Abstract
A 56-year old woman with BRCA-associated breast cancer and recurrent ovarian cancer was treated with a poly (ADP-ribose) polymerase enzyme (PARP) inhibitor, rucaparib, in the setting of dialysis-dependence which required dose modification. Rucaparib trough levels were obtained before and after dialysis and a clinically significant disease response was appreciated.
Highlights
-
•
PARPi can be used safely in a dialysis dependent BRCA mutated patient with breast and ovarian cancer.
-
•
PK levels below those described in clinical trials are associated with clinical benefit.
-
•
Toxicities seen are similar to those in subjects with normal GFR.
1. Introduction
Approximately 11–18% patients with ovarian cancer have a germline BRCA gene mutation, while up to 25% have a germline or somatic BRCA gene mutation (Balasubramaniam et al., 2017; Pennington et al., 2014; Coleman et al., 2017). The poly (ADP-ribose) polymerase enzymes (PARP) represent novel targets, with increased activity in patients with BRCA mutations. PARP enzymes are recruited to sites of single strand DNA damage and promote repair through several mechanisms, including base excision repair. Inhibition of PARP enzymes increases single strand breaks and leads to double strand DNA breaks during replication; double strand DNA breaks are the specific targets in patients with BRCA mutations as these breaks are unable to be repaired due to the mutation (Shulman, 2010). Rucaparib, an oral PARP inhibitor, is FDA approved as monotherapy in patients with deleterious BRCA mutated advanced ovarian cancer treated with two or more chemotherapy regimens as well as the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer after response to platinum-based therapy (Swisher et al., 2017; Weil & Chen, 2011). Rucaparib is known to be partially excreted by the kidney, and rucaparib clearance (CL) increased with increasing baseline creatinine CL based on information presented by Xiao and colleagues in 2017 (Xiao et al., n.d.). The kinetics in dialysis-dependent patients is unknown. The following is a case of recurrent, platinum sensitive high-grade serous carcinoma of the ovary in a dialysis-dependent patient with a germline BRCA2 mutation treated with rucaparib. Data describing the effects of dialysis on rucaparib pharmacokinetics is provided.
2. Case
A 55-year-old woman was diagnosed with Stage IIIC high grade papillary serous carcinoma of the ovary in August 2014. Treatment with platinum/taxane resulted in a complete clinical response in May 2015. Prior to her diagnosis she had stage III chronic kidney disease due to long-standing hypertension. Neutropenic sepsis secondary to a diverticular perforation occurred during her initial chemotherapy regimen resulting in hemodialysis dependence three times weekly, beginning in October 2014 with associated anuria. In December 2015, testing by Ambry Genetics® (Aliso Viejo, CA) was completed which revealed a p.R2502* mutation in the BRCA2 gene, for which the patient was heterozygous.
In May 2016 disease progression was noted with nodules in the right pleural space, a pleural effusion, an implant in the splenic hilum, and peritoneal nodularity on computed tomography. At that time the patient was concurrently diagnosed with T3NXM0 ER positive, PR positive, HER-2 negative grade III invasive ductal carcinoma of the right breast, for which 1 mg of anastrozole daily was initiated May 2016. A second line of chemotherapy was initiated June 9, 2016 for her recurrent ovarian cancer with fixed-dose carboplatin and docetaxel, of which she received a total of 9 cycles. Anastrozole was discontinued July 2016 due to noncompliance; it was not resumed until November 2017. In December 2016, computed tomography demonstrated stable disease of her peritoneal and splenic implants after Platinum based therapy. Approval for rucaparib was sought and started February 2, 2017 for ovarian cancer; dosing was 200 mg by mouth twice daily. This has been continued over one year, with a four separate one week holds due to Grade 1 thrombocytopenia to a nadir of 85 × 109/L.
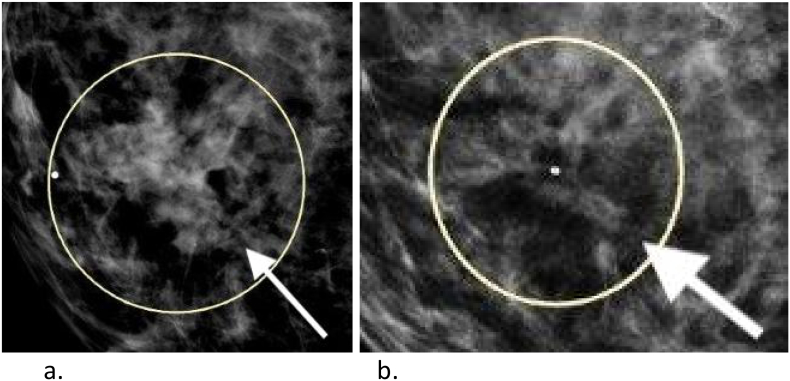
The peritoneal disease associated with her ovarian cancer had a compete response to therapy. The enhancing splenic lesion resolved as seen in Fig. 1a and b. Her breast lesion resolved as measured by mammography (Fig. 2a and b).
Fig. 1.
-
a.(12/27/16) Peritoneal implants in the medial aspect of the spleen are again noted measuring 1.2 cm.
-
b.(3/21/17) Spleen: Negative.
Fig. 2.
-
c.Speculated mass in the right breast at 4 o'clock that measures up to 45 mm on mammogram.
-
d.Interval significant favorable response to chemotherapy. Previously seen masses at right breast 4 o'clock 4 cm from the nipple and 4 o'clock 2 cm from the nipple have almost completely resolved.
Rucaparib's starting dose recommendation is 600 mg twice daily (Swisher et al., 2017), with no dose adjustments required for patients with a creatinine clearance (CrCl) between 30 and 89 mL/min (as estimated by Cockcroft-Gault equation). Patients with a CrCl less than 30 mL/min or who were receiving dialysis were excluded from clinical trials (Weil & Chen, 2011). Based upon pharmacokinetic data from Phase 1 studies, rucaparib's steady-state area under the curve (AUC) is increased by 15% in patients with mild renal impairment (CrCl 60 to 89 mL/min) and 32% in patients with moderate renal impairment (CrCl 30 to 59 mL/min) (Swisher et al., 2017). The pharmacokinetics of rucaparib in patients with severe renal impairment and dialysis dependent patients remain unknown. Given rucaparib's pharmacokinetics in patients with varying degrees of renal function, it was hypothesized that the AUC in patients with severe renal impairment would be approximately double that of patients with moderate impairment. Therefore, given the patient's dialysis dependence, a dose of 200 mg twice daily (a 66% reduction from recommended initial dose) was started.
In the ARIEL2 trial, pharmacokinetic analysis was performed on 196 patients (Swisher et al., 2017). In this analysis, the mean trough plasma concentration was 2026 ng/mL across all patients with a standard deviation of 1147 ng/mL. Although there was a trend for subjects with a BRCA mutation to have a lower AUC, there was no consistent difference in trough concentrations between BRCA mutant and BRCA wild-type patients (Swisher et al., 2017).
To determine the effects that renal failure and dialysis had on the trough plasma concentrations, we measured patient rucaparib levels at times prior to and after dialysis and at similar times on a non-dialysis day. After receiving approval through the local Institutional Review Board and obtaining patient consent, blood samples were drawn to determine the pharmacokinetics of rucaparib (see Table 1). For this patient, hemodialysis was scheduled on Monday, Wednesday, and Friday, and typically took 4.5 h. Blood sampling occurred at 8:30 am and 3:50 pm on Monday and at 8:10 am and 3:30 pm on the following Thursday. The Thursday functioned as a non-dialysis control draw. Typically, her rucaparib dosing is done at 9 am and 9 pm. For the purposes of this study, the patient was instructed not to take her first daily rucaparib dose until after the am PK blood draw, in an effort to best elucidate the effects of dialysis on rucaparib pharmacokinetics. The patient then took her twice daily rucaparib 5 h apart. As the patient underwent dialysis on Mondays, Wednesdays, and Fridays, the pre-dialysis levels represent true troughs. The patient has been taking rucaparib at 200 mg twice daily for 57 weeks prior to the study ensuring steady state levels. The samples were run on chromatograms from patient plasma by Q2 Solutions (Ithaca, NY). Runs were taken in duplicate and quality control samples were taken for precision and accuracy.
Table 1.
Rucaparib Pharmacokinetic Analysis.
| Day | Time | Concentration (ng/mL) |
|---|---|---|
| Monday-dialysis day, pre | 8:30 am | 323 |
| Monday-dialysis day, post | 3:50 pm | 287 |
| Thursday-non-dialysis day | 8:10 am | 726 |
| Thursday-non-dialysis day | 3:10 pm | 603 |
This single patient analysis reveals non-toxic concentrations of rucaparib in the setting of renal failure and chronic hemodialysis when rucaparib dose was reduced by 66% from the FDA approved dose. Additionally, the results suggest minimal filtration of rucaparib by hemodialysis with post-dialysis decreases of 36 ng/mL and 123 ng/mL on the two days assessed. The Monday levels represent the longest continuous exposure to rucaparib without dialysis.
3. Conclusion
In this single patient study, we demonstrate the relative safety of dosing rucaparib in a patient with dialysis-dependent renal failure. Although the drug may be cleared hepatically, impaired renal function was shown to affect rucaparib levels based on phase I data (Swisher et al., 2017). Trough levels in this study were below the levels reported in ARIEL2, but the lower levels on the day of dialysis could be secondary to the instructions given to the patient to hold her morning dose of rucaparib. Despite lower levels, treatment resulted in clinically effective therapy for both her serous ovarian and invasive ductal carcinomas. The patient was taking Anastrozole for a brief period of time during treatment with Rucaparib, which could confound the clinical response in the patient's breast cancer. Thrombocytopenia was the only toxicity, requiring 4 single week holds, which was a common toxicity in ARIEL3 with 28% of subjects experiencing Grade 1–4 thrombocytopenia [7].
Conflict of interest
All authors declare no conflict of interest.
Funding source
Clovis Oncology provided funding source for PK draws, analysis, and production costs related to publication.
References
- Balasubramaniam S., Beaver J.A., Horton S., Fernandes L.L., Tang S., Horne H.N. FDA Approval Summary: Rucaparib for the Treatment of patients with Deleterious BRCA Mutation–Associated Advanced Ovarian Cancer. Clin. Cancer Res. 2017 Dec 1;23(23):7165–7170. doi: 10.1158/1078-0432.CCR-17-1337. [DOI] [PubMed] [Google Scholar]
- Coleman R.L., Oza A.M., Lorusso D., Aghaijanian C., Oaknin A., Dean A. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled phase 3 trial. The Lancet. 2017;390:1949–1961. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington K.P., Walsh T., Harrell M.I., Lee M.K., Pennil C.C., Rendi M.H. Germline and Somatic Mutations in Homologous Recombination Genes Predict platinum Response and Survival in Ovarian, Fallopian Tube, and Peritoneal Carcinomas. Clin. Cancer Res. 2014 Feb 1;20(3):764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman L.P. Hereditary Breast and Ovarian Cancer (HBOC): Clinical Features and counseling for BRCA1 and BRCA2, Lynch Syndrome, Cowden Syndrome, and Li-Fraumeni Syndrome. Obstet. Gynecol. Clin. N. Am. 2010 Mar;37(1):109–133. doi: 10.1016/j.ogc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Swisher E.M., Lin K.K., Oza A.M., Scott C.L., Giordano H., Sun J. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 part 1): an international, multicentre, open-label, phase 2 trial. The Lancet Oncology. 2017 Jan;18(1):75–87. doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- Weil M.K., Chen A.P. PARP Inhibitor Treatment in Ovarian and Breast Cancer. Curr. Probl. Cancer. 2011 Jan;35(1):7–50. doi: 10.1016/j.currproblcancer.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, J.J, Green, M., Ma, S. Population Pharmacokinetics (PK) of Rucaparib (CO-338) in Patients with Advanced Ovarian Cancer (AOC) or Other Solid Tumors. [DOI] [PubMed]