Abstract
Elevated intraocular pressure (IOP) is an important risk factor for glaucoma. Mechanisms involved in its homeostasis are not well understood, but associations between metabolic factors and IOP have been reported. To investigate the relationship between levels of circulating metabolites and IOP, we performed a metabolome-wide association using a machine learning algorithm, and then employing Mendelian Randomization models to further explore the strength and directionality of effect of the metabolites on IOP. We show that O-methylascorbate, a circulating Vitamin C metabolite, has a significant IOP-lowering effect, consistent with previous knowledge of the anti-hypertensive and anti-oxidative role of ascorbate compounds. These results enhance understanding of IOP control and may potentially benefit future IOP treatment and reduce vision loss from glaucoma.
Keywords: Ascorbate metabolism, Intraocular pressure, Multi-omics
Highlights
-
•
Vitamin C and its metabolites are highly concentrated in human brain and eye tissues.
-
•
Multi-omics analysis finds evidence for association between ascorbic acid metabolism and intraocular pressure.
-
•
O-methylascorbate lowers intraocular pressure in the general population.
-
•
O-methylascorbate's role in intraocular pressure regulation is likely mediated by its anti-photooxidative properties.
1. Introduction
Glaucoma is a leading cause of irreversible blindness and an important public health concern. Better understanding of its pathophysiology is important because it might lead to earlier detection and improved management strategies. Glaucoma and intraocular pressure (IOP) are tightly correlated genetically and epidemiologically [1], but our understanding of cell and tissue-level processes underlying elevated IOP and glaucoma are not well understood.
The eyes share cellular metabolic pathways and physiological mechanisms with other organs and tissues. Genes associated with IOP and POAG are involved, among others, in systemic lipid metabolism [1], [2], lysosomal endocytosis [3] and angiogenesis [1], [4]. Additionally, both IOP [5] and POAG [6] are strongly associated with the components of the metabolic syndrome (hyperglycemia, hyperlipidemia and high systemic blood pressure).
The purposes of this work were to investigate the relationship between circulating metabolites and IOP, by performing a metabolome-wide association study, and to examine the causality direction of such relationships, using Mendelian Randomization (MR) in independent population-based cohorts.
2. Materials and methods
2.1. Study design
This work followed two stages. First, associations between circulating metabolite levels of individual metabolites and IOP were identified in a population-based cohort (TwinsUK). Subsequently, Mendelian Randomization (MR) analyses in two independent populations were used to validate the relationship between circulating metabolite levels and IOP and assess causality.
2.2. Populations and subjects
2.2.1. TwinsUK
This is a volunteer cohort recruited from the general population in the United Kingdom [7]. Included in this study are 1763 adults (684 twin pairs and 395 singletons), for whom both metabolite levels and eye measurements including IOP were available.
The IOP measurements were taken using a non-contact air-puff tonometer (Ocular Response Analyzer, ORA, Reichert, Buffalo, NY). The mean IOP was calculated from four readings (two from each eye). Subjects who were receiving IOP-lowering medications or had IOP-altering surgery were excluded from the analyses.
2.2.2. UK Biobank
UK Biobank is a large multisite cohort study of UK residents aged 40–69 years. Participants’ IOP was measured once per eye using ORA. Participants with a history of eye surgery or injury and with IOP measurements in the top and bottom 0.5 percentiles were excluded. The pre-treatment IOP of the 1571 participants under IOP-lowering medication was imputed as 130% of the measured mean IOP to allow for medication effect as previously recommended [8], [9], [10]. IOP was calculated as the mean of right and left eye ORA IOPcc parameter values for each participant. Effect size estimations for subsequent MR analyses were extracted from the results of association between genotypes and IOP, described elsewhere [1].
2.2.3. EPIC-Norfolk
EPIC-Norfolk is one of the UK arms of the European Prospective Investigation into Cancer (EPIC) study [11]. Detailed ophthalmic assessments using ORA and genotypes were available for 8623 participants. The quality control, inclusion and exclusion criteria, QC steps and linear regression methods that were used to generate results, are described in detail elsewhere [1].
2.2.4. Ethical approvals
This study was conducted in accordance with the principles of the Declaration of Helsinki and the Research Governance Framework for Health and Social Care. All participants gave informed consent after appropriate ethics committee approval: Guy's and Saint Thomas (GSTT) for the TwinsUK, the North-West Research Ethics Committee for the UK Biobank and the Norfolk Local Research Ethics Committee and East Norfolk & Waveney NHS Research Governance Committee for the EPIC-Norfolk participants.
2.3. Metabolite measurements
Non-targeted metabolite detection and quantification was conducted using the platform provided by Metabolon Inc. (Durham, USA) on fasting plasma samples as previously described [12]. Quality control steps for the 529 measured metabolites are reported elsewhere [12], and they included batch-effect data normalization, outlier (>4 SD) removal and subsequently inverse-normalization [13]. Only 313 metabolites that were measured in at least 90% of subjects were included in analyses.
2.4. Statistical analyses
2.4.1. Random Forest analysis of metabolite effects on IOP
Although metabolites may be univariably associated with genetic factors, their ratios and other higher-order forms of interaction between more than one metabolite have physiologic relevance and are under tight genetic control [12]. Simple linear regression models therefore are incapable of fully modelling these interactions. Here, we employed a Random Forest [14] machine learning technique (RF) which agnostically identifies the metabolites that are most influential over an outcome, regardless of the specific model through which the effect is mediated. These models implicitly capture higher order interactions between variables [15]. We used RF to rank all available metabolites according to the Breiman-Cutler “VIMP” values [14]. Importance ranking has no associated probabilities, or formal thresholds of significance, nor any need for multiple-testing correction as long as all variables are tested jointly at the same time. To control for bias from predictor variables’ (metabolites) differing variances [16], all variables were standard inverse-normalized as previously described [13]. The ‘mtry’ parameter was fine-tuned to minimize the out-of-bag errors. The parameters of the RF analyses were set as nTree = 10,000 and maxNodes = 10 and mtry = 140. The models also included confoundants such as ages at IOP measurement and when the blood samples were drawn, body height and weight, but only the relative importance of metabolites over IOP measurements is being reported. Specifically, the model included a mixed model adjustment term to address the family relationships among the participants of the TwinsUK cohort.
Analyses reported here were conducted using all available metabolites that passed QC; analyses on subsets of unrelated metabolites (not shown) did not produce fundamental alterations in the importance ranking of the metabolites representing their clusters. Analyses were run in the ‘randomForestSRC’ package, version 2.5.1 in R 3.4.1(www.cran.r-project.org).
2.4.2. Mendelian randomization comparisons of genetic effects
We aimed to validate the findings and assess causality for the most important metabolite identified in the RF analysis stage, through an MR model. We used as instrumental variables (IV) SNPs that associated with plasma metabolite levels (exposure) on IOP (outcome).
Effects of genetic variants over metabolite levels were obtained from a published study [12]. We used SNPs that showed association at either GWAS-significant (p < 10−08), but also at suggestive levels (p < 10−06) in the final published joint meta-analyses [12]. Estimates of effect sizes and standard errors for the association between the selected SNPs and metabolites were from the Kooperative Gesundheitsforschung in der Region Augsburg (KORA) cohort and were obtained from previously published reports [17]. Only SNPs that were independent (on different chromosomes or at least 4 million base pairs apart and r2 < 0.1) were used for the analyses. Estimated effect sizes and standard errors for association of the SNPs with IOP were obtained from a GWAS of 103,382 European participants of the UK Biobank and separately from 6595 participants in the EPIC-Norfolk study, as reported elsewhere [1].
Three MR methods were used: inverse variance weighted median, inverse-variance weighted and MR-Egger. These analyses are usually interpreted together to jointly evaluate the relationship between exposure and outcome [18], [19] and don’t require multiple testing correction. The MR-Egger regression test intercept evaluates evidence for directional pleiotropy; intercepts significantly different from the origin suggest directional pleiotropy, where the underlying Instrument Strength Independent of Direct Effect (InSIDE) assumption may not be satisfied [20]. Analyses were performed using the ‘MendelianRandomization’ R package [21].
3. Results
3.1. O-methylascorbate levels are associated with IOP
We studied the plasma levels of 313 metabolites in the dataset of 1772 TwinsUK participants, for whom IOP measurements were also available. The main demographic and clinical characteristics of the sample are summarized in Table 1.
Table 1.
Main demographic and clinical characteristics of the participating cohorts. Mean and standard deviations are given for each parameter; missing values (“NA”) are used for variables not measured in a particular cohort.
| TwinsUK |
UK Biobank |
EPIC-Norfolk |
||||
|---|---|---|---|---|---|---|
| Variable | Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation |
| Age at the time IOP was measured (years) | 55 | 9.13 | 54.4 | 7.8 | 68.8 | 8 |
| Age when blood samples were taken (years) | 58.4 | 9.87 | NA | NA | NA | NA |
| Sex (women: men) | 1755: 8 | NA | 55,103: 48,279 | NA | 3725:3059 | NA |
| Mean intraocular pressure (mmHg) | 15.6 | 3.18 | 16.1 | 3.5 | 16.8 | 3.6 |
| Central corneal thickness (µm) | 544.9 | 39.58 | NA | NA | – | – |
| Weight (kg) | 69.45 | 13.6 | 77.98 | 15.9 | 74.2 | 14.1 |
| Height (cm) | 161.8 | 6.16 | 170.1 | 9.4 | 166.4 | 9.1 |
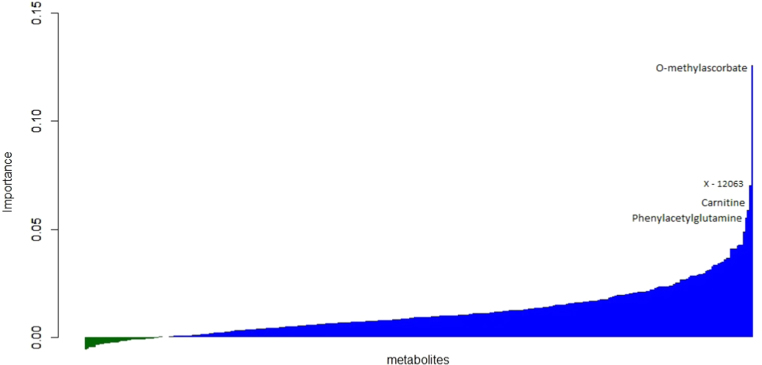
A random forest (RF) analysis ordered metabolites according to the importance of their association with IOP (Fig. 1). The highest-ranking metabolite in order of importance was O-methylascorbate [22]. This is a known metabolic product of the L-ascorbic acid (Vitamin C) [23]. Polymorphic changes of the sequences of the COMT, but also KLF12, SIL1, FDFT1 and PPPC5 genes are associated with O-methylascorbate levels [12]. The second ranking metabolite from the RF analysis was alpha-hydroxyvalerate, an amino acid metabolite. High in the rankings (Supplementary Table 1) were also carnitine, involved in lipid transfer across the mitochondrial membrane [24] and phenylacetylglutamine, a metabolite of glutamate, an antioxidative stress marker.
Fig. 1.
Plot of the VIMP parameter (relative importance) of the associations with IOP of 313 metabolite variables tested in the Random Forest analysis. The metabolites with highest importance are labeled (X- 12063 uncharacterized metabolite, identity unknown).
3.2. O-methylascorbate reduces IOP in independent populations
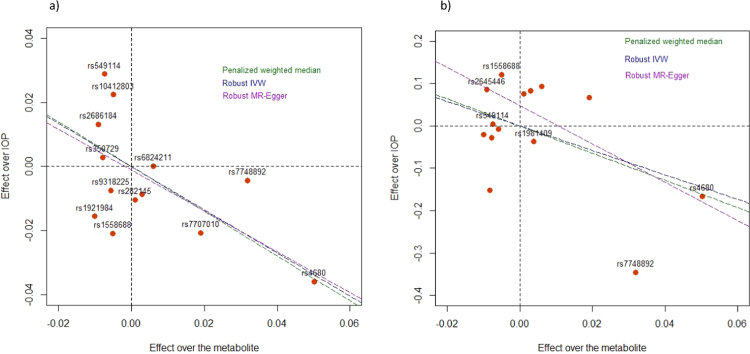
We followed up on the highest-ranking metabolite from the RF analysis. To validate results, we explored the relationship between O-methylascorbate and IOP in two independent populations. We used as genetic instruments single nucleotide polymorphisms (SNPs) that were significantly associated with O-methylascorbate levels in the KORA cohort [12], and examined their association with IOP, initially, in the UK Biobank cohort (Supplementary Table 2). A Mendelian Randomization (MR) model found a significant relationship between exposure (the O-methylascorbate levels) and IOP. All three models (Table 2, Fig. 2a) showed a statistically significant inverse relationship between the circulating levels of this metabolite and IOP (weighted median p = 0.02, robust IVW p = 2.4 ×10−10 and RM-Egger p = 3.33 × 10−06). There was no statistical evidence of pleiotropy (MR-Egger Intercept = 0.00). We further used the same instrumental variables(IVs) to build a second MR model in another independent dataset. The MR results in the EPIC-Norfolk dataset were consistent with the results obtained in the UK Biobank, with statistically significant effects of O-methylascorbate on IOP (Table 2 and Fig. 2b).
Table 2.
Mendelian Randomization (MR) study results for IOP in the UK Biobank and EPIC-Norfolk cohorts. For each of the three methods used, the β estimate, standard errors (SE) and associated p-values are reported. The Penalized robust MR-Egger intercept is not a MR model, but if different from 0 would provide evidence of directional pleiotropy and potential violation of the instrumental variable assumptions.
| UK Biobank |
EPIC |
|||||
|---|---|---|---|---|---|---|
| Method | Beta | SE | p-value | Beta | SE | p-value |
| Penalized weighted median | −0.696 | 0.304 | 0.022 | −3.219 | 1.371 | 0.019 |
| Robust inverse-variance weighted | −0.674 | 0.106 | 2.04 × 10−10 | −2.891 | 0.678 | 2.5 × 10−05 |
| Robust MR-Egger | −0.637 | 0.137 | 3.33 × 10−06 | −4.536 | 0.689 | 4.6 × 10–11 |
| Penalized robust MR-Egger (Intercept) | −0.001 | 0.006 | 0.855 | 0.048 | 0.026 | 0.071 |
Fig. 2.
Relationship of observed effect sizes of the instrumental variable SNP on IOP in the UK Biobank (a) and the EPIC-Norfolk (b) cohorts with the effect sizes of the same SNPs on O-methylascorbate levels in the KORA population. The lines represent the regression slopes for the different models, as specified in the legend.
To further exclude pleiotropy, we reversed our MR models to use IOP as the putative risk, SNPs significantly associated with IOP, described elsewhere [1] (Supplementary Table 3) as IVs, IOP as exposure and O-methylascorbate as the outcome of interest. In contrast to the previous results, none of the tests were statistically significant (Supplementary Table 4), which further suggests that O-methylascorbate levels causally affect IOP and not vice versa.
4. Discussion
Here we report, for the first time, that O-methylascorbate, a Vitamin C metabolite, is part of metabolic mechanisms that control IOP in the general population. Previous works suggested that levels of Vitamin C are reportedly inversely correlated with systemic [25] and pulmonary [26], [27] blood pressure, as well as IOP [28], [29]. Although it also enhances endothelial function [30], much of its anti-hypertensive effects are likely mediated by its powerful antioxidative properties, which provide protection from the radical oxygen species [31].
Photooxidative stress in the eye leads to trabecular meshwork degradation [32], elevated IOP due to increased aqueous outflow resistance [33] and ultimately glaucoma [34]. Vitamin C is highly concentrated in the aqueous humor and forms the first line of defense against free radicals in the eyes [35]. The O-methylascorbate, a naturally occurring metabolite, is less cytotoxic [36] and has a strong reductive capacity against photooxidative stress [37].
Our study combined metabolomic and genetic data to identify metabolic processes that modulate IOP in healthy populations. Our MR results shows that genetic factors that raise O-methylascorbate levels are associated with lower IOP. For example, the rs4680 G allele leads to higher COMT activity [38], and increased enzymatic conversion of Vitamin C into O-methylascorbate [12]. This variant is also associated with lower IOP, likely through the antioxidant properties of its enzymatic reaction product. The correlations of effects observed over several genes that independently control O-methylascorbate levels suggests that O-methylascorbate effect on IOP is real and not the result of confounding.
Several considerations are needed for the correct interpretation of these findings. First, O-methylascorbate effects over IOP homeostasis are likely modest and not deterministic. The metabolome platform that we used, only provides semi-quantitative results, but its standard-normalized output may be used to assess the strength of statistical associations, but not reliable effect size estimation. Second, the metabolomic platform we used only assesses a fraction of the metabolites present in complex organisms and could have overlooked metabolites equally or more relevant to IOP homeostasis. Third, the TwinsUK discovery cohort had power limitations and is almost exclusively female, while associations between oxidative biomarkers and glaucoma are reportedly stronger in men [29]. Finally, while the causal inference statistical methods suggest a causative role for O-methylascorbate in IOP, MR methodologies critically rely on several assumptions, whose violations would change the interpretation of causality [19]. Until further experimental confirmation, the relationship of O- methylascorbate with IOP is simply probabilistic.
Our study demonstrates that Vitamin C metabolism is involved in the control of intraocular pressure. These findings provide an additional insight into the role antioxidative stress-related mechanisms in intraocular, and maybe blood pressure homeostasis. Further work will be necessary to establish the exact mechanisms of pressure reduction via ascorbate metabolites and establish whether these mechanisms may have any role for the clinical management of IOP or glaucoma.
Acknowledgements
EPIC-Norfolk infrastructure and core functions are supported by grants from the Medical Research Council (G1000143) and Cancer Research UK (C864/A14136). The clinic for the third health examination was funded by Research into Ageing (262). Genotyping was funded by the Medical Research Council (MC_PC_13048). We thank all staff from the MRC Epidemiology laboratory team for the preparation and quality control of DNA samples. Mr. Khawaja is supported by a Moorfields Eye Charity fellowship. Professor Foster has received additional support from the Richard Desmond Charitable Trust (via Fight for Sight) and the Department for Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital and the UCL Institute of Ophthalmology for a specialist Biomedical Research Centre for Ophthalmology.
TwinsUK is funded by the Wellcome Trust, Medical Research Council, European Union, the National Institute for Health Research (NIHR) – funded BioResource, Clinical Research Facility and Biomedical Research Centre based at Guy’s and St. Thomas’ NHS Foundation Trust in partnership with King’s College London. CH and PH acknowledge the support from the TFC Frost Charitable Trust.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.10.004.
Appendix A. Supplementary material
Supplementary material
References
- 1.Khawaja A.P., Cooke Bailey J.N., Wareham N.J., Scott R.A., Simcoe M., Igo R.P., Jr., Song Y.E., Wojciechowski R., Cheng C.Y., Khaw P.T., Pasquale L.R., Haines J.L., Foster P.J., Wiggs J.L., Hammond C.J., Hysi P.G., Eye U.K.B., Vision C., Consortium N. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat. Genet. 2018;50(6):778–782. doi: 10.1038/s41588-018-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiggs J.L., Pasquale L.R. Genetics of glaucoma. Hum. Mol. Genet. 2017;26(R1):R21–R27. doi: 10.1093/hmg/ddx184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y., Allingham R.R. Major review: molecular genetics of primary open-angle glaucoma. Exp. Eye Res. 2017;160:62–84. doi: 10.1016/j.exer.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choquet H., Thai K.K., Yin J., Hoffmann T.J., Kvale M.N., Banda Y., Schaefer C., Risch N., Nair K.S., Melles R., Jorgenson E. A large multi-ethnic genome-wide association study identifies novel genetic loci for intraocular pressure. Nat. Commun. 2017;8(1):2108. doi: 10.1038/s41467-017-01913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh S.W., Lee S., Park C., Kim D.J. Elevated intraocular pressure is associated with insulin resistance and metabolic syndrome. Diabetes Metab. Res. Rev. 2005;21(5):434–440. doi: 10.1002/dmrr.529. [DOI] [PubMed] [Google Scholar]
- 6.Newman-Casey P.A., Talwar N., Nan B., Musch D.C., Stein J.D. The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology. 2011;118(7):1318–1326. doi: 10.1016/j.ophtha.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moayyeri A., Hammond C.J., Valdes A.M., Spector T.D. Cohort Profile: TwinsUK and healthy ageing twin study. Int. J. Epidemiol. 2013;42(1):76–85. doi: 10.1093/ije/dyr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Valk R., Webers C.A., Schouten J.S., Zeegers M.P., Hendrikse F., Prins M.H. Intraocular pressure-lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trials. Ophthalmology. 2005;112(7):1177–1185. doi: 10.1016/j.ophtha.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 9.van Koolwijk L.M., Ramdas W.D., Ikram M.K., Jansonius N.M., Pasutto F., Hysi P.G., Macgregor S., Janssen S.F., Hewitt A.W., Viswanathan A.C., ten Brink J.B., Hosseini S.M., Amin N., Despriet D.D., Willemse-Assink J.J., Kramer R., Rivadeneira F., Struchalin M., Aulchenko Y.S., Weisschuh N., Zenkel M., Mardin C.Y., Gramer E., Welge-Lussen U., Montgomery G.W., Carbonaro F., Young T.L., Group D.E.R., Bellenguez C., McGuffin P., Foster P.J., Topouzis F., Mitchell P., Wang J.J., Wong T.Y., Czudowska M.A., Hofman A., Uitterlinden A.G., Wolfs R.C., de Jong P.T., Oostra B.A., Paterson A.D., Wellcome Trust Case Control C., Mackey D.A., Bergen A.A., Reis A., Hammond C.J., Vingerling J.R., Lemij H.G., Klaver C.C., van Duijn C.M. Common genetic determinants of intraocular pressure and primary open-angle glaucoma. PLoS Genet. 2012;8(5):e1002611. doi: 10.1371/journal.pgen.1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hysi P.G., Cheng C.Y., Springelkamp H., Macgregor S., Bailey J.N.C., Wojciechowski R., Vitart V., Nag A., Hewitt A.W., Hohn R., Venturini C., Mirshahi A., Ramdas W.D., Thorleifsson G., Vithana E., Khor C.C., Stefansson A.B., Liao J., Haines J.L., Amin N., Wang Y.X., Wild P.S., Ozel A.B., Li J.Z., Fleck B.W., Zeller T., Staffieri S.E., Teo Y.Y., Cuellar-Partida G., Luo X., Allingham R.R., Richards J.E., Senft A., Karssen L.C., Zheng Y., Bellenguez C., Xu L., Iglesias A.I., Wilson J.F., Kang J.H., van Leeuwen E.M., Jonsson V., Thorsteinsdottir U., Despriet D.D.G., Ennis S., Moroi S.E., Martin N.G., Jansonius N.M., Yazar S., Tai E.S., Amouyel P., Kirwan J., van Koolwijk L.M.E., Hauser M.A., Jonasson F., Leo P., Loomis S.J., Fogarty R., Rivadeneira F., Kearns L., Lackner K.J., de Jong P., Simpson C.L., Pennell C.E., Oostra B.A., Uitterlinden A.G., Saw S.M., Lotery A.J., Bailey-Wilson J.E., Hofman A., Vingerling J.R., Maubaret C., Pfeiffer N., Wolfs R.C.W., Lemij H.G., Young T.L., Pasquale L.R., Delcourt C., Spector T.D., Klaver C.C.W., Small K.S., Burdon K.P., Stefansson K., Wong T.Y., Group B.G., Consortium N., Wellcome Trust Case Control C., Viswanathan A., Mackey D.A., Craig J.E., Wiggs J.L., van Duijn C.M., Hammond C.J., Aung T. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat. Genet. 2014;46(10):1126–1130. doi: 10.1038/ng.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riboli E., Kaaks R. The EPIC Project: rationale and study design. European prospective investigation into cancer and nutrition. Int. J. Epidemiol. 1997;26(Suppl. 1):S6–S14. doi: 10.1093/ije/26.suppl_1.s6. [DOI] [PubMed] [Google Scholar]
- 12.Shin S.Y., Fauman E.B., Petersen A.K., Krumsiek J., Santos R., Huang J., Arnold M., Erte I., Forgetta V., Yang T.P., Walter K., Menni C., Chen L., Vasquez L., Valdes A.M., Hyde C.L., Wang V., Ziemek D., Roberts P., Xi L., Grundberg E., Multiple Tissue Human Expression Resource C., Waldenberger M., Richards J.B., Mohney R.P., Milburn M.V., John S.L., Trimmer J., Theis F.J., Overington J.P., Suhre K., Brosnan M.J., Gieger C., Kastenmuller G., Spector T.D., Soranzo N. An atlas of genetic influences on human blood metabolites. Nat. Genet. 2014;46(6):543–550. doi: 10.1038/ng.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menni C., Fauman E., Erte I., Perry J.R., Kastenmuller G., Shin S.Y., Petersen A.K., Hyde C., Psatha M., Ward K.J., Yuan W., Milburn M., Palmer C.N., Frayling T.M., Trimmer J., Bell J.T., Gieger C., Mohney R.P., Brosnan M.J., Suhre K., Soranzo N., Spector T.D. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes. 2013;62(12):4270–4276. doi: 10.2337/db13-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breiman L. Random forests. Mach. Learn. 2001;45(1):5–32. [Google Scholar]
- 15.Wright M.N., Ziegler A., Konig I.R. Do little interactions get lost in dark random forests? BMC Bioinform. 2016;17:145. doi: 10.1186/s12859-016-0995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strobl C., Boulesteix A.L., Zeileis A., Hothorn T. Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinform. 2007;8:25. doi: 10.1186/1471-2105-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holle R., Happich M., Lowel H., Wichmann H.E., Group M.K.S. KORA–a research platform for population based health research. Gesundheitswesen. 2005;67(Suppl. 1):S19–S25. doi: 10.1055/s-2005-858235. [DOI] [PubMed] [Google Scholar]
- 18.Burgess S., Bowden J., Fall T., Ingelsson E., Thompson S.G. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017;28(1):30–42. doi: 10.1097/EDE.0000000000000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess S., Thompson S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017;32(5):377–389. doi: 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowden J., Davey Smith G., Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yavorska O.O., Burgess S. Mendelian randomization: an R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017;46(6):1734–1739. doi: 10.1093/ije/dyx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krumsiek J., Suhre K., Evans A.M., Mitchell M.W., Mohney R.P., Milburn M.V., Wagele B., Romisch-Margl W., Illig T., Adamski J., Gieger C., Theis F.J., Kastenmuller G. Mining the unknown: a systems approach to metabolite identification combining genetic and metabolic information. PLoS Genet. 2012;8(10):e1003005. doi: 10.1371/journal.pgen.1003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blaschke E., Hertting G. Enzymic methylation of L-ascorbic acid by catechol O-methyltransferase. Biochem. Pharmacol. 1971;20(7):1363–1370. doi: 10.1016/0006-2952(71)90263-2. [DOI] [PubMed] [Google Scholar]
- 24.Flanagan J.L., Simmons P.A., Vehige J., Willcox M.D., Garrett Q. Role of carnitine in disease. Nutr. Metab. 2010;7:30. doi: 10.1186/1743-7075-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigo R., Prat H., Passalacqua W., Araya J., Guichard C., Bachler J.P. Relationship between oxidative stress and essential hypertension. Hypertens. Res. 2007;30(12):1159–1167. doi: 10.1291/hypres.30.1159. [DOI] [PubMed] [Google Scholar]
- 26.Duvall M.G., Pikman Y., Kantor D.B., Ariagno K., Summers L., Sectish T.C., Mullen M.P. Pulmonary hypertension associated with scurvy and vitamin deficiencies in an autistic child. Pediatrics. 2013;132(6):e1699–e1703. doi: 10.1542/peds.2012-3054. [DOI] [PubMed] [Google Scholar]
- 27.Kupari M., Rapola J. Reversible pulmonary hypertension associated with vitamin C deficiency. Chest. 2012;142(1):225–227. doi: 10.1378/chest.11-1857. [DOI] [PubMed] [Google Scholar]
- 28.Leite M.T., Prata T.S., Kera C.Z., Miranda D.V., de Moraes Barros S.B., Melo L.A., Jr. Ascorbic acid concentration is reduced in the secondary aqueous humour of glaucomatous patients. Clin. Exp. Ophthalmol. 2009;37(4):402–406. doi: 10.1111/j.1442-9071.2009.02046.x. [DOI] [PubMed] [Google Scholar]
- 29.Benoist d′Azy C., Pereira B., Chiambaretta F., Dutheil F. Oxidative and anti-oxidative stress markers in chronic glaucoma: aa systematic review and meta-analysis. PLoS One. 2016;11(12):e0166915. doi: 10.1371/journal.pone.0166915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashor A.W., Lara J., Mathers J.C., Siervo M. Effect of vitamin C on endothelial function in health and disease: a systematic review and meta-analysis of randomised controlled trials. Atherosclerosis. 2014;235(1):9–20. doi: 10.1016/j.atherosclerosis.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Raaz U., Toh R., Maegdefessel L., Adam M., Nakagami F., Emrich F.C., Spin J.M., Tsao P.S. Hemodynamic regulation of reactive oxygen species: implications for vascular diseases. Antioxid. Redox Signal. 2014;20(6):914–928. doi: 10.1089/ars.2013.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarado J., Murphy C., Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91(6):564–579. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- 33.Stamer W.D., Acott T.S. Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol. 2012;23(2):135–143. doi: 10.1097/ICU.0b013e32834ff23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sacca S.C., Pascotto A., Camicione P., Capris P., Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch. Ophthalmol. 2005;123(4):458–463. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- 35.Sacca S.C., Izzotti A., Rossi P., Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp. Eye Res. 2007;84(3):389–399. doi: 10.1016/j.exer.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Tsao C.S., Young M. Molecular structure-dependent cytotoxic effect of ascorbate derivatives. Vitr. Cell. Dev. Biol. Anim. 1995;31(2):87–90. doi: 10.1007/BF02633967. [DOI] [PubMed] [Google Scholar]
- 37.Shick J.M., Dunlap W.C. Mycosporine-like amino acids and related Gadusols: biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002;64:223–262. doi: 10.1146/annurev.physiol.64.081501.155802. [DOI] [PubMed] [Google Scholar]
- 38.Chen J., Lipska B.K., Halim N., Ma Q.D., Matsumoto M., Melhem S., Kolachana B.S., Hyde T.M., Herman M.M., Apud J., Egan M.F., Kleinman J.E., Weinberger D.R. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet. 2004;75(5):807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material