Abstract
Objective:
The goal of the study was to analyze the incidence and patterns of failure in patients with gastric cancer who received D2 dissection and adjuvant chemoradiotherapy (CRT).
Methods:
From January 2004 to October 2015, 324 patients with gastric cancer who underwent radical D2 resection followed by postoperative CRT were enrolled. Clinicopathological characteristics and patterns of failure were retrospectively reviewed to identify factors associated with survival and recurrence.
Results:
After a median follow-up of 30 months, the 3-year overall survival and 3-year disease-free survival rates of these patients were 60.3 and 51.1%, respectively. 117 patients had recurrence or metastasis, with peritoneal recurrence as the most frequent (20.7%), followed by distant metastasis (14.2%). The most commonly involved distant organs were the liver (5.9%) and bone (4.9%). Locoregional failure occurred in 39 patients (12.0%), with isolated regional failure occurring in only 23 (7.1%). Further multivariate Cox regression analysis revealed N stage to be an independent risk factor for distant failure-free survival (p = 0.012). Independent risk factors for peritoneal metastasis were tumor differentiation (p = 0.022), T stage (p =0.035) and vascular invasion (p = 0.016).
Conclusion:
Postoperative CRT has a potential effect on optimizing locoregional control, resulting in only 12.0% of locoregional failure. In patients after D2 resection and adjuvant CRT, peritoneal metastasis was the leading pattern of failure, followed by distant metastasis.
Advances in knowledge:
Peritoneal recurrence was the most common pattern of failure after D2 dissection and adjuvant CRT, followed by distant metastasis, whereas locoregional relapse was relatively rare. Selection of patients based on the predicted risk of each recurrence pattern may be a reasonable approach to the optimization of treatment strategies.
Introduction
Gastric cancer is the fifth most common malignant cancer and the third cause of cancer-related death worldwide.1, 2 Radical surgery with lymph node dissection is considered the most crucial treatment strategy for gastric carcinoma. However, even after radical resection, 21.8–63.4% of patients experience locoregional recurrence, peritoneal dissemination, or distant metastasis, resulting in an unfavorable prognosis.3–5
The role of chemoradiation has been reported by several randomized trials, including intergroup trial 0116 (INT 0116), particularly for local advanced disease.6 A more recent Phase III trial, known as the ARTIST trial, analyzed patients with D2 lymphadenectomy and showed that the locoregional relapse rate was reduced by the addition of adjuvant CRT.7 However, this trial failed to demonstrate a benefit for survival due to the extension of lymph node dissection. In addition, the CRITICS study incorporated both preoperative chemotherapy and postoperative CRT and illustrated that postoperative CRT did not improve overall survival (OS) in patients treated with adequate preoperative chemotherapy and surgery.8 Thus, the status of radiotherapy for treating gastric carcinoma remains controversial. Although the superiority of D2 lymphadenectomy compared with D1 dissection has not been consistently illustrated, D2 lymphadenectomy is the most widely accepted surgical procedure in Asian and European countries. Accordingly, clarifying the patterns of failure in patients after D2 dissection may contribute to the selection of patients who might benefit from adjuvant CRT.
Although several studies have reported the recurrence rate following curative resection of gastric cancer, most of these studies were performed in Western countries. Evidence from patients after D2 lymphadenectomy and adjuvant CRT is still insufficient, especially with respect to risk factors for different types of failure.
Therefore, the purpose of this study was to characterize the patterns of failure in patients after D2 resection and adjuvant CRT. By investigating the relationship between clinicopathologic factors and recurrence, additional evidence may be provided for the selection of patients based on the predicted risk of each recurrence pattern.
Materials and methods
Patient identification
Patients with curatively resected gastric carcinoma who received postoperative CRT at Fudan University Shanghai Cancer Center between January 2005 and December 2015 were retrospectively identified. The eligibility criteria included the following: underwent R0 gastrectomy and D2 lymphadenectomy, with no fewer than 15 lymph nodes dissected; no clinical evidence of distant metastasis (M0) according to the American Joint Committee on Cancer TNM staging, seventh edition;9 received a postoperative combination of chemotherapy and chemoradiotherapy (CRT); followed up regularly after treatment; and complete medical record data were available. The exclusion criteria included the following: residual neoplasms at the resection margin; received preoperative chemotherapy or radiotherapy; inadequate function of the liver, kidneys, or any other major organs. All patients in the study signed informed consent forms.
Treatment
The surgical requirement for eligibility was radical resection with negative margins proven by pathology (R0 resection). All patients had undergone D2 lymphadenectomy with resections of perigastric lymph node stations along the lesser curvature (stations 1, 3, and 5) and greater curvature (stations 2, 4, and 6), lymph nodes along the left gastric artery (station 7), common hepatic artery (station 8), celiac artery (station 9), and splenic artery (stations 10 and 11). Chemotherapy was administered at 3–8 weeks after surgery, followed by chemoradiation beginning at 8–18 weeks after surgery. Radiotherapy was delivered using 3D conformal radiotherapy (3D-CRT), intensity modulated radiation therapyor volumetric modulated arc therapy. Patients were treated with a median dose of 45 Gy (range 45.0–50.4 Gy) delivered at 1.8 Gy/fraction. The radiation fields encompassed the tumor bed, anastomosis site, duodenal stump, and selected regional lymph nodes. The tumor bed was defined by preoperative and postoperative CT imaging. The selection of regional lymph nodes, including perigastric, celiac, splenic, hepatoduodenal or hepatic-portal, pancreaticoduodenal and para-aortic lymph nodes, depended on the location of the tumor.10 Concurrent chemotherapy regimens were included as follows: 225 mg m–2 d–1 5-fluorouracil weekly intravenously or 625 mg m–2 d–1 capecitabine bid d1-5 weekly or 40 mg m–2 d–1 S1 bid d1-5 weekly. All eligible patients received 5-fluorouracil or platinum-based drugs after surgery, and chemotherapy was delivered in 1–3 cycles before and in 3–5 cycles after CRT.
Follow-up
After the completion of adjuvant CRT, regular follow-up was conducted in accordance with the institutional surveillance strategy, including medical history, physical examination, serum biochemical, tumor biomarkers, CT scans or MRI of the chest, abdomen and pelvis (or positron-emission tomographic scans if necessary and within a budget limit) at each visit, as well as endoscopy. Patients were followed up every 3 months for the first 2 years, every 6 months until 5 years and yearly thereafter.
Recurrence analyses
Local recurrence was defined as recurrence at the anastomosis site, duodenal stump, tumor bed, or remnant stomach. Regional recurrence was defined as recurrence at regional lymph nodes such as the perigastric, porta hepatis, peripancreatic and para-aortic lymph nodes. Peritoneal dissemination was considered to include metastasis of the peritoneum, colorectum, ovary, and ureter. Distant metastasis was defined as metastasis to a distant organ such as liver, bone, or lung or lymph node recurrence, except for regional lymph nodes.5, 11 All of the patients’ medical records were reviewed, and any relapse or metastasis was documented. If two or more failure sites developed at the same time, they were counted separately. Two experienced radiation oncologists specializing in the gastrointestinal tract reviewed the imaging analyses to further investigate patterns of regional recurrence according to the criteria of Japanese Gastric Cancer Association (Version 14). OS was defined as the time from surgery to death, including tumor-specific death or any other cause. Disease-free survival (DFS) was considered as the time from surgery to initial recurrence or death, and local failure-free survival (LFFS)/regional failure-free survival (RFFS)/peritoneal failure-free survival (PFFS)/distant failure-free survival (DFFS) were defined as the time from surgery to local/regional/peritoneal/distant failure.
Statistical analysis
Data were recorded as categorical and continuous variables. Actuarial curves of LFFS, RFFS, PFFS, DFFS, DFS, and OS were plotted using Kaplan-Meier estimates. Univariate and multivariate Cox regression analyses were used to identify prognostic factors. The variables included age, gender, family history, tumor location, differentiation, Borrmann classification, T stage, N stage, vascular invasion and perineural invasion. All p-values were two-sided, and a p-value < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS v. 22.0 (SPSS Inc., Chicago, IL).
Results
Study population and clinicopathological characteristics
A total of 619 patients with gastric cancer received postoperative CRT. Of these, 295 individuals were excluded due to the following: 40 patients had distant metastasis before gastrectomy, 12 patients had positive resection margins, 27 patients received preoperative therapy, no complete pathologic reports and medical record data were obtained for 84 patients, and 132 patients underwent D0 or D1 lymphadenectomy. Ultimately, 324 patients met the criteria and were included in the analysis (Figure 1). The first patient underwent radical surgery in June 2005, and the last patient was treated in October 2015. The loss to follow-up rate was 19.2%.
Figure 1.
Flow diagram of patient selection according to the eligibility criteria and exclusion criteria.
The patient characteristics and surgical results are listed in Table 1. Nearly two-thirds (65.1%) of the 324 patients were male. The median age was 54 years (range, 19–77 years). Most of the enrolled patients were at pathological Stage III (82.4%), and more than half of the enrolled patients had N3 disease according to the American Joint Committee on Cancer TNM staging, seventh edition.9 The median number of dissected lymph nodes was 22 (range, 16–55).
Table 1.
Characteristics of the patients (n = 324)
| Characteristics | No. of patients (%) | |
| Age (years) | Median | 54 (19–77) |
| ≥65 | 40 (12.3%) | |
| <65 | 284 (87.7%) | |
| Sex | Male | 211 (65.1%) |
| Female | 113 (34.9%) | |
| Family history of gastrointestinal | Yes | 63 (19.4%) |
| Location of primary tumor | Upper 1/3 | 68 (21.0%) |
| Middle 1/3 | 108 (33.3%) | |
| Lower 1/3 | 141 (43.5%) | |
| Total stomach | 7 (2.2%) | |
| Tumor size (cm) | Median | 4.0 |
| Range | 1.0–15.5 | |
| Pathologic T stage |
T1 | 19 (5.9%) |
| T2 | 22 (6.8%) | |
| T3 | 115 (35.5%) | |
| T4 | 168 (51.9%) | |
| Pathologic N stage |
N0 | 19 (5.9%) |
| N1 | 42 (13.0%) | |
| N2 | 84 (25.9%) | |
| N3 | 179 (55.2%) | |
| Stage | I | 6 (1.9%) |
| II | 51 (15.7%) | |
| III | 267 (82.4%) | |
| No. of positive LNs | Median | 7 |
| Range | 0–38 | |
| No. of dissected LNs | Median | 22 |
| Range | 16–55 | |
Treatment delivery
Patients were treated with a median dose of 45 Gy (range, 45.0–50.4 Gy), with 1.8 Gy daily fractions. The median duration of radiation was 35 days (range, 28–45 days). Interruption or incomplete radiation was recorded for only 26 patients, and among them, the lowest number of fractions received was 15. In total, 287 (88.6%) individuals received concurrent chemotherapy, and among them, 67 (23.3%) experienced dose delay or reduction. The reasons for interrupted or unfinished treatment were mainly toxic effects such as myelosuppression or gastrointestinal reaction. All enrolled patients received adjuvant chemotherapy before or after radiotherapy. The chemotherapy regimens included the following: EOF (epirubicin, oxaliplatin and 5-FU) (28.4%); XELOX (capecitabine and oxaliplatin) (19.4%); FOLFOX (oxaliplatin and 5-FU) (14.5%); SOX (S-1 and oxaliplatin) (12.3%); EOX (epirubicin, oxaliplatin and capecitabine) (5.6%); ECF (epirubicin, cisplatin and 5-FU) (4.6%) and other or unknown agents.
Survival and prognostic factors
The patients were followed up until December 2016, and the median time was 30.0 months (range, 4–145 months). During the follow-up period, a total of 113 (34.9%) deaths occurred, and 117 patients (36.1%) experienced relapse. The 3-year OS and 3-year DFS were 60.3 and 51.1%, respectively (Figure 2). Univariate analysis identified a family history of gastrointestinal cancer, location, size, differentiation, perineural invasion, pathological T stage and N stage as related to OS and DFS, whereas further multivariate analysis indicated only pathological T and N stage to be independent prognosis factors.
Figure 2.
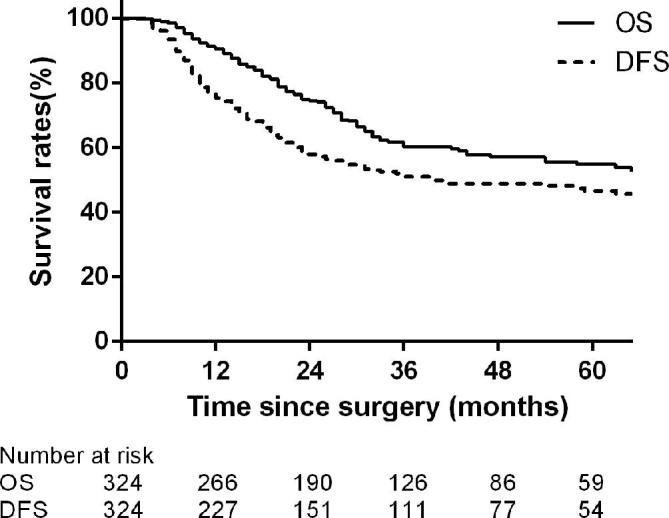
Kaplan–Meier estimate of the OS and DFS. DFS, disease free survival; OS, overall survival.
Overall patterns of failure
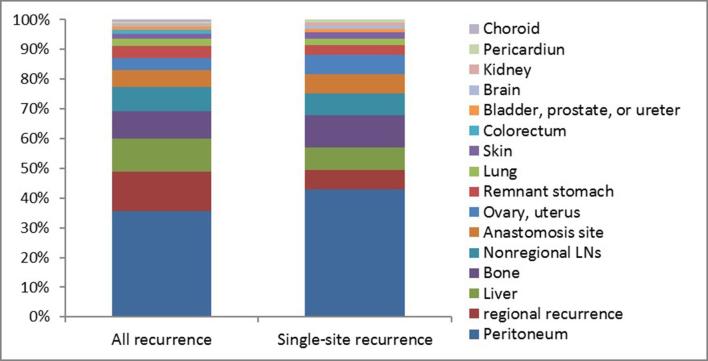
During the follow-up period, 117 patients (36.1%) showed relapse at 153 sites, and among them, single-site recurrence was noted in 84 (71.8%) and multisite recurrence in 33 (28.2%) at the time of diagnosis (Table 2). As a single pattern, peritoneal dissemination was observed most frequently (52.4%), followed by distant metastasis (29.8%). In contrast, local failure and regional recurrence were notably rare (10.7 and 7.1%). As shown in Table 3, involvement of the peritoneum, ovary, colorectum, and ureter as any component of metastasis occurred in 52.1% (61), 6.0% (7), 1.7% (2) and 1.7% (2) cases, respectively. Peritoneal dissemination was the most common site for either all recurrence or single-site recurrence (Figure 3). Moreover, the liver was the most commonly involved distant organ (16.2%). The most common combined pattern was regional recurrence with distant metastasis, and peritoneal dissemination with distant metastasis occurred in nine patients.
Table 2. .
Patterns of recurrence
| Recurrence site | No. of patients | % of recurrence patients (n = 117) |
| Single site | 84 | 71.8 |
| Local recurrence | 9 | 7.7 |
| Regional recurrence | 6 | 5.1 |
| Peritoneal metastasis | 44 | 37.6 |
| Distant metastasis | 25 | 21.4 |
| Two sites | 30 | 25.6 |
| Peritoneal + distant failure | 9 | 7.7 |
| Regional + distant failure | 9 | 7.7 |
| Peritoneal + local failure | 7 | 6.0 |
| Peritoneal + regional failure | 4 | 3.4 |
| Local + regional failure | 1 | 0.9 |
| Three or more sites | 3 | 2.6 |
Table 3. .
Recurrence sites of 117 patients
| Recurrence site | No. of patients | % of recurrence patients (n = 117) |
| Local recurrence | 17 | 14.5 |
| Remnant stomach | 7 | 6.0 |
| Anastomosis site | 10 | 8.5 |
| Regional failure | 23 | 19.7 |
| Peritoneal metastasis | 67 | 57.3 |
| Peritoneum | 61 | 52.1 |
| Ovary, uterus | 7 | 6.0 |
| Colorectum | 2 | 1.7 |
| Bladder, prostate, or ureter | 2 | 1.7 |
| Distant metastasis | 46 | 39.3 |
| Liver | 19 | 16.2 |
| Bone | 16 | 13.7 |
| Lung | 4 | 3.4 |
| Brain | 1 | 0.9 |
| Kidney | 1 | 0.9 |
| Skin | 3 | 2.6 |
| Pericardium | 1 | 0.9 |
| Choroid | 1 | 0.9 |
| Extra abdominal LNs | 14 | 12.0 |
Figure 3.
Proportion of site-specific recurrence in gastric cancer patients following curative gastrectomy and adjuvant chemoradiotherapy.
Patterns of regional recurrence
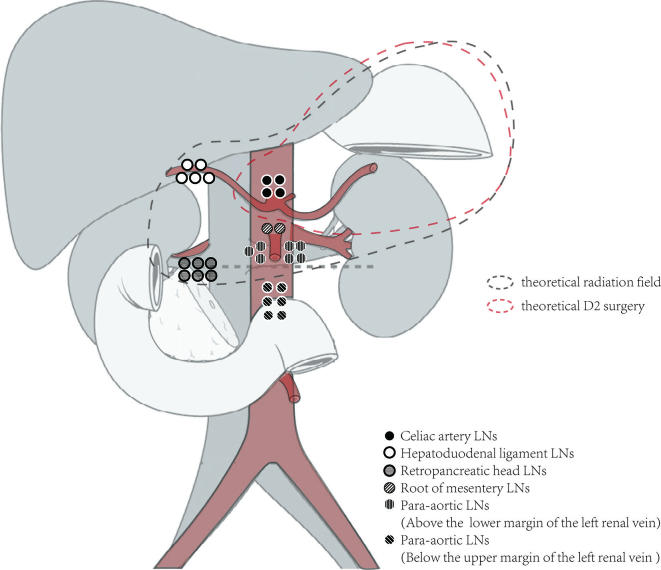
Only 23 patients (7.1%) experienced regional recurrence, and among them, 6 showed recurrence at a single site, and the remaining 17 showed simultaneous relapse at other sites. The median RFFS was 12 months (range, 1–36). However, clear imaging data were not available for 5 patients. For only 2 patients (13.3%), the areas involved were confined to the D2 lymph node dissection field, whereas the lymph nodes involved in recurrence were outside of the D2 field in 86.7% patients. As shown in Figure 4, commonly involved regional lymph nodes were the No. 16a (7 in 18 patients), No. 16b (6 in 18), No.13 (6 patients) and No. 12 (5 patients) nodes. The remaining recurrence areas mainly converged on the branch of the abdominal aorta, such as lymph nodes around the celiac artery (4 patients) and around the superior mesenteric artery (2 patients). In addition, no recurrence was observed in perigastric lymph nodes (Nos. 1–6), around the hilus lienis (No. 10) and along the middle colic artery (No. 15). When the recurrent site was analyzed with respect to the radiation treatment field (Table 4), most of the evaluable regional recurrences (23 of 30, 76.7%) were defined as in-field relapse.
Figure 4.
Patterns of regional recurrence. The recurrence regional lymph nodes of over 80% patients were out of the D2 field. Commonly involved regional lymph nodes were the No.16a nodes, No 16b nodes, No 13 nodes, No.12 nodes, No. 9 nodes and No.14 nodes.
Table 4. .
Correlation between site of regional recurrence and radiation field
| In-field | Marginal | Out-field | |||
| Site | n | Site | n | Site | n |
| Celiac artery LNs | 4 | Hpatoduodenal ligament LNs | 1 | Para-aortic LNs (16b) | 4 |
| Hpatoduodenal ligament LNs | 4 | Para-aortic LNs (16b) | 2 | ||
| Peripancreatic LNs | 6 | ||||
| Superior mesenteric vessel LNs | 2 | ||||
| Para-aortic LNs (16a) | 7 | ||||
LNs, lymph nodes.
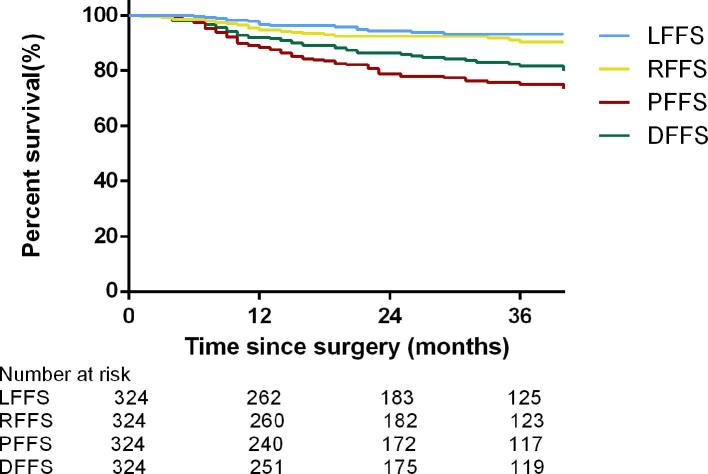
Survival rate for each failure pattern
Figure 5 depicts the survival rate for each failure pattern. The 3-year survival rates are listed as follows: 93.3% for LFFS, 90.3% for RFFS, 75.0% for PFFS, and 81.6% for DFFS.
Figure 5.
Kaplan–Meier estimate of LFFS, DFFS, RFFS, and PFFS of patients treated with adjuvant chemoradiation. DFFS, distant failure-free survival; LFFS, local failure-free survival; PFFS, peritoneal failure-free survival;RFFS, regional failure-free survival.
Uni- and multivariate analyses were performed to assess relationships between clinicopathological characteristics and prognosis (Tables 5 and 6), with each clinicopathological factor analyzed statistically among different types of recurrence. In all types of recurrence, the pathological stage was the most important factor. Univariate analysis revealed N stage as an influencing factor for all types of failure, whereas T stage only affected LFFS, RFFS and PFFS. The incidence of failure increased in proportion to the N stage. In addition, Borrmann classification, a characteristic describing the growth pattern of the primary tumor, was found to be a common factor influencing regional recurrence and distant metastasis. Furthermore, differentiation was associated with PFFS, and vascular invasion was related to RFFS, PFFS and DFFS. Moreover, a family history of gastrointestinal cancer also appeared to affect local relapse and distant metastasis. However, no relationship with respect to age, location of the primary tumor and perineural invasion was noted.
Table 5. .
Uni- and multivariate analysis of prognosis factors associated with peritoneal metastasis
| Factors | Univariate analysis | Multivariate analysis | ||
| HR | 95% CI | p value | ||
| Sex | 0.108 | 1.316 | 0.766–2.260 | 0.320 |
| Differentiation | 0.014 | 5.252 | 1.265–21.803 | 0.022 |
| T stage | 0.057 | 1.502 | 1.030–2.190 | 0.035 |
| Vascular invasion | 0.054 | 0.523 | 0.309–0.886 | 0.016 |
HR, hazard ratio; CI, confidence interval.
The P < 0.05 was considered statistically significant and was represented as bold.
Table 6. .
Uni- and multivariate analysis of prognosis factors associated with distant metastasis
| Factors | Univariate analysis | Multivariate analysis | ||
| HR | 95% CI | p value | ||
| Family history | 0.054 | 0.495 | 0.216–1.135 | 0.097 |
| Gross type | 0.046 | 1.279 | 0.857–1.909 | 0.228 |
| N stage | 0.005 | 1.835 | 1.141–2.953 | 0.012 |
| Vascular invasion | 0.157 | 0.541 | 0.278–1.053 | 0.071 |
HR, hazard ratio; CI, confidence interval.
The P < 0.05 was considered statistically significant and was represented as bold.
Upon further multivariate Cox regression analysis, N stage was shown to be an independent risk factor only for DFFS (p = 0.012). Independent risk factors involved in peritoneal metastasis were tumor differentiation (p = 0.022), T stage (p = 0.035) and vascular invasion (p = 0.016). However, multivariate analyses were not performed to identify the prognostic factors of local or regional failure due to the small number of recurrence. No statistically significant differences were found for the time of recurrence, survival after recurrence and OS in patients with each type of recurrence.
Discussion
Even after curative gastrectomy with lymph node dissection, 21.8–63.4% of patients with advanced gastric cancer patients experience progression, resulting in poor prognosis.3–5 Although the benefits of adjuvant CRT have been reported in patients with resected gastric carcinoma in Western countries,4 a Phase III randomized clinical trial known as ARTIST failed to demonstrate the survival benefit in patients after R0 gastrectomy with D2 lymphadenectomy.7 However, given the limitations in its trial design, controversy remained over the utility of radiation after extended D2 lymphadenectomy. To determine the role of adjuvant CRT, an analysis of failure patterns is needed.
The current analysis offers important implications in term of adjuvant CRT in patients after D2 dissection. First, respective 3-year OS and DFS of 60.3 and 51.1% were reported in this study, showing superior outcomes of patients in the INT-0116 study but slightly inferior to those in the ARTIST trial.4, 7 These results might be due to the earlier stage and more aggressive lymph node dissection in the ARTIST study in Korea. Only 40% of the patients enrolled in the ARTIST trial were at Stage III, but in this study cohort, over 80% of the patients had Stage III disease, which is a universal phenomenon in China due to the lack of a national screening project.
Second, local relapse or regional recurrence is a rare event occurring in only 5.2 and 7.1% of all patients, which is much lower than previous analyses from Western countries and similar to the outcomes in Korea, offering further evidence that postoperative CRT might be useful in optimizing locoregional control. In the current study, peritoneal recurrence was the most common pattern of failure, followed by distant metastasis. Several studies reported locoregional relapse as the leading pattern of failure, including the INT-0116 trial and a large-volume study from MSKCC.6, 12 However, three large-scale analyses performed in Korea, including the ARTIST trial, contradicted the proposal that peritoneal/distant metastasis was the most common site of failure in patients after postoperative CRT.7, 11,13 These three studies from Korea are noteworthy because the extent of lymphadenectomy was D2 dissection, as in the current study. Furthermore, previous studies investigating patterns of failure in patients after adjuvant chemotherapy alone demonstrated that the incidence of locoregional relapse various from 7.8 to 29.3%.5, 7,14,15 According to the reports from China, local and regional recurrences developed in 20.5 and 14.3% of all treated patients respectively, and a study performed in Korea which enrolled 382 patients with N3 disease reported a local relapse of 9.7% and a regional recurrence of 27.5%.5, 15 Although the constitution of failure pattern was similar, the incidence of local/regional recurrence in the current study were slightly lower than those in previous studies, suggesting the potential benefit of CRT for local control. In addition to the diversity of extended lymph node dissection between Eastern and Western countries, another reason for the lingering controversy is the difference in the definition of failure patterns, especially for regional recurrence. In the INT0116 study, regional recurrence was defined as recurrence detected in the peritoneal cavity, including the liver, intra-abdominal lymph nodes, and peritoneum.4 Lim defined regional lymph nodes in terms of the radiation field, and lymph node recurrence outside the radiation field was categorized as distant metastasis.11 However, the definition used in the current study was slightly different. Because the No. 16b lymph nodes (around the abdominal aorta and below the inferior margin of the left renal vein) were not routinely included within the radiation field, involvement of the No. 16b lymph nodes was defined as distant metastasis in this study, whereas involvement of the remaining regional nodes, including Nos. 1-16a, was defined as regional recurrence.
In addition, the distribution of regional nodal relapse was similar to a recent report on the failure patterns in N3 gastric cancer after D2 resection.5 The most commonly involved recurrent lymph nodes were stations 16, 13, 12, 9 and 14. In contrast, perigastric lymph nodes (Stations 1–6) and regions around the splenic hilus (Stations 10, 11) were less commonly involved. This finding might offer a clue to defining the target volume in adjuvant CRT and suggests that stations around the abdominal aorta and its main branches, as well as regions around the hepatic hilar and head of the pancreas (stations 16, 13, 12, 9 and 14), should be the most important radiotherapy targets.
This study reviewed a relatively large sample of patients with locally advanced gastric cancer in a Chinese cohort. Another potential advantage of this study is the homogeneity of the radiological assessment. All images were re-evaluated by two experienced radiation oncologists specializing in the gastrointestinal tract. In addition, patterns of failure, particularly for the distribution of regional relapse, were also illustrated in detail.
The current study has several limitations, one of which is the retrospective and single-arm design. Information from the Lauren classification in the pathological reports was missing in a subset of patients, and the authors could not retest the pathological samples to evaluate the Lauren type due to the limitation of the retrospective study. Moreover, analysis of recurrence was based on clinical and radiological examinations, and the possibility of misestimating the recurrence pattern exists. In addition, because of the small number of local or regional recurrences, multivariate analyses were not applied to identify the relationships between clinicopathological characteristics and local/regional failure.
Conclusion
In summary, the study shows the outcomes of postoperative CRT in patients after D2 dissection with favorable prognosis and local control. Peritoneal recurrence was the most common pattern, followed by distant metastasis, whereas locoregional relapse was relatively rare (12%), supplying further evidence that adjuvant CRT might optimize locoregional control. The benefit of radiotherapy in adjuvant or neoadjuvant treatment requires further validation such as ongoing ARTIST II trial and TOPGEAR trial, and the authors hope that prospective randomized controlled trials will supply answers with respect to the role of radiotherapy.
Footnotes
Ethical standards: The study was approved by the Institutional Review Board of our center, and all patients gave informed consent prior to study enrollment.
The authors Wang Yang and Ran Hu contributed equally to the work.
Contributor Information
Wang Yang, Email: yangw09@fudan.edu.cn.
Ran Hu, Email: 09301010181@fudan.edu.cn.
Gui-chao Li, Email: 09301010178@fudan.edu.cn.
Meng-long Zhou, Email: 09301010178@fudan.edu.cn.
Yan Wang, Email: wangxyyan@sina.com.
Li-jun Shen, Email: yeahslj@163.com.
Li-ping Liang, Email: llp_8521@hotmail.com.
Zhen Zhang, Email: zhen_zhang@fudan.edu.cn.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 3.Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg 2000; 87: 236–42. doi: 10.1046/j.1365-2168.2000.01360.x [DOI] [PubMed] [Google Scholar]
- 4.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001; 345: 725–30. doi: 10.1056/NEJMoa010187 [DOI] [PubMed] [Google Scholar]
- 5.Chang JS, Lim JS, Noh SH, Hyung WJ, An JY, Lee YC, et al. Patterns of regional recurrence after curative D2 resection for stage III (N3) gastric cancer: implications for postoperative radiotherapy. Radiother Oncol 2012; 104: 367–73. doi: 10.1016/j.radonc.2012.08.017 [DOI] [PubMed] [Google Scholar]
- 6.Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012; 30: 2327–33. doi: 10.1200/JCO.2011.36.7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, Sung K, Se H, Min GC, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012; 30: 268–73. doi: 10.1200/JCO.2011.39.1953 [DOI] [PubMed] [Google Scholar]
- 8.Cats A, Jansen EPM, van Grieken NCT, Sikorska K, Lind P, Nordsmark M, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol 2018; 19: 616–628. doi: 10.1016/S1470-2045(18)30132-3 [DOI] [PubMed] [Google Scholar]
- 9.S L, G M, W C. International union against cancer (UICC) TNM classification of malignant tumours, 7th ed New York: The British Institute of Radiology.; 2010. [Google Scholar]
- 10.Smalley SR, Gunderson L, Tepper J, Martenson JA, Minsky B, Willett C, et al. Gastric surgical adjuvant radiotherapy consensus report: rationale and treatment implementation. Int J Radiat Oncol Biol Phys 2002; 52: 283–93. doi: 10.1016/S0360-3016(01)02646-3 [DOI] [PubMed] [Google Scholar]
- 11.Lim DH, Kim DY, Kang MK, Kim YI, Kang WK, Park CK, et al. Patterns of failure in gastric carcinoma after D2 gastrectomy and chemoradiotherapy: a radiation oncologist’s view. Br J Cancer 2004; 91: 11–17. doi: 10.1038/sj.bjc.6601896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 2004; 240: 808–16. doi: 10.1097/01.sla.0000143245.28656.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S, Lim DH, Lee J, Kang WK, MacDonald JS, Park CH, et al. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys 2005; 63: 1279–85. doi: 10.1016/j.ijrobp.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 14.Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011; 29: 4387–93. doi: 10.1200/JCO.2011.36.5908 [DOI] [PubMed] [Google Scholar]
- 15.Deng J, Liang H, Wang D, Sun D, Pan Y, Liu Y. Investigation of the recurrence patterns of gastric cancer following a curative resection. Surg Today 2011; 41: 210–5. doi: 10.1007/s00595-009-4251-y [DOI] [PubMed] [Google Scholar]