Abstract
Objective:
To test the feasibility of two-dimensional fast Fourier transforms (FFT)-based imaging metrics in differentiating solid, non-macroscopic fat containing, enhancing renal masses using contrast-enhanced CT images. We quantify image-based intratumoral textural variations (indicator of tumor heterogeneity) using frequency-based (FFT) imaging metrics.
Methods:
In this Institutional Review Board approved, Health Insurance Portability and Accountability Act -compliant, retrospective case-control study, we evaluated 156 patients with predominantly solid, non-macroscopic fat containing, enhancing renal masses identified between June 2009 and June 2016. 110 cases (70%) were malignant RCC, including clear cell, papillary and chromophobe subtypes and, 46 cases (30%) were benign renal masses: oncocytoma and lipid-poor angiomyolipoma. Whole lesions were manually segmented using Synapse 3D (Fujifilm, CT) and co-registered from the multiphase CT acquisitions for each tumor. Pathological diagnosis of all tumors was obtained following surgical resection. Matlab function, FFT2 was used to perform the image to frequency transformation.
Results:
A Wilcoxon rank sum test showed that FFT-based metrics were significantly (p < 0.005) different between 1. benign vs malignant renal masses, 2. oncocytoma vs clear cell renal cell carcinoma and 3. oncocytoma vs lipid-poor angiomyolipoma. Receiver operator characteristics analysis revealed reasonable discrimination (area under the curve >0.7, p < 0.05) within these three groups of comparisons.
Conclusion:
In combination with other metrics, FFT-metrics may improve patient management and potentially help differentiate other renal tumors.
Advances in knowledge:
We report for the first time that FFT-based metrics can differentiate between some solid, non-macroscopic fat containing, enhancing renal masses using their contrast-enhanced CT data.
Introduction
The incidental diagnosis of renal masses has increased in the last decades owing to the widespread use of radiological imaging within the clinical workflow.1 Multiphase cross-sectional imaging, including contrast-enhanced CT (CECT) and MRI, is the current standard in diagnostic and therapeutic imaging for lesion characterization, staging, surgical planning, and tumor subtype assessment.2 Apart from lipid-rich angiomyolipomas (lp-AMLs) and simple or minimally complex cysts, it is difficult to differentiate a localized renal lesion as benign or malignant or characterize its subtype based on imaging.3 Quantitative metrics derived from cross-sectional imaging, including pixel mapping,4 contrast enhancement pattern analysis,5 chemical shift MRI,6 and histogram analysis,7, 8 have been studied as potential markers to differentiate renal lesions. However, to date, none of these metrics been proven to be a consistent or reliable discriminator as benign and malignant lesions demonstrate significant overlap in quantitative imaging characteristics.9, 10 Currently, an invasive procedure, either surgical resection or percutaneous biopsy, is required to differentiate benign and malignant solid, non-macroscopic fat containing renal tumors.11
It is important to differentiate between benign and malignant renal masses and possibly characterize its subtype, given differences in prognosis and tumor behavior. Despite these differences, the current initial treatment of localized benign or malignant lipid poor solid renal masses is either partial or radical nephrectomy depending on size and location of the tumor given the difficulty in accurately diagnosing these lesions in the preoperative setting. If benign masses can be reliably differentiated from malignant masses preoperatively, particularly when the size of the tumor is small or in patients who are poor surgical candidates, this information can be used in deciding between nephrectomy (partial or radical), ablative techniques (cryoablation, radiofrequency ablation), or active surveillance as initial treatment options.12
Texture analysis involves the study of pixel image intensity variation.13 Within the imaging domain, the image intensities can be transformed to the frequency components for the purpose of texture analysis. Extracting latent information that correlates with tumor behavior (aggressiveness and type) from standard-of-care images (here, CECT) would be an invaluable tool for physicians to improve their non-invasive diagnostic accuracy of tumor classification, particularly without the need for invasive biopsies and the associated physical and financial burden. Various mathematical techniques have been developed to quantify image texture, including statistical, Fourier, and wavelet-based methods and have been applied to numerous pathologies, such as multiple sclerosis,14 brain tumors,15 and liver diseases.16 Non-frequency based textural methods have been reported to produce promising results in segregating renal tumor types using CECT and multiparametric MRI.17–21
The purpose of our study is to evaluate the feasibility of using fast Fourier transform (FFT)-based metrics, to differentiate between different solid, non-macroscopic fat containing, enhancing renal masses on preoperative CECT. By design, FFT magnitude captures the energy captured with the individual frequencies making up the image and FFT phase captures the positioning of these frequencies. Here, we used a standard implementation of FFT (FFT2 function in Matlab®) to extract FFT magnitude and phase, which are present metrics that capture tumor texture such as complexity index (CI), a measure of the sum of all the harmonic amplitudes making up a tumor image. CI provides us an overall metric of the heterogeneity of texture within the tumor. A larger CI means higher spatial frequency/textural heterogeneity. Another FFT-based metric is entropy of the FFT magnitude, which is a measure of the randomness in the spatial frequency variation. The measure would capture the transition of texture from high to low frequency and vice-versa within the tumor. A larger entropy of FFT magnitude means higher variation in spatial frequency/textural heterogeneity. Finally, entropy of the FFT phase is an additional frequency-based metric that measures the randomness in the spatial frequency variation (phase). The measure would capture the transition of texture from high to low phase and vice-versa within the tumor. Larger entropy of FFT phase indicates higher variation in spatial frequency/ textural heterogeneity. Here, we report the performance of the FFT-metrics extracted from CECT data in segregating three groups of comparisons, i.e. 1. benign vs malignant renal masses, 2. oncocytoma vs clear cell renal cell carcinoma (ccRCC) and 3. oncocytoma vs lp-AML.
methods and Materials
Patient data
156 patients with predominantly solid, non-macroscopic fat containing, enhancing renal masses, 110 cases were malignant renal cell carcinoma (RCC), including clear cell, papillary and chromophobe subtypes and, 46 cases were benign renal masses: oncocytoma, and lp-AML, who underwent partial nephrectomy for clinically localized disease, were included in this Institutional Review Board-approved and Health Insurance Portability and Accountability Act-compliant study (Table 1) (Figure 1a,b). Patients were identified by retrospectively querying the prospectively maintained surgical database for all patients who had pre-operative multiphase CECT of the abdomen and pelvis between June 2009 and June 2016. Specialized genitourinary pathologists performed pathologic evaluation of all tumors. In the current study, we assessed only the primary tumor, and none of the lesions had intralesional calcifications in the areas assessed. Multicentric [synchronous] or metachronous RCCs were not included. The internal morphology of all lesions were predominantly solid. A few of the lesions (<3%) were described on histopathological evaluation as having a few areas of necrosis but this was not quantified. None of them showed necrotic areas on gross radiologic or pathologic evaluation. In addition, our cohort did not include predominantly cystic lesions such as Bosniak 3 or Bosniak 4 lesions. The ccRCC tumors were classified into different grades using the Fuhrman nuclear grade system (Table 1). The mean and median tumor size of the ccRCC dataset was 2.79 cm and 2.2 cm respectively with a standard deviation of 1.36 cm.
Table 1.
Tumor type distribution within the acquired CECT data
| Tumor type | No. of tumors | Class | Tumor grade | |||
| 1 | 2 | 3 | 4 | |||
| ccRCC | 70 | Malignant 100 | 5 | 61 | 28 | 6 |
| pRCC | 20 | |||||
| chRCC | 10 | Malignant 10 | NA | NA | NA | NA |
| lpAML | 18 | Benign 46 | NA | NA | NA | NA |
| Oncocytoma | 28 | |||||
Here, ccRCC, clear cell RCC; chRCC, chromophobe RCC; lpAML, lipid-poor angiomyolipoma; pRCC, papillary RCC and oncocytoma: renal oncocytoma. Please note that chromophobe RCC, although malignant is NOT subclassified using Fuhrman grade.
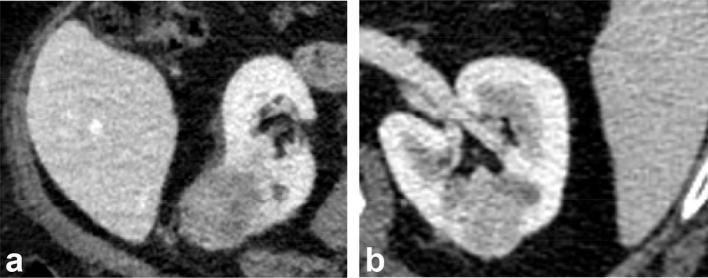
Figure 1.
(a) (Left) Axial nephrographic phase CT image of a heterogeneous hypodense, exophytic tumor, subsequently proven to be a clear cell RCC in a 65-year-old male patient. (b) Axial nephrographic phase CT image of a relatively homogenous, primarily endophytic tumor in the left renal interpolar region, proven to be an oncocytoma. RCC, renal cell carcinoma.
CECT settings
CT (Brilliance 64, Philips Healthcare, CT) imaging was performed using patient breath-holding technique with the following parameters: 120 kVp, variable tube current, slice thickness of 0.5 mm with reconstruction interval of 2 mm. Post-contrast CT scans were acquired based on fixed time delay to scanning post-injection technique for corticomedullary phase images (30 s), nephrographic phase images (90 s), and excretory phase images (5 min). Approximately, 100–150 ml of nonionic water soluble intravenous iodinated contrast Iopamidol (Isovue®−300, Bracco Diagnostics, Inc, Princeton, NJ, USA) dosed to weight was administered with a power injector at a rate of 5 ml/s.
Tumor segmentation
Manual segmentation was used to extract the renal tumor contours and performed by an experienced radiologist in Synapse 3D as three-dimensional (3D) regions of interest (ROIs).22 Nephrographic phase provided the best delineation of the tumor, and hence it was used as the reference template to co-register the other phases. The series of images were then co-registered using normalized mutual information cost function implemented in Statistical Parametric Mapping 8.0 software. Custom MATLAB® (Matlab R2012b, Mathworks, Natick, MA) code was used to extract voxel data corresponding to the ROI. Two-dimensional (2D) FFT analysis was conducted on the cross-sectional image that provided the largest tumor diameter across each of the three orthogonal imaging planes, i.e. axial, coronal, and sagittal planes, respectively and, for each of the four CECT phases. Using this strategy, we had 3 imaging plane x 4 co-registered CECT phases = 12 images to be analyzed per tumor. The segmented 3D tumor volume allowed the imaging plane with the largest tumor diameter to be easily and consistently identified using automated imaging processing workflow.
CT-based texture analysis:
From the voxel data corresponding to the segmented ROI for each tumor, across its three orthogonal imaging planes and four co-registered CECT phases, texture, defined here as the spatial arrangement of the grayscale intensities making up the tumor ROI, was extracted using an FFT-based texture characterization algorithm. Variation in the spatial arrangement of grayscale intensities create fast varying intensities (sharp features such as edges) and slow varying intensities (non-sharp features such as tumor background), which create the texture of the tumor ROI. Of the various CT-based texture methods, we choose FFT, where the spatial arrangement is converted into frequency content, i.e. fast varying intensities forming the high frequency content and slow varying intensities forming the low frequency content.
FFT analysis
FFT analysis was performed as previously described.22 Specifically, a 512-point FFT was applied to all tumor images (and CECT phases). Using the in-built Matlab implementation of the 2D FFT algorithm (FFT2), we extracted the individual frequencies, its amplitude (how much frequency of a given type is present) and phase (where in the image the frequency is present), of the original image. The resultant magnitude and phase of the FFT across all images were analyzed. Three metrics were defined (Equation 1–3). In all cases, the harmonics analysis was limited between 15 and 95% of maximum spatial frequency within the tumor. These cutoffs were chosen to avoid inclusion of low frequency content, i.e. tumor size effect and noise, and the high frequency noise. The selected band-pass frequencies correspond to the spatial frequencies within the tumor.
-
Entropy of FFT mag (E_FFT_Mag): Diversity (Randomness) measure in the magnitude of FFT harmonics
where Pk is each harmonic from the FFT transformed (magnitude) tumor image [Equation 1].
The E_FFT_Mag of a homogenous texture should be a smaller compared to the E_FFT_Mag of aheterogeneous texture.
-
Entropy of FFT phase (E_FFT_Phase): Diversity (Randomness) measure in the phase of FFT harmonics.
where is every harmonic from the FFT transformed (phase) tumor image [Equation 2].
The E_FFT_Phase of a homogenous texture should be a smaller compared to the E_FFT_Phase of a heterogeneous texture.
-
CI: sum of the amplitude of all FFT harmonics.
where Pk is every harmonic from the FFT transformed (amplitude) tumor image. [Equation 3].
The CI of a homogenous texture should be a smaller value compared to the CI of a heterogeneous texture.
Preliminary results (Supplementary material 1 and 2) were obtained by running the proposed FFT-based metrics on a 2D image of beach sand (rough texture), obtained from the “Brodatz texture collection” (which is the de facto standard for evaluating texture algorithms) in successfully discriminating homogenous profiles (smooth texture) from heterogeneous profiles (rough texture).23, 24 Considering tumor heterogeneity is a function of tumor behavior, we applied the FFT-based metrics to differentiate renal masses using their CECT data. The FFT texture metrics were applied across the cross-sectional CECT image of each renal mass that provided the largest tumor diameter across each of the three orthogonal planes (axial, coronal, and sagittal) and across the four co-registered CECT phases. The final texture was quantified as the average of the three local textures in each of the orthogonal planes for a given CECT phase. Therefore, for each tumor, we reported the average of the three proposed FFT measures obtained across the three imaging planes per CECT phase.
Statistical analysis
An independent t-test or a Wilcoxon rank sum test (data distribution dependent) was used to compare the averaged FFT-based metrics i.e. CI, E_FFT_Mag and E_FFT_Phase, that would produce statistically significant differences between the following three groups of comparisons, i.e. 1. benign vs malignant renal masses, 2. oncocytoma vs ccRCC and 3. oncocytoma vs lp-AML, for a given CECT phase. CECT phases in which significant differences in the FFT-metrics were observed for each of these comparisons have been reported in the results.
Receiver operating characteristic (ROC) curves were used to evaluate the discrimination power of FFT-based metrics within these groups. A p-value of <0.05 was used to indicate statistical significance. SAS9.4 was used for all statistical analysis.
Results
In this study, we extracted the magnitude and the phase spectrum of the spatial frequencies present within the segmented tumor image using FFT. In general, the implementation of the magnitude spectrum of FFT is such that the magnitude of the image-mean (low frequency: slowing varying components such as the background etc.) is displayed in the center of the FFT magnitude image. The magnitude of the high frequency: quickly varying components, such as texture, is displayed away from the center of the image in a radial fashion. The higher the radial distance of the frequency band from the center, the higher its frequency. Therefore, ideally, for an image with non-varying details, we would expect a large mean value in the center, with no high frequency bands encircling the center point.
The phase image, which is a distribution of the phase information of the frequencies making up the image, does not yield much new information about the structure of the spatial domain image compared to the magnitude image. We implemented it, to evaluate if it can serve as an alternate source of assessing texture.
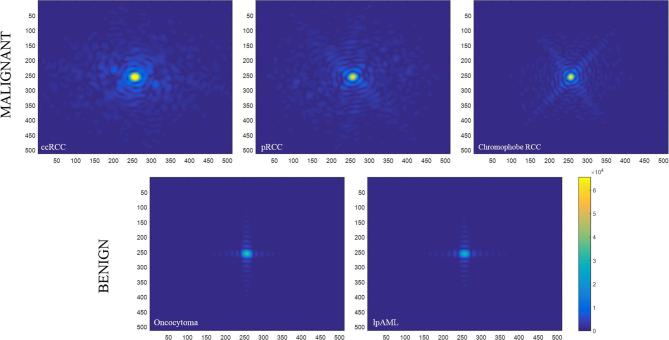
On applying FFT to the segmented tumor images, the magnitude spectrum (Figure 2, top panel) of malignant tumors (here, RCCs) showed increased intensity of high spatial frequencies (shades of blue away from the center of the image) compared to (Figure 2, bottom panel) benign tumors (here, oncocytoma and lp-AML). The magnitude spectrum (Figure 2, top panel) of malignant tumors also increased low spatial frequencies (yellow center) compared to (Figure 2, bottom panel) benign tumors. Considering the malignant tumors have both frequency components (high as well as low) in increasing amounts compared to benign, it is indicative of a comparatively more heterogeneous (comprising of various frequencies in large amount) texture profile. Benign tumors feature a more homogeneous (comprising of one strong frequency) texture profile. Visual differences in the FFT magnitude spectrums between oncocytoma and lp-AML were hard to find. The phase spectrum did not show significant differences with the malignant vs benign groups or within the other two groups (oncocytoma vs ccRCC and oncocytoma vs lp-AML).
Figure 2.
Magnitude spectrum of the malignant renal masses (top) and benign renal masses (bottom), clearly demonstrating the increase in high frequency content in the malignant tumors and the lack of the same in the benign tumors.
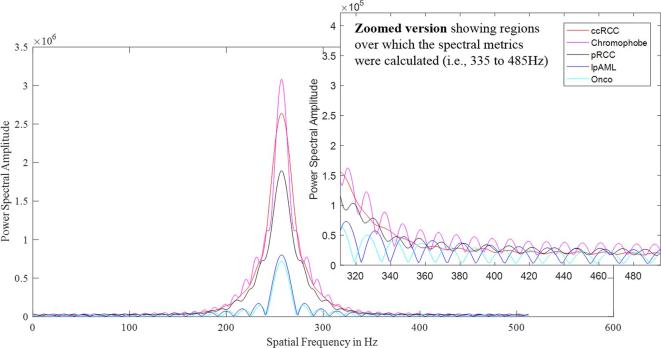
For better visualization of the variation in the magnitude spectrum, rather than the 2D representation of the magnitude spectrum as shown in Figure 2, an one-dimensional representation of the magnitude spectrum (Figure 3) of malignant and benign tumor images has been presented. The graphed plot shows the increased presence of high spatial frequencies in malignant compared to benign tumors particularly in the spatial frequency window ranging from 335 to 485 Hz (this region has been zoomed out for better visualization).
Figure 3.
Power spectrum of the malignant renal masses (red and pink curves) and benign renal masses (black, blue and cyan curves), clearly demonstrating the increase in high frequency content in the malignant tumors and the lack of the same in the benign tumors. For easy visualization, the high frequency end of the spectrum ranging from a spatial frequency of 335 to 485 Hz has been zoomed out.
FFT analysis was performed across all imaging planes and CECT phases of the segmented tumor images and the three proposed FFT-metrics measured as the average of these metrics in each of the orthogonal planes for a given CECT phase were extracted. Results of the Wilcoxon rank sum test on the final texture metrics of three groups of comparisons i.e. (1) benign vs malignant renal masses, (2) oncocytoma vs ccRCC and (3) oncocytoma vs lp-AML, where significant (p < 0.005) differences within the groups were observed have been tabulated in Tables 2–4, respectively.
Table 2.
Statistically significant (p < 0.005) difference in FFT-based texture metrics were observed between benign renal masses comprising (oncocytoma and lp-AML) and malignant renal masses comprising (clear cell RCC, papillary RCC and chromophobe)
| Benign vs malignant renal masses | ||||
| Benign (N = 46) | Malignant (N = 110) | CECT phase | FFT Predictor | p-value |
| 2.54 ± 0.09, 2.56 (2.5 to 2.6) | 2.6 ± 0.06, 2.6 (2.55 to 2.65) | Precontrast | E_FFT_Mag | 0.0001 |
| 3.72e7 ±3.85e7, 2.67e7 (1.788e7 to 3.69e7) | 7.66e7 ±8.49e7, 4.59e7 (2.17e7 to 9.78e7) | Precontrast | CI | 0.0003 |
| 2.22 ± 0.07, 2.22 (2.18 to 2.28) | 2.26 ± 0.08, 2.27 (2.21 to 2.33) | Corticomedullary | E_FFT_Mag | 0.0021 |
| 6.44e7 ±4.78e7, 4.39e7 (3.29e7 to 7.44e7) | 1.00e8 ±7.65e7, 7.46e7 (4.74e7 to 1.21e8) | Nephrographic | CI | 0.0002 |
| 5.45e7 ±3.87e7, 4.23e7 (3.37e7 to 5.28e7) | 7.92e7 ±7.21e7, 5.83e7 (3.83e7 to 9.55e7) | Excretory | CI | 0.0049 |
CECT, contrast-enhanced CT; CI, complexity index; lp-AML, lipid-poor angiomyolipoma; RCC, renal cell carcinoma.
Wilcoxon rank sum test showing that FFT-based texture metrics: CI and entropy in the amplitude of frequency (E_FFT_Mag) harmonics making up the tumor were statistically significantly (p < 0.005) different when comparing benign to malignant renal masses. Here, the mean of the FFT predictor values along with the median and range (in brackets) are reported in the first and second columns. The associated p-values are reported in the last column.
Table 3.
Statistically significant (p < 0.005) difference in FFT-based texture metrics were observed between oncocytoma and clear cell renal cell carcinoma
| Oncocytoma vs clear cell RCC | ||||
| Oncocytoma (N = 28) | ccRCC (N = 70) | CECT phase | FFT predictor | p- value |
| 3.62e7 ±3.47e7, 2.54e7 (1.78e7 to 3.3e7) | 7.99e7 ±9.46e7, 4.52e7 (2.15e7 to 9.72e7) | Precontrast | CI | 0.0048 |
| 6.477e7 ±5.32e7, 3.66e7 (3.00e7 to 7.61e7) | 1.06e8 ±8.28e7, 7.49e7 (4.98e7 to 1.38e8) | Corticomedullary | CI | 0.0002 |
| 2.42 ± 0.06, 2.43 (2.36 to 2.47) | 2.48 ± 0.07, 2.47 (2.43 to 2.53) | Nephrographic | E_FFT_Mag | 0.0002 |
| 2.18 ± 0.06, 2.17 (2.16 to 2.21) | 2.25 ± 0.07, 2.25 (2.21 to 2.3) | Excretory | E_FFT_Mag | 0.0001 |
| 4.764e7 ±2.46e7, 3.74e7 (3.27e7 to 4.94e7) | 8.66e7 ±8.15e7, 6.22e7 (3.87e7 to 1.04e8) | Excretory | CI | 0.0012 |
CECT, contrast-enhanced CT; CI, complexity index ; FFT, fast Fourier transform; lp-AML, lipid-poorangiomyolipoma; RCC, renal cell carcinoma.
Here, the mean of the FFT predictor values along with the median and range (in brackets) are reported in the first and second columns. The associated p-values are reported in the last column. Wilcoxon rank sum test showing that FFT-based texture metrics: CI and entropy in the amplitude of frequency (E_FFT_Mag) harmonics making up the tumor were statistically significantly (p < 0.005) different when comparing oncocytoma to ccRCC. Here, the mean of the FFT predictor values along with the median and range (in brackets) are reported in the first and second columns. The associated p-values are reported in the last column.
Table 4.
Statistically significant (p < 0.005) difference in FFT-based texture metrics were observed between oncocytoma and lipid-poor angiomylipoma
| Oncocytoma vs lp-AML | ||||
| Oncocytoma (N = 28) | lp-AML (N = 18) | CECT Pphase | FFT predictor | p-value |
| 2.4 ± 0.07, 2.41 (2.35 to 2.45) | 2.47 ± 0.06, 2.47 (2.44 to 2.51) | Nephrographic | E_FFT_Mag | 0.0015 |
| 2.43 ± 0.08, 2.43 (2.4 to 2.46) | 2.5 ± 0.08, 2.51 (2.46 to 2.56) | Excretory | E_FFT_Mag | 0.0023 |
CECT, contrast-enhanced CT; CI, complexity index ; FFT, fast fourier transform; lp-AML, lipid-poorangiomyolipoma; RCC, renal cell carcinoma.
Wilcoxon rank sum test showing that FFT-based texture metric: entropy in the amplitude of frequency (E_FFT_Mag) making up the tumor was statistically significantly (p < 0.005) different when comparing oncocytoma to lp-AML. Here, the mean of the FFT predictor values along with the median and range (in brackets) are reported in the first and second columns. The associated p-values are reported in the last column.
We report that:
FFT-metric CI and E_FFT_Mag were statistically significantly (p < 0.005) different when comparing benign to malignant renal masses (Table 2). The best separation was observed in the corticomedullary phase using the E_FFT_Mag metric.
FFT-metrics CI and E_FFT_Mag were statistically significantly (p < 0.005) different when comparing oncocytoma to ccRCC (Table 3). The best separation was observed in the excretory phase using the CI metric.
FFT-metric E_FFT_Mag was statistically significantly (p < 0.005) different when comparing oncocytoma to lp-AML (Table 4). The best separation was observed in the excretory phase.
FFT-metric E_FFT_Phase did not show any significant differences between the three groups of comparisons in any of the CECT phases.
To assess the discrimination power of the FFT-metrics that showed significant differences within the three groups, an ROC analysis of each FFT-based metric and a linear combination of the FFT-based metrics were performed. Results showed that various FFT-metrics showed reasonable to good [0.7 < area under the curve) AUC < 0.85] segregation between the aforementioned three groups of comparisons (Table 5). Here the combined metric, defined as the linear combination of the proposed FFT-metrics, i.e. E_FFT_Mag, E_FFT_Phase and CI performed the best in discriminating (AUC > 0.8) between benign vs malignant, oncocytoma vs ccRCC and oncocytoma vs lp-AML, respectively using the texture information latent within the excretory phase images of the renal masses.
Table 5.
Statistically significant (p < 0.05) FFT-based texture metrics identifying various renal mass subtypes
| Performance of the FFT-metrics based on their AUC in segregating the various renal mass subtypes (p < 0.05) | |||
| Comparison | CECT phase | FFT predictor | AUC |
| Oncocytoma vs clear cell | Corticomedullary | CI | 0.73, 95% CI, (0.62, 0.84) |
| Oncocytoma vs clear cell | Corticomedullary | E_FFT_Phase | 0.71, 95% CI, (0.6, 0.82) |
| Oncocytoma vs clear cell | Excretory | Combined | 0.81, 95% CI, (0.72, 0.9) |
| Oncocytoma vs clear cell | Excretory | E_FFT_Mag | 0.78, 95% CI, (0.68, 0.88) |
| Oncocytoma vs lp-AML | Nephrographic phase | Combined | 0.77, 95% CI, (0.63, 0.92) |
| Oncocytoma vs lp-AML | Nephrographic phase | E_FFT_Mag | 0.76, 95% CI, (0.61, 0.91) |
| Oncocytoma vs lp-AML | Excretory | Combined | 0.83, 95% CI, (0.71, 0.95) |
| Malignant vs benign | Excretory | Combined | 0.80, 95% CI, (0.73, 0.88) |
| Malignant vs benign | Excretory | E_FFT_Mag | 0.73, 95% CI, (0.65, 0.81) |
CECT, contrast-enhanced CT; CI, complexity index ; FFT, fast Fourier transform; lp-AML, lipid-poorangiomyolipoma; RCC, renal cell carcinoma.
Combined: E_FFT_Mag + E_FFT_Phase + CI. FFT-based metrics that provided AUC > 0.7 have been tabulated. A cutoff of AUC > 0.7 has used based on similar peer-reviewed studies in the literature. The combinedmetric of E_FFT_Mag, E_FFT_Phase and CI showed the best performance AUC > 0.8 insegregating oncocytoma from clear cell RCC and lipid-poor AML and also, insegregating benign renal masses comprising (oncocytoma and lipid-poor AML) from malignant renal masses comprising (clear cell RCC, papillary RCC andchromophobe). Values in the AUC column are reported in the following format: (AUC, confidence level, AUC range). The FFT predictors with the three highest AUC values have been highlighted in bolded text.
Discussion
Quantitative metrics such as tumor attenuation (gray-level intensity within a tumor image) extracted from non-contrast CT and enhancement pattern extracted from multiphase CT have shown promising discrimination between lp-AML and RCC and, particularly, lp-AML and ccRCC, respectively.9, 25 Texture metrics have also shown promising discrimination between the lp-AML and other tumor types.19 Similar quantitative metrics have also shown promising results for the diagnosis of oncocytoma.26, 27 Although promising, the lack large scale validation of the results observed in lp-AML and oncocytoma limits the statistical power of these results.10, 27 Here, we try to bridge the gap by evaluating the performance of FFT-based metrics in discriminating between 1. benign vs malignant renal masses, 2. oncocytoma vs ccRCC and 3. oncocytoma vs lp-AML.
A statistically significant difference in CI between benign and malignant renal masses, p < 0.005 on all the phases of CECT acquisition, was observed. In all CECT phases, malignant masses had a higher CI and E_FFT_Mag compared to benign, indicating increased heterogeneity of malignant compared to benign renal masses. Similar outcomes of predicting tumor heterogeneity have been reported in literature where increased lesion heterogeneity using CT and MRI texture analysis has been associated to malignant as compared to benign masses.8, 28,29 The increased heterogeneity may be attributed to the altered recruitment of cells to counter genomic instability. Also, the best separation between malignant and benign renal masses was observed in the corticomedullary phase. This is possibly due to the altered enhancement patterns of the neovascularity in the tumors compared to the normal enhancement in the renal cortex, which accentuates the underlying textural differences between malignant and benign renal masses in the corticomedullary phase. About 4.4% of the small renal masses (SRMs) showed near homogenous enhancement but the texture showed a high CI.
Work by Huhdanpaa et al30 indicate that absolute enhancement, as well as attenuation and residual enhancement in nephrographic phase, are more heterogeneous for lower grade tumors. Previous publications22 using FFT-metrics also report that there was a significant difference in CI between the different tumor grades, p < 0.005 at all the four phases of CECT acquisition. In all cases, a positive correlation was observed between tumor grade and CI. ROC analysis revealed the importance of the entropy of FFT amplitude, FFT phase, and CI and their ability to identify grade 1 and grade 4 tumors from the rest of the population. Extending that work here, to discriminate between benign and malignant renal masses, we observed that the heterogeneity of ccRCC is higher than pRCC across all grades. This is in agreement with the literature.31, 32 Here, in addition, we also observe that the heterogeneity of pRCC increases with the increase in grade, potentially due to increased areas of necrosis etc. (which has not been studied separately here, but warranted), which may theoretically decrease the capability of FFT-based metrics to differentiate higher-grade ccRCC from pRCC.
Corcoran et al analyzed 26 representative studies incorporating a total of 27,272 patients and found that the frequency of benign tumors ranged from 7 to 33% (mean 14.5%, median 13.9% and standard deviation 5.2%).33 Approximately, 2–6% of the benign solid masses surgically excised from the kidney are AMLs.34 Of these, poor or no fat can be observed on CT scans in ~4–5% of AMLs making it difficult to characterize.35 Renal oncocytomas constitute 3–7% of all primary epithelial neoplasms of the adult kidney.36 Oncocytomas have a high possibility to be misread as RCCs, with only up to 9.0% of blinded reviews classifying them as “suspicious for oncocytomas”. In addition, even non-blinded third reviews by an experienced abdominal radiologist found classic CT findings only in 13.5% of oncocytomas.37
Despite the identification of tumor features such as central scar, segmental enhancement inversion etc. which are characteristic of an oncocytoma, to date, there are no reliable features that can differentiate oncocytoma from RCC.10 Enhancement thresholds and texture features have showed some promise26, 27 and therefore, we evaluate the use of our FFT-metrics to distinguish oncocytoma from ccRCC and lp-AML. Here, a statistically significant (p < 0.005) difference in CI and E_FFT_Mag between oncocytoma and ccRCC was observed. The best separation was observed in the excretory phase using the CI metric. The value of CI metric was higher in ccRCC compared to oncocytoma, indicating the increased heterogeneity of ccRCC texture compared to oncocytoma. This is in agreement with other studies in literature that report that oncocytomas tended to be slightly more homogeneous than clear cell on both visual evaluation and assessment of textural heterogeneity based on entropy evaluation.27 In addition, here, a statistically significant difference in E_FFT_Mag between oncocytoma and lp-AML, p < 0.005, was observed. The best separation was observed in the excretory phase, with slightly higher E_FFT_Mag value in lp-AML compared to oncocytoma. Other studies from the literature have shown that lp-AML most commonly show rapid enhancement on the corticomedullary phase with washout of the contrast agent on the nephrographic or excretory phases.38 Here, we observe that the difference between lp-AML and oncocytoma are most prominent in the nephrographic and excretory phases, which may be related to distinctive contrast enhancement dynamics of lp-AML.
Previously published studies using texture analysis to differentiate chRCC from pRCC reported significantly lower mean and mean value of positive pixels (MPP) at fine texture scale (p = 0.002). An MPP less than 56.06 at fine texture scale on corticomedullary images was used as the identifier of pRCC from chRCC.39 Authors of the same group also showed significant differences between texture metrics such as standard deviation, MPP and kurtosis between Type 1 and Type 2 pRCC.40 However, in our study, FFT-metrics did not show significant difference between chRCC and pRCC in any of the CECT phases.
Supportive ROCs analysis revealed strong discrimination represented by a high area under the ROC curve (AUC), AUC > 0.7, within the aforementioned three groups of comparisons using FFT-based metrics. Considering the non-invasive and post-processing quantitative nature of the proposed CECT-based frequency assessment of tumor texture, the method has the potential being more objective and widely used with no additional radiation exposure to patients, particularly in early stage assessment.
In general, texture analysis can provide quantitative information regarding tumor texture, margins and shape, which may correlate with genomic data of RCC.41 In a recent study conducted by Leng et al using 158 resected small renal masses (<4 cm), comprising of clear cell RCC (n = 98), papillary RCC (n = 36), and lp-AMLs (n = 24), the authors report that ccRCC was more heterogeneous (measured using texture features such as standard deviation, entropy, and uniformity) than papillary RCC or lp-AMLs.42 These results demonstrate that even a simple first-order statistical texture metric such as histogram analysis of the tumor gray-level intensities may add complementary value to routine CT measurements and augment the diagnosis and management of SRMs. In our study, we did not specifically focus on SRMs; however, a large proportion (~75.64% i.e. 118 of 156) of the masses in our database (N = 156) were within the definition of a T1a mass (<4 cm). The mean, median and standard deviation of the distribution were 2.86 cm, 2.2 cm and 1.42 cm respectively.
Lesion morphometrics including its margin (smoothness) and lesion—normal parenchyma interface impact texture. Recent studies by Yap et al,43 investigating whether quantitative contour analysis of renal masses using 11 shape descriptors can demonstrate different shape parameters between benign and malignant renal masses, report that convex hull perimeter (CHP) in the sagittal dimension and elliptic compactness in the coronal dimension demonstrated significant differences between malignant and benign masses (p = 0.04 for both). These differences in the convex hull perimeter and elliptic compactness shape metrics signify that malignant tumors are more concave and less ellipsoid respectively than benign tumors in at least one imaging plane. Shape analysis or assessment of tumor margin characteristics have also been used to characterize the contours of breast,44 lung45 and oral squamous cell46 carcinomas as a biomarker of malignancy or aggressiveness. Studies evaluating pulmonary nodules show that benign nodules usually show a well-defined smooth edge and malignant nodules present with irregular and spiculated and ill-defined margins.47 However, there is a 33% overlap between the two nodule types using either spiculation or smoothness as a discriminating factor.
Some of the limitations of the study are that the number of renal masses within some categories, especially that of benign renal masses, was less than ideal for a thorough analysis of CT features. This limitation arises from the significantly lower incidence of the benign subtypes compared with malignant cases. Nonetheless, we chose this classification strategy, i.e. benign vs malignant, oncocytoma vsccRCC and oncocytoma vs lp-AML to increase the impact of the results. The classification of renal masses into benign vs malignant plays the most crucial role in treatment planning. To date, reliably discriminating lp-AML from RCC, and identifying oncocytoma is a challenge. In addition, our analysis was retrospective, and the study population was limited to patients with preoperative multiphase CT examination. Typically, quantification of heterogeneity must take into account the potential impact of variation in CT image acquisition parameters including tube voltage, tube current, pixel resolution etc. Here, care was taken to use the same scanner, thereby adopting the same imaging settings, noise levels and resolution to avoid variation in results due to these confounding variables. Currently, the segregation power of some of the FFT-based metrics, particularly entropy of phase (E_FFT_Phase), did not generate significant AUC values that warrant the need to validate the method on a larger scale prior to implementation into clinical practice.
Texture analysis has the potential to provide the radiologist with a quantitative assessment of tumor heterogeneity, which may improve their diagnostic confidence. As with other quantitative metrics, it is not our intention for these metrics to be a stand-alone assessment, but rather part of larger panel of imaging features assessing tumor shape, size, and additional texture metrics. Following validation of texture analysis in larger studies and the incorporation of statistical modeling, it is possible that texture analysis can be integrated into clinical practice. In the work presented here, we use FFT-metrics to assess tumor texture of predominantly solid non-macroscopic fat containing tumors and characterize them into subtypes using their clinical CECT data. We note that we could identify differences between benign (lp-AML and oncocytoma) and malignant (RCC) renal masses. In addition, we also show that the FFT-metrics can differentiate between oncocytoma from ccRCC and, oncocytoma from lp-AML. In general, the FFT metrics have a lower value in the benign masses compared to the malignant masses.
It is recognized that ccRCC has significant intratumoral heterogeneity, which may lead to misdiagnosis due to sampling errors. Considering the fact that phenotypic differences in gross ccRCC pathology are likely to be reflected on imaging as differences in heterogeneity, a heterogeneity assessment technique such as texture analysis will aid in overcoming the limitations of sampling errors. In the current study, we performed 2D FFT analysis; however, we are exploring the possibility of performing 3D FFT analysis. While outside the scope of this article, when techniques such as these are validated on volumetric tumor data using 3D texture analysis, the results would potentially identify which areas could be targeted for a biopsy.
Future studies might focus on the application of this tool in masses from other sites of the body and using it in combination with other radiomic tumors metrics48 derived from CECT that can be used to separate malignant from benign renal masses or even tumor subtyping within a sample population.
Conclusion
This work represents, to the best of our knowledge, the first instance of the use of FFT- analysis for segregating solid, non-macroscopic fat containing, enhancing renal masses on pre-operative CECT. Following validation using a larger cohort of renal masses, these results imply the ability to non-invasively discriminate renal mass subtypes using FFT-based texture features.
Footnotes
Informed consent: This project has secured all the required IRB approvals.
Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Contributor Information
Bino A Varghese, Email: bino.varghese@med.usc.edu.
Frank Chen, Email: Frank.Chen@med.usc.edu.
Darryl H Hwang, Email: Darryl.Hwang@med.usc.edu.
Steven Y Cen, Email: cen@usc.edu.
Inderbir S Gill, Email: igill@med.usc.edu.
Vinay A Duddalwar, Email: vinay.duddalwar@med.usc.edu.
REFERENCES
- 1.Gill IS, Aron M, Gervais DA, Jewett MA, mass Srenal. Clinical practice. Small renal mass. N Engl J Med 2010; 362: 624–34. doi: 10.1056/NEJMcp0910041 [DOI] [PubMed] [Google Scholar]
- 2.Kim JK, Kim TK, Ahn HJ, Kim CS, Kim KR, Cho KS. Differentiation of subtypes of renal cell carcinoma on helical CT scans. AJR Am J Roentgenol 2002; 178: 1499–506. doi: 10.2214/ajr.178.6.1781499 [DOI] [PubMed] [Google Scholar]
- 3.Bassignani MJ. Understanding and interpreting MRI of the genitourinary tract. Urol Clin North Am 2006; 33: 301–17. doi: 10.1016/j.ucl.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 4.Catalano OA, Samir AE, Sahani DV, Hahn PF. Pixel distribution analysis: can it be used to distinguish clear cell carcinomas from angiomyolipomas with minimal fat? Radiology 2008; 247: 738–46. doi: 10.1148/radiol.2473070785 [DOI] [PubMed] [Google Scholar]
- 5.Young JR, Coy H, Douek M, Lo P, Sayre J, Pantuck AJ. Clear cell renal cell carcinoma: identifying the loss of the Y chromosome on multiphasic MDCT. AJR Am J Roentgenol 2017; 15: 1–6. doi: 10.2214/AJR.16.17010 [DOI] [PubMed] [Google Scholar]
- 6.Jhaveri KS, Elmi A, Hosseini-Nik H, Hedgire S, Evans A, Jewett M, et al. . Predictive value of chemical-shift MRI in distinguishing clear cell renal cell carcinoma from non-clear cell renal cell carcinoma and minimal-fat angiomyolipoma. AJR Am J Roentgenol 2015; 205: W79–86. doi: 10.2214/AJR.14.13245 [DOI] [PubMed] [Google Scholar]
- 7.Chaudhry HS, Davenport MS, Nieman CM, Ho LM, Neville AM. Histogram analysis of small solid renal masses: differentiating minimal fat angiomyolipoma from renal cell carcinoma. AJR Am J Roentgenol 2012; 198: 377–83. doi: 10.2214/AJR.11.6887 [DOI] [PubMed] [Google Scholar]
- 8.Chen F, Gulati M, Hwang D, Cen S, Yap F, Ugwueze C, et al. . Voxel-based whole-lesion enhancement parameters: a study of its clinical value in differentiating clear cell renal cell carcinoma from renal oncocytoma. Abdom Radiol 2017; 42: 552–60. doi: 10.1007/s00261-016-0891-8 [DOI] [PubMed] [Google Scholar]
- 9.Yang CW, Shen SH, Chang YH, Chung HJ, Wang JH, Lin AT, et al. . Are there useful CT features to differentiate renal cell carcinoma from lipid-poor renal angiomyolipoma? AJR Am J Roentgenol 2013; 201: 1017–28. doi: 10.2214/AJR.12.10204 [DOI] [PubMed] [Google Scholar]
- 10.Choudhary S, Rajesh A, Mayer NJ, Mulcahy KA, Haroon A. Renal oncocytoma: CT features cannot reliably distinguish oncocytoma from other renal neoplasms. Clin Radiol 2009; 64: 517–22. doi: 10.1016/j.crad.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 11.Jewett MA, Rendon R, Lacombe L, Karakiewicz PI, Tanguay S, Kassouf W, et al. . Canadian guidelines for the management of small renal masses (SRM). Can Urol Assoc J 2015; 9:160. doi: 10.5489/cuaj.2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marconi L, Dabestani S, Lam TB, Hofmann F, Stewart F, Norrie J, et al. . Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol 2016; 69: 660–73. doi: 10.1016/j.eururo.2015.07.072 [DOI] [PubMed] [Google Scholar]
- 13.Drabycz S, Stockwell RG, Mitchell JR. Image texture characterization using the discrete orthonormal S-transform. J Digit Imaging 2009; 22: 696. doi: 10.1007/s10278-008-9138-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajeswari R, Anandhakumar P. Image Segmentation and Identification of Brain Tumor using FFT Techniques of MRI Image. ACEEE Int J on Commission 2011; 2. [Google Scholar]
- 15.Devos A, Lukas L, Suykens JA, Vanhamme L, Tate AR, Howe FA, et al. . Classification of brain tumours using short echo time 1H MR spectra. J Magn Reson 2004; 170: 164–75. doi: 10.1016/j.jmr.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 16.Hirata M, Akbar SM, Horiike N, Onji M. Noninvasive diagnosis of the degree of hepatic fibrosis using ultrasonography in patients with chronic liver disease due to hepatitis C virus. Eur J Clin Invest 2001; 31: 528–35. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Kwon YS, Labib M, Foran DJ, Singer EA. Magnetic resonance imaging as a biomarker for renal cell carcinoma. Dis Markers 2015; 2015: 1–9. doi: 10.1155/2015/648495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu H, Scalera J, Khalid M, Touret AS, Bloch N, Li B, et al. . Texture analysis as a radiomic marker for differentiating renal tumors. Abdom Radiol 2017; 42: 1–9. doi: 10.1007/s00261-017-1144-1 [DOI] [PubMed] [Google Scholar]
- 19.Hodgdon T, McInnes MD, Schieda N, Flood TA, Lamb L, Thornhill RE. Can quantitative CT texture analysis be used to differentiate fat-poor renal angiomyolipoma from renal cell carcinoma on unenhanced CT images? Radiology 2015; 276: 787–96. doi: 10.1148/radiol.2015142215 [DOI] [PubMed] [Google Scholar]
- 20.Schieda N, Vakili M, Dilauro M, Hodgdon T, Flood TA, Shabana WM. Solid renal cell carcinoma measuring water attenuation (-10 to 20 HU) on unenhanced CT. AJR Am J Roentgenol 2015; 205: 1215–21. doi: 10.2214/AJR.15.14554 [DOI] [PubMed] [Google Scholar]
- 21.Haider MA, Vosough A, Khalvati F, Kiss A, Ganeshan B, Bjarnason GA. CT texture analysis: a potential tool for prediction of survival in patients with metastatic clear cell carcinoma treated with sunitinib. Cancer Imaging 2017; 17: 4. doi: 10.1186/s40644-017-0106-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varghese BA, Hwang DH, Cen SY, Desai BB, Yap FY, Gill I, et al. . Fast fourier transform based analysis of renal masses on contrast-enhanced computed tomography images for grading of tumor. In12th International symposium on medical information processing and analysis 2017 Jan 27 (pp. 101600J-101600J). International Society for Optics and Photonics. [Google Scholar]
- 23.Picard RW, Kabir T, Liu F. I ELECTRICALNSTITUTE ENGINEERS OF INC (IEEE). IEEE Computer Society Conference on Computer Vision and Pattern Recognition 1993 Jun 15 In: Real-time recognition with the entire Brodatz texture database; 1993. pp 638–638. [Google Scholar]
- 24.Brodatz P. Textures: a photographic album for artists and designers. Mineola, NY: The British Institute of Radiology.; 1966. [Google Scholar]
- 25.Schieda N, Hodgdon T, El-Khodary M, Flood TA, McInnes MD. Unenhanced CT for the diagnosis of minimal-fat renal angiomyolipoma. AJR Am J Roentgenol 2014; 203: 1236–41. doi: 10.2214/AJR.14.12630 [DOI] [PubMed] [Google Scholar]
- 26.Kim JI, Cho JY, Moon KC, Lee HJ, Kim SH. Segmental enhancement inversion at biphasic multidetector CT: characteristic finding of small renal oncocytoma. Radiology 2009; 252: 441–8. doi: 10.1148/radiol.2522081180 [DOI] [PubMed] [Google Scholar]
- 27.Sasaguri K, Takahashi N, Gomez-Cardona D, Leng S, Schmit GD, Carter RE, et al. . Small (< 4 cm) renal mass: differentiation of oncocytoma from renal cell carcinoma on biphasic contrast-enhanced CT. AJR Am J Roentgenol 2015; 205: 999–1007. doi: 10.2214/AJR.14.13966 [DOI] [PubMed] [Google Scholar]
- 28.Zhang C, Li X, Hao H, Yu W, He Z, Zhou L. The correlation between size of renal cell carcinoma and its histopathological characteristics: a single center study of 1867 renal cell carcinoma cases. BJU Int 2012; 110(11 Pt B): E481–E485. doi: 10.1111/j.1464-410X.2012.11173.x [DOI] [PubMed] [Google Scholar]
- 29.Ganeshan B, Miles KA. Quantifying tumour heterogeneity with CT. Cancer Imaging 2013; 13: 140–9. doi: 10.1102/1470-7330.2013.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huhdanpaa H, Hwang D, Cen S, Quinn B, Nayyar M, Zhang X, et al. . CT prediction of the Fuhrman grade of clear cell renal cell carcinoma (RCC): towards the development of computer-assisted diagnostic method. Abdom Imaging 2015; 40: 3168–74. doi: 10.1007/s00261-015-0531-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue L-Y, Lu Q, Huang B-J, Li C-X, Yan L-X, Wang W-P. Differentiation of subtypes of renal cell carcinoma with contrast-enhanced ultrasonography. Clin Hemorheol Microcirc 2016; 63: 361–71. doi: 10.3233/CH-152024 [DOI] [PubMed] [Google Scholar]
- 32.Chen F, Huhdanpaa H, Desai B, Hwang D, Cen S, Sherrod A, et al. . Whole lesion quantitative CT evaluation of renal cell carcinoma: differentiation of clear cell from papillary renal cell carcinoma. Springerplus 2015; 4: 66. doi: 10.1186/s40064-015-0823-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corcoran AT, Russo P, Lowrance WT, Asnis-Alibozek A, Libertino JA, Pryma DA, et al. . A review of contemporary data on surgically resected renal masses—benign or malignant? Urology 2013; 81: 707–13. doi: 10.1016/j.urology.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 34.Milner J, McNeil B, Alioto J, Proud K, Rubinas T, Picken M, et al. . Fat poor renal angiomyolipoma: patient, computerized tomography and histological findings. J Urol 2006; 176: 905–9. doi: 10.1016/j.juro.2006.04.016 [DOI] [PubMed] [Google Scholar]
- 35.Jinzaki M, Tanimoto A, Narimatsu Y, Ohkuma K, Kurata T, Shinmoto H, et al. . Angiomyolipoma: imaging findings in lesions with minimal fat. Radiology 1997; 205: 497–502. doi: 10.1148/radiology.205.2.9356635 [DOI] [PubMed] [Google Scholar]
- 36.Amin MB, Corless CL, Renshaw AA, Tickoo SK, Kubus J, Schultz DS. Papillary (chromophil) renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 62 cases. Am J Surg Pathol 1997; 21: 621–35. doi: 10.1097/00000478-199706000-00001 [DOI] [PubMed] [Google Scholar]
- 37.Shin T, Duddalwar VA, Ukimura O, Matsugasumi T, Chen F, Ahmadi N, et al. . Does computed tomography still have limitations to distinguish benign from malignant renal tumors for radiologists? Urol Int 2017; 99: 229–36. doi: 10.1159/000460303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hakim SW, Schieda N, Hodgdon T, McInnes MD, Dilauro M, Flood TA. Angiomyolipoma (AML) without visible fat: ultrasound, CT and MR imaging features with pathological correlation. Eur Radiol 2016; 26: 592–600. doi: 10.1007/s00330-015-3851-8 [DOI] [PubMed] [Google Scholar]
- 39.Hodgdon T, McInnes MD, Xue H, Jin MD. Can quantitative CT texture analysis be used to differentiate subtypes of renal cell carcinoma on multiphasic multidetector CT images? RSNA 2016, Chicago. 2016.
- 40.Kaya D, Ganeshan B, PhD BA, PhD MD, Pheroze Tamboli MD, Wood CG. Differentiation of papillary type 1 and type 2 RCC on CT textural analysis CT images? RSNA 2016, Chicago. 2016.
- 41.Karlo CA, Di Paolo PL, Chaim J, Hakimi AA, Ostrovnaya I, Russo P, et al. . Radiogenomics of clear cell renal cell carcinoma: associations between CT imaging features and mutations. Radiology 2014; 270: 464–71. doi: 10.1148/radiol.13130663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leng S, Takahashi N, Gomez Cardona D, Kitajima K, McCollough B, Li Z, et al. . Subjective and objective heterogeneity scores for differentiating small renal masses using contrast-enhanced CT. Abdom Radiol 2017; 42: 1485–92. doi: 10.1007/s00261-016-1014-2 [DOI] [PubMed] [Google Scholar]
- 43.Yap FY, Hwang DH, Cen SY, Varghese BA, Desai B, Quinn BD, et al. . Quantitative contour analysis as an image-based discriminator between benign and malignant renal tumors. Urology 2018; 114: 121–7. doi: 10.1016/j.urology.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 44.Kilday J, Palmieri F, Fox MD. Classifying mammographic lesions using computerized image analysis. IEEE Trans Med Imaging 1993; 12: 664–9. doi: 10.1109/42.251116 [DOI] [PubMed] [Google Scholar]
- 45.Iwano S, Nakamura T, Kamioka Y, Ikeda M, Ishigaki T. Computer-aided differentiation of malignant from benign solitary pulmonary nodules imaged by high-resolution CT. Comput Med Imaging Graph 2008; 32: 416–22. doi: 10.1016/j.compmedimag.2008.04.001 [DOI] [PubMed] [Google Scholar]
- 46.Pektas ZO, Keskin A, Günhan O, Karslioğlu Y. Evaluation of nuclear morphometry and DNA ploidy status for detection of malignant and premalignant oral lesions: quantitative cytologic assessment and review of methods for cytomorphometric measurements. J Oral Maxillofac Surg 2006; 64: 628–35. doi: 10.1016/j.joms.2005.12.010 [DOI] [PubMed] [Google Scholar]
- 47.Hirakata K, Nakata H, Haratake J. Appearance of pulmonary metastases on high-resolution CT scans: comparison with histopathologic findings from autopsy specimens. AJR Am J Roentgenol 1993; 161: 37–43. doi: 10.2214/ajr.161.1.8517317 [DOI] [PubMed] [Google Scholar]
- 48.Hwang DH, Varghese BA, Chang M, Deng C, Ugweze C, Cen SY et al. . Radiomics-Based Quantitative Biomarker Discovery: Development of a Robust Image Processing Infrastructure. In12th International Symposium on Medical Information Processing and Analysis 2017 Jan 27 (pp. 1016017-1016017): The British Institute of Radiology.. [Google Scholar]