Abstract
Several species of non-indigenous planktonic invertebrates have historically been introduced to the Laurentian Great Lakes. Previous introductions of non-indigenous planktonic invertebrates to the Great Lakes have been crustacean zooplankton, specifically Cladocera and Copepoda. This report documents the first known occurrence of Brachionus leydigii var. tridentatus (Zernov, 1901) in Lake Erie and possibly the first detection of a non-indigenous rotifer species in the Laurentian Great Lakes. The specimen was collected from a U.S. EPA monitoring station in the western basin of Lake Erie on April 4, 2016.
Keywords: Zooplankton, Great Lakes, New species record, Biological monitoring
Introduction
Brachionidae belongs to the Rotifera order Ploima and after Nottomatidae (with 23 genera) and Dicranophoridae (12 genera) is the third family with most numerous (eight) genera, and the fifth most specious family with 105 known species. Among the 8 genera of Brachionidae the genus Brachionus is the most specious with 37 known species. Brachionus is characterized by strong polymorphism resulting with many morphs in each species. Brachionus leydigii (Cohen, 1862) belongs to the sixth most polymorphic species of the genus and to the seventh largest species exceeding the length of 300 μm. Brachionus leydigii has four subspecies (leydigii, quadratus, tridentatus, rotundus) out of them B. leydigii var. tridentatus (Zernov, 1901) is known from the most diverse aquatic habitats including small and large reservoirs, large rivers, ponds, canals, ditches, polders, estuaries and in the littoral of lakes (Błędzki, 1989; De Ridder and Segers, 1997). The morphology of the Lake Erie B. leydigii specimen warrants assignment to the subspecies B. leydigii var. tridentatus. Reports regarding the introduction of non-indigenous rotfers in the western hemisphere are sparse (De Paggi, 2002; Bezerra-Neto et al., 2004; Nicholls, 2016). We hereby report the first known occurrence of B. leydigii from Lake Erie and possibly, the first documented detection of a non-indigenous rotifer species in the Laurentian Great Lakes.
Methods
Sample collection and analysis
Zooplankton samples were collected across all five Laurentian Great Lakes aboard the U.S. Environmental Protection Agency’s (U.S. EPA) R/V Lake Guardian as part of the U.S. EPA Great Lakes National Program Office’s (GLNPO) long-term biological monitoring program. Samples were collected with 0.5 m diameter 63 μm and 153 μm mesh nets towed vertically through the water column following a standard operating procedure (EPA SOP, 2013) for field sampling. In the shallow western basin of Lake Erie, samples using both meshes were collected from 2 m above the lake bottom to the surface. Samples were preserved in a 4% buffered sugar formalin solution treated with rose bengal dye.
Rotifers are typically counted from the tow taken with the 63 μm mesh net as the larger 153 μm mesh net does not capture sufficient numbers of these small taxa. The single B. leydigii specimen was an exception, being found in the 153 μm mesh net sample during crustacean zooplankton enumeration. A Folsom plankton splitter was used to divide the sample in half and repeated until 200–400 individuals per sub-sample were reached. These two smallest subsamples were analyzed first. Second, two additional larger subsamples representing 2 and 4 times the split factor of the smallest subsample were analyzed for sub-dominant and rare taxa following a standard operating procedure (EPA SOP, 2016) for crustacean zooplankton sample analysis. After detecting B. leydigi in a subsample, the corresponding 63 μm mesh net sample from the same date and location was processed (methods below) for additional B. leydigii specimens without success.
In addition to the crustacean enumeration described above, the 63 μm mesh net sample was analyzed for microzooplankton following a different method. These organisms were counted from separate 1 mL aliquots withdrawn with a Hensen-Stempel pipette from the appropriate, thoroughly homogenized split. The goal is to enumerate 200–400 rotifers and copepod nauplii (dreissenid veliger are enumerated as well but are not included toward the 200–400 total) in an original count as well as a duplicate count. These are referred to as A and B counts. The 1 mL aliquot is placed in a Sedgwick-Rafter cell and covered with a glass coverslip. All microzooplankton are identified and enumerated under a compound microscope at 100× magnification following a standard operating procedure (EPA SOP, 2016) for microzooplankton sample analysis. Microphotographs were taken with an ACCU-SCOPE Excelis HD camera attached to an OLYMPUS CX41 compound microscope, extended depth of field images were created using CaptaVision PC Imaging Software. Specimen measurements were taken using an OLYMPUS CX41 compound microscope with a drawing tube and a GTCO CalComp DrawingBoard VI.
Results
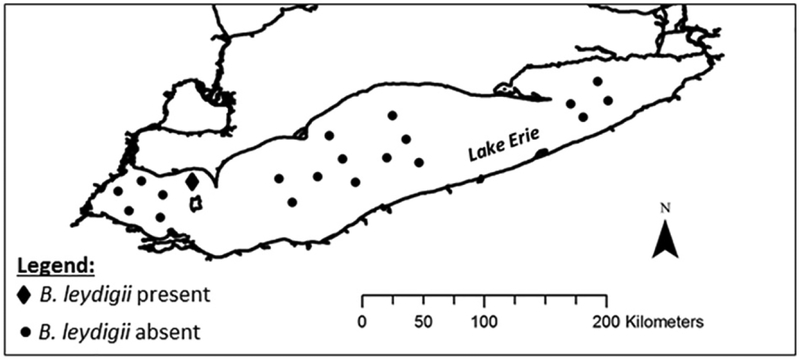
A single female specimen of B. leydigii var. tridentatus was detected in a preserved plankton sample collected from the U.S. EPA monitoring station ER92 (41.951°N/82.68701°W) on April 4, 2016 at 01:36 AM Eastern Standard Time. Net tows at station ER92 were collected at a depth of 11 m to the water’s surface. Twenty open water monitoring stations were sampled across Lake Erie and only one B. leydigii specimen was detected at a single station (Fig. 1). No additional B. leydigii specimens were detected at EPA monitoring stations in Lake Erie in the spring or summer of 2016. And no additional specimens of B. leydigii were collected from ER92 on April 5, 2017. Environmental conditions at the time of sample collection were as follows: water column was iso-thermal, water temperature was 5.3 °C, and chlorophyll-a concentration was 3.6 μg/L.
Fig. 1.
U.S. EPA GLNPO long-term monitoring stations in Lake Erie.
Based on the condition of the specimen’s internal anatomy it is our judgement that this animal was alive at the time of collection. Lorica length of the specimen as measured from the anterior margin of the dorsal plate (not including the anterior spines) to the posterior margin of the dorsal plate (not including the posterior spines) was 222 μm. Lo-rica width of the specimen as measured at the widest point of the lorica was 215 μm. The lengths of the anterior spines as measured from the base to the distal ends were as follows; anteriomedian spines measured 37 μm (left) and 43 μm (right), anteriointermediate spines measured 24 μm (left) and 25 μm (right), anteriolateral spines measured 17 μm (left) and 22 μm (right). The length of the medial posterior spine as measured from the base to the distal end was 14 μm. The foot while only partly extended measured 86 μm long and the toes measured 14 μm (left) and 19 μm (right) long.
Morphological description
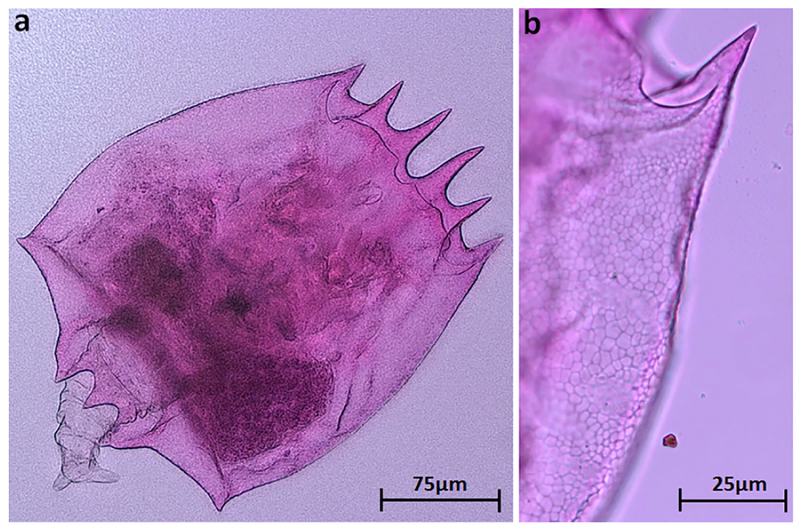
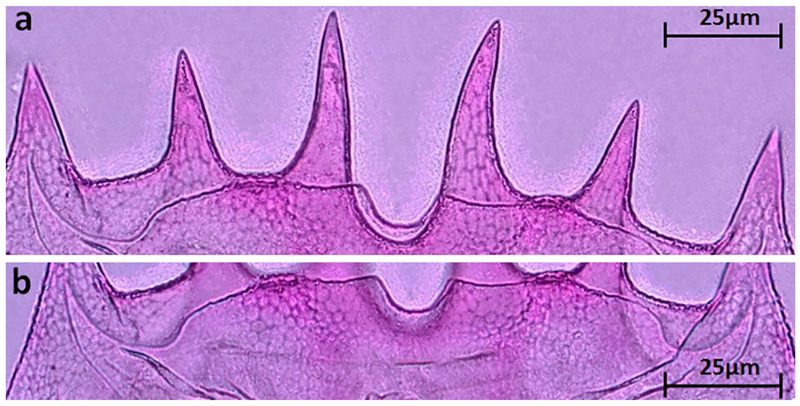
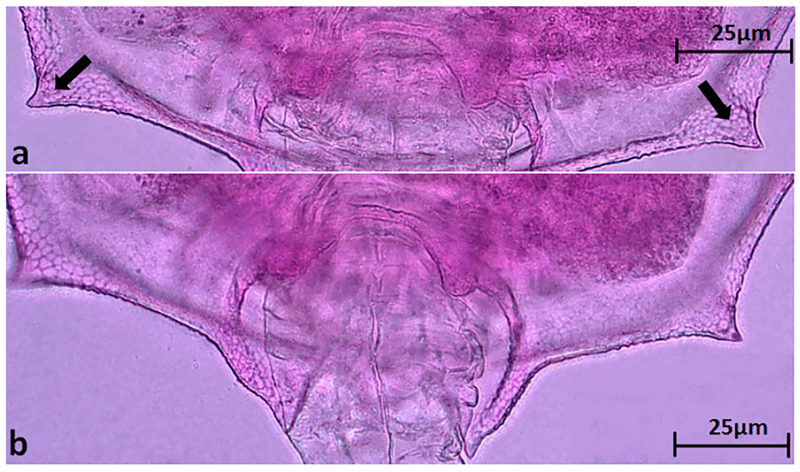
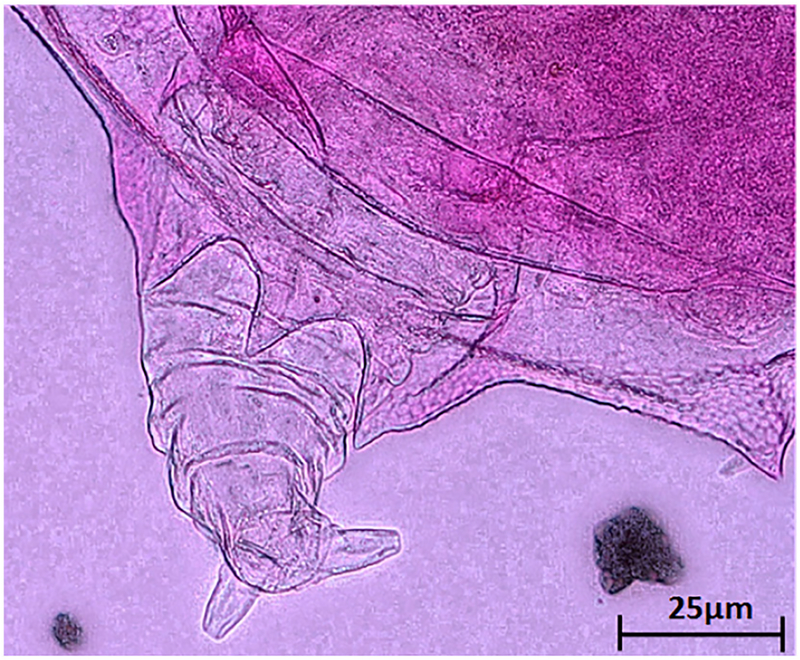
The morphology of B. leydigii is fairly unique and recognizable within the Great Lakes Brachionus assemblage. In comparison to native Great Lakes Brachionus species, B. leydigii var. tridentatus has a somewhat square lorica (Fig. 2a), comprised of 3 plates: dorsal, ventral, and basal. The lorica surface is finely textured with a distinctive but relatively subtle polygonal pattern which may also help distinguish it from the nominal form (Fig. 2b). The anteriodorsal margin is characterized by 6 spines (Fig. 3a) with the anteriomedian spines longest and curved somewhat outward. The anterioventral margin is characterized by a relatively shallow medially placed U-shaped sinus (Fig. 3b). The posterior margin is characterized by small posteriolaterally placed spines (Fig. 4a). The ventral foot opening is relatively large and club-shaped (Fig. 4b). Three broad based posterior spines are placed directly below the foot opening, the medial spine being the shortest (Fig. 5). The foot is annulated and fairly long if fully extended and terminates in two toes (Fig. 5).
Fig. 2.
(a) Extended depth of field image of Lake Erie B. leydigii var. tridentatus specimen. (b) Polygonal pattern on lorica surface of B. leydigii var. tridentatus.
Fig. 3.
(a) Anterior spines on dorsal plate of B. leydigii var. tridentatus. (b) Anterior margin of ventral plate of B. leydigii var. tridentatus.
Fig. 4.
(a) Small posteriolateral spines on B. leydigii var. tridentatus. (b) Club-shaped foot opening on posterior margin of ventral plate of B. leydigii var. tridentatus.
Fig. 5.
Posterior spines on posterior margin of dorsal plate of B. leydigii var. tridentatus; Partly extended foot and toes of B. leydigii var. tridentatus.
Discussion
The Lake Erie specimen was initially identified as B. leydigii (Cohen, 1862) based on Ahlstrom (1940). Subsequently, the specimen was determined to be the infrasubspecific variant B. leydigii var. tridentatus based on Kutikova (1970) and Koste (1978). The nomenclature B. var. tridentatus (Zernov, 1901) was assigned to the specimen in question in reference to the Rotifer World Catalog website (Jersabek and Leitner, 2013) and Segers et al. (2015) which both noted Brachionus leydigii var. tridentatus as a “junior subjective synonym” of Brachionus leydigii (Cohn, 1862). The genus Brachionus is large, specious and well known for morphological variability; in the Laurentian Great Lakes 12 species have previously been reported (Grothe and Grothe, 1977; Stemberger, 1979; Jersabek et al., 2003). The Great Lakes native B. variabilis could potentially be mistaken for B. leydigii based on the 6 anterior spines, somewhat similar shaped lorica, and posterior protuberance placed dorsally to the foot opening. But B. variabilis is characterized by a flattened or rounded posterior protuberance rather than pointed or triangular broad based posterior spines (Fig. 5) as present in B. leydigii. In open water areas of the Great Lakes, Brachionus species are most abundant during summer months in the western basin of Lake Erie (Barbiero and Warren, 2011). Members of the genus are primarily littoral and often associated with eutrophic environments (Stemberger, 1979). Only a single female specimen of B. leydigii var. tridentatus was detected from Lake Erie in 2016 and no evidence of reproduction such as carried eggs were observed associated with the specimen. If the species is newly introduced it is likely that planktonic densities are still very low and may elude detection with most subsampling enumeration methods. For these reasons the establishment status of this species in Lake Erie cannot be determined and is therefore unknown. However, it should be stated that the ability of rotifers to reproduce parthenogenetically can allow for rapid colonization of new habitats (Wallace et al., 2006).
B. leydigii is widely distributed in the eastern hemisphere including reports from Europe (Sládeček, 1983), Asia (Jersabek and Bolortsetseg, 2010), and Australia (Koste and Shiel, 1987). B. leydigii has even been reported from some saltwater environments including the Baltic Sea, the North Sea (Fontaneto et al., 2006) and saline lakes (Viayeh and Špoljar, 2012). Kutikova (1970) reported the infrasubspecific variant B. leydigii var. tridentatus from large lakes including Lake Baikal. De Ridder and Segers (1997) suggested B. leydigii var. tridentatus is only known to the Palearctic and is widely distributed in this zoogeographic region; across much of Europe into the Caspian region.
The native distribution of B. leydigii and its subspecies are poorly understood in the western hemisphere, but some reports are likely credible. Kofoid (1908) reported B. leydigii from the Illinois River as occurring from May until August. However, with no sketches associated with the Kofoid (1908) report, the species cannot be verified with certainty. Ahlstrom (1934) reported B. leydigii (Cohen, 1862) from Florida within a list of rotifers surveyed in the state but did not include descriptions or sketches of the specimens collected. Ahlstrom (1940) in reference to B. leydigii remarked “I have seen material from England, Sweden, India, China, and? Ohio” but made no mentions of his 1934 reference to the species presence in Florida. Ahlstrom (1940) included sketches and measurements of all B. leydigii specimens mentioned except for the specimens reported from Ohio, making it impossible to verify the Ohio note. Dumont (1983) did report B. leydigii (Cohen, 1862) as present in North America but based on Ahlstrom’s uncertain 1940 Ohio report. Reports of B. leydigii in North America are sparse and date primarily to the early twentieth century (Kofoid, 1908; Ahlstrom, 1934; Ahlstrom, 1940). B. leydigii has not been previously reported from South American waters (Dumont, 1983). Ahlstrom’s, 1934 inclusion of B. leydigii (Cohen, 1862) on a list of rotifers surveyed in the state of Florida suggests B. leydigii may be native to North America. Additionally, B. leydigii has been reported from the River Antigua in Vera-cruz, Mexico further supporting the presence of this species in the Western Hemisphere (Nandini et al., 2017).
Ballast associated collections of B. leydigii have been reported from the Great Lakes in recent years. A single specimen of B. leydigii (Cohen, 1862) was collected in ballast water from the upper wing ballast tanks of a trans-oceanic vessel in Hamilton Harbor, Lake Ontario (Bailey et al., 2005b). Additionally, specimens of B. leydigii were successfully hatched from the residual ballast sediment of four trans-oceanic vessels entering the Great Lakes (Bailey et al., 2005a). The detection of B. leydigii var. tridentatus from plankton in the western basin of Lake Erie may possibly be the result of a ballast related introduction. If so, this and other recent detections of non-indigenous species (Connolly et al., 2017) in Great Lakes may indicate that the Great Lakes are still vulnerable to species introductions. Microscopic taxa in particular, may be easily transported by various vectors and present a risk for introductions which may go unnoticed without regular biological monitoring efforts.
Acknowledgements
This study was supported by a grant from U.S. EPA’s Great Lakes National Program Office, Cooperative Agreement GL 00E01184-0 and GL 00E02259-0 to Cornell University, the research described in this article has not been subjected to U.S. EPA review. Any opinions expressed in this publication are those of the authors and do not necessarily reflect the views or policies of the U.S. EPA. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. EPA, USGS, NOAA, or U.S. Fish and Wildlife Service. We thank the following people for their guidance, co-operation, information, correspondence, and support of research activities: the captain and crew of the R/V Lake Guardian, Elizabeth Hinchey, Todd Nettesheim, Glenn Warren, Rick Barbiero, Sarah Bailey, Patrick Hudson, Christian Jersabek, Beth Whitmore, and Gabriella Doud. Also we recognize the U.S. Fish and Wild-life Service for performing an Ecological Risk Screening Summary for B. leydigii, USGS NAS and GLANSIS for maintaining publicly available databases on non-indigenous species, and the Rotifer World Catalog for their inclusion of this record of B. leydigii var. tridentatus into their online database.
References
- Ahlstrom EH, 1934. Rotatoria of Florida. Trans. Am. Microsc. Soc 53, 251–266. [Google Scholar]
- Ahlstrom EH, 1940. A revision of the Rotatorian genera Brachionus and Platyias with descriptions of one new species and two varieties. Bull. Am. Mus. Nat. Hist 77, 143–184. [Google Scholar]
- Bailey SA, Duggan IC, Jenkins P, MacIsaac HJ, 2005a. Invertebrate resting stages in residual ballast sediment of transoceanic ships. Can. J. Fish. Aquat. Sci 62, 1090–1103. [Google Scholar]
- Bailey SA, Nandakumar K, Duggan IC, van Overdijk CDA, Johengen TH, Reid DF, MacIsaac HJ, 2005b. In situ hatching of invertebrate diapausing eggs from ships’ ballast sediment. Divers. Distrib 11, 453–460. [Google Scholar]
- Barbiero RP, Warren GJ, 2011. Rotifer Communities in the Laurentian Great Lakes, 1983–2006 and factors affecting their composition. J. Great Lakes Res 37, 528–540. [Google Scholar]
- Bezerra-Neto JF, Aguila LR, Landa GG, Pinto-Coelho RM, 2004. The exotic rotifer Kellicottia bostoniensis (Rousselet, 1908) (Rotifera: Brachionidae) in the zooplankton community in a tropical reservoir. Lundiana 5, 151–153. [Google Scholar]
- Błędzki LA, 1989. Ekologia zooplanktonu Zbiornika Włocławskiego. N. Copernicus University, Toruń (Poland) (PhD thesis, 171). [Google Scholar]
- Connolly JK, Watkins JM, Hinchey EK, Rudstam LG, Reid JW, 2017. New cyclopoid copepod (Thermocyclops crassus) reported in the Laurentian Great Lakes. J. Great Lakes Res 43, 198–203. [Google Scholar]
- De Paggi SJ, 2002. New data on the distribution of Kellicottia bostoniensis (Rousselet, 1908) (Rotifera: Monogononta: Brachionidae): its presence in Argentina. Zool. Anz 241, 363–368. [Google Scholar]
- De Ridder M, Segers H, 1997. Monogonont Rotifera Recorded in the World Literature (Except Africa) From 1960 to 1992. 88 Koninkijk Belgisch Institut voor Natuurwetenschappen, Gent, pp. 1–481. [Google Scholar]
- Dumont HJ, 1983. Biogeography of rotifers. Hydrobiologia 104, 19–30. [Google Scholar]
- Fontaneto D, De Smet WH, Ricci C, 2006. Rotifers in saltwater environments, reevaluation of an inconspicuous taxon. J. Mar. Biol. Assoc. U. K 86, 626–656. [Google Scholar]
- Grothe DW, Grothe DR, 1977. An Illustrated Key to the Planktonic Rotifers of the Laurentian Great Lakes. U.S. Environmental Protection Agency, Chicago, IL. [Google Scholar]
- Jersabek CD, Bolortsetseg E, 2010. Mongolian rotifers (Rotifera, Monogononta) – a checklist with annotations on global distribution and autecology. Proc. Acad. Natl. Sci. Phila 159, 119–168. [Google Scholar]
- Jersabek CD, Leitner MF, 2013. The Rotifer World Catalog. World Wide Web Electronic Publication; http://rotifera.hausdernatur.at/Species/Index/2324#TabStripSpecies-4, Accessed date: 10 June 2018. [Google Scholar]
- Jersabek CD, Segers H, Dingmann BJ, 2003. The Frank J. Myers Rotifera collection at the Academy of Natural Sciences: the whole collection in digital images. (CD-ROM). The Academy of Natural Sciences of Philadelphia, Special Publication 20. [Google Scholar]
- Kofoid CA, 1908. The plankton of the Illinois River, 1894–1899. Part II. Constituent Organisms and Their Seasonal Distribution. Bulletin of the Illinois State Laboratory of Natural History. 8, p. 361. [Google Scholar]
- Koste W, 1978. Rotatoria Die Rädertiere Mitteleuropas.Ein Bestimmungswerk begründet von Max Voigt. Überordnung Monogononta. Gebr der Borntraeger, Berlin, Stuttgart: (1.Textband & 2.Tafelband: 1–673 & 234 Tafeln). [Google Scholar]
- Koste W, Shiel RJ, 1987. Rotifera from Australian Inland Waters. II. Epiphanidae and Brachionidae (Rotifera: Monogononta). Invertebr. Syst 7, 949–1021. [Google Scholar]
- Kutikova LA, 1970. Kolovratki fauny CCCP (Rotatoria), Podklass Eurotatoria (Otriady Ploimida, Monimotrichida, Paedotrochida). 104 Izdatelstvo Nauka, Leningrad, pp. 1–744. [Google Scholar]
- Nandini S, Sarma SSS, Gulati RD, 2017. A seasonal study reveals the occurrence of exotic rotifers, the river Antigua, Veracruz, close to the Gulf of Mexico. River Res. Appl 33, 970–982. [Google Scholar]
- Nicholls KH, 2016. The pantropical rotifer Lecane monostyla new to Canada. J. Great Lakes Res 42, 728–730. [Google Scholar]
- Segers H, De Smet WH, Fischer C, Fontaneto D, Michaloudi E, Wallace RL, Jersabek CD, 2015. Towards a list of available names in zoology, partim phylum Rotifera. Zootaxa 3179 (231 (A-List). Revised version 04). [DOI] [PubMed] [Google Scholar]
- Sládeček V, 1983. Rotifers as indicators of water quality. Hydrobiologia 100, 169–201. [Google Scholar]
- Stemberger RS, 1979. A Guide to Rotifers of the Laurentian Great Lakes. U.S. Environmental Protection Agency, Cincinnati, OH. [Google Scholar]
- [US EPA] United States Environmental Protection Agency, 2013. Standard operating procedure for zooplankton sample collection and preservation and secchi depth measurement field procedures, LG402, revision 11. https://www.epa.gov/sites/production/files/2017-01/documents/sop-for-zooplankton-sample-collection-preservation-and-secchi-depthmeasurement-field-procedures-201303-7pp.pdf, Accessed date: 30 January 2018.
- [US EPA] United States Environmental Protection Agency, 2016. Standard operating procedure for zooplankton analysis, LG403, revision 7. https://www.epa.gov/sites/production/files/2017-01/documents/sop-for-zooplankton-analysis-201607-22pp.pdf, Accessed date: 30 January 2018.
- Viayeh RM, Špoljar M, 2012. Structure of rotifer assemblages in shallow waterbodies of semi-arid northwest Iran differing in salinity and vegetation cover. Hydrobiologia 686, 73–89. [Google Scholar]
- Wallace RL, Snell TW, Ricci C, Nogrady T, 2006. Rotifera In: Segers H (Ed.), Biology, Ecology, and Systematics. Volume 1 Backhuys Publishers; Ghent, Kenobi Productions 23, Leiden, pp. 1–299. [Google Scholar]