsequently, leukotrienes and platelet activating factor participate in the activation of integrins by increasing their avidity and expression, thus helping to maintain rolling and firm adhesion. On the other hand, cytokines and chemokines induce the transcription of endothelial CAMs such as selectins, intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1). Activated endothelial cells also produce an excess of ROS , which results in the activation of oxidative stress-sensitive transcription factors such as the nuclear factor-kappa B (NF-κB) or the activator protein 1 (AP-1). Interestingly, AP-1 also directs endothelial cells towards a pro-adhesive leukocyte phenotype in an inflammatory environment.17 Conversely, NO, prostaglandin I2 (PGI2) and adenosine are endogenous anti-adhesive factors.17
Importance of the endothelial glycome in endothelial-immune cell interactions
Modifications of the glycan profile, whose entire spectrum defines the glycome, alter the function of proteins and participate in cellular functions, notably adhesion and cellular communication in endothelial cells. Selectins are specialised protein receptors for leukocyte glycoconjugates that mediate tethering and rolling of leukocytes under flow in inflamed vascular beds.18 Endothelial cells have many glycoconjugates on their surface that are regulated in an inflammatory context and are involved in interactions with circulating cells (Figure 1).19 These glycan structures can thus be considered as entry points for the immune system. In addition, the endothelial glycocalyx, a covering composed of a layer of polysaccharides covalently bound to the lipids and proteins of the membrane, also plays an important role in the immune-inflammatory response, especially via its role in the leukocyte-endothelial interaction.20 Following an acute or chronic injury, pro-inflammatory stimuli, such as TNF-α, induce shedding of glycosaminoglycans, thereby decreasing the width and size of the endothelial glycocalyx.21 This allows greater accessibility of endothelial glycoprotein epitopes, on which circulating leukocytes roll and to which they adhere.
RADIOBIOLOGY AND RADIOPATHOLOGY OF THE VASCULAR ENDOTHELIUM
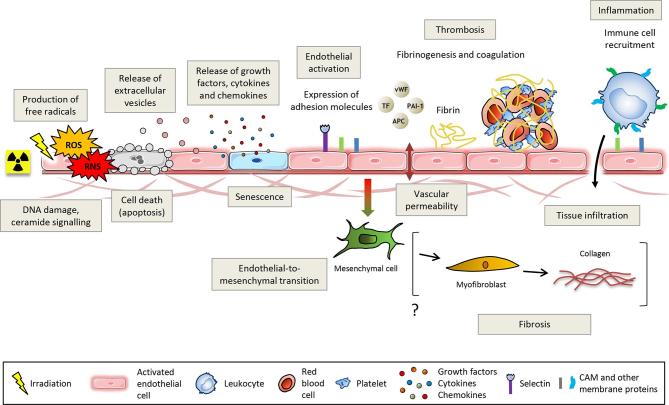
Ionising radiation has multiple effects on the endothelium, which is thought to contribute to the initiation and progression of radiation-induced damage to healthy tissues following RT.22 Among the main effects are radiation-induced endothelial cell death, loss of thromboresistance, cell activation and secretion of soluble factors such as cytokines and growth factors.23 Vessels of normal tissues surrounding the tumours and of the tumours themselves are both impacted by ionising radiation during RT. In most cases, normal and pathological tissues comprise genetically normal endothelial cells. In tumours, however, vascular cells undergo the influence of the tumour environment and display different phenotypes, and sometimes different genotypes, from those of endothelial cells from normal tissues. Figure 2 shows a schematic view of the effects of ionising radiation on the vascular endothelium, which are detailed below.
Figure 2.
Ionising radiation injures the vascular endothelium. DNA damage and ceramide production lead to cell death, stress-induced premature senescence, cell activation mainly characterised by the overexpression of adhesion molecules and disruption of the endothelial barrier. Endothelial activation promotes a pro-thrombotic and pro-inflammatory phenotype that ultimately leads to thrombosis and recruitment of leukocytes. Irradiated endothelial cells can also undergo an endothelial-to-mesenchymal transition that potentially contributes to fibrosis via a final differentiation into activated myofibroblasts capable of secreting collagens. APC, antigen-presenting cells; CAM, cell adhesion molecules; PAI-1, plasminogen activator inhibitor-type 1; TF, tissue factor; vWF, von Willebrand factor.
Differential effects of various forms of radiotherapy on endothelial damage
External beam RT using photon radiation, and especially X-rays, is used in almost all RT treatments. However, particular indications can use high linear transfer energy (LET) particle RT with protons or carbon ions (hadrontherapy), as well as internal RT with radioactive sources of gamma-rays (brachytherapy) and systemic RT using targeted radionuclide therapy (radioimmunotherapy). Although not investigated in depth, the effects of gamma- and X-rays on endothelium are supposed to be similar because of their equal relative biological effectiveness. On the other hand, it is conceivable that differences in endothelial cell response may occur as a function of dose rate, energy spectrum and fractionation schedule. More likely, radioimmunotherapy and hadrontherapy may have very different effects on endothelium compared to photon radiation, because of their high LET radiation properties. However, most data are available on the effects of low LET radiation and data on high LET radiation are scarce. For example, the impact of carbon and Fe ion irradiation on the response of endothelial cells has been compared to X-ray irradiation, showing a differential effect of high LET radiation compared to photon radiation.24, 25 Interestingly, C-ions seemed to be more effective in damaging endothelial cells than X-rays shortly after exposure, but late damage was not found at the doses used (0.25–1.5 Gy).25 On the other hand, Fe ions did not significantly induce inflammation, in contrast to X-rays, but the radiation impact on gene and protein expression was more marked and longer lasting for Fe ions than for X-rays.24
Endothelial cell apoptosis and senescence
Apoptosis is a frequent cell death process after irradiation.26 Exposure of healthy tissues to a high dose of ionising radiation (≥10 Gy) induces an acute endothelial reaction characterised by a rapid wave of endothelial apoptosis.22, 27 Yet, the key role and the existence itself of radiation-induced endothelial apoptosis in normal tissue lesions remain controversial. Endothelial apoptosis has been shown to be the primary event responsible for gastrointestinal syndrome after whole-body irradiation at 15 Gy in mice.28 Despite contradictory results, which have been discussed elsewhere,29 the participation of endothelial apoptosis in the deleterious tissue effects after irradiation is still widely accepted.
Endothelial cells also experience stress-induced premature senescence in vitro and likely in vivo.30 Senescent irradiated endothelial cells can accumulate in tissues, promoting age- and therapy-related disorders, including cardiovascular diseases. Through secretion of many inflammatory mediators and extracellular proteases, called the senescence-associated secretory phenotype (SASP), the senescent phenotype causes chronic inflammation and disruption of tissue structure and function. The surviving irradiated cells likely participate in the development of a dysfunctional vascular phenotype. In the early phase, this is marked by excessive secretion of pro-inflammatory cytokines, increased recruitment of circulating cells such as platelets and lymphocytes, activation of the coagulation system and increased vascular permeability.22 In the late phase, a collapse of the vessels is observed, associated with a reduction in the thickness of the basal membrane and the persistence of a pro-coagulating, pro-inflammatory and potentially senescent endothelial phenotype.31
Endothelial activation
Ionising radiation triggers changes in the phenotype of endothelial cells that lead to endothelial activation and then potentially to endothelial dysfunction. Endothelial activation is defined as the endothelial expression of adhesion molecules, such as VCAM-1, ICAM-1 and E-selectin.32 It is usually triggered by pro-inflammatory cytokines such as tumour necrosis factor (TNF)-α or interleukin (IL)−6. Loss of NO also leads to increased endothelial cell activation, which can then cause endothelial impairment, including dysfunction of vascular tone and chronic activation of the coagulation and immune-inflammatory systems. Radiation-induced endothelial activation results in increased expression of adhesion proteins such as VCAM-1, ICAM-1, PECAM-1, E-selectin and P-selectin which participate in the recruitment of circulating cells.33–36 Irradiation leads to increased platelet-endothelial interactions both in vitro and in vivo36, 37 by a mechanism that involves PECAM-1. Recently, like chronic inflammation induced by TNF-α or in the context of atherosclerosis,38, 39 it has been shown that irradiation modifies the glycosylation pattern of endothelial cells, causing an increase in monocyte adhesion.40 In vivo, an increase in endothelial expression of ICAM-1 and VCAM-1 has been shown in a model of intestinal inflammation induced by radiation.41 Interestingly, ICAM-1 knock-out mice exhibited less severe pulmonary and intestinal inflammation than wild-type mice,42 suggesting that cell infiltration could be deleterious in this context. In human subjects, the endothelium can be activated by therapeutic radiation43, 44 through activation of NF-κB, which is likely essential in the genesis of cardiovascular disease induced by RT.45, 46 These studies have overall demonstrated that the increase in expression of adhesion molecules by endothelial cells after irradiation plays a decisive role in the recruitment of circulating cells and thus in the radiation-induced inflammation of the tissue and/or the tumour, with a potential deleterious effect on normal tissues.
Endothelial cell-derived extracellular vesicles
Cell-derived extracellular vesicles, comprising exosomes and microvesicles, have emerged as important mediators of a new mode of intercellular communication.47, 48 Like all cells, endothelial cells are able to secrete extracellular vesicles. These endothelial vesicles have been shown to play a variety of roles in the human body, ranging from contributing to cardiovascular disease to promoting endothelial cell survival.49 Ionising radiation has been shown to induce production of extracellular vesicles by both primary and tumour cells.50–54 Endothelial injury by ionising radiation elicits the release of microparticles, in correlation with apoptosis and ROS formation, which can be inhibited by anti-oxidative treatment.55 It is now crucial to clarify the role of these endothelial microparticles in the context of irradiation as they may act locally or far from the irradiation site as mediators of angiogenesis, inflammation, coagulation or injury, certainly through their protein and/or microRNA content.56
Disruption of the endothelial cell-cell barrier after radiotherapy
RT is known to induce vascular permeability. Several studies are concordant with an increase of albumin leakage after single-dose irradiation from 2 to 30 Gy or more. The increase in permeability is transitory, varies from one day to one week after RT and penetration depth from vessels varies by a factor 1.5 to 6, depending on the dose.57, 58 In microvascular endothelial cells, cytoskeletal changes mediated by RhoA-guanosine triphosphatase and Rho kinase are implicated in the redistribution of basal VE-cadherin and intercellular junctions.59 Interestingly, mast cells may contribute to cutaneous microvascular hyperpermeability, as shown in an animal model of localised irradiation.58 Fractionated RT has also been shown to increase vascular permeability, but not as a result of a lack of junction proteins. In contrast, ICAM-1 and Zo-1, which are involved in the endothelial barrier, are more expressed after fractionated RT.60, 61 It should be noted that microvascular permeability induced by fractionated RT seems to be more durable than that induced by single-dose RT.62, 63
Endothelial tight junctions form the impermeable blood-brain barrier (BBB). In BBB models, microvascular permeability is increased in a dose-dependent manner after single-dose irradiation.64 This increase is not directly explained by a change in tight junction protein expression or by basal lamina lesion, since the expressions of Zo-1 and the endothelial barrier antigen are not affected at doses below 20 Gy.64, 65 The increase in permeability could be explained by changes in the cytoskeleton of endothelial cells. In a clinical context of fractionated RT for the treatment of brain tumours, the radiation-induced disruption of the BBB has been widely demonstrated.66–68
Radiotherapy and vascular changes in tumours
RT significantly affects the state and function of blood vessels. The severity of vascular damages depends on the RT protocol used, i.e. the number of fractions, the dose rate and the total dose of radiation applied.69 In solid tumours, the overproduction of pro-angiogenic molecules in response to RT results in the dilatation of microvessels, the disruption of the endothelial lining, more branches and overall a very irregular blood vessel architecture. At the cell level, the blood capillaries are incompletely matured, the perivascular cells are absent or detached, the basement membrane is absent or abnormally thick and endothelial cell junctions are absent. This impaired vascularisation is associated with deregulation of tissue oxygenation which finally leads to hypoxic areas in the tumour. As a consequence, the lack of oxygen lowers the amount of radiation-induced ROS, ultimately leading to decreased RT efficiency.70
Observations that transplanting cancer cells into a previously irradiated site in mice resulted in slower growth of the subsequent tumour led to the concept of “tumour bed effect”,71 especially following large doses of radiation (10 to 20 Gy and more). Suggestions were made that vascular damage could impact the ability to regrow after irradiation. More recently, it was demonstrated that microvascular damage, i.e. endothelial cell apoptosis, regulates the response of tumour cells to radiation at the clinically relevant dose range.72, 73 However, the involvement of endothelial cells in the tumour response remains controversial.74 In SCID mice, in which the scid mutation radiosensitises endothelial cells, tumour growth was not affected by radiation compared with wild-type mice, suggesting that the vasculature did not play a significant role in tumour response to radiation in this model.75 Also, a specific deletion of atm in endothelial cells made them more sensitive to ionising radiation in sarcomas,76 but failed to enhance sarcoma eradication, unlike specific deletion of atm in tumour cells which increased sarcoma eradication.77 These studies have strongly suggested that tumour cells, rather than endothelial cells, are critical targets for sarcoma eradication by RT delivered in high single dose, like doses delivered in stereotactic body radiation therapy (SBRT), although an enhanced growth delay was observed in the tumours with the more sensitive endothelial cells.
As a consequence of endothelial sensitivity to radiation, the irradiated tumour microenvironment (TME) undergoes changes in vasculature and oxygenation rates. For large single doses over 10 Gy, microvascular density is impaired at least in a transitory manner. The decrease in vessel density corresponds to an alteration of blood perfusion and to an increase in hypoxic zones as shown in different tumour models.78–81 Nevertheless, for repeated doses, the radiation schedule seems to matter in a more important way. A vascular density reduction is also observed but is not systematically followed by an increase in tumour hypoxia. In a clinically relevant pattern of repeated doses from 2 to 15 Gy per fraction, the microvascular density reduction resembles vascular normalisation rather than destruction. Unlike using large single doses, repeated RT leads to transitory or durable hypoxia reduction in pulmonary, prostate and head and neck cancer models.78, 80,82
On the other hand, fractionated RT impacts pericyte coverage of blood vessels and blood vessel functionality in the TME. Pericytes are helpful to stabilise microvessels and regulate their permeability in healthy tissues.83 They are less frequent and more loosely associated with endothelial cells in tumours. Also, pericytes are involved in tumour hypoxia reduction84 and cancer metastatic diffusion.85 Interestingly, pericytes are also involved in transmigration and trafficking of immune cells into tumours.86 Several studies show that after repeated doses from 2 to 12 Gy, pericyte coverage and blood perfusion are improved despite microvascular density reduction.61, 78,80,87 As a consequence, immune infiltrate and immunotherapy efficacy should be enhanced.88 Tumour blood perfusion is likely multifactorial and dependent on the quality of blood supply as well as cell density and metabolism.89 The consequences of single-dose RT on pericyte coverage remain unclear and deserve to be better known. After 12 Gy, pericyte coverage and perfusion decreases in a neuroblastoma model79 and after 20 Gy in a fibrosarcoma model,81 whereas it increases after 12 or 14 Gy in pulmonary tumour models.80, 90
Radiation-induced vascular changes in normal tissues
Endothelial apoptosis, increased vascular permeability, cell activation and recruitment of inflammatory cells as well as activation of the coagulation system91 are all early phenomena contributing to the induction and progression of radiation-induced tissue damage. Late vascular lesions, such as fibrosis and luminal reduction, also generate areas of tissue hypoxia that contribute to and amplify radiation-induced healing.91 On the morphological level, the vascular lesions are different depending on the size of the vessels. Microvasculature is considered to be the most radiosensitive part of the vasculature. Capillary ruptures and dilatations, hypertrophy and detachment of endothelial cells from the basal lamina as well as thrombosis are observed.91 In the case of large vessels, it is very difficult to distinguish, at the morphological level, the vascular lesions of conventional atherosclerosis from radiation-induced vascular lesions. The latter are characterised by vascular fibrosis with luminal reductions, excessive extracellular matrix deposition in the media and adventitia, neointimal hyperplasia and thrombus formation.92 The histological similarities between radiation-induced vascular lesions and atherosclerotic lesions suggest that similar initiation and progression mechanisms could be involved.93 At the endothelial cell surface, thrombomodulin downregulation, observed very early after irradiation, persists chronically in both rats and humans.94–96 The endothelium is in a chronic pro-coagulant state, which may contribute to the long-term persistence of deleterious effects of irradiation. The induction of ICAM-1 expression in irradiated endothelial cells and an increase in neutrophil adhesion are maintained more than 10 days after irradiation, suggesting a pro-inflammatory endothelial cell phenotype that persists over time.97In vivo studies also suggest that vascular damage contributes to radiation-induced fibrosis. In a pulmonary radiation-induced fibrosis model in rats, significant hypoxia associated with severe fibrosis 6 months after irradiation could result from damage to endothelial cells, interstitial oedema and vascular dysfunction.98 Recently, it has been shown that endothelial hypoxia-inducible factor (HIF)−1α deletion confers resistance to radiation-induced enteritis, whereas similar deletion in intestinal epithelium does not, suggesting new functions of endothelial HIF-1α-signalling pathways as mediators of mucosal-inflammatory processes.99 On the other hand, it has been demonstrated in vivo that the endothelium is directly involved in the progression of radiation-induced enteritis by using a mouse model harbouring an endothelium-specific deletion of the serpinE1 gene, which encodes the plasminogen activator inhibitor-type 1 (PAI-1).100
IMMUNOLOGICAL CONSEQUENCES OF RADIOTHERAPY
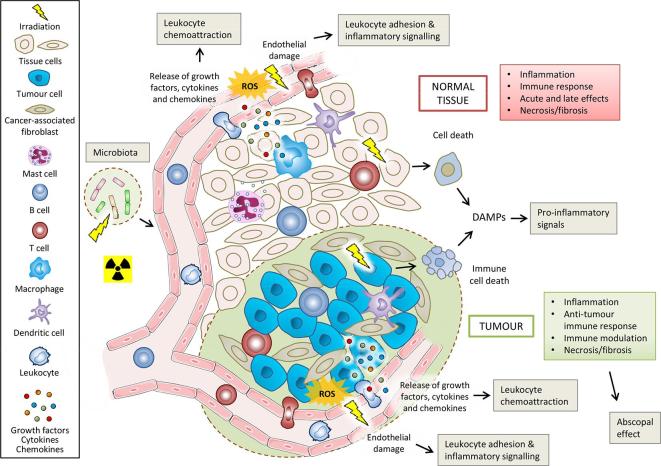
RT has immune-inflammatory effects on both tumours and healthy tissues. Figure 3 summarises these effects, which are discussed below.
Figure 3.
Immune-inflammatory effects of radiation exposure on normal tissues, tumours and tumour microenvironment. Ionising radiation causes tumour and normal cell damage, cell death and release of DAMPS, which act as pro-inflammatory signals. Radiation activates cells that then release cytokines/chemokines. Activated endothelial cells acquire a pro-inflammatory phenotype which promotes the leukocyte adhesion cascade. These initial responses finally lead to the recruitment and activation of diverse immune cells, which can then also participate in the abscopal effect of radiotherapy. Delayed mitotic death and proliferation of immune cells in the tissue then cause environmental changes that ultimately contribute to necrosis or fibrosis. Irradiation of the microbiota of the intestinal tract can also influence the responses of normal tissues and tumours through immune-vascular crosstalk. DAMPS, damage-associated molecular patterns; ROS, reactive oxygen species.
Consequences in tumours and healthy tissues
Radiation induces changes to the tumour cell immunophenotype and immunogenicity by damaging DNA and membranes, and by production of cytoplasmic ROS, which activate many transcription factors and signalling pathways.101 RT promotes the presentation of tumour antigens by tumour cells by increasing the cell pool of specific antigens and by stimulating the expression of the major Type 1 histocompatibility complex (MHC class 1).102 It also generates immunogenic cell death, i.e. the dispersion of immune-stimulating tumour antigens from dying cells into the surrounding milieu,103 through the release of damage-associated molecular patterns (DAMPs).104, 105 The activated dendritic cells then present the tumour antigens to the naive T lymphocytes in the lymph nodes allowing the formation of cytotoxic T lymphocytes specific for the tumour antigen.106 RT facilitates the recruitment of these effector T lymphocytes by generating the secretion of the chemokine CXCL16 and endothelial expression of the cell adhesion ICAM-1, VCAM-1 and E –selectin.97, 107 These processes finally trigger an immune response, which is accompanied by a pro-inflammatory reaction, with release of IL-1, IL-2, IL-6, IL-12, interferon (INF)-α and IFN-β and TNF-α, which are involved in amplification of the anti-tumour immune response.105
In normal tissues injured by radiation, release of DAMPs and secretion of cytokines and chemokines also activate the immune system. This phase moves to an acute inflammatory phase characterised by an activated pro-inflammatory response and vascular leakage. In this phase, the recruitment of diverse immune cells of myeloid and lymphoid origin, which is associated with a perpetual cytokine/chemokine cascade,108, 109 leads to various degrees of inflammation and symptoms. Lymphocyte subpopulations such as TH1, TH17 and possibly innate lymphoid cells, can contribute to inflammation, while Treg could be needed to control damaging and excessive pro-inflammatory responses. An excessive response, sustained by activation, proliferation of these cells and cytokine secretion can then shape the microenvironment of normal tissues towards the development of severe inflammation such as, for example, severe pneumonitis in the case of irradiation of the lung.110 Mitotic cell death occurs later and can subsequently trigger tissue hypoxia leading to the release of DAMPs and cytokines/chemokines from resident cells thereby modifying the microenvironment in the tissue. These changes impact the tissue-resident immune cells that then release cytokines. Finally, epithelial-to-mesenchymal and endothelial-to-mesenchymal transitions, recently found to be induced by irradiation,111, 112 mesenchymal stem cell differentiation and the altered microenvironment contribute to myofibroblast activation and collagen deposition, which ultimately leads to fibrosis.
In irradiated normal tissues, the recruitment of immune cells has a dual effect. Resolution of inflammation and repair progression are concomitant with late mitotic cell death, which results from the initial damage, hypoxia and release of DAMPs, cytokines and growth factors, representing the chronic phase of radiation-induced injury of many normal tissues. These environmental changes may contribute to immunomodulation. For example, different populations of lymphocytes such as TH2, TH9, Treg and possibly innate lymphoid cells display both anti-inflammatory and pro-fibrotic effects, thereby potentially promoting the induction of pathological myofibroblasts and fibrosis.110 It is believed that lymphocytes play a complex role in radiation diseases in which, depending on the disease stage and the environmental conditions, specific subpopulations of lymphocytes could exert beneficial or adverse effects.110 For instance, in the case of radiation-induced pulmonary fibrosis, a disturbed balance between tissue inflammation and repair processes, with an involvement of lymphocytes, may participate in the development of the syndrome as described for other fibrotic diseases.113 A question is whether immune cells contribute directly to radiation diseases or only modulate disease progression. In addition, it is not yet established if innate lymphoid cells also contribute to radiation-induced late injuries. These questions have been nicely explored in several reviews that we recommend as further reading in the context of radiation-induced pulmonary acute and late effects.110, 113,114
Influence of the adaptive immune response by the tumour endothelium in the context of radiotherapy
Normal endothelial cells participate in adaptive immune responses by recruiting circulating T-cells into inflamed tissues.115 In contrast, tumour endothelial cells play a role in immune cell exclusion and inhibition of lymphocyte activation,116 causing the development of intratumoural immunosuppression, which is involved in tumour escape from immunity and conventional cancer therapies.14, 117 Mechanisms underlying this dysfunction involve the absence or expression at low levels of ICAM-1, VCAM-1 and E-selectin on tumour vasculature, despite an inflammatory environment.118 This lack of response to inflammatory stimuli, called endothelial anergy,119 may be due at least in part to angiogenic factors such as vascular endothelial growth factor-A (VEGF-A), VEGF-C, VEGF-D and basic fibroblast growth factor (bFGF),120, 121 which are expressed in response to tissue hypoxia through activation of the HIF pathway. Therefore, approaches that improve the recruitment and activation of lymphocytes by tumour endothelial cells are being investigated.14, 118,122 On the other hand, RT is immunostimulatory and has the potential to enhance anti-tumour response by the immune system.123 For instance, in this way, vascular normalisation by VEGFR2 blockade has been shown to cause an increase in oxygenation able to govern brain tumour response to radiation by restoring anti-tumour T-cell activity.124 Also, low doses of irradiation in the range of a fraction dose of conventional RT have been shown to cause aberrant vascular system normalisation and efficient recruitment of tumour-specific T-cells in human pancreatic carcinomas, as well as T-cell-induced rejection of tumours and prolonged survival in spontaneous and xenograft tumour models.125 These effects were in part related to the suppression of the production of angiogenic, immunosuppressive and tumour growth factors such as VEFG. Lastly, RT increases the expression of adhesion molecules on tumour endothelial cells, including ICAM-1, VCAM-1 and E-selectin,35,125–127 which helps to normalise the tumour vasculature and makes it easier to infiltrate.
Radiation-induced abscopal effects and their cross-talk with vascular remodelling
Tumour regression may occur in lesions far from the radiation field of the tumour site by a systemic effect (abscopal effect).126 This effect is currently being observed in clinics and could be the result of immunogenic cell death, which triggers tumour vaccination.127 It requires efficient infiltration of the primary tumour by immune cells, subsequent extravasation of these cells so they can act away from the irradiated site and ultimately infiltration of the secondary tumour by the educated immune cells. Many barriers, including the vascular barrier, prevent radiation-induced in situ tumour vaccination,128 which explains why only a few distant responses in non-irradiated sites have been reported.129 In solid tumours, poor vascularisation may impair this process by decreasing leukocyte infiltration and effector function, otherwise leading to hypoxia, which can contribute to immune tolerance by regulating immunosuppressive cell populations.130–135 On the other hand, RT induces a pro-inflammatory environment which can enhance lymphocyte infiltration and adaptive immune system activation by normalising the vasculature.136 This infiltration may be caused by upregulation of ICAM-1 correlated with RT.33
Alteration of microbiota following radiotherapy and its impact on vascular and immune systems
The microbiome plays a critical role in the maintenance of vascular health and the development of vascular disease.137 Changes in intestinal microbiota composition after RT have been reported in several studies.138 The major changes were reduced diversity of Firmicutes and Bacteroidetes and increase of Proteobacteria. In a pre-clinical model of radiation proctitis, recent findings have demonstrated that local radiation treatment induces microbial dysbiosis.139 Remarkably, this radiation-induced dysbiotic microbiota was able to transmit inflammatory susceptibility and to render germ-free recipient mice more susceptible to ionising radiation through a host cytokine induction. Conversely, faecal microbiota transplantation from healthy mice to total body irradiated mice improved gastrointestinal tract function and epithelial integrity of the small intestine, and facilitated angiogenesis.140 These two studies suggest that the microbiota may be manipulated to improve or prevent radiation-induced tissue damage.
Evidence suggests that interactions between the enteric microbiota and the innate immune system are important in modulating the response to radiation,141 especially through triggering of innate immune receptors by the microbiota.142 After exposure to ionising radiation, the integrity of the intestinal barrier is decreased, allowing intestinal bacteria and their components (pathogen-associated molecular patterns) to translocate to the lamina propria where they are recognised by Toll-like receptors in host antigen-presenting cells (APCs).143, 144 Activated APCs then secrete pro-inflammatory cytokines and prime donor T-cells. On the other hand, endothelial apoptosis has been shown to be lower in germ-free than in conventionally raised mice.145 The microbiota-associated enhancement of endothelial radiosensitivity did not require mature lymphocytes since Rag1 knock-out mice did not show differences in the development of lethal radiation enteropathy.145
Little is known about the effect of microbiota on the regulation of tumour response to RT.142 Yet, recently, the influence of the gut microbiome composition on the response to anti-programmed cell death protein (PD)−1 immunotherapy in cancer patients was described in three outstanding articles.146–148 These studies suggest that the microbiota should be considered when assessing therapeutic intervention. In the same way that the gut microbiota affects the immune response induced by immunogenic cell death in chemotherapy and immunotherapy,149–151 it can therefore be assumed that the gut microbiota also plays a role in the immunostimulatory effects of RT.
ENDOTHELIAL-ORIENTED STRATEGIES FOR THERAPEUTIC GAIN IN RADIATION ONCOLOGY
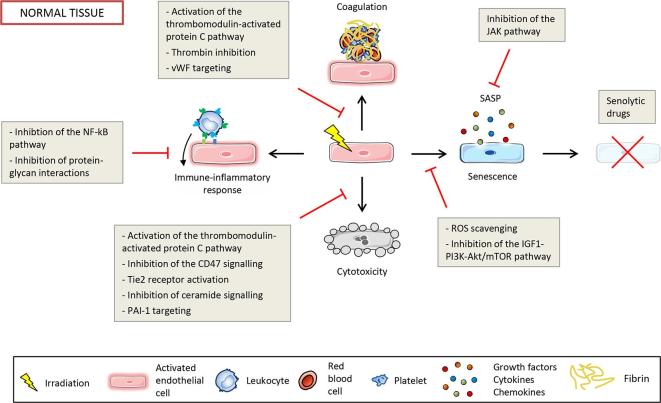
Endothelium-based strategies for therapeutic gain in radiation oncology attempt either to increase injuries in the tumour vascular system or to protect the vasculature of normal tissue from radiation injuries, or ideally both. Many studies have attempted to preserve the endothelial barrier of normal tissues by protecting endothelial cells from death, or by inhibiting vascular inflammation and endothelial cell activation. Apart from approaches that aim to radiosensitise tumours by directly targeting cancer cells, the manipulation of the TME based on the tumour vasculature and on the immune system has aroused great interest. The different endothelial-oriented strategies to improve RT are detailed below and illustrated in Figure 4 for normal tissues and in Figure 5 for tumours.
Figure 4.
Potential endothelial-oriented strategies to spare normal tissue by targeting cytotoxicity, coagulation, activation of the immune-inflammatory response and senescence. PAI-1, plasminogen activator inhibitor-type 1; ROS, reactive oxygen species; SASP, senescence-associated secretory phenotype; vWF, von Willebrand factor.
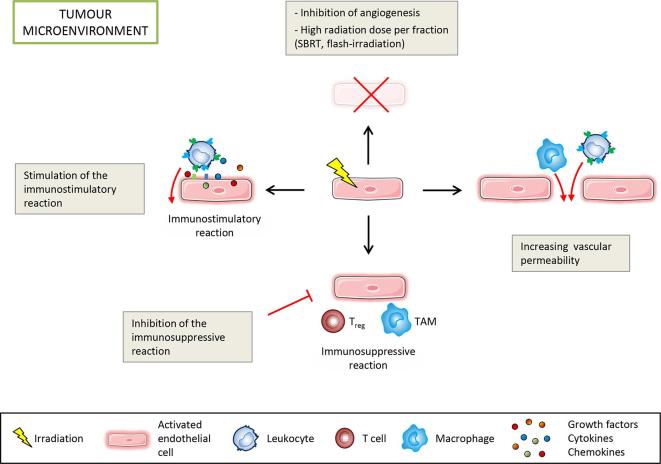
Figure 5.
Endothelial-oriented strategies to enhance tumour control by targeting the immunostimulatory and immunosuppressive reactions, inducing cell death and increasing vascular permeability to allow immune cell infiltration. SBRT, stereotactic body radiation therapy, TAM, tumour-associated macrophage.
Endothelial-oriented strategies to spare normal tissue
Preventing endothelial cytotoxicity
Among effective modifiers of radiation-induced endothelial cytotoxicity in vivo and in vitro, bFGF has generated great interest in the protection of endothelial cells from apoptosis.28, 152,153 Administration of bFGF increases the endothelial expression of thrombomodulin and is effective in reducing fibrosis, for instance within the urinary bladder.154 Without going through bFGF, direct activation of the thrombomodulin-activated protein C (TM-APC) pathway has beneficial biological effects on the vasculature because of its anti-inflammatory, cytoprotective, anti-fibrinolytic, anti-oxidant and anti-coagulant properties.155 By using a pharmacological strategy to activate this pathway, mitigation of radiation toxicity was achieved in a relevant model of RT in which a loop of rat small bowel was exposed to nine daily doses of 5 Gy.156 On the other hand, the targeting of CD47, a thrombospondin-1 receptor, has demonstrated that inhibiting CD47 signalling maintains the viability of normal tissues following irradiation, likely through radioprotection of endothelial cells, while increasing the radiosensitivity of tumours.157 Also, an angiopoietin-1 mimic significantly reduces skin radiation toxicity, potentially by increasing survival and function of irradiated endothelial cells through tyrosine kinase with immunoglobulin and epidermal growth factor homology domains 2 (Tie2) receptor activation.158 In addition, the use of a ceramide-targeting antibody to prevent ceramide platform formation in endothelial cells protects against gastrointestinal syndrome.159 Also, a strategy that targets PAI-1 to prevent endothelial cell death has been proposed to mitigate the severity of intestinal radiation injury.160 This work showed a promising temporary protection against early lethality in a mouse model of radiation-induced enteropathy.
Decreasing coagulopathy
A radiation-induced decrease in endothelial thrombomodulin-1 leads to an increase in thrombin, which results in activation of blood clotting. In liver, lung and heart, the lumen of central veins becomes blocked by fibrillar material resulting in obstruction of irradiated vessels with platelet aggregates.161 Therefore, there has been great interest in modulating blood coagulation as a strategy to reduce radiation toxicity of normal tissue. Increased platelet adherence to irradiated endothelial monolayers can be blocked by anti-von Willebrand factor antibodies.162 The use of anti-coagulants such as heparin has been investigated, but the results to date have been mostly insignificant and generally inconsistent.163 In contrast, the TM–APC pathway is a hopeful target for preventing or treating radiation toxicity in normal tissues using strategies aimed at restoring or preserving endothelial TM or replacing protein C.156, 164,165 Also, thrombin inhibition has been investigated as a strategy to minimise the side-effects of RT. The recombinant thrombin inhibitor hirudin has shown a protective effect against small bowel radiation toxicity in a model of localised small bowel radiation in rats.166 However, the authors felt that targeting specific thrombin functions may be superior to global thrombin inhibition. On the other hand, the administration of the thrombin peptide TP508 has been shown to activate endothelial cells and stem cells to revascularise and regenerate tissues in a whole-body irradiation model,167, 168 suggesting a possible use to limit RT side-effects.
Targeting premature endothelial senescence
Approaches to target endothelial cell senescence to prevent, mitigate and treat radiation-induced cardiovascular diseases are under investigation.31 The different approaches rely on three main strategies: (i) preventing endothelial cells from becoming senescent using anti-oxidants to scavenge ROS169 or inhibitors of the IGF1/PI3K/Akt/mTOR pathway that activate radiation-induced endothelial senescence;170 (ii) suppression of SASP to prevent most of the deleterious effects of senescent cells,171 for instance by using RNAi or JAK inhibitors to target the JAK pathway,172 an upstream regulatory pathway of SASP; and (iii) clearing senescent endothelial cells with senolytic drugs to selectively kill senescent cells.173 Clearance of senescent cells in mice by ABT263, a Bcl-2/xL-specific inhibitor, effectively cleared senescent cells in several tissues, including senescent bone marrow hematopoietic stem cells and muscle stem cells, and suppressed SASP in the lungs.174 Nevertheless, inhibiting the induction of senescence could be detrimental by increasing tumorigenesis and decreasing tumour response to RT. Moreover, since senescent cells persist in vivo, their elimination or death may ultimately be worse with deleterious effects on the irradiated organ, despite their dysfunction and their key role in radiation-induced diseases, as discussed previously.175
Modulation of the immune-inflammatory response
A number of studies suggest that the recruitment of immune cells may become detrimental to healthy tissues when chronic and unresolved,22 and possibly participate in the initiation and/or the development of acute and late adverse tissue effects in the course of RT. Thus, strategies that target normal tissue-associated endothelial cells would be of interest to impair their ability to recruit immune cells. In such a strategy, the question of the timing of the modulation should be explored in depth. Many studies have aimed to identify strategies to antagonise adhesion molecule function, by either preventing interactions with receptors or inhibiting NF-κB signalling, to limit the inflammatory response. Hitherto, no clinical strategy has emerged that controls inflammation by targeting expression of the NF-κB-dependent endothelial adhesion molecule. However, mechanisms independent of the NF-κB pathway may also regulate adhesion molecule function, including post-transcriptional regulation by IL-19176 and post-translational modification by N-linked sugars.39 Since leukocyte trafficking in inflammation is actually governed by protein-glycan interaction,19 understanding of these complex interactions raises great hopes for the emergence of new therapeutic targets to treat inflammatory diseases such as atherosclerosis, intestinal bowel disease and thus also for radiation diseases.40
Endothelial-oriented strategies to target tumours
Increasing injury to tumour vasculature
The strategy of destroying the tumour vasculature has long been considered of interest in enhancing the potential of RT, although a poorly vascularised tumour may then prove to be more resistant to ionising radiation due to lower oxygen content. Using anti-VEGF antibodies (bevacizumab), anti-angiogenic therapies have an anti-vascular effect in human tumours, but are still far from being effective in monotherapy.177 On the other hand, the benefit of the combination of anti-angiogenic therapy with other conventional treatments such as RT or chemo-RT has been reported in numerous studies.177, 178 However, combined therapy was found to be associated with increased severe toxicity, indicating the need to improve timing and dose delivery in the future.178, 179Radiation-induced damage to endothelial cells has been proposed to explain the therapeutic advantage of SBRT, but the targets that allow this enhanced therapeutic response are still the subject of debate.29 In particular, the aforementioned study of Moding et al reported cancer cells as primary targets of SBRT rather than endothelial cells.77 In the same way, differences in the radiosensitivity of endothelial cells in the tumour and the normal tissues could explain the differential effect of flash-irradiation with a dose rate above 100 Gy s–1.180, 181
Cancer immunotherapy
The tumour immune response largely involves the vascular system, which allows the transport of immune cells to the tumour. Radiation facilitates the trafficking, homing and extravasation of effector cytotoxic CD8+ T-cells into the tumour, resulting in radiation-induced immunogenic cell death.182 The complex reactions of the immune system in an irradiated TME are both immunostimulatory and immunosuppressive.104 Immunostimulatory effects come from the recruitment of circulating immune cells due to the production of cytokines and other pro-inflammatory factors by the tumour and its microenvironment, antigen exposure and dendritic cell priming as well as activation of the endothelial cells present in the tumour. In contrast, tumours and their microenvironment contain immunosuppressive immune cells like tumour-associated macrophages (TAMs), which resemble the alternatively activated M2 macrophage, and TReg cells that have immunosuppressive and tolerising effects. If radiation-induced immunosuppressive effects could be overcome and immunostimulatory effects enhanced, or both, RT would promote strong responses against tumour cells. A strategy to improve the recruitment of immune cells in tumours to enhance tumour cell death would be to manipulate the tumour-associated endothelial cells. In this way, Wilson et al have shown that the delivery of miR-103 in tumour-bearing mice leads to decreased angiogenesis and tumour growth by radiosensitisation of tumour cells.183 Also, a strategy that consists in increasing the ability of endothelial cells to adhere to circulating cells would also allow immune infiltration of the tumours to promote tumour cell death. However, to date, the molecular targets and the molecules to test have yet to be discovered.
Combining immunotherapy, anti-angiogenic therapy and radiotherapy
A therapeutic perspective might consider the combination of immune checkpoint inhibitors, angiogenic inhibitors and RT, although this warrants further investigation.184 On one hand, an immunotherapy anti-angiogenic combination has given promising results.185, 186 In such therapy, the immunostimulatory effects of inhibitors of immune checkpoint regulators, such as antibodies against PD-1 or its ligand PD-ligand 1 (PD-L1), or cytotoxic lymphocyte-associated antigen-4 (CTLA-4), are combined with antibodies against VEGF, or its receptor VEGFR, which have immunosuppressive properties through inhibition of dendritic cell maturation in addition to their angiogenic properties. On the other hand, as discussed previously, RT has immunosuppressive effects and can be combined with anti-angiogenic therapy to increase efficacy. The combination of these three therapies is certainly an innovative approach with potential clinical benefits.
Opening the endothelium for drug delivery
Local tumour irradiation (≥15 Gy) has recently been shown to substantially improve the delivery of therapeutic nanoparticles in a pre-clinical study with a benefit in tumour control.187 Interestingly, TAM, which can serve as a nanoparticle drug depot,188 increased in the vicinity of the microvasculature after therapeutic priming of the TME by irradiation, which finally increased therapeutic nanoparticle delivery. In this model, radiation initiated a vascular burst of TAM extravasation through a cascade of changes to the tumour vasculature and microenvironment, leading to increased uptake of the drug in neighbouring tumour cells. Vessel thickening, tortuous vascular branching and perivascular TAM localisation were induced by radiation, all of which helped to enhance vessel permeability, allowing selective therapeutic nanoparticle delivery through TAM and improving tumour killing. These findings show that TAM were beneficial for drug delivery and, since RT stimulates an increase in TAM relative to the tumour,125, 187,189 open new interesting perspectives for combined RT and therapeutic nanoparticle treatment with broad applicability.
Since the BBB can be disrupted by RT, the modulation of BBB permeability by SBRT has been proposed to enhance drug delivery to the brain.190 For instance, the idea of combining the use of RT to open the BBB with i.v. injection of nanoparticles is an interesting new concept for the treatment of brain tumours. Nanoparticles are a potential tool in the treatment of cancer because of their low toxicity and their ability to increase vascular sensitivity to radiation.191 They significantly increase the DNA damage to blood vessels of the brain induced by ionising radiation. It has therefore been proposed that low doses of irradiation could locally increase the response of endothelial cells and thereby increase the permeability of the BBB with limited toxicity. Given the increased accuracy of RT, it would theoretically be possible to disrupt the BBB in strategic locations in the tumour bed prior to the administration of chemotherapy to increase its distribution and efficacy. However, it is not known which dose or dose schedule will best achieve the desired results or when permeability peaks after RT.
CONCLUSIONS
In the light of current knowledge, the vascular endothelium can be considered as a principal checkpoint for radiation-induced inflammatory and immunity processes following radiation exposure in both normal tissues and tumours. The endothelium could therefore be an ideal target compartment for improving the therapeutic index of RT. Future studies should focus on endothelial molecular targets of both normal tissues and tumours, but with opposite objectives: in normal tissues, therapeutic strategies will aim to modulate immune-inflammatory cell entry, at times which will have to be precisely determined for each treatment, whereas in tumours, treatments associated with RT and other treatments will aim to open the endothelium barrier so as to enhance the immune response.
Contributor Information
Olivier Guipaud, Email: olivier.guipaud@irsn.fr.
Cyprien Jaillet, Email: cyprienjaillet@gmail.com.
Karen Clément-Colmou, Email: karen.colmou@etu.univ-nantes.fr.
Agnès François, Email: agnes.francois@irsn.fr.
Stéphane Supiot, Email: Stephane.Supiot@ico.unicancer.fr.
Fabien Milliat, Email: fabien.milliat@irsn.fr.
REFERENCES
- 1.Shrieve DC, Loeffler JS. Human radiation injury. Philadelphia: The British Institute of Radiology.; 2011. [Google Scholar]
- 2.Stone HB, McBride WH, Coleman CN. Modifying normal tissue damage postirradiation. Report of a workshop sponsored by the Radiation Research Program, National Cancer Institute, Bethesda, Maryland, September 6-8, 2000. Radiat Res 2002; 157: 204–23. [DOI] [PubMed] [Google Scholar]
- 3.DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s cancer: principles & practice of oncology: The British Institute of Radiology.; 2011. [Google Scholar]
- 4.Moding EJ, Kastan MB, Kirsch DG. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat Rev Drug Discov 2013; 12: 526–42. doi: 10.1038/nrd4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 2011; 11: 239–53. doi: 10.1038/nrc3007 [DOI] [PubMed] [Google Scholar]
- 6.Liauw SL, Connell PP, Weichselbaum RR. New paradigms and future challenges in radiation oncology: an update of biological targets and technology. Sci Transl Med 2013; 5: 173sr2. doi: 10.1126/scitranslmed.3005148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer 2010; 10: 505–14. doi: 10.1038/nrc2868 [DOI] [PubMed] [Google Scholar]
- 8.Azimzadeh O, Sievert W, Sarioglu H, Merl-Pham J, Yentrapalli R, Bakshi MV, et al. Integrative proteomics and targeted transcriptomics analyses in cardiac endothelial cells unravel mechanisms of long-term radiation-induced vascular dysfunction. J Proteome Res 2015; 14: 1203–19. doi: 10.1021/pr501141b [DOI] [PubMed] [Google Scholar]
- 9.Heinonen M, Guipaud O, Milliat F, Buard V, Micheau B, Tarlet G, et al. Detecting time periods of differential gene expression using Gaussian processes: an application to endothelial cells exposed to radiotherapy dose fraction. Bioinformatics 2015; 31: 728–35. doi: 10.1093/bioinformatics/btu699 [DOI] [PubMed] [Google Scholar]
- 10.François A, Milliat F, Guipaud O, Benderitter M. Inflammation and immunity in radiation damage to the gut mucosa. Biomed Res Int 2013; 2013: 1–9. doi: 10.1155/2013/123241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14: 1014–22. doi: 10.1038/ni.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weninger W, Biro M, Jain R. Leukocyte migration in the interstitial space of non-lymphoid organs. Nat Rev Immunol 2014; 14: 232–46. doi: 10.1038/nri3641 [DOI] [PubMed] [Google Scholar]
- 13.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007; 7: 678–89. doi: 10.1038/nri2156 [DOI] [PubMed] [Google Scholar]
- 14.Mauge L, Terme M, Tartour E, Helley D. Control of the adaptive immune response by tumor vasculature. Front Oncol 2014; 4: 61. doi: 10.3389/fonc.2014.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zitvogel L, Kroemer G. Subversion of anticancer immunosurveillance by radiotherapy. Nat Immunol 2015; 16: 1005–7. doi: 10.1038/ni.3236 [DOI] [PubMed] [Google Scholar]
- 16.Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity 2014; 41: 694–707. doi: 10.1016/j.immuni.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 17.Granger DN, Senchenkova E. Inflammation and the microcirculation: The British Institute of Radiology.; 2010. [PubMed] [Google Scholar]
- 18.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 2011; 118: 6743–51. doi: 10.1182/blood-2011-07-343566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott DW, Patel RP. Endothelial heterogeneity and adhesion molecules N-glycosylation: implications in leukocyte trafficking in inflammation. Glycobiology 2013; 23: 622–33. doi: 10.1093/glycob/cwt014 [DOI] [PubMed] [Google Scholar]
- 20.Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol 2002; 283: H1282–H1291. doi: 10.1152/ajpheart.00117.2002 [DOI] [PubMed] [Google Scholar]
- 21.Henry CBS, Duling BR. TNF-α increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol 2000; 279: H2815–H2823. doi: 10.1152/ajpheart.2000.279.6.H2815 [DOI] [PubMed] [Google Scholar]
- 22.Korpela E, Liu SK. Endothelial perturbations and therapeutic strategies in normal tissue radiation damage. Radiat Oncol 2014; 9: 266. doi: 10.1186/s13014-014-0266-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milliat F, François A, Tamarat R, Benderitter M. Role of endothelium in radiation-induced normal tissue damages. Ann Cardiol Angeiol 2008; 57: 139–48. doi: 10.1016/j.ancard.2008.02.015 [DOI] [PubMed] [Google Scholar]
- 24.Baselet B, Azimzadeh O, Erbeldinger N, Bakshi MV, Dettmering T, Janssen A, et al. Differential impact of single-dose Fe ion and X-ray irradiation on endothelial cell transcriptomic and proteomic responses. Front Pharmacol 2017; 8: 570. doi: 10.3389/fphar.2017.00570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helm A, Lee R, Durante M, Ritter S. The influence of C-ions and X-rays on human umbilical vein endothelial cells. Front Oncol 2016; 6: 5. doi: 10.3389/fonc.2016.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol 2010; 31: 363–72. doi: 10.1007/s13277-010-0042-8 [DOI] [PubMed] [Google Scholar]
- 27.Corre I, Niaudet C, Paris F. Plasma membrane signaling induced by ionizing radiation. Mutat Res 2010; 704: 61–7. doi: 10.1016/j.mrrev.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 28.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001; 293: 293–7. doi: 10.1126/science.1060191 [DOI] [PubMed] [Google Scholar]
- 29.Karam SD, Bhatia S. The radiobiological targets of SBRT: tumor cells or endothelial cells? Ann Transl Med 2015; 3: 290. doi: 10.3978/j.issn.2305-5839.2015.09.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lafargue A, Degorre C, Corre I, Alves-Guerra MC, Gaugler MH, Vallette F, et al. Ionizing radiation induces long-term senescence in endothelial cells through mitochondrial respiratory complex II dysfunction and superoxide generation. Free Radic Biol Med 2017; 108: 750–9. doi: 10.1016/j.freeradbiomed.2017.04.019 [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Boerma M, Zhou D. Ionizing radiation-induced endothelial cell senescence and cardiovascular diseases. Radiat Res 2016; 186: 153–61. doi: 10.1667/RR14445.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao JK. Linking endothelial dysfunction with endothelial cell activation. J Clin Invest 2013; 123: 540–1. doi: 10.1172/JCI66843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heckmann M, Douwes K, Peter R, Degitz K. Vascular activation of adhesion molecule mRNA and cell surface expression by ionizing radiation. Exp Cell Res 1998; 238: 148–54. doi: 10.1006/excr.1997.3826 [DOI] [PubMed] [Google Scholar]
- 34.Quarmby S, Kumar P, Wang J, Macro JA, Hutchinson JJ, Hunter RD, et al. Irradiation induces upregulation of CD31 in human endothelial cells. Arterioscler Thromb Vasc Biol 1999; 19: 588–97. doi: 10.1161/01.ATV.19.3.588 [DOI] [PubMed] [Google Scholar]
- 35.Quarmby S, Hunter RD, Kumar S. Irradiation induced expression of CD31, ICAM-1 and VCAM-1 in human microvascular endothelial cells. Anticancer Res 2000; 20: 3375–81. [PubMed] [Google Scholar]
- 36.Gaugler MH, Vereycken-Holler V, Squiban C, Aigueperse J. PECAM-1 (CD31) is required for interactions of platelets with endothelial cells after irradiation. J Thromb Haemost 2004; 2: 2020–6. doi: 10.1111/j.1538-7836.2004.00951.x [DOI] [PubMed] [Google Scholar]
- 37.Mouthon MA, Vereycken-Holler V, Van der Meeren A, Gaugler MH. Irradiation increases the interactions of platelets with the endothelium in vivo: analysis by intravital microscopy. Radiat Res 2003; 160: 593–9. doi: 10.1667/3068 [DOI] [PubMed] [Google Scholar]
- 38.Chacko BK, Scott DW, Chandler RT, Patel RP. Endothelial surface N-glycans mediate monocyte adhesion and are targets for anti-inflammatory effects of peroxisome proliferator-activated receptor γ ligands. J Biol Chem 2011; 286: 38738–47. doi: 10.1074/jbc.M111.247981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott DW, Chen J, Chacko BK, Traylor JG, Orr AW, Patel RP. Role of endothelial N-glycan mannose residues in monocyte recruitment during atherogenesis. Arterioscler Thromb Vasc Biol 2012; 32: e51–e59. doi: 10.1161/ATVBAHA.112.253203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaillet C, Morelle W, Slomianny MC, Paget V, Tarlet G, Buard V, et al. Radiation-induced changes in the glycome of endothelial cells with functional consequences. Sci Rep 2017; 7: 5290. doi: 10.1038/s41598-017-05563-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mollà M, Gironella M, Miquel R, Tovar V, Engel P, Biete A, et al. Relative roles of ICAM-1 and VCAM-1 in the pathogenesis of experimental radiation-induced intestinal inflammation. Int J Radiat Oncol Biol Phys 2003; 57: 264–73. doi: 10.1016/S0360-3016(03)00523-6 [DOI] [PubMed] [Google Scholar]
- 42.Hallahan DE, Virudachalam S. Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation. Proc Natl Acad Sci U S A 1997; 94: 6432–7. doi: 10.1073/pnas.94.12.6432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halle M, Ekström M, Farnebo F, Tornvall P. Endothelial activation with prothrombotic response in irradiated microvascular recipient veins. J Plast Reconstr Aesthet Surg 2010; 63: 1910–6. doi: 10.1016/j.bjps.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 44.Halle M, Gabrielsen A, Paulsson-Berne G, Gahm C, Agardh HE, Farnebo F, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 2010; 55: 1227–36. doi: 10.1016/j.jacc.2009.10.047 [DOI] [PubMed] [Google Scholar]
- 45.Halle M, Hall P, Tornvall P. Cardiovascular disease associated with radiotherapy: activation of nuclear factor kappa-B. J Intern Med 2011; 269: 469–77. doi: 10.1111/j.1365-2796.2011.02353.x [DOI] [PubMed] [Google Scholar]
- 46.Halle M, Tornvall P. Beyond nuclear factor kappaB in cardiovascular disease induced by radiotherapy. J Intern Med 2011; 270: 486. doi: 10.1111/j.1365-2796.2011.02439.x [DOI] [PubMed] [Google Scholar]
- 47.Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 2015; 4: 27066. doi: 10.3402/jev.v4.27066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018; 19: 213–28. doi: 10.1038/nrm.2017.125 [DOI] [PubMed] [Google Scholar]
- 49.Hromada C, Mühleder S, Grillari J, Redl H, Holnthoner W. Endothelial extracellular vesicles-promises and challenges. Front Physiol 2017; 8: 275. doi: 10.3389/fphys.2017.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu S, Wang J, Ding N, Hu W, Zhang X, Wang B, et al. Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol 2015; 12: 1355–63. doi: 10.1080/15476286.2015.1100795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albanese J, Dainiak N. Modulation of intercellular communication mediated at the cell surface and on extracellular, plasma membrane–derived vesicles by ionizing radiation. Exp Hematol 2003; 31: 455–64. doi: 10.1016/S0301-472X(03)00050-X [DOI] [PubMed] [Google Scholar]
- 52.Jella KK, Rani S, O'Driscoll L, McClean B, Byrne HJ, Lyng FM. Exosomes are involved in mediating radiation induced bystander signaling in human keratinocyte cells. Radiat Res 2014; 181: 138–45. doi: 10.1667/RR13337.1 [DOI] [PubMed] [Google Scholar]
- 53.Al-Mayah AH, Irons SL, Pink RC, Carter DR, Kadhim MA. Possible role of exosomes containing RNA in mediating nontargeted effect of ionizing radiation. Radiat Res 2012; 177: 539–45. doi: 10.1667/RR2868.1 [DOI] [PubMed] [Google Scholar]
- 54.Al-Mayah A, Bright S, Chapman K, Irons S, Luo P, Carter D, et al. The non-targeted effects of radiation are perpetuated by exosomes. Mutat Res 2015; 772: 38–45. doi: 10.1016/j.mrfmmm.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 55.Szotowski B, Antoniak S, Goldin-Lang P, Tran QV, Pels K, Rosenthal P, et al. Antioxidative treatment inhibits the release of thrombogenic tissue factor from irradiation- and cytokine-induced endothelial cells. Cardiovasc Res 2007; 73: 806–12. doi: 10.1016/j.cardiores.2006.12.018 [DOI] [PubMed] [Google Scholar]
- 56.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol 2011; 31: 27–33. doi: 10.1161/ATVBAHA.110.218123 [DOI] [PubMed] [Google Scholar]
- 57.Krishnan EC, Krishnan L, Jewell B, Bhatia P, Jewell WR. Dose-dependent radiation effect on microvasculature and repair. J Natl Cancer Inst 1987; 79: 1321–5. [PubMed] [Google Scholar]
- 58.Park KR, Monsky WL, Lee CG, Song CH, Kim DH, Jain RK, et al. Mast cells contribute to radiation-induced vascular hyperpermeability. Radiat Res 2016; 185: 182–9. doi: 10.1667/RR14190.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gabryś D, Greco O, Patel G, Prise KM, Tozer GM, Kanthou C. Radiation effects on the cytoskeleton of endothelial cells and endothelial monolayer permeability. Int J Radiat Oncol Biol Phys 2007; 69: 1553–62. doi: 10.1016/j.ijrobp.2007.08.039 [DOI] [PubMed] [Google Scholar]
- 60.Cervelli T, Panetta D, Navarra T, Andreassi MG, Basta G, Galli A, et al. Effects of single and fractionated low-dose irradiation on vascular endothelial cells. Atherosclerosis 2014; 235: 510–8. doi: 10.1016/j.atherosclerosis.2014.05.932 [DOI] [PubMed] [Google Scholar]
- 61.Potiron VA, Abderrahmani R, Clément-Colmou K, Marionneau-Lambot S, Oullier T, Paris F, et al. Improved functionality of the vasculature during conventionally fractionated radiation therapy of prostate cancer. PLoS One 2013; 8: e84076. doi: 10.1371/journal.pone.0084076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burrell K, Hill RP, Zadeh G. High-resolution in-vivo analysis of normal brain response to cranial irradiation. PLoS One 2012; 7: e38366. doi: 10.1371/journal.pone.0038366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ceelen W, Smeets P, Backes W, Van Damme N, Boterberg T, Demetter P, et al. Noninvasive monitoring of radiotherapy-induced microvascular changes using dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) in a colorectal tumor model. Int J Radiat Oncol Biol Phys 2006; 64: 1188–96. doi: 10.1016/j.ijrobp.2005.10.026 [DOI] [PubMed] [Google Scholar]
- 64.Fauquette W, Amourette C, Dehouck MP, Diserbo M. Radiation-induced blood-brain barrier damages: an in vitro study. Brain Res 2012; 1433: 114–26. doi: 10.1016/j.brainres.2011.11.022 [DOI] [PubMed] [Google Scholar]
- 65.Li YQ, Chen P, Jain V, Reilly RM, Wong CS. Early radiation-induced endothelial cell loss and blood-spinal cord barrier breakdown in the rat spinal cord. Radiat Res 2004; 161: 143–52. [DOI] [PubMed] [Google Scholar]
- 66.Debbage PL, Seidl S, Kreczy A, Hutzler P, Pavelka M, Lukas P. Vascular permeability and hyperpermeability in a murine adenocarcinoma after fractionated radiotherapy: an ultrastructural tracer study. Histochem Cell Biol 2000; 114: 259–75. [DOI] [PubMed] [Google Scholar]
- 67.Lee WH, Warrington JP, Sonntag WE, Lee YW. Irradiation alters MMP-2/TIMP-2 system and collagen type IV degradation in brain. Int J Radiat Oncol Biol Phys 2012; 82: 1559–66. doi: 10.1016/j.ijrobp.2010.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Vulpen M, Kal HB, Taphoorn MJ, El-Sharouni SY. Changes in blood-brain barrier permeability induced by radiotherapy: implications for timing of chemotherapy? (Review). Oncol Rep 2002; 9: 683–8. doi: 10.3892/or.9.4.683 [DOI] [PubMed] [Google Scholar]
- 69.Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res 2012; 177: 311–27. doi: 10.1667/RR2773.1 [DOI] [PubMed] [Google Scholar]
- 70.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer 2008; 8: 180–92. doi: 10.1038/nrc2344 [DOI] [PubMed] [Google Scholar]
- 71.Milas L, Hunter N, Peters LJ. The tumor bed effect: dependence of tumor take, growth rate, and metastasis on the time interval between irradiation and tumor cell transplantation. Int J Radiat Oncol Biol Phys 1987; 13: 379–83. doi: 10.1016/0360-3016(87)90012-5 [DOI] [PubMed] [Google Scholar]
- 72.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003; 300: 1155–9. doi: 10.1126/science.1082504 [DOI] [PubMed] [Google Scholar]
- 73.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene 2003; 22: 5897–906. doi: 10.1038/sj.onc.1206702 [DOI] [PubMed] [Google Scholar]
- 74.Hill RP. The changing paradigm of tumour response to irradiation. Br J Radiol 2017; 90: 20160474. doi: 10.1259/bjr.20160474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Budach W, Taghian A, Freeman J, Gioioso D, Suit HD. Impact of stromal sensitivity on radiation response of tumors. J Natl Cancer Inst 1993; 85: 988–93. doi: 10.1093/jnci/85.12.988 [DOI] [PubMed] [Google Scholar]
- 76.Moding EJ, Lee CL, Castle KD, Oh P, Mao L, Zha S, et al. Atm deletion with dual recombinase technology preferentially radiosensitizes tumor endothelium. J Clin Invest 2014; 124: 3325–38. doi: 10.1172/JCI73932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moding EJ, Castle KD, Perez BA, Oh P, Min HD, Norris H, et al. Tumor cells, but not endothelial cells, mediate eradication of primary sarcomas by stereotactic body radiation therapy. Sci Transl Med 2015; 7: 278ra34. doi: 10.1126/scitranslmed.aaa4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen FH, Chiang CS, Wang CC, Tsai CS, Jung SM, Lee CC, et al. Radiotherapy decreases vascular density and causes hypoxia with macrophage aggregation in TRAMP-C1 prostate tumors. Clin Cancer Res 2009; 15: 1721–9. doi: 10.1158/1078-0432.CCR-08-1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jani A, Shaikh F, Barton S, Willis C, Banerjee D, Mitchell J, et al. High-dose, single-fraction irradiation rapidly reduces tumor vasculature and perfusion in a xenograft model of neuroblastoma. Int J Radiat Oncol Biol Phys 2016; 94: 1173–80. doi: 10.1016/j.ijrobp.2015.12.367 [DOI] [PubMed] [Google Scholar]
- 80.Lan J, Wan XL, Deng L, Xue JX, Wang LS, Meng MB, et al. Ablative hypofractionated radiotherapy normalizes tumor vasculature in lewis lung carcinoma mice model. Radiat Res 2013; 179: 458–64. doi: 10.1667/RR3116.1 [DOI] [PubMed] [Google Scholar]
- 81.Song C, Hong BJ, Bok S, Lee CJ, Kim YE, Jeon SR, et al. Real-time tumor oxygenation changes after single high-dose radiation therapy in orthotopic and subcutaneous lung cancer in mice: clinical implication for stereotactic ablative radiation therapy schedule optimization. Int J Radiat Oncol Biol Phys 2016; 95: 1022–31. doi: 10.1016/j.ijrobp.2016.01.064 [DOI] [PubMed] [Google Scholar]
- 82.Hu F, Vishwanath K, Salama JK, Erkanli A, Peterson B, Oleson JR, et al. Oxygen and perfusion kinetics in response to fractionated radiation therapy in FaDu xenografts head and neck cancer xenografts are related to treatment outcome. Int J Radiat Oncol Biol Phys 2016; 96: 462–9. doi: 10.1016/j.ijrobp.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011; 21: 193–215. doi: 10.1016/j.devcel.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 84.Cooke VG, LeBleu VS, Keskin D, Khan Z, O'Connell JT, Teng Y, et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 2012; 21: 66–81. doi: 10.1016/j.ccr.2011.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim J, de Sampaio PC, Lundy DM, Peng Q, Evans KW, Sugimoto H, et al. Heterogeneous perivascular cell coverage affects breast cancer metastasis and response to chemotherapy. JCI Insight 2016; 1: e90733. doi: 10.1172/jci.insight.90733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature 2008; 453: 410–4. doi: 10.1038/nature06868 [DOI] [PubMed] [Google Scholar]
- 87.Kane JL, Krueger SA, Hanna A, Raffel TR, Wilson GD, Madlambayan GJ, et al. Effect of irradiation on tumor microenvironment and bone marrow cell migration in a preclinical tumor model. Int J Radiat Oncol Biol Phys 2016; 96: 170–8. doi: 10.1016/j.ijrobp.2016.04.028 [DOI] [PubMed] [Google Scholar]
- 88.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res 2013; 73: 2943–8. doi: 10.1158/0008-5472.CAN-12-4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 1989; 49: 6449–65. [PubMed] [Google Scholar]
- 90.Wang HH, Cui YL, Zaorsky NG, Lan J, Deng L, Zeng XL, et al. Mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after stereotactic body radiation therapy. Cancer Lett 2016; 375: 349–59. doi: 10.1016/j.canlet.2016.02.033 [DOI] [PubMed] [Google Scholar]
- 91.Fajardo LF. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol 2005; 44: 13–22. doi: 10.1080/02841860510007440 [DOI] [PubMed] [Google Scholar]
- 92.Stewart FA, Heeneman S, Te Poele J, Kruse J, Russell NS, Gijbels M, et al. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE-/- mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol 2006; 168: 649–58. doi: 10.2353/ajpath.2006.050409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Borghini A, Gianicolo EA, Picano E, Andreassi MG. Ionizing radiation and atherosclerosis: current knowledge and future challenges. Atherosclerosis 2013; 230: 40–7. doi: 10.1016/j.atherosclerosis.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 94.Richter KK, Fink LM, Hughes BM, Sung CC, Hauer-Jensen M. Is the loss of endothelial thrombomodulin involved in the mechanism of chronicity in late radiation enteropathy? Radiother Oncol 1997; 44: 65–71. doi: 10.1016/S0167-8140(97)00063-7 [DOI] [PubMed] [Google Scholar]
- 95.Richter KK, Fink LM, Hughes BM, Shmaysani HM, Sung CC, Hauer-Jensen M. Differential effect of radiation on endothelial cell function in rectal cancer and normal rectum. Am J Surg 1998; 176: 642–7. doi: 10.1016/S0002-9610(98)00280-3 [DOI] [PubMed] [Google Scholar]
- 96.Wang J, Zheng H, Ou X, Fink LM, Hauer-Jensen M. Deficiency of microvascular thrombomodulin and up-regulation of protease-activated receptor-1 in irradiated rat intestine: possible link between endothelial dysfunction and chronic radiation fibrosis. Am J Pathol 2002; 160: 2063–72. doi: 10.1016/S0002-9440(10)61156-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gaugler MH, Squiban C, van der Meeren A, Bertho JM, Vandamme M, Mouthon MA. Late and persistent up-regulation of intercellular adhesion molecule-1 (ICAM-1) expression by ionizing radiation in human endothelial cells in vitro. Int J Radiat Biol 1997; 72: 201–9. doi: 10.1080/095530097143428 [DOI] [PubMed] [Google Scholar]
- 98.Vujaskovic Z, Anscher MS, Feng QF, Rabbani ZN, Amin K, Samulski TS, et al. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys 2001; 50: 851–5. doi: 10.1016/S0360-3016(01)01593-0 [DOI] [PubMed] [Google Scholar]
- 99.Toullec A, Buard V, Rannou E, Tarlet G, Guipaud O, Robine S, et al. HIF-1α deletion in the endothelium, but not in the epithelium, protects from radiation-induced enteritis. Cell Mol Gastroenterol Hepatol 2018; 5: 15–30. doi: 10.1016/j.jcmgh.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rannou E, François A, Toullec A, Guipaud O, Buard V, Tarlet G, et al. In vivo evidence for an endothelium-dependent mechanism in radiation-induced normal tissue injury. Sci Rep 2015; 5: 15738. doi: 10.1038/srep15738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015; 16: e498–e509. doi: 10.1016/S1470-2045(15)00007-8 [DOI] [PubMed] [Google Scholar]
- 102.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, K.Wansley E, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006; 203: 1259–71. doi: 10.1084/jem.20052494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Golden EB, Apetoh L. Radiotherapy and immunogenic cell death. Semin Radiat Oncol 2015; 25: 11–17. doi: 10.1016/j.semradonc.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 104.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 2015; 15: 409–25. doi: 10.1038/nrc3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schaue D, McBride WH. Links between innate immunity and normal tissue radiobiology. Radiat Res 2010; 173: 406–17. doi: 10.1667/RR1931.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Demaria S, Formenti SC. Role of T lymphocytes in tumor response to radiotherapy. Front Oncol 2012; 2: 95. doi: 10.3389/fonc.2012.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008; 181: 3099–107. doi: 10.4049/jimmunol.181.5.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys 1995; 33: 99–109. doi: 10.1016/0360-3016(95)00095-G [DOI] [PubMed] [Google Scholar]
- 109.Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat Res 2012; 178: 505–23. doi: 10.1667/RR3031.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wirsdörfer F, Jendrossek V. The role of lymphocytes in radiotherapy-induced adverse late effects in the lung. Front Immunol 2016; 7: 591. doi: 10.3389/fimmu.2016.00591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mintet E, Lavigne J, Paget V, Tarlet G, Buard V, Guipaud O, et al. Endothelial Hey2 deletion reduces endothelial-to-mesenchymal transition and mitigates radiation proctitis in mice. Sci Rep 2017; 7: 4933. doi: 10.1038/s41598-017-05389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mintet E, Rannou E, Buard V, West G, Guipaud O, Tarlet G, et al. Identification of endothelial-to-mesenchymal transition as a potential participant in radiation proctitis. Am J Pathol 2015; 185: 2550–62. doi: 10.1016/j.ajpath.2015.04.028 [DOI] [PubMed] [Google Scholar]
- 113.Todd NW, Luzina IG, Atamas SP. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair 2012; 5: 11. doi: 10.1186/1755-1536-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schaue D, McBride WH. T lymphocytes and normal tissue responses to radiation. Front Oncol 2012; 2: 119. doi: 10.3389/fonc.2012.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol 2012; 33: 49–57. doi: 10.1016/j.it.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ganss R, Hanahan D. Tumor microenvironment can restrict the effectiveness of activated antitumor lymphocytes. Cancer Res 1998; 58: 4673–81. [PubMed] [Google Scholar]
- 117.Uldry E, Faes S, Demartines N, Dormond O. Fine-tuning tumor endothelial cells to selectively kill cancer. Int J Mol Sci 2017; 18: 1401. doi: 10.3390/ijms18071401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hendry SA, Farnsworth RH, Solomon B, Achen MG, Stacker SA, Fox SB. The role of the tumor vasculature in the host immune response: implications for therapeutic strategies targeting the tumor microenvironment. Front Immunol 2016; 7: 621. doi: 10.3389/fimmu.2016.00621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Griffioen AW, Damen CA, Blijham GH, Groenewegen G. Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood 1996; 88: 667–73. [PubMed] [Google Scholar]
- 120.Dirkx AE, Oude Egbrink MG, Kuijpers MJ, van der Niet ST, Heijnen VV, Bouma-ter Steege JC, et al. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res 2003; 63: 2322–9. [PubMed] [Google Scholar]
- 121.Griffioen AW, Damen CA, Martinotti S, Blijham GH, Groenewegen G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res 1996; 56: 1111–7. [PubMed] [Google Scholar]
- 122.Lanitis E, Irving M, Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr Opin Immunol 2015; 33: 55–63. doi: 10.1016/j.coi.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin 2017; 67: 65–85. doi: 10.3322/caac.21358 [DOI] [PubMed] [Google Scholar]
- 124.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 2004; 6: 553–63. doi: 10.1016/j.ccr.2004.10.011 [DOI] [PubMed] [Google Scholar]
- 125.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS⁺/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013; 24: 589–602. doi: 10.1016/j.ccr.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 126.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol 2009; 10: 718–26. doi: 10.1016/S1470-2045(09)70082-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C, et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol 2010; 22: 113–24. doi: 10.1016/j.smim.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 128.Wennerberg E, Lhuillier C, Vanpouille-Box C, Pilones KA, García-Martínez E, Rudqvist NP, et al. Barriers to radiation-induced in situ tumor vaccination. Front Immunol 2017; 8: 229. doi: 10.3389/fimmu.2017.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015; 41: 503–10. doi: 10.1016/j.ctrv.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sceneay J, Chow MT, Chen A, Halse HM, Wong CS, Andrews DM, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res 2012; 72: 3906–11. doi: 10.1158/0008-5472.CAN-11-3873 [DOI] [PubMed] [Google Scholar]
- 131.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 2010; 207: 2439–53. doi: 10.1084/jem.20100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res 2014; 74: 24–30. doi: 10.1158/0008-5472.CAN-13-1196 [DOI] [PubMed] [Google Scholar]
- 133.Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res 2010; 70: 7465–75. doi: 10.1158/0008-5472.CAN-10-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 2011; 475: 226–30. doi: 10.1038/nature10169 [DOI] [PubMed] [Google Scholar]
- 135.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014; 211: 781–90. doi: 10.1084/jem.20131916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ganss R, Ryschich E, Klar E, Arnold B, Hämmerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res 2002; 62: 1462–70. [PubMed] [Google Scholar]
- 137.Kinlay S, Michel T, Leopold JA. The future of vascular biology and medicine. Circulation 2016; 133: 2603–9. doi: 10.1161/CIRCULATIONAHA.116.023513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ferreira MR, Muls A, Dearnaley DP, Andreyev HJ. Microbiota and radiation-induced bowel toxicity: lessons from inflammatory bowel disease for the radiation oncologist. Lancet Oncol 2014; 15: e139–e147. doi: 10.1016/S1470-2045(13)70504-7 [DOI] [PubMed] [Google Scholar]
- 139.Gerassy-Vainberg S, Blatt A, Danin-Poleg Y, Gershovich K, Sabo E, Nevelsky A, et al. Radiation induces proinflammatory dysbiosis: transmission of inflammatory susceptibility by host cytokine induction. Gut 2018; 67: 97–107. doi: 10.1136/gutjnl-2017-313789 [DOI] [PubMed] [Google Scholar]
- 140.Cui M, Xiao H, Li Y, Zhou L, Zhao S, Luo D, et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol Med 2017; 9: 448–61. doi: https://doi.org/10.15252/emmm.201606932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Packey CD, Ciorba MA. Microbial influences on the small intestinal response to radiation injury. Curr Opin Gastroenterol 2010; 26: 88–94. doi: 10.1097/MOG.0b013e3283361927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer 2017; 17: 271–85. doi: 10.1038/nrc.2017.13 [DOI] [PubMed] [Google Scholar]
- 143.Chen Y, Zhao Y, Cheng Q, Wu D, Liu H. The role of intestinal microbiota in acute graft-versus-host disease. J Immunol Res 2015; 2015: 145859–9. doi: 10.1155/2015/145859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ratikan JA, Micewicz ED, Xie MW, Schaue D. Radiation takes its Toll. Cancer Lett 2015; 368: 238–45. doi: 10.1016/j.canlet.2015.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Crawford PA, Gordon JI. Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci U S A 2005; 102: 13254–9. doi: 10.1073/pnas.0504830102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018; 359: 104–8. doi: 10.1126/science.aao3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018; 359: 97–103. doi: 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359: 91–7. doi: 10.1126/science.aan3706 [DOI] [PubMed] [Google Scholar]
- 149.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013; 342: 967–70. doi: 10.1126/science.1240527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013; 342: 971–6. doi: 10.1126/science.1240537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and anticancer immunosurveillance. Cell 2016; 165: 276–87. doi: 10.1016/j.cell.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 152.Haimovitz-Friedman A, Balaban N, McLoughlin M, Ehleiter D, Michaeli J, Vlodavsky I, et al. Protein kinase C mediates basic fibroblast growth factor protection of endothelial cells against radiation-induced apoptosis. Cancer Res 1994; 54: 2591–7. [PubMed] [Google Scholar]
- 153.Fuks Z, Persaud RS, Alfieri A, McLoughlin M, Ehleiter D, Schwartz JL, et al. Basic fibroblast growth factor protects endothelial cells against radiation-induced programmed cell death in vitro and in vivo. Cancer Res 1994; 54: 2582–90. [PubMed] [Google Scholar]
- 154.Zhang S, Qiu X, Zhang Y, Fu K, Zhao X, Wu J, et al. Basic fibroblast growth factor ameliorates endothelial dysfunction in radiation-induced bladder injury. Biomed Res Int 2015; 2015: 967680–10. doi: 10.1155/2015/967680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ito T, Maruyama I. Thrombomodulin: protectorate god of the vasculature in thrombosis and inflammation. J Thromb Haemost 2011; 9(Suppl 1): 168–73. doi: 10.1111/j.1538-7836.2011.04319.x [DOI] [PubMed] [Google Scholar]
- 156.Pathak R, Wang J, Garg S, Aykin-Burns N, Petersen KU, Hauer-Jensen M. Recombinant thrombomodulin (solulin) ameliorates early intestinal radiation toxicity in a preclinical rat model. Radiat Res 2016; 186: 112–20. doi: 10.1667/RR14408.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M, et al. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med 2009; 1: 3ra7. doi: 10.1126/scitranslmed.3000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Korpela E, Yohan D, Chin LC, Kim A, Huang X, Sade S, et al. Vasculotide, an Angiopoietin-1 mimetic, reduces acute skin ionizing radiation damage in a preclinical mouse model. BMC Cancer 2014; 14: 614. doi: 10.1186/1471-2407-14-614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rotolo J, Stancevic B, Zhang J, Hua G, Fuller J, Yin X, et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J Clin Invest 2012; 122: 1786–90. doi: 10.1172/JCI59920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Abderrahmani R, François A, Buard V, Benderitter M, Sabourin JC, Crandall DL, et al. Effects of pharmacological inhibition and genetic deficiency of plasminogen activator inhibitor-1 in radiation-induced intestinal injury. Int J Radiat Oncol Biol Phys 2009; 74: 942–8. doi: 10.1016/j.ijrobp.2009.01.077 [DOI] [PubMed] [Google Scholar]
- 161.Ward WF, Molteni A, Ts’ao CH. Endothelial-oriented strategies to spare normal tissues : Rubin D. B, The radiation biology of the vascular endothelium: The British Institute of Radiology.; 1997. 185–208. [Google Scholar]
- 162.Verheij M, Dewit LG, Boomgaard MN, Brinkman HJ, van Mourik JA. Ionizing radiation enhances platelet adhesion to the extracellular matrix of human endothelial cells by an increase in the release of von Willebrand factor. Radiat Res 1994; 137: 202–7. doi: 10.2307/3578813 [DOI] [PubMed] [Google Scholar]
- 163.Hauer-Jensen M, Fink LM, Wang J. Radiation injury and the protein C pathway. Crit Care Med 2004; 32(5 Suppl): S325–S330. doi: 10.1097/01.CCM.0000126358.15697.75 [DOI] [PubMed] [Google Scholar]
- 164.Geiger H, Pawar SA, Kerschen EJ, Nattamai KJ, Hernandez I, Liang HP, et al. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat Med 2012; 18: 1123–9. doi: 10.1038/nm.2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kennedy AR, Maity A, Sanzari JK. A review of radiation-induced coagulopathy and new findings to support potential prevention strategies and treatments. Radiat Res 2016; 186: 121–40. doi: 10.1667/RR14406.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang J, Zheng H, Ou X, Albertson CM, Fink LM, Herbert JM, et al. Hirudin ameliorates intestinal radiation toxicity in the rat: support for thrombin inhibition as strategy to minimize side-effects after radiation therapy and as countermeasure against radiation exposure. J Thromb Haemost 2004; 2: 2027–35. doi: 10.1111/j.1538-7836.2004.00960.x [DOI] [PubMed] [Google Scholar]
- 167.Kantara C, Moya SM, Houchen CW, Umar S, Ullrich RL, Singh P, et al. Novel regenerative peptide TP508 mitigates radiation-induced gastrointestinal damage by activating stem cells and preserving crypt integrity. Lab Invest 2015; 95: 1222–33. doi: 10.1038/labinvest.2015.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Olszewska-Pazdrak B, McVicar SD, Rayavara K, Moya SM, Kantara C, Gammarano C, et al. Nuclear countermeasure activity of TP508 linked to restoration of endothelial function and acceleration of DNA repair. Radiat Res 2016; 186: 162–74. doi: 10.1667/RR14409.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dong X, Tong F, Qian C, Zhang R, Dong J, Wu G, et al. NEMO modulates radiation-induced endothelial senescence of human umbilical veins through NF-κB signal pathway. Radiat Res 2015; 183: 82–93. doi: 10.1667/RR13682.1 [DOI] [PubMed] [Google Scholar]
- 170.Panganiban RA, Day RM. Inhibition of IGF-1R prevents ionizing radiation-induced primary endothelial cell senescence. PLoS One 2013; 8: e78589. doi: 10.1371/journal.pone.0078589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 2013; 123: 966–72. doi: 10.1172/JCI64098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A 2015; 112: E6301–E6310. doi: 10.1073/pnas.1515386112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011; 479: 232–6. doi: 10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 2016; 22: 78–83. doi: 10.1038/nm.4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Day RM, Snow AL, Panganiban RA. Radiation-induced accelerated senescence: a fate worse than death? Cell Cycle 2014; 13: 2011–2. doi: 10.4161/cc.29457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.England RN, Preston KJ, Scalia R, Autieri MV. Interleukin-19 decreases leukocyte-endothelial cell interactions by reduction in endothelial cell adhesion molecule mRNA stability. Am J Physiol Cell Physiol 2013; 305: C255–C265. doi: 10.1152/ajpcell.00069.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hamming LC, Slotman BJ, Verheul HMW, Thijssen VL. The clinical application of angiostatic therapy in combination with radiotherapy: past, present, future. Angiogenesis 2017; 20: 217–32. doi: 10.1007/s10456-017-9546-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kleibeuker EA, Griffioen AW, Verheul HM, Slotman BJ, Thijssen VL. Combining angiogenesis inhibition and radiotherapy: a double-edged sword. Drug Resist Updat 2012; 15: 173–82. doi: 10.1016/j.drup.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 179.Kleibeuker EA, Ten Hooven MA, Verheul HM, Slotman BJ, Thijssen VL. Combining radiotherapy with sunitinib: lessons (to be) learned. Angiogenesis 2015; 18: 385–95. doi: 10.1007/s10456-015-9476-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med 2014; 6: 245ra93. doi: 10.1126/scitranslmed.3008973 [DOI] [PubMed] [Google Scholar]
- 181.Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond JF, Petit B, et al. Irradiation in a flash: unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother Oncol 2017; 124: 365–9. doi: 10.1016/j.radonc.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 182.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31: 51–72. doi: 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 183.Wilson R, Espinosa-Diez C, Kanner N, Chatterjee N, Ruhl R, Hipfinger C, et al. MicroRNA regulation of endothelial TREX1 reprograms the tumour microenvironment. Nat Commun 2016; 7: 13597. doi: 10.1038/ncomms13597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kamrava M, Bernstein MB, Camphausen K, Hodge JW. Combining radiation, immunotherapy, and antiangiogenesis agents in the management of cancer: the Three Musketeers or just another quixotic combination? Mol Biosyst 2009; 5: 1262–70. doi: 10.1039/b911313b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Garber K. Promising early results for immunotherapy-antiangiogenesis combination. J Natl Cancer Inst 2014; 106: dju392. doi: 10.1093/jnci/dju392 [DOI] [PubMed] [Google Scholar]
- 186.McDermott D, Lebbé C, Hodi FS, Maio M, Weber JS, Wolchok JD, et al. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev 2014; 40: 1056–64. doi: 10.1016/j.ctrv.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 187.Miller MA, Chandra R, Cuccarese MF, Pfirschke C, Engblom C, Stapleton S, et al. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Sci Transl Med 2017; 9: eaal0225. doi: 10.1126/scitranslmed.aal0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Miller MA, Zheng YR, Gadde S, Pfirschke C, Zope H, Engblom C, et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat Commun 2015; 6: 8692. doi: 10.1038/ncomms9692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC, Coussens LM. TH2-polarized CD4+ T cells and macrophages limit efficacy of radiotherapy. Cancer Immunol Res 2015; 3: 518–25. doi: 10.1158/2326-6066.CIR-14-0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Appelboom G, Detappe A, LoPresti M, Kunjachan S, Mitrasinovic S, Goldman S, et al. Stereotactic modulation of blood-brain barrier permeability to enhance drug delivery. Neuro Oncol 2016; 18: 1601–9. doi: 10.1093/neuonc/now137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kunjachan S, Detappe A, Kumar R, Ireland T, Cameron L, Biancur DE, et al. Nanoparticle mediated tumor vascular disruption: a novel strategy in radiation therapy. Nano Lett 2015; 15: 7488–96. doi: 10.1021/acs.nanolett.5b03073 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]