Abstract
Objective:
Peritoneal metastasis (PM) is the most frequent form of metastasis in gastric cancer (GC). The sensitivity of detecting PM by pre-operative imaging modalities is low. Utility of positron emission tomography (PET) with 18F-fluodeoxyglucose (FDG) for GC is limited, because diffuse-type tumors are not FDG-avid. 18F-fluothymidine ([F-18]FLT) is a radiotracer that reflects cellular proliferation and the utility of [F-18]FLT-PET in GC has been reported. In this proof-of-concept study, we explored the ability of [F-18]FLT-PET/CT to detect PM of GC previously identified by other imaging modalities.
Methods:
The key eligibility criteria were as follows; (i) histologically proven gastric adenocarcinoma; (ii) evident PM detected by CT performed within 4 weeks prior to registration; (iii) no prior treatment of PM within 4 weeks before registration. [F-18]FLT-PET/CT was performed at National Cancer Center Hospital, and [F-18]FLT-PET/CT images were evaluated independently by two radiologists. Safety assessments were carried out before and after [F-18]FLT-PET/CT. The primary end point was the detection sensitivity of PM.
Results:
A total of 19 eligible patients were analyzed, of which 15 (78.9%) had diffuse-type histology. Detection sensitivity of PM, primary lesion, and lymph node metastasis were 73.7% [maximum standardized uptake value (SUVmax): 1.697–13.21], 100% (SUVmax: 2.71–22.01), and 72.7% (SUVmax: 2.079–12.61), respectively. No patients experienced adverse events during or after [F-18]FLT-PET/CT.
Conclusion:
This proof-of-concept study shows that [F-18]FLT-PET/CT is a sensitive method for detecting PM in GC, and paves the way for future studies investigating the clinical utility of this approach for the detection of clinically non-evident PM in GC.
Advances in knowledge:
This proof-of-concept study found that [F-18]FLT-PET/CT is a sensitive method for detecting peritoneal metastases in GC.
Introduction
Peritoneal metastasis (PM) is the most frequent form of metastasis in gastric cancer (GC), especially in diffuse-type tumors. The ability to detect a PM is the critical determining factor regarding tumor resectability and curability in patients with GC. The sensitivity by which current pre-operative imaging modalities can detect a PM is low,1 therefore, a clinically non-evident PM can only be accurately identified accurately by staging laparoscopy.1–3
Positron emission tomography (PET) with 18F-fluodeoxyglucose (FDG) is a powerful, non-invasive metabolic imaging modality to evaluate various tumors.4, 5 However, the utility of metabolic imaging in GC is limited, because diffuse-type tumors are not FDG-avid and the sensitivities of FDG-PET and FDG-PET combined with CT for detection of a PM in GC are low.6–13
18F-fluothymidine ([F-18]FLT) is a radiotracer that reflects cellular proliferation.14–17 Previous reports showed higher sensitivity of [F-18]FLT-PET compared with FDG-PET in GC, regardless of the histological type.18–20 In contrast to FDG and despite its low SUV, [F-18]FLT can visualize primary or metastatic GC lesions with sufficient contrast because of its lower physiological accumulation in the intestinal tract.18–21 Therefore, [F-18]FLT-PET is expected to be a potentially useful, non-invasive metabolic imaging modality that can identify PM in GC with high sensitivity. To date, however, no reports have investigated the utility of [F-18]FLT using PET/CT for the detection of PM in GC.
In this proof-of-concept study, we explored the sensitivity of [F-18]FLT-PET/CT for detecting evident PM of GC previously identified by other imaging modalities.
Methods and Materials
Patient eligibility
The eligibility criteria for inclusion in the study were as follows; (i) histologically proven gastric adenocarcinoma; (ii) evident PM detected by CT performed within 4 weeks prior to registration (CT positivity was judged according the findings of irregular peritoneal nodules or mass formation enhanced by the contrast agent); (iii) no prior treatment of PM within 4 weeks before registration; (iv) age 20 years or older; (v) Eastern Cooperative Oncology Group performance status 0–2; (vi) hemoglobin (≥8.0 g dl−1, (vii) white blood cell counts ≥2000 mm–3,(viii) platelet counts ≥7.5 ×104 /mm3, (ix) total bilirubin ≤2.0 mg dl−1, (x) aspartate aminotransferase and alanine aminotransferase ≤150 IU l−1, (xi) creatinine ≤2.0 mg dl−1); and (xii) written informed consent.
The key exclusion criteria were as follows: (i) history of alcohol intolerance (as [F-18]FLT contains a low amount of alcohol as a solvent), and (ii) marked acute infection. This study was approved by the Institutional Review Boards of the National Cancer Center Hospital, was monitored by an independent data and safety monitoring committee, and was conducted in accordance with the Helsinki Declaration and Japanese Ethical Guidelines for Clinical Studies. This study is registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR), Number 000009329.
Synthesis of [F-18]FLT
[F-18]FLT was synthesized by using a cyclotron at the National Cancer Center Hospital as follows. Target water containing [18F] fluoride ions was added to an AG1 × 8 anion exchange resin (prepared by the standard method) in order to trap [18F] fluoride ions. After desorption with 66 mM potassium carbonate aqueous solution (0.35 ml), as [18F] potassium fluoride, the solution was injected into a reactor containing 35 mM K.222 acetonitrile solution (1.5 ml). The solvent was removed by heating (120 °C, 15 min). Dimethylsulfoxide solution (1 ml) was injected in order to dissolve 5′-O-(4,4′-dimethoxytrityl) −2,3'-anhydrothymidine into the reactor and fluorinate it (200 °C, 10 min). Hydrochloric acid (1 M, 0.35 ml) was added to the reactor and hydrolyzed (65°C, 10 min). After the reaction, 0.5 M sodium acetate water solution (1.5 ml) was added and the reaction was transferred to a reservoir containing 10 ml water for injection. The solution was passed through a Sep-Pak C18 cartridge in order to trap [F-18]FLT. After removing unreacted [18F] fluoride ions and water-soluble impurities by water injection (20 ml), [F-18]FLT was eluted in dimethyl sulfoxide (1.5 ml) and purified by high-performance liquid chromatography. The [F-18]FLT was separated and transferred to flask containing 25% ascorbic acid injection solution, and the solvent was removed by evaporation. Distilled water for injection into the flask was added, and [F-18]FLT for injection was then derived after passing through a sterilized filter (0.22 µm).
PET/CT imaging protocol
All scans were acquired on a PET/CT device (Discovery 600: General Electric Healthcare, Waukesha, WI) equipped with a 16 section CT scanner with whole-body mode and with the standard software installed. The patients were fasted for at least 6 h prior to the procedure. Acquisition of emission scans from the head to the mid-thigh started 60 min after intravenous administration of [F-18]FLT at a mean dose of 306.6 MBq (range 296.7–317.6 MBq). CT scan was performed from the head to the mid-thigh in accordance with a standardized protocol with the following settings: axial 1.25 mm collimation × 16 modes; 120 kVp; auto-regulated tube current; 0.5 s tube rotation; and table speed 37.52 mm s–1. Patients maintained normal shallow respiration during the acquisition of the three-dimensional CT scans. No iodinated contrast material was administered. The acquisition time for PET was 3 min per table position. Images were reconstructed automatically with time decay correction and with attenuation-corrected ordered-subset expectation maximization with 2 iterations and 16 subsets using emission scans and CT data.
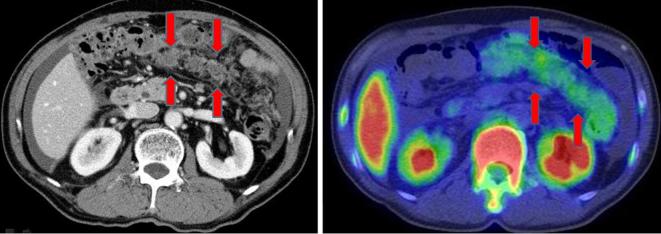
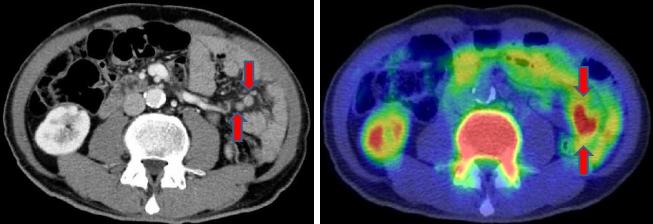
Interpretation of the [F-18]FLT-PET/CT images was performed independently by two radiologists (TT and UT) who were blind to any clinical information related to the patients. The patterns of PM were classified into either omental-cake-type (oPM; Figure 1) or nodule-type (nPM; Figure 2).
Figure 1.
Omental-cake-type peritoneal metastasis.
Figure 2.
Nodule-type peritoneal metastasis.
Safety analysis of [F-18]FLT-PET/CT
A safety assessment was made just before and after [F-18]FLT-PET/CT by a physician, followed by a general health check and blood sampling 1 week after [F-18]FLT-PET/CT. Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events v. 4.0. Blood samples were analyzed for: (a) sodium, potassium, chloride, and glucose level; (b) aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, albumin, and total bilirubin; (c) creatinine and blood urea nitrogen; and (d) hemoglobin, hematocrit, white blood cell, and platelet count.
Statistics
The primary end point for this exploratory study was the detection sensitivity (DS) of PM. DS was defined as positive if at least one of the lesions detected by prior CT imaging was visualized by [F-18]FLT-PET/CT. The DS value was expected to be at least 50%, and 30% or lower DS was considered unacceptable for the future studies to investigate the clinical utility of [F-18]FLT-PET/CT in detecting clinically non-evident PM in GC before surgery. A precision-based sample size calculation was performed so that the one-sided 80% confidence interval (80% CI) for the estimated sensitivity would be no greater than +15%. Assuming a 15% of dropout rate, the total number of patients was set at 20.
Results
Patient characteristics
A total of 20 patients were enrolled in this study. One patient was diagnosed with gastric lymphoma by pathological review after [F-18]FLT-PET/CT, and was thus excluded from the analysis. The median age was 67 years (range: 48-79), and 14 of 19 (73.7%) patients were male. Eastern Cooperative Oncology Group performance status was 1 in 14 patients (73.7%) and 0 in 5 patients (26.3%). 11 of 19 patients (57.9%) had initial Stage IV disease, and other 8 patients had recurrent disease. 11 of 19 patients (57.9%) had primary lesions, lymph node metastases were present in 11 patients (57.9%), liver metastases in 1 patient (5.3%), and lung metastases in 2 patients (10.5%). 14 patients (73.7%) had a diffuse-type histology; non-solid type poorly differentiated adenocarcinoma in 8, signet-ring cell carcinoma in 4, and mucinous adenocarcinoma in 2. Other five patients had intestinal histology; well-differentiated adenocarcinoma in two, moderately differentiated adenocarcinoma in two, and papillary adenocarcinoma in one.
Detection sensitivity
PM was detected by [F-18]FLT-PET/CT in 14 of 19 patients (maximum standardized uptake value [SUVmax]: 1.697–13.21, median SUVmax: 2.937, DS: 73.7%, 80% CI: 59–84%). [F-18]FLT uptake in the PM was more evident in patients with Stage IV disease than in those with recurrent disease (Table 1). There was no difference in the PM SUVmax values between: (i) intestinal-type or diffuse-type histology; (ii) Stage IV or recurrent disease; (iii) patients with normal or abnormal CEA levels; and (iv) those of normal or abnormal CA19-9 levels (Table 2). All primary lesions in the eleven affected patients, were detected by [F-18]FLT-PET/CT (DS: 100%, SUVmax: 2.71–22.01, median SUVmax: 5.427). The DS of [F-18]FLT-PET/CT for lymph node metastases in the 11 affected patients was 72.7% (8 of 11 patients, SUVmax for positive cases: 2.08–12.61, median SUVmax: 4.385).
Table 1.
Patient characteristics between no accumulation and accumulation of [F-18]FLT in PM
| Characteristic | No accumulation (n = 5) | Accumulation (n = 14) | p-value | ||
| Tumor histology ― no. (%) | 0.570 | ||||
| Diffuse type | 3 | (60.0) | 11 | (78.6) | |
| Intestinal type | 2 | (40.0) | 3 | (21.4) | |
| Disease status ― no. (%) | 0.005 | ||||
| Recurrent disease | 5 | (100.0) | 3 | (21.4) | |
| Stage IV disease | 0 | (0.0) | 11 | (78.6) | |
| CEA level ― no. (%) | 1.000 | ||||
| Normal (≤5.0 ng ml−1) | 4 | (80.0) | 10 | (71.4) | |
| Abnormal (>5.0 ng ml−1) | 1 | (20.0) | 4 | (28.6) | |
| CA19-9 level ― no. (%) | 0.628 | ||||
| Normal (≤37 U ml−1) | 2 | (40.0) | 8 | (57.1) | |
| Abnormal (>37 U ml−1) | 3 | (60.0) | 6 | (42.9) | |
[F-18]FLT, 18F-fluothymidine; CEA, carcino embryonic antigen; PM, peritoneal metastasis.
Table 2.
SUVmax level of PM in accumulation-[F-18]FLT-PET/CT positive cases
| Characteristics | Intestinal type histology (N = 3) | Diffuse type histology (N = 11) | p-value |
| SUVmax | 0.353 | ||
| Median | 2.813 | 3.467 | |
| Range | 1.70–13.21 | 2.51–6.52 | |
| Characteristics | Stage IV disease (N = 11) | Recurrent disease (N = 3) | P-value |
| SUVmax | 0.278 | ||
| Median | 2.760 | 4.766 | |
| Range | 1.70–13.21 | 3.01–6.52 | |
| Characteristics | Nodular type (N-7) | Omental cake type (N = 7) | P-value |
| SUVmax | 0.646 | ||
| Median | 3.007 | 2.886 | |
| Range | 1.70–6.52 | 1.77–13.21 | |
| Characteristics | Normal CEA (N = 10) | Abnormal CEA (N = 4) | P-value |
| SUVmax | 0.800 | ||
| Median | 2.813 | 3.255 | |
| Range | 1.70–13.21 | 1.91–4.08 | |
| Characteristics | Normal CA19-9 (N = 8) | Abnormal CA19-9 (N = 6) | P-value |
| SUVmax | 1.000 | ||
| Median | 2.884 | 2.866 | |
| Range | 1.70–13.21 | 1.91–4.08 | |
[F-18]FLT, 18F-fluothymidine; CEA, carcino embryonic antigen; PET, positron emission tomography; SUVmax, maximum standardizeduptake value.
7 of 19 patients (36.8%) had oPM, of which all were detected by [F-18]FLT-PET/CT (SUVmax: 1.771–13.21, median SUVmax: 2.886, DS: 100%). Of the 42 nodules detected by CT in the 12 patients with nPM, 20 were detected by [F-18]FLT-PET/CT (SUVmax for positive cases: 1.697–6.524, median SUVmax: 3.007, DS: 47.6%).
Safety analysis
All 20 patients who received [F-18]FLT-PET/CT underwent a safety analysis. No adverse events were observed at three predefined time points: immediately before and immediately after [F-18]FLT-PET/CT, and 1 week after [F-18]FLT-PET/CT. Analysis of blood samples taken between the two predefined checkpoints (before registration and 1 week after [F-18]FLT-PET/CT) found no evidence of any adverse effects.
Discussion
To best of our knowledge, this study is the first to explore the ability of [F-18]FLT-PET/CT to detect evident PM in GC. In this study, CT performed within the 4 weeks prior to registration was mandatory as inclusion criteria, because it was necessary to secure the comparability between CT and [F-18]FLT-PET/CT. We found that [F-18]FLT-PET/CT met its primary end point (DS of PM was 73.7%, 80% CI: 59–84%) and is a sensitive method to detect primary lesions (DS: 100%) and lymph node metastases (DS: 72.7%) in GC compared to other imaging modalities.
FDG-PET is a useful, non-invasive metabolic imaging modality to evaluate various tumors,4, 5 but its utility in GC is limited for two main reasons. First, FDG physiologically accumulates in the intestinal tract with a SUVmax of ~2.5, rendering it difficult to obtain clear images with sufficient contrast in the lesions around this area.21 Secondly, GCs (especially those with diffuse-type histology) are not FDG-avid tumors. Previous reports revealed that FDG-PET and FDG-PET/CT have low sensitivity and high specificity as detection modalities in GC as follows: (i) sensitivity: 34.3–94%; specificity:>90% in primary lesions6, 7 ; (ii) sensitivity: 17–88%; specificity:>90% in lymph node metastases;8, 9 (iii) sensitivity: 34.3–67%; specificity: 93.2–97% in distant metastases;22, 23 and (iv) sensitivity: 30–72.7%; specificity: 63–99% in PM.10, 11 According to tumor histology, the sensitivity of FDG-PET is 33.3% in intestinal type GC, 42.6% in diffuse-type GC, and 15% in tumors with signet ring cell carcinoma.9 A systematic review of FDG-PET in GC by the Japanese Gastric Cancer Association Task Force also revealed that FDG-PET has limitations such as frequent false-negatives in signet-ring cell carcinoma and non-solid type poorly differentiated carcinoma, and the diagnostic impact of FDG-PET for the detection of PM was low.13
[F-18]FLT is an alternative radiotracer to FDG and reflects the activity of thymidine kinase 1, which is the key enzyme of the salvage pathway for thymidine monophosphate production.14, 15 [F-18]FLT has thus been developed to image cellular proliferation status.16, 17 Previous reports identified that [F-18]FLT-PET has a lower SUV and higher sensitivity (95-100%) than FDG-PET in GC, regardless of the histological sub type.18–20 Despite its low SUV, [F-18]FLT is capable of identifying primary or metastatic lesions in GC with sufficient contrast because it does not physiologically accumulate in the intestinal tract.18–21 Conversely, [F-18]FLT-PET is unsuitable for the detection of liver and bone metastases, as the tracer physiologically accumulates at very high levels in these two organs. In our study, [F-18]FLT uptake to PM was more evident in initial Stage IV disease than recurrent disease. In metastatic GC, initial Stage IV disease (without gastrectomy) is well known as one of the strong prognostic factor, therefore [F-18]FLT uptake might reflect the malignant potential of each GC lesions. On the other hand, degree of [F-18]FLT uptake to PM detected by CT was different for each lesions even in same patient, especially in nodule- type PM. This finding might be explained by the heterogeneity of GC cells and the limitation of the diagnosis ability of CT.
We also demonstrated the safety profile of [F-18]FLT-PET/CT. FLT was initially investigated as a treatment for HIV infection in humans.24 Previous clinical trials showed that FLT-induced hematologic and hepatic toxicity as well as peripheral neuropathy in patients with HIV infection.25 However, patients with HIV infection might have latent hematologic problem, and the concentration of FLT used in our study was low, at ~1% of the dose used to treat HIV infection. Tolerance of [F-18]FLT-PET was confirmed in a previous trial.26 In this study, a slight but statistically significant decrease was noted in hemoglobin and serum albumin levels during the blood sample analysis, but the severity of symptoms did not change at before and after [F-18]FLT-PET.
Previous studies have demonstrated the utility of FDG-PET in early detection of the prognostication or chemosensitivity based on its metabolic parameters in locally-advanced GC or esophagogastric junction adenocarcinoma.27, 28 Previously, [F-18]FLT has been assessed successfully for imaging drug-related inhibition of thymidylate synthase.29, 30 Given the very high sensitivity of [F-18]FLT-PET/CT for detecting primary lesions in this study, this technique might also be used for predicting chemosensitivity in patients with GC exposed to neoadjuvant treatments containing thymidylate synthase-inhibitory fluopyrimidine.
Conclusion
This proof-of-concept study has found that [F-18]FLT-PET/CT is a sensitive method for detecting PM in GC. These data pave the way for future studies investigating the clinical utility of this approach for detecting clinically non-evident PM in GC.
Footnotes
Funding: This study was supported by Research Grant of the Princess Takamatsu Cancer Research Fund (09-24119).
Contributor Information
Yoshitaka Honma, Email: yohonma@ncc.go.jp.
Takashi Terauchi, Email: takashi.terauchi@jfcr.or.jp.
Ukihide Tateishi, Email: ttisdrnm@tmd.ac.jp.
Daisuke Kano, Email: dkano@east.ncc.go.jp.
Kengo Nagashima, Email: nshi@chiba-u.jp.
Hirokazu Shoji, Email: hshouji@ncc.go.jp.
Satoru Iwasa, Email: siwasa@ncc.go.jp.
Atsuo Takashima, Email: atakashi@ncc.go.jp.
Ken Kato, Email: kenkato@ncc.go.jp.
Tetsuya Hamaguchi, Email: thamaguc@saitama-med.ac.jp.
Narikazu Boku, Email: nboku@ncc.go.jp.
Yasuhiro Shimada, Email: yasuhiro.shimada@gmail.com.
Yasuhide Yamada, Email: yasuhide-yamada@amed.go.jp.
REFERENCES
- 1.Burbidge S, Mahady K, Naik K. The role of CT and staging laparoscopy in the staging of gastric cancer. Clin Radiol 2013; 68: 251–5. doi: 10.1016/j.crad.2012.07.015 [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa S, Nashimoto A, Yabusaki H. Role of staging laparoscopy with peritoneal lavage cytology in the treatment of locally advanced gastric cancer. Gastric Cancer 2007; 10: 29–34. doi: 10.1007/s10120-006-0406-3 [DOI] [PubMed] [Google Scholar]
- 3.Ajani JA, Bentrem DJ, Besh S, D'Amico TA, Das P, Denlinger C, et al. Gastric cancer, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw 2013; 11: 531–46. [DOI] [PubMed] [Google Scholar]
- 4.Hustinx R, Bénard F, Alavi A. Whole-body FDG-PET imaging in the management of patients with cancer. Semin Nucl Med 2002; 32: 35–46. doi: 10.1053/snuc.2002.29272 [DOI] [PubMed] [Google Scholar]
- 5.Bomanji JB, Costa DC, Ell PJ. Clinical role of positron emission tomography in oncology. Lancet Oncol 2001; 2: 157–64. doi: 10.1016/S1470-2045(00)00257-6 [DOI] [PubMed] [Google Scholar]
- 6.Terauchi T, Murano T, Daisaki H, Kanou D, Shoda H, Kakinuma R, et al. Evaluation of whole-body cancer screening using 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography: a preliminary report. Ann Nucl Med 2008; 22: 379–85. doi: 10.1007/s12149-008-0130-7 [DOI] [PubMed] [Google Scholar]
- 7.Yamada A, Oguchi K, Fukushima M, Imai Y, Kadoya M. Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med 2006; 20: 597–604. doi: 10.1007/BF02984657 [DOI] [PubMed] [Google Scholar]
- 8.Lerut T, Flamen P, Ectors N, Van Cutsem E, Peeters M, Hiele M, et al. Histopathologic validation of lymph node staging with FDG-PET scan in cancer of the esophagus and gastroesophageal junction: A prospective study based on primary surgery with extensive lymphadenectomy. Ann Surg 2000; 232: 743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging 2006; 33: 148–55. doi: 10.1007/s00259-005-1887-8 [DOI] [PubMed] [Google Scholar]
- 10.Yang QM, Bando E, Kawamura T, Tsukiyama G, Nemoto M, Yonemura Y, et al. The diagnostic value of PET-CT for peritoneal dissemination of abdominal malignancies. Gan To Kagaku Ryoho 2006; 33: 1817–21. [PubMed] [Google Scholar]
- 11.Lim JS, Kim MJ, Yun MJ, Oh YT, Kim JH, Hwang HS, et al. Comparison of CT and 18F-FDG pet for detecting peritoneal metastasis on the preoperative evaluation for gastric carcinoma. Korean J Radiol 2006; 7: 249–56. doi: 10.3348/kjr.2006.7.4.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sim SH, Kim YJ, Oh DY, Lee SH, Kim DW, Kang WJ, et al. The role of PET/CT in detection of gastric cancer recurrence. BMC Cancer 2009; 9: 73. doi: 10.1186/1471-2407-9-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimada H, Okazumi S, Koyama M, Murakami K. Japanese gastric cancer association task force for research promotion: clinical utility of ¹⁸F-fluoro-2-deoxyglucose positron emission tomography in gastric cancer. A systematic review of the literature. Gastric Cancer 2011; 14: 13–21. doi: 10.1007/s10120-011-0017-5 [DOI] [PubMed] [Google Scholar]
- 14.Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med 1998; 4: 1334–6. doi: 10.1038/3337 [DOI] [PubMed] [Google Scholar]
- 15.Coppock DL, Pardee AB. Regulation of thymidine kinase activity in the cell cycle by a labile protein. J Cell Physiol 1985; 124: 269–74. doi: 10.1002/jcp.1041240215 [DOI] [PubMed] [Google Scholar]
- 16.Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med 2002; 43: 1210–7. [PubMed] [Google Scholar]
- 17.Barthel H, Perumal M, Latigo J, He Q, Brady F, Luthra SK, et al. The uptake of 3'-deoxy-3'-[18F]fluorothymidine into L5178Y tumours in vivo is dependent on thymidine kinase 1 protein levels. Eur J Nucl Med Mol Imaging 2005; 32: 257–63. doi: 10.1007/s00259-004-1611-0 [DOI] [PubMed] [Google Scholar]
- 18.Herrmann K, Ott K, Buck AK, Lordick F, Wilhelm D, Souvatzoglou M, et al. Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med 2007; 48: 1945–50. doi: 10.2967/jnumed.107.044867 [DOI] [PubMed] [Google Scholar]
- 19.Kameyama R, Yamamoto Y, Izuishi K, Takebayashi R, Hagiike M, Murota M, et al. Detection of gastric cancer using 18F-FLT PET: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging 2009; 36: 382–8. doi: 10.1007/s00259-008-0970-3 [DOI] [PubMed] [Google Scholar]
- 20.Ott K, Herrmann K, Schuster T, Langer R, Becker K, Wieder HA, et al. Molecular imaging of proliferation and glucose utilization: utility for monitoring response and prognosis after neoadjuvant therapy in locally advanced gastric cancer. Ann Surg Oncol 2011; 18: 3316–23. doi: 10.1245/s10434-011-1743-y [DOI] [PubMed] [Google Scholar]
- 21.Kamimura K, Fujita S, Nishii R, Wakamatsu H, Nagamachi S, Yano T, et al. An analysis of the physiological FDG uptake in the stomach with the water gastric distention method. Eur J Nucl Med Mol Imaging 2007; 34: 1815–8. doi: 10.1007/s00259-007-0477-3 [DOI] [PubMed] [Google Scholar]
- 22.Podoloff DA, Ball DW, Ben-Josef E, Benson AB, Cohen SJ, Coleman RE, et al. NCCN task Force: clinical utility of PET in a variety of tumor types. J Natl Compr Canc Netw 2009; 7(Suppl 2): S-1–0. doi: 10.6004/jnccn.2009.0075 [DOI] [PubMed] [Google Scholar]
- 23.Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer 2009; 12: 6–22. doi: 10.1007/s10120-008-0492-5 [DOI] [PubMed] [Google Scholar]
- 24.Bardich-Crovo PA, Kornhauser DM, Petty BG. Phase I pharmacokinetic evaluation of 3’-deoxy-3’-fluorothymidine (FLT), a new potent anti-HIV nucleoside [abstract 2114]. In VII International Conference on AIDS, Florence, Italy; 1991.
- 25.Flexner C, van der Horst C, Jacobson MA, Powderly W, Duncanson F, Ganes D, et al. Relationship between plasma concentrations of 3’-deoxy-3’-fluorothymidine (alovudine) and antiretroviral activity in two concentration-controlled trials. J Infect Dis 1994; 170: 1394–403. doi: 10.1093/infdis/170.6.1394 [DOI] [PubMed] [Google Scholar]
- 26.Turcotte E, Wiens LW, Grierson JR, Peterson LM, Wener MH, Vesselle H. Toxicology evaluation of radiotracer doses of 3'-deoxy-3'-[18F]fluorothymidine (18F-FLT) for human PET imaging: Laboratory analysis of serial blood samples and comparison to previously investigated therapeutic FLT doses. BMC Nucl Med 2007; 7: 3. doi: 10.1186/1471-2385-7-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Na SJ, o JH, Park JM, Lee HH, Lee SH, Song KY, et al. Prognostic value of metabolic parameters on preoperative 18F-fluorodeoxyglucose positron emission tomography/ computed tomography in patients with stage III gastric cancer. Oncotarget 2016; 7: 63968–80. doi: https://doi.org/10.18632/oncotarget.11574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.zum Büschenfelde CM, Herrmann K, Schuster T, Geinitz H, Langer R, Becker K, et al. (18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial. J Nucl Med 2011; 52: 1189–96. doi: 10.2967/jnumed.110.085803 [DOI] [PubMed] [Google Scholar]
- 29.Weber G, Nagai M, Natsumeda Y, Ichikawa S, Nakamura H, Eble JN, et al. Regulation of de novo and salvage pathways in chemotherapy. Adv Enzyme Regul 1991; 31: 45–67. doi: 10.1016/0065-2571(91)90008-A [DOI] [PubMed] [Google Scholar]
- 30.Wells P, Aboagye E, Gunn RN, Osman S, Boddy AV, Taylor GA, et al. 2-[11C]thymidine positron emission tomography as an indicator of thymidylate synthase inhibition in patients treated with AG337. J Natl Cancer Inst 2003; 95: 675–82. doi: 10.1093/jnci/95.9.675 [DOI] [PubMed] [Google Scholar]