Abstract
In patients with silicone breast implants, implant rupture can occur, which can be intra- or extracapsular. Following implant rupture, silicone can travel through the lymphatic system into regional and distant lymph nodes. The purpose of this pictorial essay is to present findings of silicone implant rupture with intramammary and systemic silicone deposition as seen on dual energy CT, ultrasound, mammogram, PET/CT and MRI. We include imaging findings of silicone deposition in the breast in cases of intra- and extracapsular rupture. We also present silicone deposition in mediastinal, axillary, and internal mammary lymph nodes, as well as in the liver and spleen. To our knowledge, deposition of silicone in the liver and spleen has not been previously demonstrated on cross-sectional imaging. While all imaging modalities were able to detect silicone in the spleen, ultrasound appeared to be more sensitive than dual energy CT or MRI for detection of silicone deposition in the liver.
Introduction
Breast reconstruction with silicone implants is commonly performed following breast cancer surgery and for breast augmentation. These implants can be composed entirely with silicone or they can be dual lumen implants, consisting of an inner saline construct and outer silicone shell. The median life expectancy of silicone implants is approximately 10–16 years.1 The prevalence of silicone breast implant rupture in a population-based study has been reported to be as high as 55%, with 22% of ruptured implants showing extracapsular spread of silicone.1 Clinical signs and symptoms of patients with implant rupture include breast pain, wrinkling, asymmetry, scarring, and rarely infection. Local complications and adverse outcomes include capsular contracture, reoperation and removal.
The purpose of this pictorial essay is to present imaging findings of silicone implant rupture with intramammary and systemic silicone deposition as noted on mammogram, ultrasound, dual energy CT (DECT), PET/CT, and MRI. We include imaging findings of intracapsular and extracapsular silicone rupture in the breast. In addition, we present silicone deposition in mediastinal, axillary, and internal mammary (IM) lymph nodes, as well as in the liver and spleen. To our knowledge, deposition of silicone in the liver and spleen has not been previously demonstrated on cross sectional imaging.
Types of implant rupture
Following placement of silicone breast implants, the body forms a fibrous capsule around the breast implant. Thus, when implant rupture occurs, it can be intracapsular or extracapsular. Intracapsular rupture is defined as disruption of the implant shell without extrusion of silicone through the fibrous capsule. Extracapsular rupture is defined as macroscopic silicone extending beyond the fibrous capsule. Another phenomenon called “gel bleed” can occur where small unpolymerized silicone molecules permeate through the intact elastomer shell of the implant and can travel through the lymphatics. In each of these cases, silicone outside of the implant can travel through the lymphatic system into regional and distant lymph nodes.
Imaging modalities for evaluation of implant rupture
Mammography has a reported sensitivity of 11–69% for detection of implant rupture.2 Extracapsular rupture of silicone can be recognized mammographically as dense silicone in breast tissue. Intracapsular rupture is difficult to identify on mammogram and often requires ultrasound or MRI.
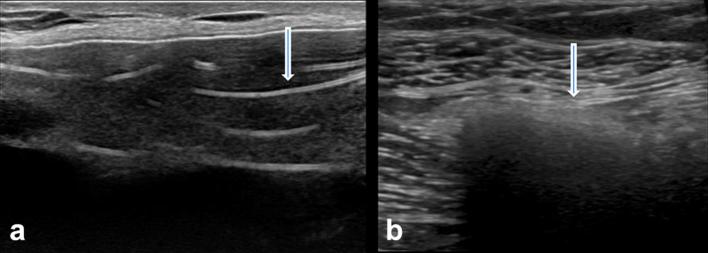
Ultrasound is slightly better than mammography with a reported sensitivity of 30–75%.2 A key sonographic feature of intracapsular rupture is the “stepladder” sign where linear hyperechoic lines are noted corresponding to the collapsed portion of the implant shell.3 The “snowstorm” appearance is seen with extracapsular silicone deposition within the breast tissue, lymph nodes or systemic organs (Figure 1).
Figure 1.
(a) The “stepladder” sign of intracapsular implant rupture on ultrasound with multiple linear hyperechoic areas (arrow) within the implant. (b) The “snowstorm” appearance refers to dense shadowing as seen with extracapsular silicone deposition in breast tissue and lymph nodes (arrow).
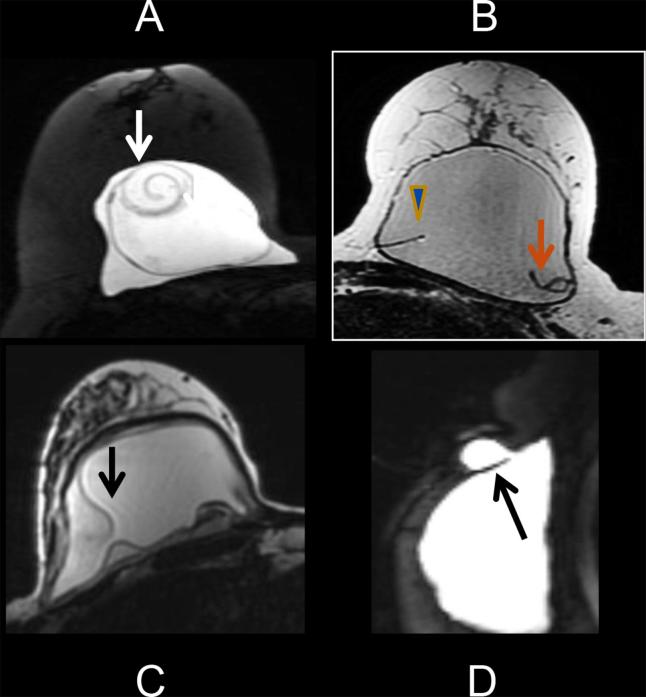
MRI is considered the most accurate imaging modality for evaluation of silicone implant rupture, with reported sensitivity of 72–94%.2 MRI signs of intracapsular rupture include the linguine sign (most specific), teardrop/keyhole appearance, or a subcapsular line. Extracapsular rupture appears as parenchymal silicone outside the fibrous capsule (Figure 2). The Food and Drug Administration has mandated a surveillance MRI screening examination for silent rupture in patients at 3 years following implantation of silicone breast implants and every 2 years thereafter.4
Figure 2.
(a) The linguine sign of intracapsular rupture on MRI is noted as linear swirling components of the silicone shell floating within the implant. (c) The keyhole appearance (arrow) of intracapsular rupture on MRI. (d) MRI showing extracapsular rupture where the silicone extends beyond the fibrous capsule (arrow) into the adjacent breast parenchyma.
DECT has been described as an alternative technique for evaluation of silicone implant rupture5, 6 and may be a reasonable option in patients with contraindication to MRI. DECT uses two different energies to delineate structures based on differences in their physical density (g/cm3) and atomic number (Z). Silicone, which contains the atomic element silicon (atomic number 14), has a different physical density and atomic number compared to surrounding soft tissues which are predominantly made up of hydrogen (atomic number 1) and oxygen (atomic number 8). Using these differential properties, tissue decomposition can identify silicone as a separate entity from other soft tissue structures on CT.5
DECT can identify calcification within fibrous capsule from longstanding implant placement. It can identify radial folds of the intact implant envelope seen adjacent to the fibrous capsule. It can also identify the “keyhole” appearance of silicone within the radial folds suggesting “gel bleed” where silicone transudates through an intact shell.
We present cases of intact silicone implants, gel bleed, free silicone from injections, intracapsular rupture, and extracapsular rupture on multimodality imaging in Figures 3–9.
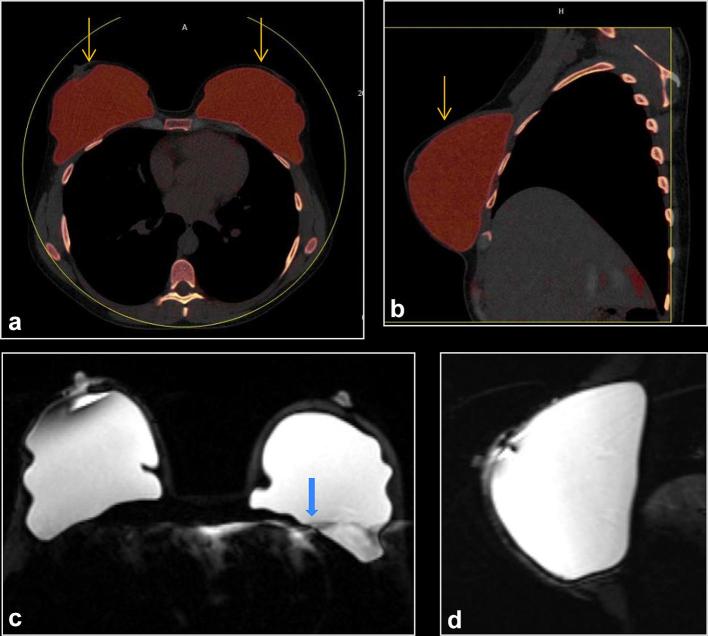
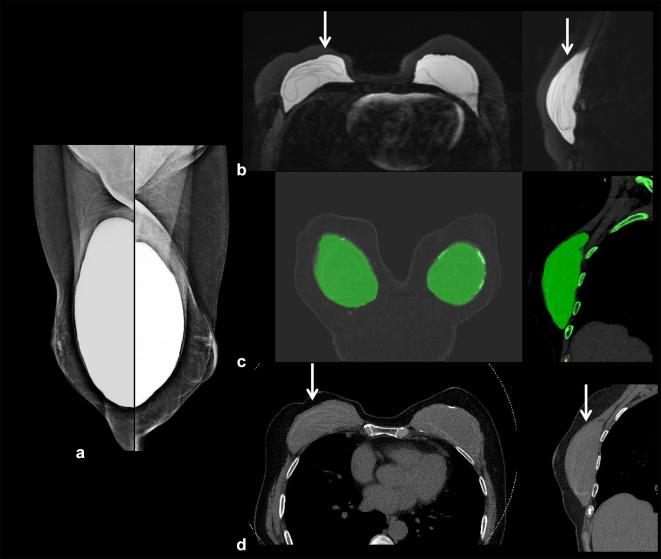
Figure 3. .
A 34-year-old female with prophylactic nipple sparing mastectomies at age 32. (a, b) Axial and left sagittal DECT, with silicone color mapped as red, shows intact bilateral subpectoral silicone implants (arrows). The implant is held in place by a surrounding fibrous capsule. No silicone is noted outside the breast fibrous capsule. (c, d) Axial and left sagittal MR silicone sensitive sequences show silicone within the fibrous capsule without signs of rupture. There is artifact (arrow). DECT, dual energy CT.
Figure 4.
A 76-year-old female with bilateral mastectomy with implant reconstruction who presented with clinical asymmetry. (a, b) DECT silicone series show radial folds and focal silicone outside the silicone envelope but contained within the fibrous capsule, consistent with gel bleed (arrows). No extracapsular silicone is present. (c, d) Sagittal and coronal CT reformats show radial folds and keyhole appearance of gel bleed (arrows). Surgical findings were consistent with gel bleed without implant rupture. DECT, dual energy CT.
Figure 5.
A 70-year-old female with bilateral mastectomies. (a) Mammograms show intact implants. (b) Silicone sensitive MRI shows “linguine” sign of intracapsular rupture (arrows). Correlating DECT (c) and CT (d) show similar findings. No silicone is noted outside the fibrous capsule. DECT, dual energy CT.
Figure 6.
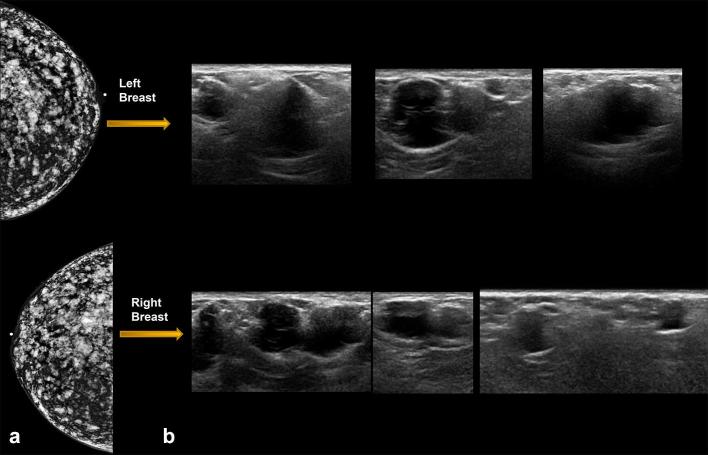
Patient with history of free silicone injections in the breasts bilaterally, presents with breast pain. (a) Mammogram showing high density free silicone throughout the breast. (b) On ultrasound, focal silicone aggregates can appear as innumerable irregular complex cystic masses in both breasts, some of which corresponded with sites of pain in this patient.
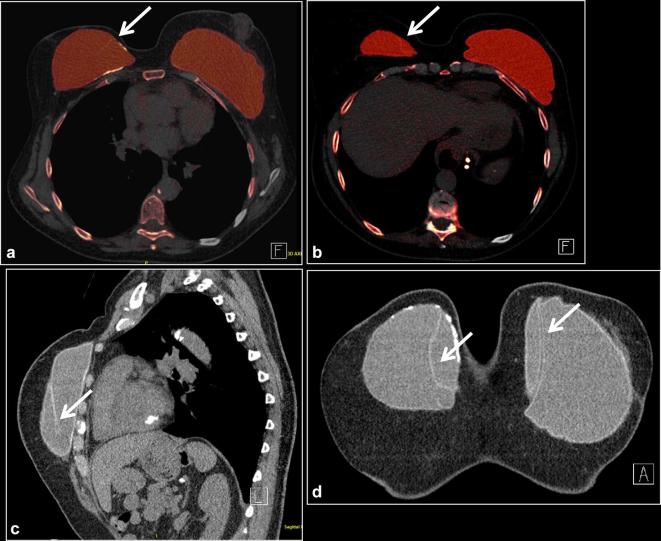
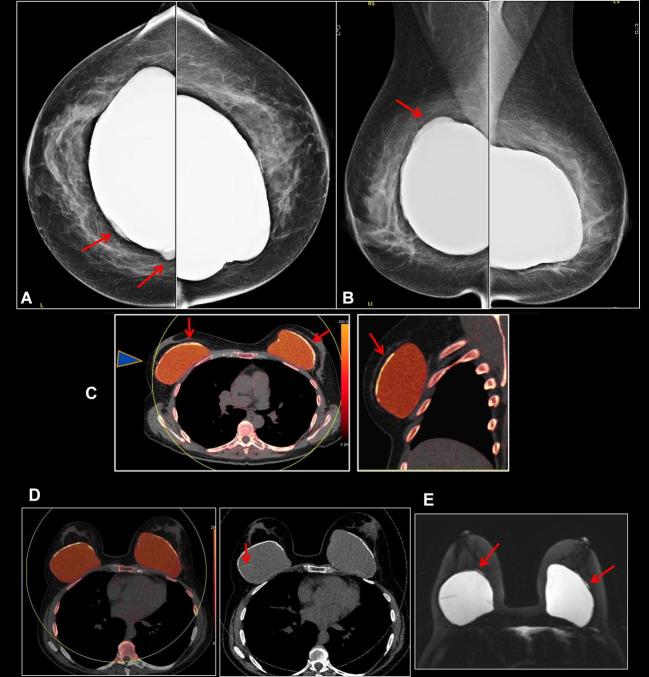
Figure 7. .
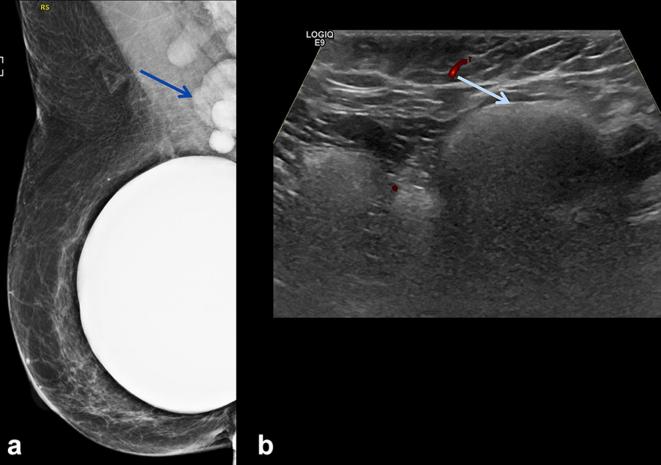
A 64-year-old female with bilateral subglandular silicone implant placement over 30 years ago. (a, b) Bilateral screening mammogram shows focal bulges of both implants (arrows), suspicious for implant rupture. C: DECT scanned SUPINE. Some breast tissue not included in scan circle (arrowhead). Sliver of extra-capsular silicone noted outside the calcified capsule bilaterally (arrows). (d) DECT scanned prone. All the tissue included in the scan circle. Radial fold is noted in the right breastarrow), an incidental finding. (e) Silicone sensitive MR sequence shows sliver of silicone outside the fibrous capsule bilaterally (arrows). Findings are consistent with bilateral extracapsular rupture. DECT, dual energy CT.
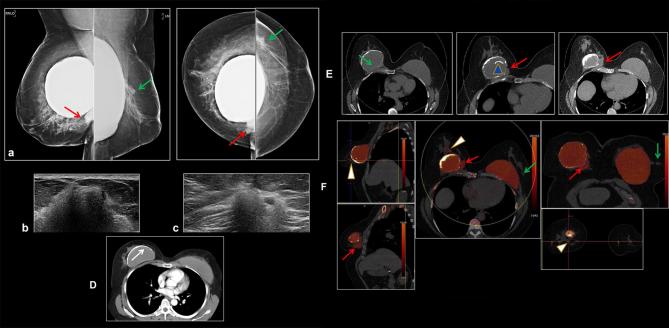
Figure 8.
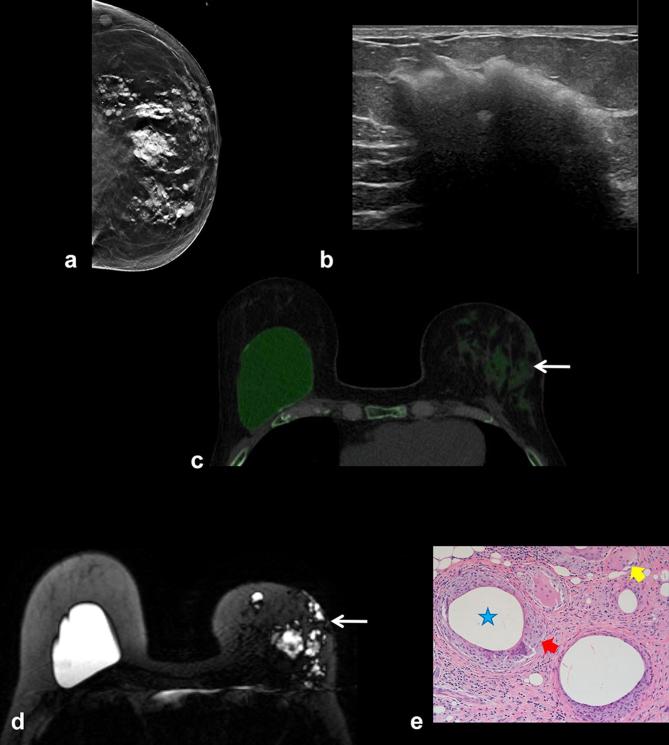
A 65-year-old female with 2-week history of painful new lump in the medial right breast. She had bilateral subglandular silicone implants placed over 40 years ago with history of left implant rupture 4 years after placement, which was replaced with a subpectoral silicone implant. (a) Right mammogram shows high density material adjacent to the calcified fibrous capsule of the right subglandular implant (arrow) suspicious for extracapsular silicone. This corresponded to the palpable mass. Left mammogram also demonstrated extracapsular high density material (arrow), suspicious for implant rupture. (b) Ultrasound in the area of palpable right breast mass demonstrates dense shadowing with a snowstorm appearance of silicone. (c) Ultrasound of left lateral breast shows snowstorm appearance with focal cyst corresponding to extracapsular silicone noted on mammogram. (d) Old CT scan from 20 years ago shows intact right calcified fibrous capsule but the implant envelope is displaced from the capsule medially consistent with intracapsular rupture (arrow). (e). Monoenergetic DECT shows heavily calcified right fibrous capsule with collapsed and calcified implant envelope (arrowhead). Indeterminant new soft tissue density tissue medial to the implant corresponding to the palpable mass (arrows). (f) DECT silicone color mapping images. In the right breast, palpable mass corresponds to extra-capsular silicone (arrows). There is addditional silicone anteriorly in the right breast (arrowhead). In the left breast, there is an intact left subpectoral implant. Extra-capsular silicone laterally in the left breast (arrow) is from prior implant rupture. This was biopsied and showed fat necrosis and multiple cysts filled with foreign material which would be consistent with silicone, associated inflammation and foreign body macrophages. DECT, dual energy CT.
Figure 9.
Patient with extracapsular silicone rupture in the left breast. (a) Mammogram shows high density free silicone throughout the breast parenchyma. (b) Ultrasound shows snowstorm appearance of extracapsular silicone in the breast parenchyma. (c) DECT demonstrates extracapsular silicone in the left breast as green color mapping (arrow). The right breast silicone implant is intact. (d) Silicone sensitive MRI sequence demonstrates ruptured extracapsular silicone as high signal intensity in the left breast (arrow). An intact silicone implant is noted in the contralateral right breast. (e) Pathology demonstrates benign breast parenchyma with dense fibrosis, fat necrosis (star), and foreign body multinucleated giant cell reaction (arrows) consistent with silicone implant rupture. DECT, dual energy CT.
Silicone in the chest, including axillary, internal mammary, mediastinal, and supraclavicular lymph nodes
There have been prior case reports of silicone deposition in the brachial plexus, upper extremity, anterior abdominal wall, and mediastinum.7, 8 It has been hypothesized that systemic silicone deposition primarily occurs through hematogenous or lymphatic routes. There have been prior case reports of systemic complications from silicone, including silicone pneumonitis and pulmonary embolism, but those are primarily due to subcutaneous injections of silicone.9, 10 Systemic complications from silicone breast implants are extremely rare. There has been one prior case report to our knowledge of silicone pneumonitis secondary to breast implants.11
Silicone deposition within lymph nodes can present as lymphadenopathy. Imaging is important in distinguishing reactive lymphadenopathy related to silicone deposition from metastatic disease, since some of these patients may have a history of breast cancer. Silicone within lymph nodes can appear dense on mammogram, can have a snowstorm appearance on ultrasound, can demonstrate color mapping on DECT, and can be hyperintense on silicone-sensitive MRI sequences.
Axillary lymph nodes may be seen on mammogram if included in the field of view. In patients with newly diagnosed breast cancer, axillary lymph nodes are considered suspicious for metastatic disease if cortical thickness is >3 mm or abnormal morphology is present. However, in patients with history of silicone breast implants, silicone granulomas can cause reactive enlargement of these lymph nodes. From our personal experience, the most accurate method to distinguish reactive vs metastatic lymphadenopathy is with ultrasound, as it can show a classic snowstorm appearance in cases of silicone deposition within the node. Silicone-sensitive MRI may not always exhibit high signal intensity as silicone may variably infiltrate the node.2
IM nodes can be identified on ultrasound, PET/CT, DECT or MRI. Recent studies have shown that IM nodes are present in approximately 50% of high risk screening MRI patients without implants.12 IM nodes in patients without breast cancer should have a short axis dimension of <6 mm. In patients with breast cancer, any visualized IM node needs to be further evaluated. Silicone-sensitive MRI or PET/CT can be used to identify the source of lymphadenopathy.13
Similar to IM nodes, supraclavicular lymph nodes can be identified on ultrasound, DECT or MRI while mediastinal nodes can be identified on DECT or MRI.
We present multiple cases of silicone deposition in mediastinal, supraclavicular, IM and axillary lymph nodes on multimodality imaging in Figures 10–13.
Figure 10. .
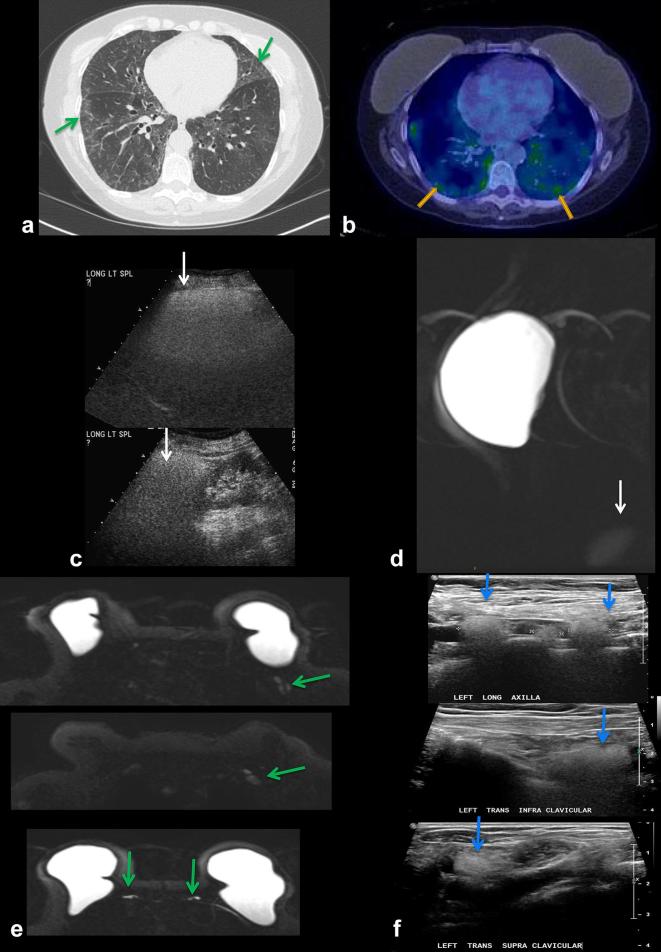
A 70-year-old female with history of prior silicone implant rupture and implant exchange presents with palpable right axillary mass. (a) Mammogram shows high density axillary adenopathy (arrow) and an intact silicone implant. (b) Ultrasound of the right axilla shows “snowstorm” appearance (arrow) of silicone lymphadenitis.
Figure 11.
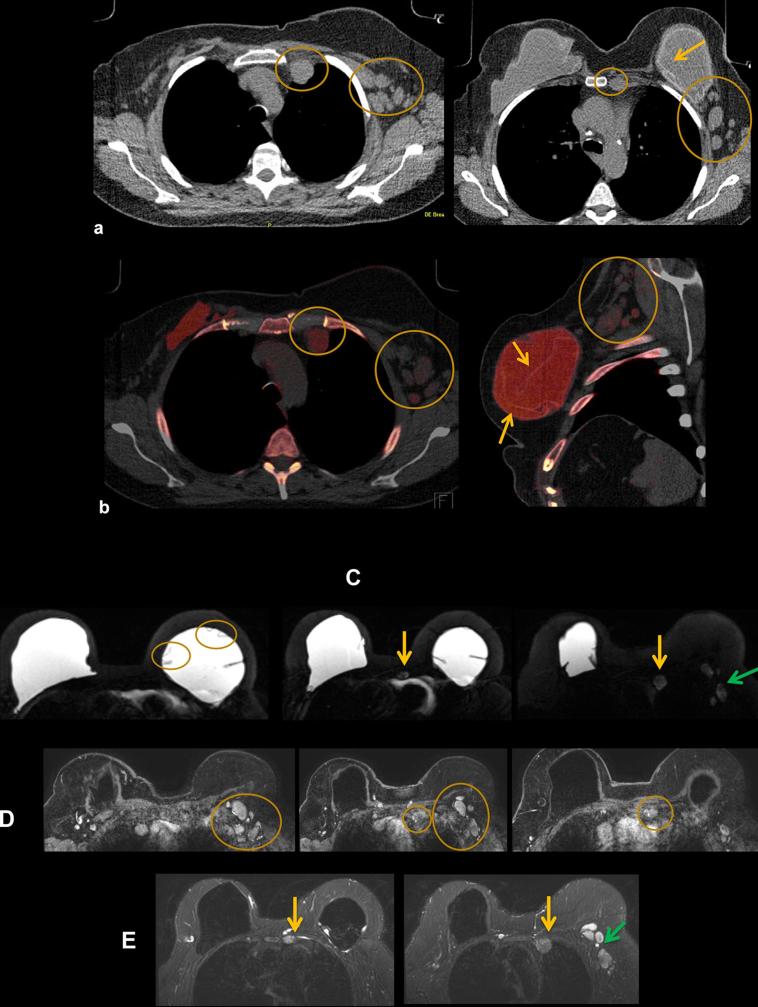
60-year-old woman with history of implant explantation (a) Indeterminate right breast soft tissue masses and left axillary lymphadenopathy seen on monoenergetic CT (arrows) (b) DECT makes definitive diagnosis of residual silicone (red color) on DECT silicone images in the right breast and palpable left axillary node (arrows) as demonstrated by increased red signal. (c) MR silicone sensitive sequence showing high signal silicone in the right breast (arrow) and left axillary lymph node (arrow). (d) US of the left axilla show snowstorm appearance of silicone within the left axillary node (arrow). DECT, dual energy CT.
Figure 12.
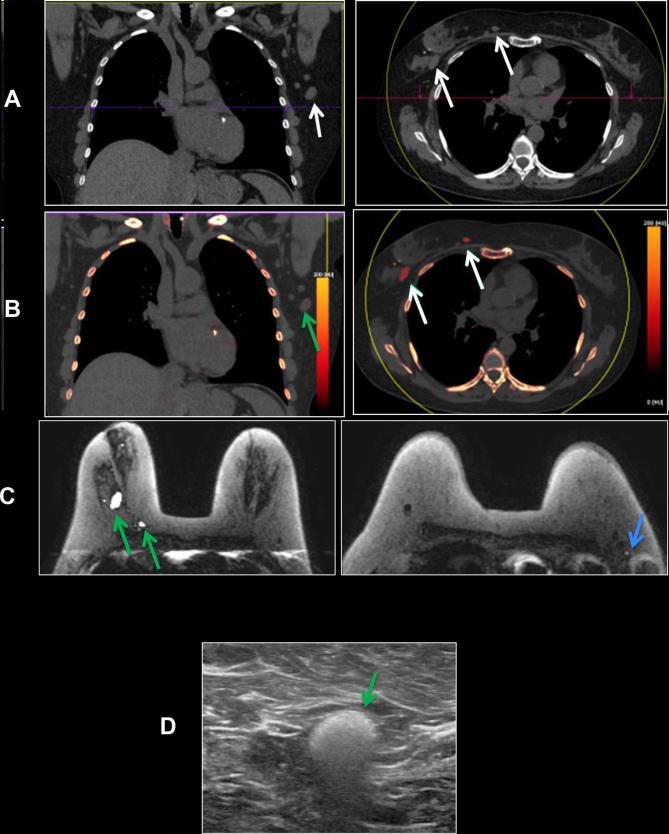
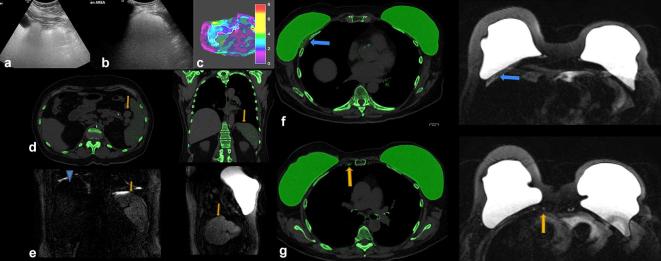
A 50-year-old female with of headaches. Patient had a history of Wilms tumor as a child, status post-nephrectomy and radiation. Patient also had right breast cancer, status post-bilateral mastectomies in over 15 years ago. A PET-CT was obtained to evaluate for recurrent disease. (a) Axial FDG PET-CT: FDG uptake in bilateral IM nodes (circles) and axillary nodes (not shown), highly suspicious for breast cancer metastases. (b) Axial silicone selective MRI shows intact dual lumen silicone implants, with an inner saline shell (low signal intensity) and an outer silicone shell (high signal intensity). High signal is present in IM nodes, suggestive of silicone. (c) Axial silicone selective MRI (top) shows silicone in bilateral IM nodes (arrows). Axial T1 post-contrast MRI (bottom) shows enlarged low signal intensity IM nodes (arrows); silicone appears as low intensity on post-gadolinium images. (d) Axial DECT images show multiple enlarged IM nodes containing silicone as noted by green color mapping (circle) corresponding to PET-CT. (e) Patient also had mild green color mapping in right axillary lymph nodes, suggestive of silicone (circle). (f) Ultrasound of right axillary node demonstrated typical snowstorm appearance of silicone within nodes (arrow). Ultrasound-guided biopsy of the right axillary lymph node was performed for confirmation, which revealed silicone granuloma. Patient underwent explantation of the bilateral breast implants and has done well since, without new pain or symptoms. DECT, dual energy CT; FDG, fludeoxyglucose; IM, .
Figure 13. .
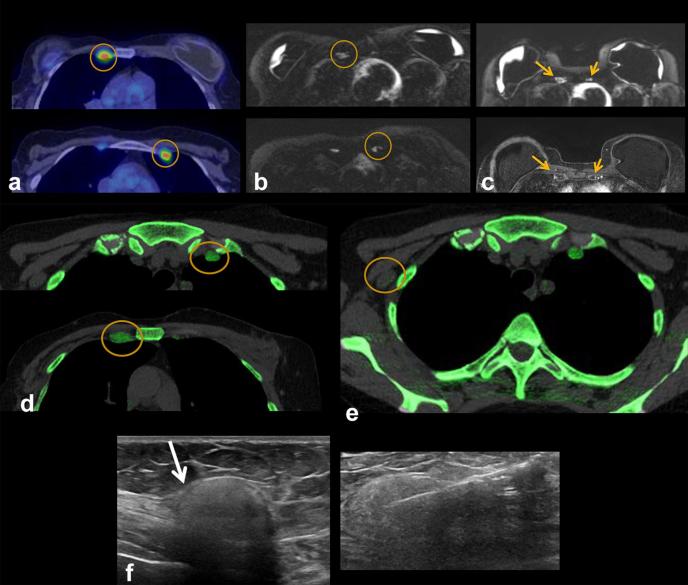
A 58-year-old female presents with tenderness in left chest wall. She has a history of left breast invasive ductal carcinoma, status post-bilateral mastectomy and silicone implant placement 20 years ago, with three implant revisions since. (a) Axial (right) and sagittal (left) non-contrast CT shows enlarged left IM nodes and multiple enlarged left axillary nodes (circles). There are also findings of left implant intracapsular rupture (arrow). (b) Axial (right) and sagittal (left) DECT shows silicone within the left IM and axillary nodes as noted by red color mapping. Intracapsular rupture is also present on DECT as noted by the silicone shell floating within the implant (arrows). DECT, dual energy CT; IM, internal mammary.
Silicone in the liver and spleen
Following implant rupture, silicone can migrate through the lymphatic system to deposit in the spleen and liver. Silicone in the liver has previously been reported in rat models implanted with silicone.14, 15 There are two articles regarding silicone deposition in the liver and spleen in human subjects with silicone breast implants based on H-1 MR spectroscopy findings. It was noted in these studies that silicone in the liver could be detected as early as 3–4 years after breast implant placement, and higher concentration of silicone was detected in the liver in cases of implant rupture.15
Silicone in the liver and spleen is best seen on ultrasound as a snowstorm appearance. Silicone-selective DECT and MR can also identify silicone deposition in the liver and spleen. However, in our experience, silicone-selective DECT and MR showed equivocal findings which were suggestive but not conclusive of silicone within the liver.
Figure 14 presents a case of silicone deposition within the spleen, and Figure 15 describes a case of silicone deposition in both the liver and spleen.
Figure 14.
A 75-year-old patient with chronic pulmonary infiltrates. History of metastatic breast cancer status post-bilateral mastectomies and silicone implant reconstruction 25 years ago, and several implant revisions since. (a) CT chest shows ground-glass opacities in the lungs bilaterally (arrows). Multifocal lymphadenopathy was also noted on CT (not shown) (b) PET/CT shows hypermetabolic FDG-avid pulmonary infiltrates bilaterally (arrows). Patient underwent lung biopsy showing silicone pneumonitis. (c) Interestingly, an ultrasound of the spleen performed 10 years prior to presentation shows hyperechoic shadowing, suggestive of silicone deposition, not appreciated at the time. (d) Recent silicone sensitive MRI demonstrates incidental hyperintense signal in the spleen (arrow), confirming silicone deposition in the spleen. (e) Silicone sensitive MRI sequences showing hyperintense signal in left axillary lymph nodes (top), supraclavicular and infraclavicular lymph nodes (middle) and IM nodes (bottom), compatible with silicone deposition in these nodes (arrows). (f) Ultrasound of left axillary (top), infraclavicular (middle) and supraclavicular (bottom) lymph nodes demonstrating the “snowstorm” appearance of silicone lymphadenopathy . FDG, fludeoxyglucose;IM, internalmammary.
Figure 15.
A 67-year-old female presented with abdominal discomfort. She had a history of left infiltrative lobular carcinoma T1N0 status post-bilateral total mastectomies and left axillary dissection with bilateral dual implant reconstructions over 20 years ago. She subsequently underwent three separate implant revisions , most recently having replacement of her subpectoral silicone implants. Initial blood work, including CBC and a chemistry panel, showed normal liver function tests. (a) An abdominal ultrasound was initially performed which showed dense echogenic shadowing throughout the liver, suggestive of the “snowstorm” appearance of silicone. (b) Ultrasound of the spleen demonstrated similar dense echogenic shadowing diffusely. (c) Abdominal MRI with elastography was performed which showed normal liver stiffness and no suspicious abnormality. White line denotes the ROI drawn to measure mean liver stiffness. (d) DECT was performed due to suspicion of silicone deposition. Axial and coronal DECT of the abdomen shows silicone in the spleen and splenules, as noted by green color mapping (arrow). Not much silicone is seen in the liver. (e) Silicone sensitive MRI was performed. Coronal and sagittal sequences show hyperintense signal in spleen (arrow), compatible with silicone deposition. The liver (arrowhead) is minimally hyperintense without definitive findings of silicone deposition on MRI. DECT (f) and silicone sensitive MRI (g) sequences also show silicone in thickened right pectoralis muscle (blue arrow) and in normal sized right internal mammary lymph nodes (arrow). Muscle thickening was likely post-operative from prior implant revision. In this case, silicone was detected in the spleen on ultrasound, DECT, and MRI, while silicone in the liver was best detected on ultrasound. CBC, complete blood count; DECT, dual energy CT; ROI, region of interest.
Conclusion
We present multimodality imaging correlation of intracapsular and extracapsular silicone rupture within the breast as well as systemic silicone deposition. While all imaging modalities were able to detect silicone in the spleen, in our experience, ultrasound was helpful in the detection of silicone in the liver.
Contributor Information
Naziya Samreen, Email: Samreen.Naziya@mayo.edu.
Katrina N Glazebrook, Email: glazebrook.katrina@mayo.edu.
Asha Bhatt, Email: Bhatt.Asha@mayo.edu.
Sudhakar K Venkatesh, Email: venkatesh.sukhakar@mayo.edu.
Brendan P McMenomy, Email: mcmenomy.brendan@mayo.edu.
Anupam Chandra, Email: chandra.anupam@mayo.edu.
Shuai leng, Email: leng.shuai@mayo.edu.
Kalie E Adler, Email: adler.kalie@mayo.edu.
Cynthia H McCollough, Email: McCollough.Cynthia@mayo.edu.
REFERENCES
- 1.Berg WA, Nguyen TK, Middleton MS, Soo MS, Pennello G, Brown SL. MR imaging of extracapsular silicone from breast implants: diagnostic pitfalls. AJR Am J Roentgenol 2002; 178: 465–72. doi: 10.2214/ajr.178.2.1780465 [DOI] [PubMed] [Google Scholar]
- 2.Seiler SJ, Sharma PB, Hayes JC, Ganti R, Mootz AR, Eads ED, et al. Multimodality imaging-based evaluation of single-lumen silicone breast implants for rupture. Radiographics 2017; 37: 366–82. doi: 10.1148/rg.2017160086 [DOI] [PubMed] [Google Scholar]
- 3.Venta LA, Salomon CG, Flisak ME, Venta ER, Izquierdo R, Angelats J. Sonographic signs of breast implant rupture. AJR Am J Roentgenol 1996; 166: 1413–9. doi: 10.2214/ajr.166.6.8633455 [DOI] [PubMed] [Google Scholar]
- 4.Chung KC, Malay S, Shauver MJ, Kim HM. Economic analysis of screening strategies for rupture of silicone gel breast implants. Plast Reconstr Surg 2012; 130: 225–37. doi: 10.1097/PRS.0b013e318254b43b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glazebrook KN, Leng S, Jacobson SR, McCollough CM. Dual-energy CT for evaluation of intra- and extracapsular silicone implant rupture. Case Rep Radiol 2016; 2016: 6323709–4. doi: 10.1155/2016/6323709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson TR, Himsl I, Hellerhoff K, Mayr D, Rjosk-Dendorfer D, Ditsch N, et al. Dual-energy CT for the evaluation of silicone breast implants. Eur Radiol 2013; 23: 991–6. doi: 10.1007/s00330-012-2667-z [DOI] [PubMed] [Google Scholar]
- 7.Ahn CY, Shaw WW. Regional silicone-gel migration in patients with ruptured implants. Ann Plast Surg 1994; 33: 201–8. doi: 10.1097/00000637-199408000-00014 [DOI] [PubMed] [Google Scholar]
- 8.Bauer PR, Krajicek BJ, Daniels CE, Shah SS, Ryu JH. Silicone breast implant-induced lymphadenopathy: 18 Cases. Respir Med CME 2011; 4: 126–30. doi: 10.1016/j.rmedc.2011.01.001 [DOI] [Google Scholar]
- 9.de March Ronsoni R, Schwingel FL, Melo LH, de Albernaz Muniz RZ, Lourenço KC, Magalhães PSC, et al. Pulmonary embolism due to liquid silicone: Case report. Respiratory Medicine Extra 2007; 3: 172–4. doi: 10.1016/j.rmedx.2007.09.002 [DOI] [Google Scholar]
- 10.Rosioreanu A, Brusca-Augello GT, Ahmed QA, Katz DS. CT visualization of silicone-related pneumonitis in a transsexual man. AJR Am J Roentgenol 2004; 183: 248–9. doi: 10.2214/ajr.183.1.1830248 [DOI] [PubMed] [Google Scholar]
- 11.García Hernández MJ, López Milena G, Ruiz Carazo E. Subacute silicone pneumonitis after silent rupture of breast implant. Arch Bronconeumol 2016; 52: 397–8. doi: 10.1016/j.arbr.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 12.Mack M, Chetlen A, Liao J. Incidental Internal Mammary Lymph Nodes Visualized on Screening Breast MRI. AJR Am J Roentgenol 2015; 205: 209–14. doi: 10.2214/AJR.14.13586 [DOI] [PubMed] [Google Scholar]
- 13.Sutton EJ, Watson EJ, Gibbons G, Goldman DA, Moskowitz CS, Jochelson MS, et al. Incidence of Internal Mammary Lymph Nodes with Silicone Breast Implants at MR Imaging after Oncoplastic Surgery. Radiology 2015; 277: 381–7. doi: 10.1148/radiol.2015142717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfleiderer B, Ackerman JL, Garrido L. Migration and biodegradation of free silicone from silicone gel-filled implants after long-term implantation. Magn Reson Med 1993; 30: 534–43. doi: 10.1002/mrm.1910300503 [DOI] [PubMed] [Google Scholar]
- 15.Pfleiderer B, Campbell T, Hulka CA, Kopans DB, Lean CL, Ackerman JL, et al. Silicone gel-filled breast implants in women: findings at H-1 MR spectroscopy. Radiology 1996; 201: 777–83. doi: 10.1148/radiology.201.3.8939231 [DOI] [PubMed] [Google Scholar]