Abstract
Objective:
To determine the diagnostic value of combining conventional MRI, diffusion-weighted imaging (DWI) and dynamic contrast enhanced MRI (DCE-MRI) in salivary gland tumors.
Methods:
45 patients with salivary gland tumors were evaluated with conventional MRI, DWI and DCE-MRI prior to surgery and confirmed by pathologic findings. The apparent diffusion coefficient (ADC) was calculated from DWI that was obtained with a factor of 0 and 1000 s mm–2. A time-intensity curve (TIC) was obtained from DCE-MRI.
Results:
In conventional MRI, benign tumors often showed well-defined and clear margins, malignant tumors showed irregular margins or infiltration into the surrounding tissue. There were significant differences with regard to the ADC values between pleomorphic adenoma (1.72 ± 0.29 × 10−3 mm2 s–1) and malignant tumors (0.95 ± 0.09 × 10−3 mm2 s–1, p < 0.05) and between adenolymphoma (0.74 ± 0.05 × 10−3 mm2 s–1) and malignant tumors (p < 0.05). However, there was no significant differences in term of the ADC values between benign tumors (1.33 ± 0.52×10−3 mm2 s–1) and malignant tumors. DCE-MRI showed benign tumors with A-type, B-type and D-type of TICs, and the malignant tumors with C-type TICs. A combination of all of these parameters yielded sensitivity, specificity, accuracy, and positive and negative predictive values of 90%, 97%, 95%, 90 and 97%, respectively.
Conclusion:
An evaluation combining MRI morphologic findings and functional MRI (ADCs and TIC) appears to be useful in differentiating benign from malignant tumors in salivary gland tumors.
Advances in knowledge:
The study firstly dealt with the combination of conventional MRI, DWI-MRI with DCE-MRI in salivary gland tumors.
Introduction
Salivary gland tumors account for 5% of all head and neck tumors, with the epithelial tumor being in the majority and mesenchyme tissues tumor being relatively rare.1 Salivary gland tumors mainly arise from parotid gland, submandibular gland and minor salivary gland. Parotid gland and palate are the high incidence site of major and minor salivary gland tumors, respectively.2 The most of salivary gland tumors are usually benign, whereas, the minority of salivary gland tumors tend to be malignant.1 Histologically, the most common benign tumor is pleomorphic adenoma, followed by adenolymphoma, and the most common malignant tumor is mucoepidermoid carcinoma, followed by adenoid cystic carcinoma.3 The most common benign and malignant tumors in glandulae salivariae majores and minor salivary glands are pleomorphic adenomas and mucoepidermoid carcinoma, pleomorphic adenomas and adenoid cystic carcinoma, respectively.2
Many studies have been reported fine-needle aspiration cytology (FNAC) is a useful tool in the preoperative diagnosis of salivary gland tumors.4 However, it may be difficult to the biopsies of salivary gland tumors, which can lead to dissemination of the tumors, due to tumors are often covered by normal mucosa and biopsies are performed deep within the mucosa.4 In addition, due to the complex pathological types of salivary gland tumors, it is often difficult to obtain a definite diagnosis from the biopsy. Furthermore, it is clinically important for surgeons to know preoperatively whether a salivary gland tumor is benign or malignant preoperatively, which strongly affect the surgical procedure. Therefore, the preoperative imaging plays an important role in surgical planning.
Previously, the conventional MRI had been widely used in the clinical assistant diagnosis of salivary gland tumors, it could determine the location of the salivary gland tumor and the anatomical relationship between tumors and the surrounding tissue.5 Yabuuchi et al6 and Ahuja et al7 reported MRI played a useful role in diagnosing and evaluating extent of local and distant involvement for patient with salivary gland tumors. In addition, over the past decades, diffusion-weighted imaging (DWI), dynamic contrast-enhanced MRI (DCE-MRI) solely or the combination of both also have been evaluated in salivary gland tumors. DWI quantifies the diffusional mobility of water protons with apparent diffusion coefficient (ADC); DCE-MRI provides information related to the physiology of the microcirculation and vascularity. Eissa et al8 reported the combined of DWI and dynamic contrast enhancement curves could provide valuable data in characterization of salivary glandular tumors. Nakamura et al9 reported that the ADC of DWI may provide pre-operative tissue characterization of the salivary gland tumors. Asaumi et al3 reported that DCE-MRI are useful in diagnosing salivary gland tumors on the basis of the combined assessment of CImax (CImax, maximum contrast index) and Tmax (Tmax, time of CImax) or washout ratio (WR). Pirayesh et al1 reported that the combination of DCE-MRI and DWI might be effective for separation of salivary gland tumors by using time-intensity curve (TIC) analysis plus apparent diffusion coefficient (ADC) map.
However, there have been few reports concerning the diagnostic value of conventional MRI combined with DWI and DCE-MRI for salivary gland tumors. The purpose of this work was to evaluate the potential value of combining conventional MRI, DWI and DCE-MRI in preoperative characterization of salivary glandular tumors.
Methods and Materials
Subjects
The project was approved by the ethics committee of Jining NO.1 People’s Hospital, and informed consent was obtained from all patients. The retrospective analysis included 45 patients with salivary gland tumors, examined with conventional MRI, DWI and DCE-MRI and treated surgically at our hospital, from September 2011 to December 2012. The patients’ inclusion criteria were nonsmokers, not having fixed partial dentures, not addicted to alcohol, no previous history of head or neck disease or radiation therapy, not having had head or neck surgery, not under chemotherapy for systemic malignancy, not taking medication or invasive procedures at the time of imaging examinations, high MRI imagines without motion and susceptibility artifacts. The patients were 18 females and 27 males with a mean age of 62.3 years (range, 15–79 years). The tumors were located in the parotid (38), submandibular gland (5), sublingual gland (1), and small salivary gland (1). The patients’ histopathological diagnoses included 34 benign tumors (18 pleomorphic adenoma, 13 adenolymphoma, 1 schwannoma, 1 lymphangioma and 1 basal cell adenoma), and 11 malignant tumors (3 acinar cell carcinoma, 2 mucoepidermoid carcinoma, 1 adenoid cystic carcinoma, 1 carcinoma ex pleomorphic adenoma, 1 infiltrating carcinoma, 1 poorly differentiated carcinoma, 1 parotid branchial cleft cyst canceration and 1 carcinoma sareomatodes). The final diagnoses were made based on the pathological findings of the specimens obtained during surgical resection.
MRI
MRIs were performed by using a Siemens Trio 3.0 T Magnetom Symphony imager (Siemens, Erlangen, Germany) with head and neck array coils. The conventional MRI sequences included axial and coronal T1 weighted imaging (T1WI) and fat suppressed T2 weighted imaging (T2WI). T1WI MRI sequences were performed with 689 ms repetition time (TR), 23 ms echo time (TE), 320 × 240 matrix and 90 s scanner time. Coronal T2 short-tau inversion-recovery (T2-STIR) MRI sequences were performed with 3300 ms TR, 34 ms TE, 256 × 192 matrix and 163 s scanner time. Axial T2 fat saturation (T2-FS) MR sequences with were performed with 5280 TR, 75 ms TE, 320 × 240 matrix, and 100 s scanner time. Moreover, other same parameters included 24 × 24 cm field of view (FOV), 3 mm section thickness, 0.3 mm intersection gap, and 3 times of excitations.
DWI was performed using a single-shot spin echo echoplanar imaging (SS SE-EPI) with 4200 ms TR, 94 ms TE, FOV, 24 × 24 cm, 0.3 mm intersection gap, 3 times excitations, 192 × 173 matrix, 3 mm section thickness. Sensitizing diffusion gradients were applied sequentially in three directions with b-values between 0 and 1000 s mm−2, and the scan time was 102 s.
DCE-MRI was performed using a gradient-recalled echo volumetric interpolated breathhold examination sequencea in coronal turbo spin echo T2WI. The scanning parameters were as follows: TR/TE, 4.9/1.74 ms; FOV, 26 × 26 cm; matrix, 256 × 192; section thickness, 3.6 mm; intersection gap, 0.72 mm; 1 time of excitation and bandwidth of 250 Hz Px–1. The scanner time was 262 s, and 24 phases were collected, and the scanning time of each phase was 11 s. Gadopentate dimeglumine pentaacetic acid (Magnevist, Schering, Berlin, Germany) was injected intravenously at a dose of 0.1 mmol kg–1 body weight and at an injection rate of 2.5 ml s−1. Contrast-enhanced transverse and coronal fat suppression-fast spin echo T1WI images were also obtained.
Image analysis
All MRIs were interpreted and consensus was achieved by two experienced radiologists with 13 and 20 years’ experience, respectively, in head and neck MRI diagnosis, who were blinded to the histopathologic results and the clinical information. In addition, the two experienced radiologists were blinded to each other. The interpretation of the conventional MRI was based on the shape, margin, capsule and T1WI, T2WI signal intensity (SI) of tumors. The signals intensity was divided into hyper-, hypo- and isointense signals referred to the surrounding tissue and adjacent muscles.10
The DWI data and DCE-MRI were post-processed using the MRWP workstation of Siemens. The DWI used the ADC value as an indicator of the magnitude of the diffusion. For ADC measurements, three ROIs (mean, 0.32 cm2) was drawn with an electronic cursor and were randomly selected from the parenchymal portion of tumor avoiding the cystic–necrotic or calcification areas. ADC was calculated with linear regression analysis.
For post-processing of DCE-MRI, the ROIs were drawn in the shape of circular (area >0.1 cm2; diameter, 3–4 mm), avoiding the vessels and cystic regions within the tumors, and TIC for each ROI was generated. The mean SI on the ROI of each lesion was calculated using the MRWP workstation of Siemens. The parameters SIpre, SI4min, SImax, WR and time to peak (Tpeak) were derived from the TICs for classification of tumors. The Tpeak represents the microvessel count, and the threshold between malignant and benign tumors, which has been reported in previous studies to be about 120 s.11 The WR is considered to represent the difference in the concentration of contrast medium between the intravascular and extravascular phases.11 SIpre represented the pre-contrast SI, SI4min was the SI at 4 min after injection of the contrast agent, SImax was defined as the SI at maximal contrast enhancement. SIpeak was defined as the first SI measurement that satisfied the inequality SI> (0.9 (SImax–SIpre))+SIpre. WR, expressed as a percentage, was defined as follows: WR = (SImax–SI4min)/(SImax–SIpre) × 100%. Tpeak represented the time that corresponded to the SIpeak, and was used as a dynamic parameter for differentiation between benign and malignant tumors. We did not define Tpeak as the time that corresponded to the SImax, as was done in earlier studies. When we have defined Tpeak as the time that corresponded to the SImax, we have found it difficult to determine a reliable Tpeak in cases in which the TIC showed an early peak and plateau enhancement pattern, because SI can change to a very small degree within an error during the plateau phase. In this study, according to Tpeak and WR, the TICs were categorized into four patterns for salivary gland tumor: (1) Type A (persistent pattern): Tpeak > 120 s; (2) Type B (washout pattern): Tpeak ≤ 120 s, WR ≥ 30%; (3) Type C (plateau pattern): Tpeak ≤ 120 s, WR <30%; (4) Type D (flat pattern): flat, without prominent enhancing.6
Statistical analysis
Statistical analyses were performed using the SPSS 13.0 statistical software package (SPSS, Chicago, IL). All statistical analyses were performed by using non-parametric statistical tests because of relatively small sample size. A p-value < 0.05 was considered as statistically significant. For the indexes with statistical significance, receiver operating characteristic curve was plotted to determine the threshold value and calculate sensitivity, specificity, positive-, negative-predictive value and accuracy rate.
Result
MRI findings
Among benign salivary tumors, pleomorphic adenoma (n = 18) were round or oval mass with clear margin, there are two cases with no clear capsule, the rest with hypointense envelope. T1WI images showed hypointense signal in 14 cases, equal signal in 2 cases, hyperintense signal in 2 cases; T2WI images showed hyperintense signal in all cases (n = 13, homogeneous signal; n = 5, heterogeneous signal). Enhanced scan showed 18 cases of lesions were significantly enhanced (Table 1, Figure 1). Adenolymphoma (n = 13, 3 multiple tumors and 10 solitary tumors) showed round shape, clear border and smooth outline. T1WI images showed slightly hypointense signal in 10 cases, isointense signals in 2 cases, hyperintense signal in 1 case; T2WI images showed hypointense signal in 1 case, isointense signals in 2 cases, hyperintense signal in 10 cases. Enhanced scan showed 13 cases of lesions were mildly enhanced (Table 1, Figure 2). Schwannoma (n = 1) with multiple small cysts presented clear margin, hypointense signal on T1WI, inhomogeneous hyperintense signal on T2WI (Table 1). Basal cell adenoma (n = 1) showed cystic solid changes, hypointense signal on T1WI, hyperintense signal on T2WI of cystic part, isointense signals on T1WI and hyperintense signal on T2WI in solid part showed, enhanced scan showed the solid part and cyst wall of the lesions were enhanced (Table 1). Lymphangioma (n = 1), with many hypointensive internal septations and small liquid–liquid planes on MR, showed similar-circular mass, clear margin, low T1WI signal, and hyperintense T2WI signal. The enhanced scan showed no obvious enhancement (Table 1).
Table 1.
Conventional MRI findings of 45 cases of salivary gland tumors
| Characteristic | Benign tumors (n = 34) | Malignant tumor (n = 11) | ||||
| Pleomorphic adenoma | Adenolymphoma | Angiolymphoma | Schwannoma | Basal cell adenoma | ||
| Clear margin | 18/18 | 13/13 | 1/1 | 1/1 | 1/1 | 2/11 |
| Regular shape | 18/18 | 13/13 | 1/1 | 1/1 | 1/1 | 2/11 |
| Displayed envelope | 16/18 | 11/13 | 1/1 | 1/1 | 1/1 | 0/11 |
| T1WI signal | ||||||
| Hypointense | 14/18 | 10/13 | 1/1 | 1/1 | 1/1 | 11/11 |
| isointense | 2/18 | 2/13 | 0 | 0 | 0 | 0 |
| Hyperintense | 2/18 | 1/13 | 0 | 0 | 0 | 0 |
| T2WI signal | ||||||
| Hypointense | 0 | 1/13 | 0 | 0 | 0 | 0 |
| Isointense | 0 | 3/13 | 0 | 0 | 0 | 0 |
| Hyperintense | 18/18 | 9/13 | 1/1 | 1/1 | 1/1 | 11/11 |
T1WI,T1 weighted imaging; T2WI,T2 weighted imaging.
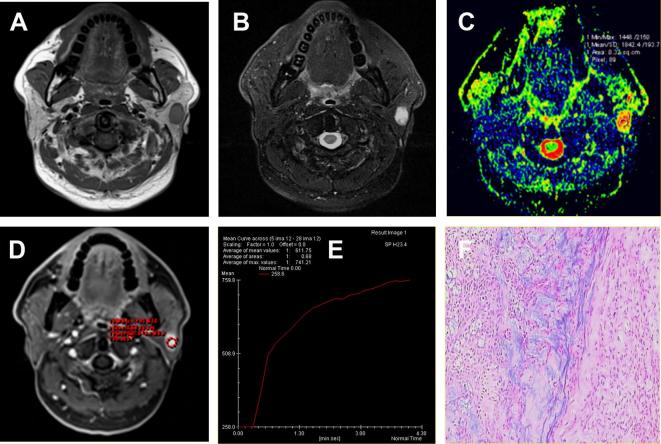
Figure 1.
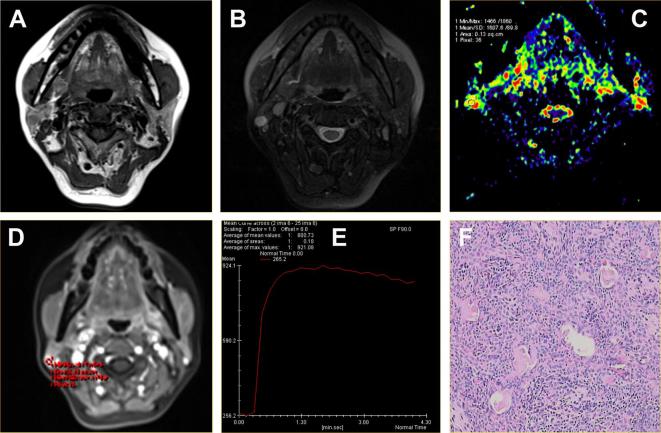
A pleomorphic adenoma located in the superficial lobe of the left parotid gland of a 63-year-old male. (A) Axial T1WI showed a round mass with isointense signal intensity and hypointense capsule. (B) Axial T2WI sequences with fat suppression showed the lesions with inhomogeneous hyperintense signal. (C) ADC map showed the mass alternated with red or yellow signal. The ADC value was 1.84 × 10−3 mm2 s–1. (D-E) Time-signal intensity curve showed a persistent pattern (Type A). (F) HE staining: original magnification, 100×. ADC, apparent diffusion coefficient; HE, hematoxylin; T1WI, T1 weighted imaging.
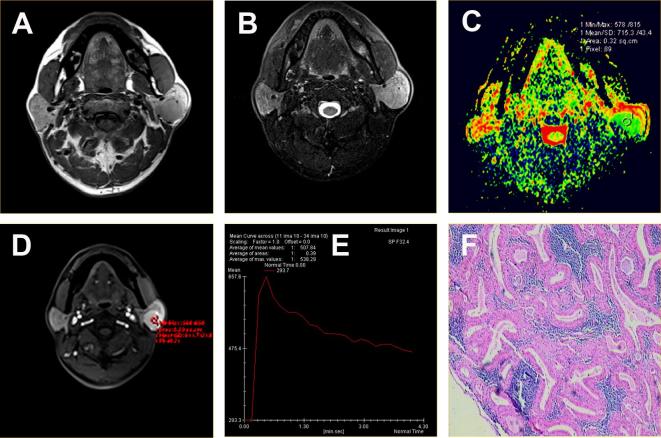
Figure 2.
Adenolymphoma located in the superficial lobe of the left parotid gland of a 49-year-old male. (A) Axial T1WI showed lesion with homogeneous isointense signal and hypointense capsule. (B) Axial T2WI sequences with fat suppression showed left parotid gland superficial lobe with round mass and homogeneous isointense signal. (C) ADC map showed the mass with green signal. The ADC value was 0.72 × 10−3 mm2 s–1. (D-E) Time-signal intensity curve showed a washout enhancement pattern (Type B). (F) HE staining: original magnification, 100×. ADC, apparent diffusion coefficient ; HE, hematoxylin;T1WI, T1 weighted imaging.
All malignant tumors (n = 11) were almost with irregular shape, no obvious capsule, unclear margins and some lesions invading surrounding soft tissues, hyperintense T1WI signal, hyperintense T2WI signal. The signals were heterogeneous, the delayed scanning showed inhomogeneous enhancement (Table 1, Figure 3). One case of acinar cell carcinoma, with similar-circular mass and clear margin, was difficult to distinguish from the benign tumor.
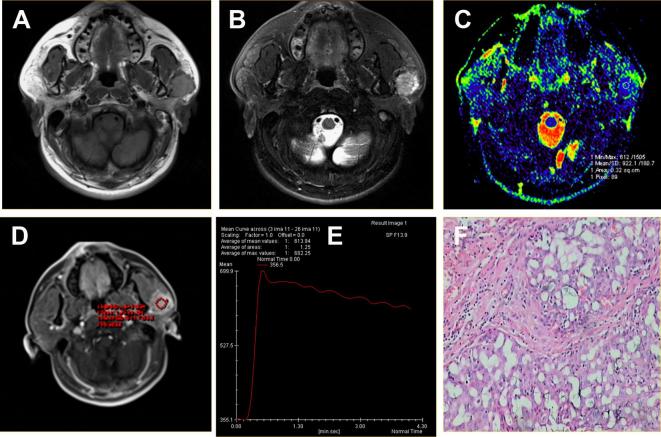
Figure 3.
Acinar cell carcinoma located in the superficial lobe of the left parotid gland of a 40-year-old male. (A) Axial T1WI showed a round mass with homogeneous isointense signal and unclear margin. (B) Axial T2WI sequences with fat suppression showed the lesions with hyperintense signal and radial hypointense signal shadow. (C) ADC map showed the mass alternated with blue signal. The ADC value was 0.92 × 10−3 mm2 s–1. (D-E) Time-signal intensity curve showed a plateau enhancement pattern (Type C). (F) HE staining: original magnification, 400×. ADC, apparent diffusion coefficient; HE, hematoxylin; T1WI, T1 weighted imaging; T2WI, T2 weighted imaging.
DWI findings
The mean ADC values for the different mass types were as follows: 1.72 ± 0.29×10−3 mm2 s–1 for pleomorphic adenomas, 0.74 ± 0.05×10––3 mm2 s–1 for adenolymphoma, and 0.95 ± 0.09 × 10−3 mm2 s–1 for malignant tumors. There were significant differences with regard to the ADC values between pleomorphic adenoma and malignant tumors (p < 0.05) and between adenolymphoma and malignant tumors (p < 0.05) (Table 2). The ADC thresholds derived from the receiver operating characteristic curve-based positive test were 1.29 × 10–3 mm2 s–1 between pleomorphic adenoma and malignant salivary gland tumor (Figure 4A), and 0.78 × 10−3 mm2 s–1 between adenolymphoma and malignant tumors (Figure 4B). There was no significant difference regarding the mean ADC values between the benign tumors and malignant tumors (1.33 ± 0.52 × 10−3 mm2 s–1 vs 0.95 ± 0.09 × 10−3 mm2 s–1, p > 0.05, Table 2).
Table 2.
The mean ADCs (×10−3 mm2 s–1) for four types of salivary gland tumors
| Mass type | Means ± SD | p-value |
| Adenolymphoma | 0.74 ± 0.05 | <0.01 |
| Pleomorphic adenoma | 1.72 ± 0.29 | <0.01 |
| Benign tumor | 1.33 ± 0.52 | >0.05 |
| Malignant tumor | 0.95 ± 0.09 | – |
α, adenolymphoma vs malignant tumor; β, pleomorphic adenoma vs malignant tumor; γ, benign tumor vs malignant tumor. ADC, apparent diffusion coefficient; SD, standard deviation.
Figure 4.
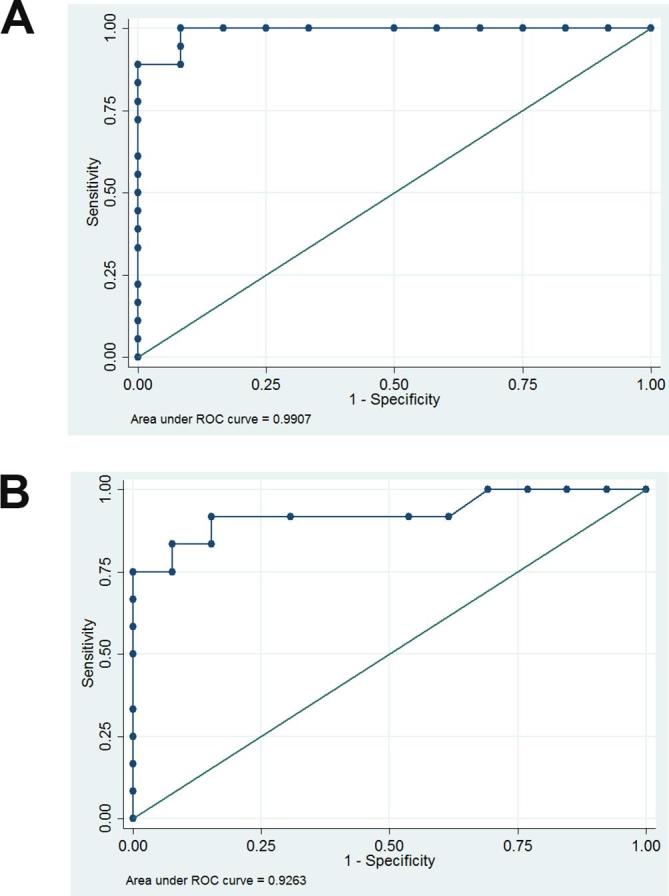
(A) Graph shows the results of the ROC-based positive test between pleomorphic adenoma and malignant tumors at variable ADC levels. The cutoff level of ADC values between pleomorphic adenoma and malignant tumor was 1.29 × 10−3 mm2 s–1. (sensitivity, 100%; specificity, 91.67%; AUC = 0.9907). (B) Graph shows the results of the ROC-based positive test between adenolymphoma and malignant tumor at variable ADC levels. The cutoff level of ADC values between lymphoma and malignant tumor was 0.78 × 10–3 mm2 s–1. (sensitivity, 91.67%; specificity, 84.62%; AUC = 0.9263). ADC, apparent diffusion coefficient; AUC, area under the curve; ROC, receiver operating characteristic.
DCE-MRI findings
Among the 45 salivary gland tumors, 16 pleomorphic adenoma and 1 schwannoma showed progressive enhancement and A-type curve (Figure 1E); 13 adenolymphoma, 1 basal cell adenoma, 1 acinar cell carcinoma and 1 mucoepidermoid carcinoma showed rapid enhancement, rapid clearance and B-type curve (Figures 2E, 3E and 5E); 9 malignant tumors and 2 pleomorphic adenoma showed rapid enhancement, slow clearance and C-type curve (Figure 3F); 1 lymphangioma showed no significantly enhanced and D-type curve. In brief, the TIC findings of 45 salivary gland tumors included 17 A-type, 16 B-type, 11 C-type and 1 D-type (Table 3). The distribution of Tpeak values were 22.4 ± 12.1 s in 13 adenolymphoma, and 42.5 ± 15.5 s in 11 malignant tumors. Statistically significant differences in Tpeak were seen between adenolymphoma and malignant tumors (p < 0.01, Table 4). The mean Tpeak of adenolymphoma was earlier than that of malignant tumors, and both were shorter than 120 s. There was no statistically significant difference in WR between 13 adenolymphoma (WR = 57.5% ± 8.1) and 11 malignant tumors (WR = 17.2% ± 13.2, p < 0.01, Table 4)
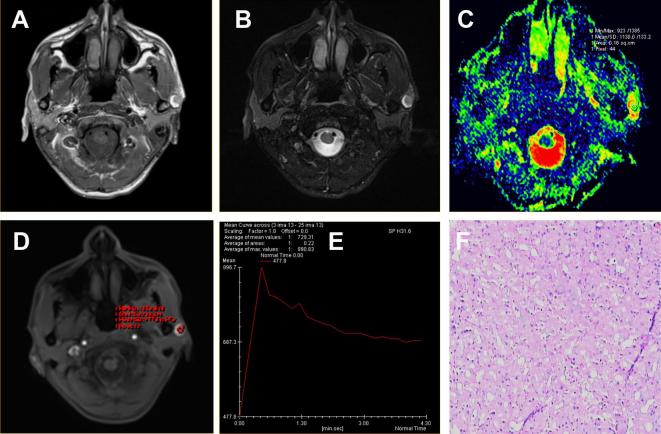
Figure 5.
Acinar cell carcinoma located in the superficial lobe of the left parotid gland of a 50-year-old male. (A-B) The axial T1WI and T2WI fat suppression sequences showed cystic solid lesions, and the solid part with homogeneous slightly equal and clear margin. (C) ADC map showed the mass alternated with green signal. The ADC value was 1.13 × 10−3 mm2 s–1. (D-E) Time-signal intensity curve showed a washout enhancement pattern (Type B), which showed the tumors was benign. However, the combination of TIC and ADC value showed the tumor was malignant. (F) HE staining: original magnification, 200×. ADC, apparent diffusion coefficient; HE, hematoxylin;T1WI, T1 weighted imaging; T2WI, T2 weighted imaging; TIC, time-intensity curve.
Table 3.
TIC patterns among histopathologic diagnoses
| Diagnosis | TIC type | |||
| A | B | C | D | |
| Benign(n = 34) | ||||
| Pleomorphic adenoma (18) | 16 | 0 | 2 | 0 |
| Adenolymphoma (13) | 0 | 13 | 0 | 0 |
| Basal cell adenoma (1) | 0 | 1 | 0 | 0 |
| Schwannoglioma (1) | 1 | 0 | 0 | 0 |
| Lymphangioma (1) | 0 | 0 | 0 | 1 |
| Malignant (n = 11) | ||||
| Acinar cell carcinoma (3) | 0 | 1 | 2 | 0 |
| Mucoepidermoid carcinoma (2) | 0 | 1 | 1 | 0 |
| Adenoid cystic carcinoma (1) | 0 | 0 | 1 | 0 |
| Carcinoma ex pleomorphic adenoma (1) | 0 | 0 | 1 | 0 |
| Infiltrating carcinoma (1) | 0 | 0 | 1 | 0 |
| Parotid branchial cleft cyst canceration (1) | 0 | 0 | 1 | 0 |
| Carcinoma sareomatodes (1) | 0 | 0 | 1 | 0 |
| Poorly differentiated carcinoma (1) | 0 | 0 | 1 | 0 |
TIC, time-intensity curve.
Table 4.
The mean Tpeak and WR of adenolymphoma and malignant tumor
| Parameter | Adenolymphoma | Malignant tumor | p-value |
| Tpeak (s) | 22.4 ± 12.1 | 42.5 ± 15.5 | <0.01 |
| WR (%) | 57.5 ± 8.1 | 17.2 ± 13.2 | <0.01 |
WR, washout ratio.
Combined MRI finding
According to the characteristic of dynamic enhancement curve of salivary gland tumor, we regarded the tumors with TIC types A, B and D as benign and Type C as malignancy, and combined with the conventional MRI findings, the sensitivity, specificity, accuracy, positive- and negative-predictive value were 82%,94%,91%,82 and 94%, respectively. The sensitivity, specificity, accuracy, positive- and negative-predictive value of types B and C curves were 90%, 97%, 95%, 90%, 97%, respectively, after modified diagnosis with ADC. For the tumors with TIC types B and C, it could improve the qualitative diagnosis of salivary gland tumors when conventional MRI, DCE-MRI combined with ADC value. Figures 5 and 6 showed two representative cases in which we combined conventional MRI, DWI-DCE-MRI with ADC values to assign the correct diagnosis. In general, according to the above mentioned findings of four items (shape, capsule, ADC value, TIC), irregular shape, with no capsule, mean ADC < 0.78 × 10–3 mm2 s–1 and TIC Type C were valuable for malignant tumors.
Figure 6.
Pleomorphic adenoma located in the superficial lobe of the right parotid gland of a 50-year-old female. (A) Axial T1WI sequences showed a mass lesion with hypointense signal and clear margin. (B) The T2WI fat suppression sequence showed a round mass with high and homogeneous signal. (C) ADC map showed the mass alternated with blue signal. The ADC value was 1.61 × 10−3 mm2 s–1. (D-E) Time-signal intensity curve showed a plateau enhancement pattern (Type C), which showed the tumors was malignant. However, the combination of TIC and ADC value showed the tumor was benign. (F) HE staining: original magnification, 200×. ADC, apparent diffusion coefficient; HE, hematoxylin;T1WI, T1 weighted imaging; T2WI, T2 weighted imaging; TIC, time-intensity curve.
Discussion
Salivary gland tumors are peculiar and represent the most heterogeneous histopathological results.12 These two facts have great challenge to the diagnosis of salivary gland tumors. FNAC and imaging are two methods to acquire accurate information from a tumor and for the clinician before any treatment. Although FNAC is a common method, there are several limitations for the detection of salivary gland tumors. For instance, due to diversification of salivary gland tumors and small sample size, FNAC, as a minimally invasive procedure, with non-diagnostic rate about 6%, shows sampling errors.13, 14 Therefore, pre-operative imaging can help to make up for these deficiencies.1 There are several imaging techniques such as type-B ultrasound, CT and MRI for diagnostic of salivary gland tumors. Compared with the first two imaging techniques, MRI has a good ability to differentiate various soft tissue types due to its superb spatial resolution. In recent years, functional MRI techniques such as DCE-MRI and DWI have significantly contributed to the differentiation between benign and malignant salivary gland tumors.15
Conventional MRI of benign tumors displayed homogeneous mass with clear margin, typical performance of hypointense signal on T1WI and hyperintense signal on T2WI. Som et al16 reported the pre-operative MRI diagnosis of 35 patients with parotid gland tumor, they suggested the hyperintense signal on T2WI was related to tissue cystic changes and mesenchymal mucin. In this study, 18 cases of pleomorphic adenoma showed clear margin, homogeneous mass, a hypointense signal on T1WI and a uneven hyperintense signal T2WI, and obvious enhancement after enhancement scanning. For the 13 adenolymphomas (16 lesions), included 1 unilateral multiple tumor and 2 bilateral multiple tumors, all lesions were located in the posterior inferior pole of the parotid gland, with clear margin and hyperintense signal or hypointense signal on T2WI. The hypointense signal on T2WI confirmed by many studies might be associated with epithelial tissue.17 Highly malignant tumors, such as mucoepidermoid carcinoma, undifferentiated carcinoma, adenocarcinoma and squamous cell carcinoma will show hypointense signal on T1WI and T2WI, with no clear margin due to high percentage of cellular components and lack of serous and mucin. Malignant tumors usually showed infiltrative growth, unclear margin, uneven signal, surrounding soft tissue and facial nerve invasion. Freling et al18 performed the conventional MRI of 116 patients with clinical suspicion of a parotid tumor. They finally suggested we should rely on tumor invasion of deep tissue for diagnosis of malignant tumors, such as adjacent muscles, bones and parapharyngeal space rather than margin, heterogeneity and SI. Meanwhile, Som et al16 found that low-graded malignant tumors, such as low-grade mucoepidermoid carcinoma, acinic cell carcinoma, also showed hypointense signal on T1WI and hyperintense signal on T2WI, which were not easy to distinguish from the benign tumors. In this study, among 11 malignant tumors, 1 acinic cell carcinoma also showed round and clear margin, abnormal hyperintense signal on T1WI and T2WI, leading to difficulties in differentiating benign tumors with malignant tumors in conventional MRI. Therefore, in addition to the conventional MRI, more advanced methods were needed to improve the accuracy of the diagnosis of salivary gland tumors.
For DWI, the ADC value could reflect the diversity and histological features of salivary gland tumors, such as cell proliferation, myxoid tissue, fibrosis, necrosis, cystic degeneration, and lymphoid tissue. The change of ADC value is greatly influenced by the relative increase in proliferation of tumor cells and water capacity. The different diffusion sensitive coefficients (b-value) could also cause differences of ADC values,9 so we chose the appropriate b-value (b = 1000 s mm–2) on the basis of satisfying imaging diagnosis. The histological types of salivary gland tumors are diverse, so the DWI of tumors are largely affected by the proportion of various components of the tumor, different salivary gland tumors have different ADC values. In this study, we reported that the mean ADC value of adenolymphoma was significantly lower than that of malignant tumors, which was consistent with the report of Ikeda et al.19 They studied the DWI-MRI of 17 patients with 19 adenolymphoma and 17 patients with 17 malignant parotid tumors, and found that the ADC values of adenolymphoma (0.96 ± 0.13 × 10−3 mm2 s–1) were significantly lower (p < 0.01) than those of malignant tumors (1.19 ± 0.19 × 10−3 mm2 s–1).19 Motoori et al reviewed MR studies of 33 pleomorphic adenomas and 13 malignant tumors in the major salivary glands, and reported the MRI features of pleomorphic adenoma overlap with that of some malignant salivary gland tumors due to the diverse composition.20 Some experts used the mucoid tissue to identify pleomorphic adenoma and malignant tumors on MRI, due to the ADC value of pleomorphic adenoma increased with the ADC value of the corresponding myxoid tissue.21 In addition, the cystic changes could lead to the ADC values of pleomorphic adenoma increased , which was rare in malignant tumors.22 Matsushima et al23 performed the DWI and ADC values in 32 patients with a wide spectrum of major salivary gland tumors (17 benign, 15 malignant). They reported that mean ADC values increased with the degree of extracellular components, which could be used as an important criterion for the differentiation between benign and malignant salivary gland tumors.23 In this study, there was no significant difference in mean ADC value between benign and malignant tumors, however, the average ADC value of pleomorphic adenoma was higher than that of malignant tumors (p < 0.05), the average ADC value of adenolymphoma was lower than the malignant tumor (p < 0.05). The results were different from those of previous studies might due to the small sample size, few kinds of salivary gland, low incidence of other tumors, and different devices in this study.
DCE-MRI monitors the entrance of diffusible contrast agents into the tumor tissue over time. The ROI of DCE-MRI could be used for qualitative and quantitative analysis of lesions. This method could estimate hemodynamic changes of different tissues and show the temporal and spatial distribution of contrast agents in different pathological tissues. By measuring the SI of one or more ROIs, the TICs of the region could be plotted. A flat or persistent TIC pattern on DCE-MRI indicated benign disease. On DCE-MRI, malignant tumors are supposed to typically show rapid/slow washout and rapid enhancement.24 Several studies including our study have reported the usefulness of DCE-MRI parameters and TIC for the differential diagnosis of salivary gland tumors.25–29 For example, Yabuuchi et al evaluated the diagnostic value of DCE-MRI of 29 patients with salivary gland tumors (22 benign and 11 malignant tumors). According to the four TIC types that are classified on the basis of a Tpeak of 120 s and a WR of 30%, their study showed nine pleomorphic adenomas (9/12) were Type A, eight adenolymphoma (8/9) were Type B, the malignant tumors except one lymphoma were Type C. In this study, as was shown in Tables 3 and 4, the results suggested the characteristics of its dynamic curve coincided with the report of Yabuuchi.6 In addition, adenolymphomas (13) had higher WR due to it has more microvascular and cell matrix and there was a certain relationship between flow rate and cell matrix classification, according to the report of Yabuuchi et al.6 Furthermore, in this study, pleomorphic adenoma (16) showed A-type of TIC, due to intact capillary endothelial cells and more component and complex structure of stromal in tumor, and the contrast-medium flowed slowly and could remain in the extracellular space for a long time. Yabuuchi et al reported 2 pleomorphic adenoma showed C-type of TIC, due to it with abundant epithelium and a small myxoid stroma, among 12 cases of malignant salivary gland tumor, 9 cases showed C-type of TIC, which were related to abundant microvessel counts and low-grade interstitial cells in the tumor. Our results suggested that there were partial overlap between benign and malignant salivary gland tumors when TIC showed B-type or C-type on DCE-MRI, the combined of ADC value and TIC was helpful to improve the qualitative diagnosis of salivary gland tumors. The use of ADC thresholds could avoid the misdiagnosis of malignant tumors, and unnecessary surgical or biopsy punctures in benign tumors. Adenolymphoma (13), acinic cell carcinoma (1) and mucoepidermoid carcinoma (1) showed B-type TICs, in which the adenolymphoma were need to identify from malignant tumor combined ADC value. Most of pleomorphic adenoma showed A-type TICs, few showed C-type TICs which were needed to distinguish from malignant tumor. One pleomorphic adenoma and one acinar cell carcinoma presenting with B-type TICs were correctly diagnosed by the combination of DWI and DCE-MRI with ADC value and TICs.
This study had some limitations. First, a small sample size were included in this study. Second, patients with the rare type of salivary gland tumors were not included. We did not consider the gland signal change with patient age. The ADC value varies depending on equipment and hospital imaging method. In future studies, we will pay more attention to the collection of special cases (cases of low incidence) and a larger sample size are needed to confirm the combining MRI morphologic findings and functional MRI (ADCs and TIC) for salivary gland tumors.
Conclusion
In conclusion, if salivary gland tumors are shown on MRI as being of irregular shape, with no capsule, mean ADC < 0.78 × 10−3 mm2 s–1 and TIC type C, a malignancy should be strongly considered. In summary, an evaluation combining MRI morphologic findings (shape, capsule and SI) and functional MRI (ADCs and TIC) appears to be useful in the diagnosis of salivary gland tumors. The combination would compensate for the shortcomings of each and, thus, improved the diagnostic accuracy for differentiating benign and malignant salivary gland tumors.
Footnotes
The authors Ning Zheng and Rui Li contributed equally to the work.
Wenjuan Liu and Shuo Shao have contributed equally to this study and should be considered as senior authors.
Contributor Information
Ning Zheng, Email: zhengning_369@163.com.
Rui Li, Email: liruizuoyou@163.com.
Wenjuan Liu, Email: wenjuanliu@163.com.
Shuo Shao, Email: doubleshaoshuo@163.com.
Shan Jiang, Email: ShanJiang_369@163.com.
REFERENCES
- 1.Assili S, Fathi Kazerooni A, Aghaghazvini L, Saligheh Rad HR, Pirayesh Islamian J. Dynamic contrast magnetic resonance imaging (DCE-MRI) and diffusion weighted MR imaging (DWI) for differentiation between benign and malignant salivary gland tumors. J Biomed Phys Eng 2015; 5: 157–68. [PMC free article] [PubMed] [Google Scholar]
- 2.Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg 1986; 8: 177–84. doi: 10.1002/hed.2890080309 [DOI] [PubMed] [Google Scholar]
- 3.Hisatomi M, Asaumi J, Yanagi Y, Unetsubo T, Maki Y, Murakami J, et al. . Diagnostic value of dynamic contrast-enhanced MRI in the salivary gland tumors. Oral Oncol 2007; 43: 940–7. doi: 10.1016/j.oraloncology.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 4.Das DK, Petkar MA, Al-Mane NM, Sheikh ZA, Mallik MK, Anim JT. Role of fine needle aspiration cytology in the diagnosis of swellings in the salivary gland regions: a study of 712 cases. Med Princ Pract 2004; 13: 95–106. doi: 10.1159/000075637 [DOI] [PubMed] [Google Scholar]
- 5.Sumi M, Van Cauteren M, Sumi T, Obara M, Ichikawa Y, Nakamura T. Salivary gland tumors: use of intravoxel incoherent motion MR imaging for assessment of diffusion and perfusion for the differentiation of benign from malignant tumors. Radiology 2012; 263: 770–7. doi: 10.1148/radiol.12111248 [DOI] [PubMed] [Google Scholar]
- 6.Yabuuchi H, Fukuya T, Tajima T, Hachitanda Y, Tomita K, Koga M. Salivary gland tumors: diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation. Radiology 2003; 226: 345–54. doi: 10.1148/radiol.2262011486 [DOI] [PubMed] [Google Scholar]
- 7.Lee YY, Wong KT, King AD, Ahuja AT. Imaging of salivary gland tumours. Eur J Radiol 2008; 66: 419–36. doi: 10.1016/j.ejrad.2008.01.027 [DOI] [PubMed] [Google Scholar]
- 8.Eissa L, Abou Seif S, El Desooky S, Eid M, Koraitim T. Accuracy assessment of combined diffusion weighed and dynamic gadolinium MR sequences in characterization of salivary gland tumors. The Egyptian Journal of Radiology and Nuclear Medicine 2016; 47: 127–39. doi: 10.1016/j.ejrnm.2015.11.011 [DOI] [Google Scholar]
- 9.Eida S, Sumi M, Sakihama N, Takahashi H, Nakamura T. Apparent diffusion coefficient mapping of salivary gland tumors: prediction of the benignancy and malignancy. AJNR Am J Neuroradiol 2007; 28: 116–21. [PMC free article] [PubMed] [Google Scholar]
- 10.Tao X, Yang G, Wang P, Wu Y, Zhu W, Shi H, et al. . The value of combining conventional, diffusion-weighted and dynamic contrast-enhanced MR imaging for the diagnosis of parotid gland tumours. Dentomaxillofac Radiol 2017; 46: 20160434. doi: 10.1259/dmfr.20160434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitamoto E, Chikui T, Kawano S, Ohga M, Kobayashi K, Matsuo Y, et al. . The application of dynamic contrast-enhanced MRI and diffusion-weighted MRI in patients with maxillofacial tumors. Acad Radiol 2015; 22: 210–6. doi: 10.1016/j.acra.2014.08.016 [DOI] [PubMed] [Google Scholar]
- 12.Arrangoiz R, Papavasiliuo P, Sarcu D, Galloway TJ, Ridge JA, Lango M. Current Thinking on Malignant Salivary Gland Neoplasms. Journal of Cancer Treatment and Research 2013; 1: 8–24. doi: https://doi.org/10.11648/j.jctr.20130101.12 [Google Scholar]
- 13.He Y, Zhang ZY, Tian Z. The diagnostic value of fine-needle aspiration cytology (FNAC) for lesions in the parotid gland. Shanghai Kou Qiang Yi Xue 2003; 12: 410–3. [PubMed] [Google Scholar]
- 14.Mihashi H, Kawahara A, Kage M, Kojiro M, Nakashima T, Umeno H, et al. . Comparison of preoperative fine-needle aspiration cytology diagnosis and histopathological diagnosis of salivary gland tumors. Kurume Med J 2006; 53(1-2): 23–7. doi: 10.2739/kurumemedj.53.23 [DOI] [PubMed] [Google Scholar]
- 15.Eida S, Sumi M, Nakamura T. Multiparametric magnetic resonance imaging for the differentiation between benign and malignant salivary gland tumors. J Magn Reson Imaging 2010; 31: 673–9. doi: 10.1002/jmri.22091 [DOI] [PubMed] [Google Scholar]
- 16.Som PM, Biller HF. High-grade malignancies of the parotid gland: identification with MR imaging. Radiology 1989; 173: 823–6. doi: 10.1148/radiology.173.3.2813793 [DOI] [PubMed] [Google Scholar]
- 17.Minami M, Tanioka H, Oyama K, Itai Y, Eguchi M, Yoshikawa K, et al. . Warthin tumor of the parotid gland: MR-pathologic correlation. AJNR Am J Neuroradiol 1993; 14: 209–14. [PMC free article] [PubMed] [Google Scholar]
- 18.Freling NJ, Molenaar WM, Vermey A, Mooyaart EL, Panders AK, Annyas AA, et al. . Malignant parotid tumors: clinical use of MR imaging and histologic correlation. Radiology 1992; 185: 691–6. doi: 10.1148/radiology.185.3.1438746 [DOI] [PubMed] [Google Scholar]
- 19.Ikeda M, Motoori K, Hanazawa T, Nagai Y, Yamamoto S, Ueda T, et al. . Warthin tumor of the parotid gland: diagnostic value of MR imaging with histopathologic correlation. AJNR Am J Neuroradiol 2004; 25: 1256–62. [PMC free article] [PubMed] [Google Scholar]
- 20.Motoori K, Yamamoto S, Ueda T, Nakano K, Muto T, Nagai Y, et al. . Inter- and intratumoral variability in magnetic resonance imaging of pleomorphic adenoma: an attempt to interpret the variable magnetic resonance findings. J Comput Assist Tomogr 2004; 28: 233–46. [DOI] [PubMed] [Google Scholar]
- 21.Terra GT, Oliveira JX, Hernandez A, Lourenço SV, Arita ES, Cortes AR. Diffusion-weighted MRI for differentiation between sialadenitis and pleomorphic adenoma. Dentomaxillofac Radiol 2017; 46: 20160257. doi: 10.1259/dmfr.20160257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motoori K, Iida Y, Nagai Y, Yamamoto S, Ueda T, Funatsu H, et al. . MR imaging of salivary duct carcinoma. AJNR Am J Neuroradiol 2005; 26: 1201–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Matsushima N, Maeda M, Takamura M, Takeda K. Apparent diffusion coefficients of benign and malignant salivary gland tumors. Comparison to histopathological findings. J Neuroradiol 2007; 34: 183–9. doi: 10.1016/j.neurad.2007.04.002 [DOI] [PubMed] [Google Scholar]
- 24.Yuan Y, Tang W, Tao X. Parotid gland lesions: separate and combined diagnostic value of conventional MRI, diffusion-weighted imaging and dynamic contrast-enhanced MRI. Br J Radiol 2016; 89: 20150912. doi: 10.1259/bjr.20150912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aghaghazvini L, Salahshour F, Yazdani N, Sharifian H, Kooraki S, Pakravan M, et al. . Dynamic contrast-enhanced MRI for differentiation of major salivary glands neoplasms, a 3-T MRI study. Dentomaxillofac Radiol 2015; 44: 20140166. doi: 10.1259/dmfr.20140166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuzaki H, Yanagi Y, Hara M, Katase N, Asaumi J, Hisatomi M, et al. . Minor salivary gland tumors in the oral cavity: diagnostic value of dynamic contrast-enhanced MRI. Eur J Radiol 2012; 81: 2684–91. doi: 10.1016/j.ejrad.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 27.Lam PD, Kuribayashi A, Imaizumi A, Sakamoto J, Sumi Y, Yoshino N, et al. . Differentiating benign and malignant salivary gland tumours: diagnostic criteria and the accuracy of dynamic contrast-enhanced MRI with high temporal resolution. Br J Radiol 2015; 88: 20140685. doi: 10.1259/bjr.20140685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts C, Jackson A, Rushton V, Parker GJ. The Use of DCE-MRI in the assessment of Lacrimal and Salivary Glands in Sjogren's Syndrome patients. Nestlã© Nutrition Institute Workshop 2003; 82: 17–25. [Google Scholar]
- 29.Attyé A, Troprès I, Rouchy RC, Righini C, Espinoza S, Kastler A, et al. . Diffusion MRI: literature review in salivary gland tumors. Oral Dis 2017; 23: 572–5. doi: 10.1111/odi.12543 [DOI] [PubMed] [Google Scholar]