Abstract
Chemical detection is key to various behaviours in both marine and terrestrial animals. Marine species, though highly diverse, have been underrepresented so far in studies on chemosensory systems, and our knowledge mostly concerns the detection of airborne cues. A broader comparative approach is therefore desirable. Marine annelid worms with their rich behavioural repertoire represent attractive models for chemosensation. Here, we study the marine worm Platynereis dumerilii to provide the first comprehensive investigation of head chemosensory organ physiology in an annelid. By combining microfluidics and calcium imaging, we record neuronal activity in the entire head of early juveniles upon chemical stimulation. We find that Platynereis uses four types of organs to detect stimuli such as alcohols, esters, amino acids and sugars. Antennae are the main chemosensory organs, compared to the more differentially responding nuchal organs or palps. We report chemically evoked activity in possible downstream brain regions including the mushroom bodies (MBs), which are anatomically and molecularly similar to insect MBs. We conclude that chemosensation is a major sensory modality for marine annelids and propose early Platynereis juveniles as a model to study annelid chemosensory systems.
Keywords: Platynereis, marine chemosensation, calcium imaging, microfluidics, neuronal activity, invertebrates
1. Introduction
Chemical signals are central to animal behaviour, including feeding, predation, courtship and mating, aggregation, defence, habitat selection and communication [1]. Adapting to variable habitats and changing chemical landscapes, animals have evolved a broad variety of chemosensory organs. Investigations of chemosensory systems in mammals, insects and nematodes have provided insights into the molecular and cellular basis of how chemical information is encoded into neuronal activity [2–4]. While similar circuit architectures can be found in distant species at some steps of information processing, this appears to be no general rule [5,6]. Genomic studies have revealed that receptor proteins are highly diverse in the animal kingdom [7] and can be entirely different between distant species—vertebrates and insects, for example, use distinct types of receptors [8,9]. Hence, a broader comparative approach will facilitate the elucidation of both general operating principles and evolutionary origins of animal chemosensation. Notwithstanding studies in fish and crustaceans [10,11], and to a lesser extent in molluscs [12,13], our current understanding of animal chemosensation still mainly concerns terrestrial and airborne cues. Marine animals thus deserve more attention.
Marine annelids, traditionally referred to as ‘polychaetes’, represent an attractive group for chemosensory studies. These worms, represented by more than 10 000 species, are typically free-living, burrow in the marine sediment or build tubes. They are known to respond to chemical signals in reproduction, feeding, aggression, avoidance, aggregation, environment probing, larval settlement and metamorphosis [14]. Marine annelids are suited for electrophysiological [15–18] as well as behavioural [19–22] studies, and their nervous system is anatomically and histologically well described [23–31]. Potential chemoreceptor proteins have been identified in the first published annelid genomes, which contain homologues for insect receptors (41 ionotropic receptors (IRs) and 12 gustatory receptor-like receptors (GRs)), but apparently not for mammalian ones (olfactory receptors (ORs)) [32,33]. Despite these advantages, the physiology of chemical sensing in annelids is scarcely known (for an up-to-date review on annelid chemosensation, see [34, ch. II]).
Unlike terrestrial annelids such as leeches and earthworms, marine annelids possess elaborate head sensory organs with diverse morphologies [35]. Nuchal organs, paired ciliated cavities located at the back of the head, are considered an annelid synapomorphy [36] and are generally regarded as chemosensory. Based on cell morphology, they are the best candidate chemosensory organs, though no physiological support yet substantiates this claim. Palpae, or palps, the most important head appendages for phylogenetic systematization, have been proposed to be chemosensory based on cell ultrastructure and activity-dependent cell labelling [31,37,38]. Similar claims based on ultrastructure were made for antennae and tentacular cirri, the two other major types of head appendages [31,39]. Gross in 1921 [19] showed that the removal of palps or antennae, and to a lesser extent of tentacular cirri, lengthens the reaction time of the nereidid Nereis virens to ionic solutions. Yet, physiological evidence is scarce, and at present no direct experimental proof of chemosensitivity exists for any of these four head organs.
We report here a comprehensive study of head chemosensory organ physiology in the marine annelid Platynereis dumerilii (figure 1a–c), which can be easily kept in the laboratory and is amenable to molecular studies. This species belongs to nereidids, the family regarded as best representing the annelid nervous system [40, p. 735]. Rather than adults, we chose to study early juveniles around the 6-days-post-fertilization (6 dpf) stage, which are already equipped with nuchal organs, palps, antennae and one pair of tentacular cirri (figure 1c). They feed, crawl, swim and display various natural behaviours such as phototaxis, startling, escaping or silk spinning. They present experimental advantages comparable to those of the nematode Caenorhabditis elegans, being transparent, developmentally synchronous, easily obtainable in high numbers and suitable for whole-body light and electron microscopy. A whole-body atlas of gene expression available for this developmental stage [41] constitutes a unique resource which facilitates the characterization of cell types. Moreover, a connectomic resource exists at a larval stage that has proved powerful in the reconstruction of whole-body neuronal circuits, notably in the context of phototaxis and ciliated locomotion [42–44].
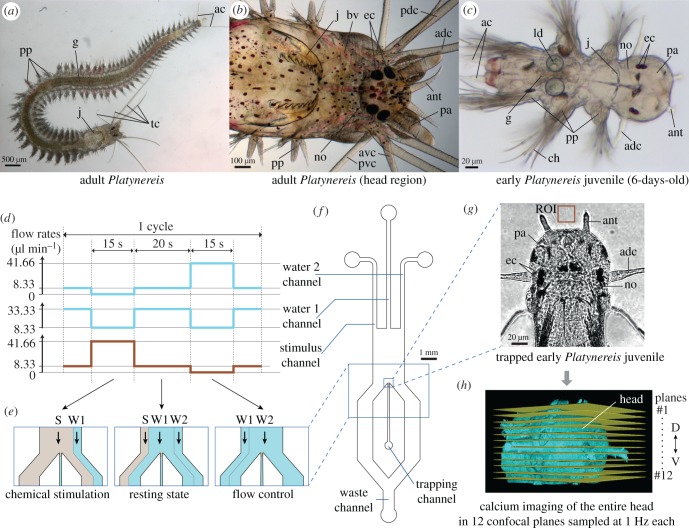
Figure 1.
Platynereis and the microfluidic device for precise chemical stimulations. (a–c) Light microscopy pictures of Platynereis at the adult (a,b) and early juvenile (c) stages, showing antennae (ant), palps (pa) and tentacular cirri (tc, adc, avc, pdc, pvc). Further abbreviations: see the abbreviations list. (a–c) Antje Fischer ©. (d) Channel flow rates used to generate (e) the different flow patterns. The sum of the three channels' flow rates is constant. (f) Schematic of the microfluidic device. The three inlet channels are operated by computer-controlled pumps. Single animals are introduced manually in the trapping channel and immobilized at its end. (g) An immobilized early juvenile, with its head freely exposed to the seawater flows. Confocal image with transmitted light illumination. (h) Calcium signals upon chemical stimulation are recorded with a confocal microscope in 12 optical planes sampling the whole head volume.
Four stimuli (1-butanol, amyl acetate, glutamate and sucrose) were chosen for their different physico-chemical properties and their likely ecological relevance to Platynereis. Short alcohols trigger behavioural reactions in Nereis [45]. Amyl acetate can act as a conditioned stimulus for associative learning in the aquatic snail Lymnea [46]. Glutamate elicits behavioural responses in Nereis [45], and amino acids, in general, are relevant aquatic chemical cues for various marine animals [12,38,47–50]. Sugars can be degradation products of the polysaccharides contained in plants such as eelgrass and seagrass, or in algae, on which nereidid polychaetes are known to feed [19,51]. However, except for a pH and salinity preferendum [52], nothing is known regarding chemoreception and relevant chemical cues in early Platynereis juveniles.
Using a customized microfluidic device for animal immobilization and precise stimulus delivery, we performed whole-head functional imaging in early juveniles ubiquitously expressing the genetically encoded calcium sensor GCaMP6s. We found that nuchal organs, palps, antennae and tentacular cirri are chemosensory, though with different degrees of specialization: for example, antennae responded to all stimulants, while nuchal organs were most sensitive to amyl acetate and sucrose, but did not respond to glutamate. We observed a chemically evoked activity in other regions including the mushroom bodies (MBs), which could potentially be involved in learning phenomena. We also described a prominent oscillatory activity in the larval apical organ, however not obviously linked to chemical stimulations. We provide the first direct evidence of chemosensory function in annelid head organs and lay the ground for future investigations of sensory integration.
2. Results
2.1. Establishment of a functional imaging assay system for chemosensation in early Platynereis juveniles
We designed a simple microfluidic device, made of the transparent polymer polydimethylsiloxane (PDMS) and fabricated by soft lithography. The device is symmetric, has a uniform height of 60 µm and consists of a single chamber in which a constant flow of natural seawater is established. Three inlet channels generate three parallel, non-mixing water streams thanks to a laminar flow regime. Changing the relative flow rates between channels allows to expose the chamber's centre to any of the streams (figure 1d,e). With one side stream being used to deliver a chemical stimulus and the other to deliver the solvent alone (seawater), a flow control is performed in each experiment that allows to separate the purely chemical sensory input from the mechanical one. An early Platynereis juvenile can be immobilized at the end of a central trapping channel (figure 1f), where its head is exposed to the water flows (figure 1g; see electronic supplementary material, figure S1A,B for a detailed description of the set-up).
We performed calibration experiments with a dye to quantify the actual changes of stimulant concentration at the level of the animal's head (see electronic supplementary material, figure S1C and Material and methods). With this set-up, a change from zero to maximum stimulant concentration (stimulus onset) can be completed within 0.9 ± 0.3 s, with a delay of 3.2 ± 0.5 s compared with the pump triggering time. The reverse change (stimulus offset) can be completed within 1.0 ± 0.2 s, with a delay of 3.0 ± 0.5 s. Slight timing differences between these two events stem from the geometrical asymmetry between central and lateral streams.
To survey calcium activity in the entire head, we immobilized juveniles ubiquitously expressing GCaMP6s and acquired images with a confocal microscope from 12 horizontal, equally spaced optical sections sampling the whole head volume (figure 1h). With the whole stack being acquired within a second, a 1 Hz temporal resolution was obtained for each of the 12 planes; hence, the precision of stimulus delivery was deemed satisfactory.
2.2. Ten distinct sets of cells show activity during chemical stimulation experiments
To comprehensively identify head regions active in the context of chemical stimulations, we imaged calcium activity across the entire head in response to four stimulants (see Introduction). For each stimulant, three to four experiments were conducted for each of nine animals (only eight for 1-butanol). A single experiment consisted of three identical cycles: each cycle comprised a chemical stimulus and a flow control, lasting 15 s each and spaced by 20 s resting intervals (figure 1d).
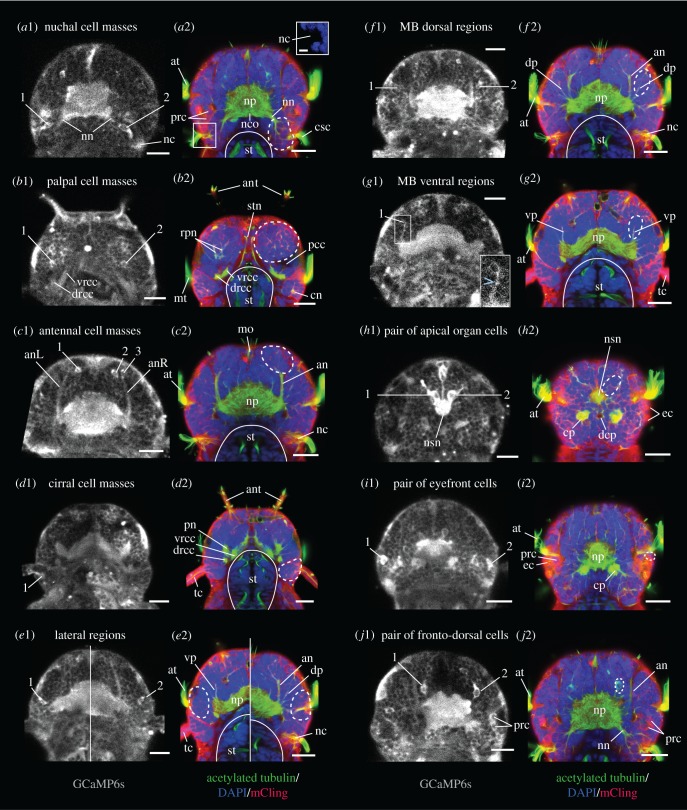
We observed activity in 10 bilaterally symmetrical spots (figure 2), including four paired regions which we identified as the cell masses of the four presumed chemosensory organs (nuchal organs, palp, antennae and tentacular cirri). We also observed activity in another paired chemosensory region, the lateral region, as well as in the dorsal and ventral mushroom body (MB) regions. Finally, we saw activity in three bilaterally symmetric pairs of cells: one in the apical organ area, the eyefront cells and the fronto-dorsal cells. The lateral regions, the eyefront cells and the fronto-dorsal cells, so far undescribed, were named for the present study. Activity was also observed in the nuchal, palpal antennal and cirral nerves, which were thus included in the analyses.
Figure 2.
Calcium images and anatomical stainings in dorsal view, showing the head regions active in the context of chemical stimulations. (a1, b1, … j1) GCaMP6s signal from single confocal planes, averaged over several consecutive time points. Individual active cells are pointed at by numbers. (a2, b2, … j2) Acetylated tubulin immunostaining (green), counterstained for nuclear DNA (DAPI, blue) and membranes (mCling, red), averaged over several consecutive confocal planes. The approximate boundaries of the active regions are indicated by white dashed lines, and the outlines of the stomodeum by white solid lines. The dorsoventral position of the planes, as well as lateral views of the anatomical stainings, are shown in the electronic supplementary material, figure S2. Two different horizontal planes have been stitched in (e1) and (e2). an, antennal nerve; ant, antenna; cn, cirral nerve; csc, cilia of the nuchal organ supporting cells; dp, dorsal peduncle of the MBs; nc, nuchal cavity; nn, nuchal nerve; np, main neuropil; nsn, neurosecretory neuropil; pn, palpal nerve; prc, photoreceptor cells; tc, tentacular cirrus; vp, ventral peduncle of the MBs. Further abbreviations: see the abbreviations list. Scale bars: 20 µm for all, 5 µm for inset in (a2).
To make sense of these signals, we first characterized the tissue context of the responding cells at the 6 dpf stage (figure 2; electronic supplementary material, figure S2). The nuchal organs (figure 2a; electronic supplementary material, figure S2A) lie posterior to the eyes and slightly more ventral. Nuchal cavities equipped with ciliated supporting cells can be recognized. The short nuchal nerves project posteriorly to the central neuropil and form a commissure. In the absence of clear anatomical boundaries, only proximity to the cavity and coactivation with the nerve can allow to attribute a cell to the nuchal cell mass. Most calcium activity was observed in a pair of cells, named ‘revolver cells’ due to their typical shape, but other cells next to the cavity were occasionally active. The palps (figure 2b; electronic supplementary material, figure S2B) lie ventrally, on each side of the mouth opening, and protrude only very little at this stage. Their mobile tip possesses clusters of cilia. The palpal nerves, short and thick, project to the central neuropil in proximity to the dorsal and ventral roots of the circum-oesophageal connectives. The palpal cell masses, delimited by membrane layers and developing coelomic cavities, constitute the largest cell masses in the head. Up to three pairs of active cells were observed rather in proximity to the nerves than to the tips. The antennae (figure 2c; electronic supplementary material, figure S2C) are slender, frontal organs. Cellular extensions and nervous fibres, but not cell bodies (electronic supplementary material, figure S3), are present inside the appendages, whose surface is equipped with clusters of cilia. The prominent antennal nerves, always identifiable in calcium recordings, project laterally to the central neuropil. The antennal cell masses, anatomically well delimited, are located at the base of the antennal appendages. Up to four pairs of active cells were observed throughout the cell masses. The mobile tentacular cirri (figure 2d; electronic supplementary material, figure S2D) possess clusters of cilia at their surface and nervous fibres. The cirral nerves project to the circum–oesophageal connectives, not to the central neuropil. The cirral cell masses, future cirral ganglia, are well delimited and occupy a lateral and posterior position in the head. Unlike the antennae, the cirral appendages contain cell bodies (electronic supplementary material, figure S3). Activity was recorded only in cells located at their base, amounting to a maximum of two pairs.
The lateral regions (figure 2e; electronic supplementary material, figure S2E) are well delimited, located between the palpal and cirral cell masses, close to the ciliary band called the akrotroch. Between one and three active cell bodies were observed in these regions, with no apparent neurite connection to the central neuropil. The MB regions consist of probably 15–20 cells each at this stage, organized around two neurite bundles called peduncles: a dorsal one, immediately lateral to the antennal nerve (figure 2f; electronic supplementary material, figure S2F), and a ventral one, ventral to the antennal nerve and dorsal to the palpal cell mass (figure 2g; electronic supplementary material, figure S2G). At 6 dpf, the strong condensation of MB cell nuclei characteristic of adult nereidid brains [53] is not yet apparent; hence, precise delimitation of MB cells is not possible. Nevertheless, active cells were observed in immediate proximity to the peduncles, and a coactivation with the peduncle was visible in ventral MB regions (inset in figure 2g1).
Single active cells were observed in the area of the apical organ (figure 2h; electronic supplementary material, figure S2H), an unpaired sensory organ present in annelid and other marine larvae and thought to be involved in their settlement, a crucial life cycle transition [54–56]. These two cells are close to the dorsal head surface, directly anterior to the akrotroch, in proximity to the neurosecretory neuropil known to be associated with the apical organ [57]. Their flask shape is typical for apical organ cells described in Platynereis at 2 dpf [58]. It should be noted that the cilia present dorsomedially at the head surface at 6 dpf do not belong to the apical organ but to another organ of unknown function, the dorsal ciliated pit (see electronic supplementary material, figure S2H1). A second pair of active cells was observed in a position immediately anterior to the eyes and therefore named eyefront cells (figure 2i; electronic supplementary material, figure S2I). Their activity, though observed only in a minority of animals, was prominent. A third pair, called fronto-dorsal cells (figure 2j; electronic supplementary material, figure S2J), was observed in a position slightly more dorsal and medial than the antennal nerves, and anterior to the central neuropil. These cells seem to have an axonal projection into the central neuropil, and their shape suggests an anterior cellular extension. A pair of tubulin-rich cells with a similar shape is present at the same position (figure 2j2), but we could not determine whether they are the same cells.
2.3. Nuchal organs, palps, antennae and tentacular cirri respond differentially to four chemical stimulants
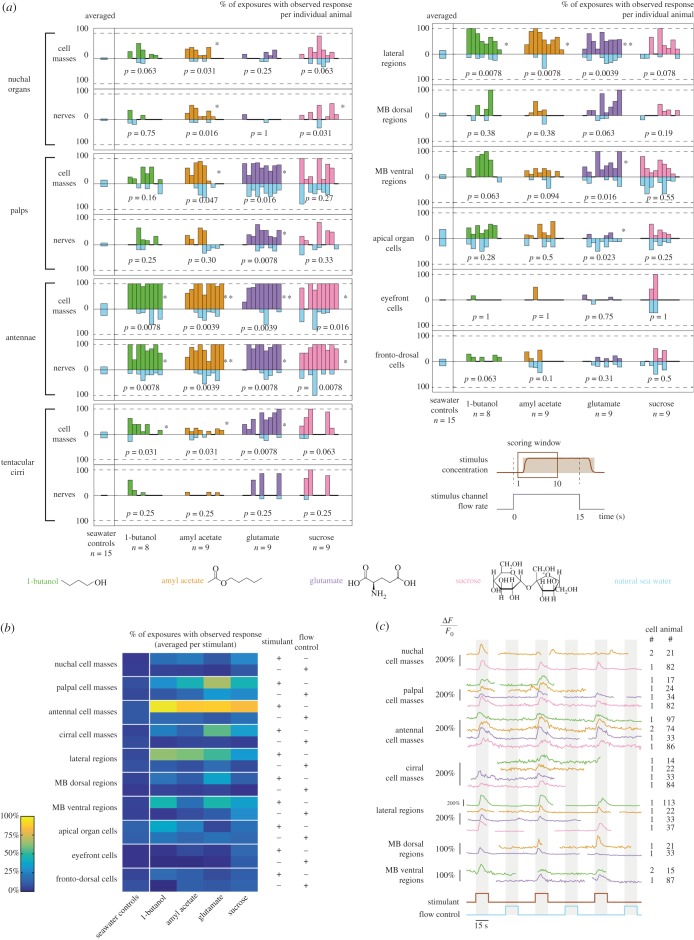
Using four distinct chemical stimulants, we quantified the occurrence of responses for all regions and cells, in each animal, over a long time window following each stimulus onset (figure 3a,b; see Material and methods). The most obvious responses were those of the antennae, which responded systematically to each of the four stimulants. By contrast, the three other organs showed more differential responses. Nuchal organs were sensitive to amyl acetate and sucrose and, to a lesser degree, to 1-butanol, but did not seem to respond at all to glutamate. Palps responded to all compounds, but responses were observed for typically two-thirds of the exposures with glutamate, as opposed to about one-third for the other compounds, indicating that palps are particularly responsive to glutamate. In tentacular cirri, responses were seen frequently with glutamate and sucrose, and seldom with 1-butanol and amyl acetate, suggesting that glutamate and sucrose can elicit stronger responses. We performed an analysis of variance to quantify these differences (electronic supplementary material, table S1). Only cirral responses to glutamate versus amyl acetate differed significantly, but more differences would probably become apparent with an increased sample size (here, nine animals per compound). These results show that chemosensitivity in nuchal organs, palps and tentacular cirri is tuned to different types of stimulants.
Figure 3.
Calcium responses of the active regions to the four stimulants. (a) Fractions of exposures with observed response for individual animals, shown as mirror barplots for chemical stimulants (green, orange, purple, pink) and flow controls (light blue). For each region, an averaged value obtained in control experiments is shown on the left (separate light blue barplots). p-Values and significance levels of a Wilcoxon signed-rank test between responses to chemical stimulants and to flow controls are shown (*p < 0.05, **p < 0.005). (b) Fractions of exposures with observed response, averaged per stimulant. (c) Calcium activity traces of individual cells; calcium snapshots and the corresponding regions of interest are shown in the electronic supplementary material, figure S4. Trace colours correspond to the stimulants used.
Different causes seem to account for the non-systematic observation of responses in nuchal organs, palps and tentacular cirri. In nuchal organs, responses were only observed in about 30% of exposures. The fact that these responses were of high amplitude (typically ΔF/F0 = 100–150%) and occurred in large cells (diameter of 8–12 µm, as opposed to 5–9 µm in the other regions) excludes that they may have been omitted by our imaging, and suggests that they were conditional. In palps, responses were seen in about 60% of exposures on average. Since the responding cells were small (less than 6 µm in diameter) and because abundant muscle fibres and neurites produce calcium signals in this area, we attribute this percentage to technical difficulties in detecting the responses, rather than true biological variability. Responses in the tentacular cirri, whenever observed, occurred in a high fraction of exposures, but were nearly absent in some animals. Since they were overall the weakest responses (typically ΔF/F0 < 50%), we concluded that the cirri did respond to all stimulants, but the low amplitude of calcium signals did not always allow their detection.
In all organs, the responses were more frequent for the stimulants than for the flow controls (see statistical significance in figure 3a), which confirmed that the observed activity was chemically evoked. Control experiments without chemical stimulants showed that all organs had comparable levels of responses to natural seawater stimulations as here (figure 3a; see Material and methods), confirming that responses to flow controls in the present experiments did correspond to a non-chemically evoked activity. An overall increased activity of the palpal cell masses was nevertheless observed in the particular cases of glutamate and sucrose stimulation.
We next investigated chemically evoked responses at the single-cell level rather than for the entire organs. In palps, antennae and tentacular cirri, we found that most cells which responded to a stimulant did so systematically and responded only after its onset. Examples are shown for each stimulant in figure 3c (calcium signal snapshots and regions of interest (ROIs) are shown in electronic supplementary material, figure S4; more examples of activity traces can be found in Chartier [34, ch. IV]). These findings indicate that these organs are indeed able to directly detect the onset of such stimulants. In nuchal organs, sucrose was the only stimulant for which we could observe reproducible responses, though only in a single animal. Two examples of activity traces are shown in figure 3c: the first is representative of responses overall observed in nuchal organs (only some of the calcium transients correlate with a stimulus onset), and the second shows repeatable responses observed in this single animal. Hence, while the nuchal organs are able to directly respond to the onset of at least some stimulants, such responses do not seem to be robust in our assay.
2.4. Chemosensory responses of the mushroom body regions and a newly identified lateral region
Similar to nuchal organs, palps, antennae and tentacular cirri, the lateral and MB regions showed an enhanced activity directly related to the onset of chemical stimuli (figure 3a,b), which identifies these regions of the early differentiated Platynereis brain as part of the chemosensory circuits. Responses were observed in about 65% of exposures for the lateral regions, 40% for the ventral MB regions and 15% for the dorsal MB regions. The lateral regions responded to all compounds, though slightly less to sucrose, indicating a broad chemosensitivity as for the antennae. The MB regions showed more differential responses, with the ventral ones being more responsive to 1-butanol and glutamate than to amyl acetate and sucrose, and the dorsal ones being mostly responsive to glutamate. Flow controls confirmed the chemically evoked nature of responses in the three regions. Only responses of the ventral MB regions upon sucrose stimulation were an exception; in this particular case, another factor than the stimulant may have led to an overall increased activity (three times higher than in control experiments). We found examples of chemically evoked single-cell responses to all stimulants for the lateral regions, to amyl acetate and glutamate for the dorsal MB regions, and to 1-butanol and glutamate but not sucrose for the ventral MB regions (figure 3c; calcium signal snapshots and ROIs are shown in electronic supplementary material, figure S4). This confirmed that the lateral regions respond to all stimulants, and the two MB regions to at least some of them.
By contrast, no evidence for stimulant-specific responses could be found in the apical organ cells, the eyefront cells or the fronto-dorsal cells.
2.5. Responses are observed with a delay in the lateral regions and mushroom body regions compared with the major chemosensory organs
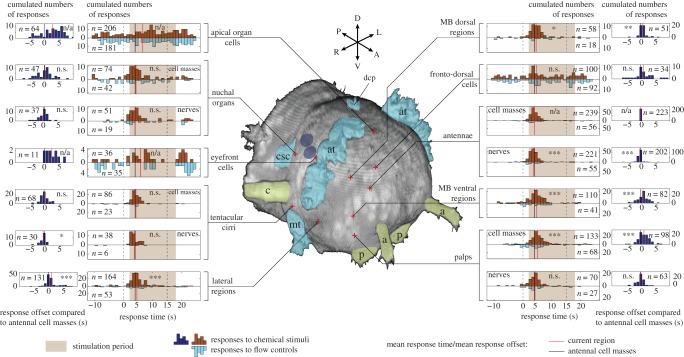
Following the observation that seven regions were activated by stimulants, we set out to determine when exactly their responses occurred with respect to the window of exposure. The cumulated distributions of all response times were calculated for each region, by pooling experiments involving all four stimulants (figure 4, inner graphs). The response times correspond to the beginning of calcium transients (see Material and methods).
Figure 4.
Distribution of response times with respect to the stimulation period. Relative response times, with the pump trigger time of the stimulus taken as t = 0 s, are shown in the inner graphs. The total numbers of available responses are indicated. Student's t-test was used to compare the mean of the distributions with the mean response time of the antennal cell masses. Delays over the antennal cell masses are shown in the outer graphs. The total numbers of responses for which a delay could be calculated (responses which co-occurred with an antennal response) are indicated. Student's t-test was used to compare the mean delay with 0 (n.s. = non-significant, *p < 0.05, **p < 0.005, ***p < 0.0005, n/a, not applied). The three-dimensional head reconstruction is obtained from the same anatomical stainings as shown in figure 2. Eyes (dark blue ovals), ciliated structures (cyan) and head appendages (green) are highlighted: a, antenna; at, akrotroch; c, tentacular cirrus; csc, cilia of the nuchal organ supporting cells; dcp, dorsal ciliated pit; mt, metatroch; p, palp.
For the nuchal organs, palps, antennae and cirri, the vast majority of responses took place within 5 s following the stimulus onset. The observed variability in these response times was at least partly attributable to variable stimulus onsets compared with the pump triggering times (electronic supplementary material, figure S1C). Activity outside of the stimulation period was seen more often in the nuchal organs than in the three other chemosensory organs, in agreement with the more erratic responses observed in single-cell activity traces (figure 3c).
For the lateral regions and MB regions, responses were likewise observed predominantly following stimulus onset, but the mean response times were slightly delayed. Taking as a reference the antennal cell masses' responses—the most abundant and robust ones—we saw indeed a statistically significant delay in the order of 1 s for the mean response time of the lateral regions and of 0.75 s for the two MB regions, but no significant delay or lead for the nuchal organs and cirri (figure 4, inner graphs). The palps did respond as fast as the antennae, because the delay observed for the cell masses was absent for the nerves.
Complementarily, the offsets of response times with respect to the antennal cell masses were assessed in individual animals, by calculating the actual offset for each response of each region (see Material and methods). The cumulated distributions of these offsets confirmed the statistical significance of the observed delays (figure 4, outer graphs). A rather wide distribution of offsets was visible for the palpal cell masses and the ventral MB regions, as opposed to the other regions responsive to stimulants. For the lateral regions, the offset distribution seemed to be bimodal, with a main peak at around +1 s and a minor one at around +5 s (15% of the responses). A small but significant lead was observed for the antennal nerves over the antennal cell masses (in the order of 0.3 s). The observation that the lateral regions, the two MB regions as well as the palpal cell masses responded with some delay with respect to the overall synchronized responses of the chemosensory organs was robust when offset distributions were alternatively calculated against the cirral cell masses, cirral nerves or palpal nerves (data not shown).
Activity in the apical organ cells and eyefront cells was rather uniformly distributed, irrespective of the chemical stimulation period. For the fronto-dorsal cells, the coexistence of a uniform activity and a peak of responses synchronized to the antennal ones suggested that these cells may show both specific and unspecific responses.
2.6. The apical organ cells show a periodic activity, synchronized with the eyefront cells
We further analysed single-cell activity in apical organs cells, eyefront cells and fronto-dorsal cells, to better understand why this activity was prominent in our experiments, though not obviously chemically induced (figure 5). Activity traces reveal that in 13 out of 35 animals, apical organ cells had slow, large-amplitude calcium fluctuations, as shown in figure 5a (for calcium signal snapshots and ROIs, see electronic supplementary material, figure S5). These fluctuations were synchronous between both cells, as well as with the closely located neurosecretory neuropil (figure 5b). We found that the eyefront cells, though rarely active, were always synchronized with the apical organ cells (figure 5a,b), which suggests that these two pairs of cells are interconnected. The calcium fluctuations’ period, typically 35 s (animals #29, 30, 33, 37, 40), matches the periodicity of the alternated stimulations with and without chemical stimulant (see Material and methods). Although the fluctuations had variable phases compared with the stimulations, they could thus have been entrained by the flow stimulations, independently of chemical stimulants; in fact, they can take place in the absence of chemical stimulant (animal #32). Yet, additional experiments, either without flow patterns or with a different periodicity of flow patterns, would be needed to exclude that these cells had an intrinsic periodic rhythm which accidentally matched the flow period used here. Nevertheless, in two animals, a different pattern of activity was observed, with beginnings of calcium transients correlating with the onsets of chemical stimuli and resulting in similar fluctuations with a period of approximately 70 s, i.e. double (animals #86, 97). This suggests that apical organ cells could still be responsive to chemical stimulants, at least to 1-butanol and sucrose. On the whole, the present experiments do not suffice to conclude whether or not chemical stimulants trigger the oscillating calcium activity in the apical organ cells and eyefront cells.
Figure 5.
Calcium activity of the apical organ cells, eyefront cells and fronto-dorsal cells. (a) Individual calcium traces showing synchronization between apical organ cells and the neurosecretory neuropil (i) and between eyefront cells and apical organ cells (ii). The stimulations are colour-coded. (b) Correlation of the calcium signals between cells. (c) Individual calcium traces showing responses of the fronto-dorsal cells in relation with stimulations (colour-coded) and with locomotor activity (kymographs). Calcium snapshots and the corresponding regions of interest for (a) and (c) are shown in the electronic supplementary material, figure S5. Dark parts of the kymographs correspond to movement.
2.7. Activation of the fronto-dorsal cells partially coincides with stimulus onset or termination of locomotor activity
In all experiments, activity was highly synchronous between the two fronto-dorsal cells (figure 5b). A closer look at single-cell activity confirms that these cells do show occasional responses to the onsets of chemical stimuli (figure 5c, animals #21, 22-1, 40; for calcium signal snapshots and ROIs, see electronic supplementary material, figure S5), as previously suggested by the distribution of response times. Responses to the onset of the flow controls were also observed (animal #22-1). Besides, activation of these two cells was seen to follow muscle contraction in the trunk or stomodeum in several animals (animals #21, 22, 40, 72, 100). The existence of overlapping calcium transients when an episode of movement and a stimulus onset rapidly follow each other (animals #21, 22-2, 34) suggests that both types of activity may add up.
3. Discussion
3.1. Platynereis possesses four types of head chemosensory organs
Our calcium imaging experiments reveal chemically evoked responses to four types of chemical compounds in nuchal organs, palps, antennae and tentacular cirri (figure 3a,b). Single-cell responses (figure 3c) immediately follow the onset, not the offset, of the chemical stimuli, and are synchronous for the four organs (figure 4). The lateral regions may constitute a fifth type of primary chemosensory organ, as suggested by their proximity to the surface (figure 2e) and their high activity upon chemical stimulations (figure 3a). However, the absence of externally obvious sensory structures associated with them (figure 2e) as well as their systematic response delay compared to the four other organs (figure 4) would speak against that, hence this question deserves further investigation.
In palps, antennae and tentacular cirri, responsive cell bodies were only seen at the base of the appendage, hence at least part of them must correspond to sensory neurons that extend a long dendritic process through the appendage until the actual site of chemical detection; in fact, such processes were sometimes visible in the calcium signal (e.g. electronic supplementary material, video S1). However, part of the active cells may well have been interneurons. In nuchal organs, only two cell types are present around the sensory cavity: sensory neurons which extend a dendritic process into the cavity and ciliated supporting cells [59,60], while interneurons described in Platynereis at 3 dpf are distant from the cavity [61]. The revolver cells are thus clearly sensory neurons (see morphology in figure 2a), and so are probably the rare other responsive cells observed close to the cavity.
We showed that nuchal organs, palps and tentacular cirri, unlike the antennae, respond differentially to the compounds, which suggests a specialization of these organs in terms of chemosensory repertoire. Our few attempts to test for functional differences between cells belonging to the same organ, using two stimulants per animal, were so far inconclusive (data not shown). Nevertheless, it is likely that at least antennae and palps could be capable of chemosensory discrimination, due to the high number of cells they possess.
Antennae appear to be central in Platynereis' chemosensation and are probably responsible for the general identification of chemical cues. It came as a surprise that their responses were by far more systematic than those of the nuchal organs, because the latter are generally thought to be important for annelid chemosensation, but not the former [24,36]. Palps, which are located close to the mouth and were more strongly activated by the amino acid and the sugar (figure 3a,b), may be specialized in the detection of directly food-related chemical cues, as is hypothesized in spionid annelids [22,38,62]. These highly musculated appendages, which adult Platynereis use for prehension of food items (TF Chartier 2015, personal observations), could also serve in the contact chemoreception of hydrophobic compounds, whose importance is often underestimated in marine animals [63,64]. The tentacular cirri sometimes showed separate response times between the left and right side (data not shown). It is likely that these long organs, which extend in different directions in adults, can provide relevant spatial information about the localization of chemical cues. These tactile organs are also photosensitive and involved in the shadow reflex in Platynereis [65]. Hence, their role is probably to collect general multisensory information about objects approaching the head, in order to produce immediate, coarse responses. Finally, there is little doubt that the highly conserved nuchal organs play an important sensory role in annelids; hence, it is likely that we did not test the most relevant cues for them.
Platynereis detects 1-butanol and glutamate, which corroborates behavioural observations made long ago in Nereis [44]. Amino acids, such as glutamate, are general chemical cues in aquatic environments, as is known in fish and crustaceans [66,67]; other known cues such as nucleotides, steroids and bile acids [68] should be tested in future experiments. The stimulants were presented here at 10 µM, but the presence notably in antennae of responses that slightly precede the earliest possible onset of this concentration (figure 4; electronic supplementary material, figure S1C) suggests that lower concentrations can be detected. The fact that 1-butanol and amyl acetate, which are odorant molecules for humans, act as distance cues in the water for Platynereis provides support to the view of Mollo et al. [1, section 8] that the traditional categories of ‘olfactory molecules’ and ‘taste molecules’ should be abandoned.
Our results in Platynereis suggest that marine annelids possess head chemosensory organs with distinct roles, adapted to sets of chemical cues relevant in different situations (feeding, escaping, reproduction, etc.), similar to what is known from crustacean chemoreception [69]. Antennae, which seem to be the main chemosensory organs in Platynereis, are present in a vast majority of annelid taxa [70] and may thus be of general importance in annelid chemosensation. Though we did not test it here, a distributed chemosensitivity of the body surface is likely for annelids, as suggested by previous behavioural and anatomical studies [19,26,71,72].
3.2. Annelid mushroom bodies as possible chemosensory integration centres
Mushroom bodies (MBs), which have long been described in annelid brains [25,27], have a high anatomical similarity with their homonyms in insects [73]. In fact, similar structures are found in several protostome phyla including flatworms, nemerteans and onychophorans [74], which suggest that they may have been inherited from the last common protostome ancestor's brain. In insects, MBs are the place where associative memories are formed, notably with odour stimuli [75–77]. In mammals, this role is endorsed by the pallium [78–80], which includes cortex and hippocampus, and develops from neuroectodermal brain regions expressing similar combinations of transcription factors as in Platynereis [81]. We observed cells located in the dorsal and ventral MB regions responding specifically to several chemical stimulants (figure 3a,c). In particular for the ventral regions, coactivation with the MB peduncles proved that these responsive cells indeed belonged to the MBs (figure 2g1, inset). Our observations represent the first physiological data available for annelid MBs and firmly establish them as part of the chemosensory system. MB cellular responses were delayed compared to the four major organs (figure 4) and may have stemmed from sensory interneurons. This would be in line with a presumed role of MBs in the representation and integration of chemical cues also in the annelid brain.
3.3. Apical organ cells, eyefront cells and fronto-dorsal cells may be part of the chemosensory circuits
We have identified three distinct pairs of cells in the head whose activity may be partially linked to chemical stimulations. Activity in the apical organ cells did correlate with the onset of chemical stimulations in at least two animals (figure 5a). This preliminary evidence for chemosensitivity calls for further exploration, as apical organs are likely important in the settlement and metamorphosis of marine larvae in general, which they are thought to trigger via the detection of environmental chemical cues [54–56]. The long duration of calcium transients in these cells, as well as their proximity to and coactivation with the neurosecretory neuropil (figure 2h, figure 5a,b), suggest a neurosecretory nature. One can hypothesize that upon detection of appropriate settlement cues, these two cells would adopt periodic patterns of neurosecretory activity as observed here, signalling to the animal that settlement can start. Alternatively, calcium fluctuations in these cells may have been merely entrained by the changing flow patterns, as would also be consistent with our data. However, we do not favour this latter hypothesis, because their prominent activity should then have been observed in most animals, not in one-third of them. Whenever the eyefront cells were visible, their activity was tightly synchronized to that of the apical organ cells; hence, both pairs of cells may belong to a common circuit involved in neurosecretion and/or larval settlement. The pair of fronto-dorsal cells, which are probably neurons due to their morphology and activity patterns (figure 2f; electronic supplementary material, figure S5), were seen to respond after at least three types of events: chemical stimulations only, chemical stimulations and flow stimulations, locomotor episodes (figure 5c). Hence, their responses to chemical stimulants were not primary sensory responses. These neurons may have an inhibitory effect, their role being either to prevent a locomotor reaction to external stimuli such as chemical cues, or to stop an ongoing locomotor episode. We hypothesize that they form part of a general circuit for locomotor inhibition receiving inputs from different sensory modalities. As such, they may represent a non-specific part of the chemosensory circuits.
3.4. Variability of responses: biological and technical factors
While antennal responses were strong and systematic for all animals and all compounds, responses in palps and tentacular cirri could not be observed in all animals or for all exposures (figure 3a). Since single-cell responses, though of lower amplitude than in antennae, were always robust (figure 3c), we interpret this fact as a consequence of their response amplitude sometimes falling below our detection threshold. We conclude that these two organs do detect these stimulants, which a more targeted imaging would allow to verify. By contrast, single-cell calcium responses to stimulants in the nuchal organs were hardly repeatable (figure 3c). The typically high amplitude of these responses, whenever observed, rules out an issue of detection threshold. While we cannot exclude that other compounds may elicit systematic responses, it seems that nuchal organs respond to chemical stimulants in a more conditional manner, though we were so far unable to tell what influences their responsiveness. For the four chemosensory organs, the nerve was seen to respond with a slight lead over the cell mass (figure 4), even though the former is located anatomically downstream of the latter. We interpret this as an effect of geometry, with local calcium concentrations increasing faster in the axons than in the somata. Finally, in most active regions, a majority of responses were visible only on one side of the head, for example, in figure 2d1 or g1 (see quantification in electronic supplementary material, figure S6). Because the imaging planes can be slightly tilted, we regard this as an artefact of imaging, occurring when a weakly responsive nerve or cell body on one side is located between two consecutive optical planes and fails to be detected. However, we are confident that all responses are indeed bilateral, notably because no anatomical asymmetry is known for the head and because the regions where most non-bilateral responses are seen are the most difficult to image and show the weakest responses (palpal and cirral nerves, MB dorsal and ventral regions; see electronic supplementary material, figure S6). Besides, an activity can appear non-bilateral for the cell masses, while it is clearly bilateral in the corresponding nerves, as is obvious for the nuchal organs and antennae (electronic supplementary material, figure S6).
3.5. Sensory cell anatomy and physiology is different between nuchal organs and head appendages
Based on our functional imaging data, nuchal organs seem to possess a different physiology than the three other chemosensory organs, and incidentally, they are known from electron microscopy studies to possess different types of sensory cells than palps, antennae and tentacular cirri. While cells of the three appendages have sensory cilia that traverse the cuticle and come directly in contact with the environment [23,26,31,39,82–84], sensory cilia of nuchal organ cells sit in a fluid-filled sensory cavity shielded from the environment by specialized cuticular or microvillar layers [59,60,85,86]. If these layers affect the diffusion of molecules, changes of chemical composition in the fluid environment may be effective with some delay inside the cavity compared with the surface of appendages. The nuchal organs' shielded anatomy suggests that they may detect the global presence and concentration of ambient chemical cues, but not capture their rapid concentration dynamics, as the three appendages probably do. A role of these organs in inter-individual communication and the detection of pheromones constitute a good working hypothesis.
3.6. A microfluidics set-up for immobilization and targeted stimulus delivery
The use of microfluidics for in vivo experiments offers several advantages over the immobilization methods used so far with Platynereis juveniles and larvae: gluing, MgCl2 or mecamylamine paralyzing, low-melting agarose embedding or slide-coverslip mounting. The present device allows both a reliable animal immobilization without any chemical agent potentially interfering with the animal's physiology and an ecologically relevant exposure to chemical stimulants. Moreover, stimulant exposures are precise and repeatable, which is key for probing single-cell activity.
Further experiments can be performed with the same set-up, notably testing discriminatory abilities of the chemosensory organs between either two concentrations or two stimulants. Any other waterborne stimulus such as pH, salinity, O2 or CO2 levels, or even small solid particles, can be used and possibly combined with a precise pharmacological treatment. Adding a dye to the stimulus solution would allow a more precise monitoring of stimulus timing, though all dyes we have tested were improper in that they triggered antennal chemosensory responses. Whole-head recordings at higher spatial and temporal resolutions could be obtained using a light-sheet microscope (as in [87]). The microfluidic device is suited for experiments on 4–7 dpf animals and can easily be adapted for younger stages. Beyond 7 dpf, muscles, hence movement artefacts, are too strong, and either a more elaborate trap or a drug treatment would be needed to achieve immobilization. The trap design could also be adapted for other marine larvae or small organisms, some of which are suited for microfluidic experiments (TF Chartier 2015, personal observations). Though our conclusions would not have been altered, absorption issues documented for PDMS devices may have reduced effective stimulant concentrations [88]; hence, alternative materials such as COC [89] or sTPE [90] may be preferred to PDMS for future experiments. Microfluidic set-ups have been successfully used to explore neuronal and motor activity in nematodes and fish larvae [4,91], and our study shows that similar experiments are possible with Platynereis juveniles.
3.7. Early Platynereis juveniles as a model for the study of annelid chemosensory systems
Platynereis sensory organs and nervous systems are representative for annelids, and early juveniles already possess all types of adult chemosensory organs. Our results show that whole-head activity upon precisely controlled chemical stimulations can be imaged in early juveniles. Hence, these juveniles can be used to test the general physiology of annelid chemosensory organs, as monitored by any fluorescent reporter. The availability of a cellular resolution expression atlas [41] in combination with single-cell sequencing and mapping onto the atlas [92,93] will enable efficient identification of candidate chemoreceptor genes. Candidates would then be validated by combining knockouts [94] of receptor proteins and functional imaging, and possible in vitro deorphanization of receptors [95]. We have demonstrated that single cells, such as the eyefront cells, fronto-dorsal cells or apical organ cells, can be identified based on their calcium activity patterns (figures 2 and 3). Mapping functional imaging data as acquired here onto the gene expression atlas will allow thorough characterization of such cells. Furthermore, the feasibility of connectomics in Platynereis has been proved at the 3 dpf stage [43,44,57,61], and a connectomic effort at 6 dpf is ongoing, which could allow to add circuit information to the molecular and functional data presented here. It will be interesting in the future to investigate Platynereis' abilities for sensory integration and associative learning, whose neuronal correlates could be directly studied with the present set-up. Finding an involvement of annelid MBs in associative learning would be of particular interest for the comparative neurobiology of learning.
4. Conclusion
We have established that nuchal organs, palps, antennae and tentacular cirri are chemosensory organs in Platynereis, responding to an alcohol, an ester, an amino acid and a sugar. This conclusion is likely to extend to annelids, for which similar sensory cells have been detected in electron microscopy. Our results show a capability to differentially respond to multiple chemosensory cues, which opens the possibility of complex chemosensory integration. With our findings, we establish 6-day-old Platynereis juveniles as an experimental system for the chemosensory physiology of marine annelids.
5. Material and methods
5.1. Device fabrication
Standard soft lithography was used to fabricate the mould [96]. The photomask was designed with AutoCAD (2014 free student version, Autodesk, Inc.) and printed at a resolution of 25 400 dpi by an external company (Selba S.A., Versoix, Switzerland); the source file is available as .dwg file in the electronic supplementary material. The trapping channel's width linearly decreases from 150 to 75 µm at its end, which constitutes the trap. The mould with a uniform height of 60 µm was obtained by spin-coating a silicon wafer (4 inches; Siltronix, France) with a negative photoresist (SU-8 2050, MicroChem Corp., Newton, MA, USA). Devices were produced by pouring onto a mould and curing at 65°C for a minimum of 4 h a prepolymer mixture of polydimethylsiloxane (PDMS, Sylgard 184 silicone elastomer kit, Dow Corning Corp.) with a 1 : 9 ratio of curing agent. PDMS blocks were then irreversibly bound to a 0.17 mm glass coverslip (#1871, 24 × 50 mm, Carl Roth GmBH, Germany) by a 1 min treatment in a plasma oven (Femto, Diener electronic GmbH & Co. KG, Germany). For a detailed fabrication protocol, see chapter IV and appendix E in Chartier [34].
5.2. Experimental set-up and procedure
Filtered natural seawater obtained with 0.22 µm sterile filters (Millipore), plastic syringes (Luer Plastipak, BD, USA), metallic needles (Microlance #20, 302200, BD, USA) and polytetrafluoroethylene tubing (PTFE, TW24, inner diameter 0.59 mm, Adtech Polymer Engineering Ltd, UK) were used in all experiments. A 1 mM stock solution was prepared weekly for each chemical stimulant. Working solutions at 10 µM were prepared on the day of the experiment, and syringes loaded with these solutions were placed in the experimental room 1 h before starting. All solutions were kept at 18°C and handled in glassware, because preliminary experiments had revealed that the animals may detect dissolved substances from plastic containers such as Falcon tubes. Water streams were generated in a laminar flow regime (7 mm s−1, Reynolds number ≈0.5). Flow rates of 8.33, 33.33 and 41.66 µl min−1 were used in the channels (figure 1d), with the total flow rate kept constant (50 µl min−1) to minimize pressure changes experienced by the animal. The device was operated by push-pull pumps (AL4000-220Z, WPI Germany GmbH), which were computer-automated via Micro Manager (v. 1.4.21, [97]). Image acquisition and stimulus delivery were synchronized with Auto Mouse Click (MurGee.com), a software for automated mouse actions. A customized metallic chip holder was built to hold the fragile device and facilitate its observation under an upright microscope. For details on the pump automation and the pumping programmes, see chapter IV and appendix D in Chartier [34].
5.3. Imaging
GCaMP6s fluorescence excited at 488 nm was detected by a hybrid detector (HyD) set to photon-counting mode in a Leica TCS-SP8 confocal microscope, equipped with a 40× (NA 1.1) water-immersion objective (water was preferred to oil for experimental convenience). Transmitted light images were recorded with a classical PMT detector, from the same excitation light as GCaMP. The head region was imaged in 12 horizontal optical sections (pinhole opened at 6.4 Airy units) sampling the whole volume at 5 µm intervals. To balance potential biases due to increased signal loss with tissue depth, approximately half of the animals were imaged from the dorsal side and the other half from the ventral side, thanks to adequate trapping. Images were acquired by confocal scanning at 8 kHz (resonant mode, phase X correction 1.32, laser powers 6–28 µW, pixel dwelling time 50 ns).
5.4. Calibration experiments
A green dye (tartrazine, E102) was dissolved in the two side streams to visualize their moving boundaries. Transmitted light intensity from a 633 nm laser illumination was measured at 10 Hz resolution in a square region of interest, constant in size and position, located just upstream of the animal's head (brown rectangle in figure 1g). Minimal and maximal intensities, which according to the Beer–Lambert law corresponded, respectively, to the absence of stimulant and maximum stimulant concentration, were normalized between 0 and 1. Edge detection allowed to quantify the beginning and ending of stimulation onsets and offsets (electronic supplementary material, figure S1C). Measurements were made successively with eight trapped animals (four experiments per animal).
5.5. Animal preparation and handling
Platynereis juveniles were obtained from a permanent culture following Hauenschild & Fischer's breeding protocol [98] and kept at 18°C with 16 L : 8 D cycles. Calcium imaging experiments were conducted at 18–20°C, between 142 and 177 h post-fertilisation (hpf), at various times of the day and night. For each chemical stimulant, animals coming from at least two distinct batches were imaged. The calcium reporter GCaMP6s [99] was transiently and ubiquitously expressed by microinjecting Platynereis eggs with mRNA (1.000 ng µl−1) between 1 hpf and the first cleavage. Capped and polyA-tailed mRNAs were synthesized with the mMESSAGE mMACHINE T7 Ultra Kit (Life Technologies) from a vector obtained from the Jékely lab (pUC57-T7-RPP2-GCaMP6 described in [43]). After micro-injection, eggs were kept in filtered natural seawater and culture conditions remained unchanged. The device was washed with filtered natural seawater for 4 min before every new animal was manually introduced with a syringe. Each animal was allowed to rest for 5 min before the experiments, and successive experiments on one animal were performed at 5–10 min intervals. After the experiments, the animal could be recovered without damage for potential further observations, by gently flushing it out of the device.
5.6. Response assessment
Cellular or nerve responses were assessed with the human eye from raw recordings. In the absence of specific genetic markers, the attribution of a responsive cell to a region relied on its position, guided by anatomical landmark recognition based on precise reference immunostainings (figure 2; electronic supplementary material, figure S2). A given region was considered to respond whenever at least one cell was seen to respond in at least one of the region's two bilaterally symmetric parts—for a given nerve, whenever at least one of the two bilaterally symmetric nerves was seen to respond. The bilaterality of observed responses is quantified in the electronic supplementary material, figure S6, where every response has been classified as ‘bilateral’, ‘left only’ or ‘right only’, and the relative fractions of such responses were plotted as barplots for each region. The time of occurrence of a response was defined as the beginning of the corresponding calcium transient, and the threshold of visual detection of such an event corresponded to a signal-to-noise ratio of approximately 3 : 1. To be noted: a permanent activity of the eye photoreceptor cells was induced by the 488 nm laser illumination. All response scorings were performed twice at a 6-month interval by the same person; the resulting data are available as .xlsx file in electronic supplementary material, table S2.
5.7. Quantification of activity
5.7.1. Occurrence of responses (figure 3)
A scoring window was defined, starting with the earliest possible onset of the stimulant (1 s after the pump trigger, see electronic supplementary material, figure S1C) and lasting 9 s. Responses of the regions were scored for each stimulus exposure, with a 1 counted if at least one response was seen during the scoring window and a 0 otherwise. The fractions of exposures with an observed response were calculated for each animal and each region, separately for exposures to stimulants and flow controls (figure 3a). The averages of these fractions for each stimulant are shown as a heatmap (figure 3b). For each set of animals assayed with a given stimulus, e.g. for the nine animals tested with glutamate, a Wilcoxon signed-rank test (significance level α = 0.05) was performed for each region to determine whether the fraction of responses over exposures statistically differed between the chemical stimulant and its flow controls (figure 3a). To quantify response occurrences in the absence of chemical stimulation, control experiments were run: 15 of the animals assayed with chemical stimulants had been beforehand imaged in one experiment with both side channels containing filtered natural seawater only, prior to any introduction of chemical stimulant in the device. The response occurrences were quantified as described above, and a global fraction of observed responses over the number of exposures was calculated for each region by pooling responses of the 15 animals (figure 3a,b).
5.7.2. Distributions of relative response times and response offsets (figure 4)
All responses of all 35 animals were pooled for each region, and the cumulated distributions of their times of occurrence with respect to the stimulation period were calculated. Student's t-test (α = 0.05) was performed to determine whether the mean of each distribution differed from the mean response time of the antennal cell masses (all means were calculated over the 9 s scoring window defined for figure 3). Additionally, for each individual response that co-occurred with a response of the antennal cell masses within this window, the time offset between the two was calculated. The cumulated distributions of these offsets for each region are shown as barplots in figure 4 (outer graphs) for responses to the chemical stimuli only, not to the flow controls. Student's t-test (α = 0.05) was performed to determine whether the means of these offsets differed from 0, i.e. whether these regions responded at a different time than the antennal cell masses.
5.7.3. Single-cell activity traces and kymographs (figure 3c, figure 5a,c)
Movement artefacts on the raw calcium recordings were first corrected in ImageJ (v. 1.50a) using the plugin StackReg [100] with rigid body transformations. Whenever needed, several parts of the recordings were registered individually and the traces obtained from each of them subsequently reassembled. Mean fluorescence intensity was then calculated from regions of interest (ROI) drawn manually in ImageJ. Further data analysis was done in MATLAB (2014a student version, The MathWorks, Inc.). Traces were plotted as ΔF/F0, with F0 calculated as the mean fluorescence value over a 10 s time window during a resting state, i.e. outside of stimulation periods. Muscle contractions (figure 5c), which are likewise reflected by calcium activity, were quantified in the same way from ROIs drawn manually on part of the trunk or stomodeal muscles, and plotted as kymographs instead of curves, with darker parts corresponding to higher fluorescence levels.
5.8. Immunohistochemistry
Animals collected at the 6-day stage (precisely 144 hpf) were fixed in 4% PFA and 0.1% Triton X-100. Tubulin structures were marked with a monoclonal mouse antibody against alpha-acetylated tubulin (Cat# T6793, Sigma-Aldrich GmbH, Germany, 1 : 250 dilution) and an Alexa 488 secondary antibody (Jackson Laboratories, USA, 1 : 500 dilution). Nuclear DNA was stained with DAPI (1 : 1000 dilution) and membranes with mCLING–ATTO 647N (Synaptic Systems GmbH, Germany, 1 : 50 dilution). DAPI, tubulin and mCling fluorescence was excited at 405, 488 and 633 nm, respectively, and recorded with a Leica TCS-SP8 confocal microscope equipped with a 63× glycerol-immersion objective. For details, see chapter III in Chartier [34].
Abbreviations list
ac: anal cirrus; adc: anterior dorsal tentacular cirrus; an: antennal nerve; ant, a: antenna; at: akrotroch; avc: anterior ventral tentacular cirrus; bv: blood vessel; cc: circum-oesophageal connectives; ch: chaetae; cn: cirral nerve; cp: ciliary photoreceptor; csc: cilia of the nuchal organ supporting cells; dcp: dorsal ciliated pit; dp: dorsal peduncle of the mushroom bodies; drcc: dorsal root of the circum-oesophageal connectives; ec: pigmented eye cup; g: gut; gc: glandular cell; j: jaw; ld: lipid droplet; mo: mechanosensory organ; mt: metatroch; nc: nuchal cavity; nco: nuchal commissure; nn: nuchal nerve; np: main neuropil; nsn: neurosecretory neuropil; pa, p: palp; pcc: palpal coelomic cavity; pdc: posterior dorsal tentacular cirrus; pm: palpal muscle; pp: parapodia; prc: photoreceptor cells; pvc: posterior ventral tentacular cirrus; rpn: roots of the palpal nerve; sog: sub-oesophageal ganglion; st: stomodeum; stn: stomodeal nerve; tc, c: tentacular cirrus; vp: ventral peduncle of the mushroom bodies; vrcc: ventral root of the circum-oesophageal connectives.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Maria Tosches, Kaia Achim, Paola Bertucci (Arendt Lab), Csaba Verasztó, Luis Alberto Bezares-Calderón and Cristina Piñeiro-Lopez (Jékely Lab) for technical help on molecular biology work, microinjections and calcium imaging. We thank Csaba Verasztó and Robert Prevedel for helpful comments on the manuscript. We further thank Nirupama Ramanathan and the Merten Lab (EMBL) for initial training on microfluidic fabrication, Christian Liebig from the MPI Tübingen Light Microscopy facility and all staff from the EMBL Advanced Light Microscopy Facility for continuous microscopy support.
Data accessibility
The datasets supporting the conclusions of this article, as well as videos showing examples of calcium activity, have been provided as the electronic supplementary material. Raw calcium recordings are available on demand.
Authors' contributions
T.F.C. and G.J. designed the experiments. T.F.C. and J.D. built the set-up. T.F.C. performed the experiments and analysed the data. W.D. performed the immunostainings. T.F.C. and D.A. interpreted the results and wrote the manuscript. All authors commented and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Funding
The work was supported by the FP7 Marie Curie Initial Training Network ‘NEPTUNE’—grant no. 317172 (T.F.C., G.J. and D.A.), by the European Molecular Biology Laboratory (T.F.C., J.D., W.D. and D.A.) and by the Deutsche Forschungsgemeinschaft—DFG JE 777/3-1 (G.J.).
References
- 1.Mollo E, Garson MJ, Polese G, Amodeo P, Ghiselin MT. 2017. Taste and smell in aquatic and terrestrial environments. Nat. Prod. Rep. 34, 496–513. ( 10.1039/c7np00008a) [DOI] [PubMed] [Google Scholar]
- 2.Hansson BS, Stensmyr MC. 2011. Evolution of insect olfaction. Neuron 72, 698–711. ( 10.1016/j.neuron.2011.11.003) [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Liberles SD. 2015. Aversion and attraction through olfaction. Curr. Biol. 25, R120–R129. ( 10.1016/j.cub.2014.11.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, Bargmann CI. 2007. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450, 63–70. ( 10.1038/nature06292) [DOI] [PubMed] [Google Scholar]
- 5.Hildebrand JG, Shepherd GM. 1997. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu. Rev. Neurosci. 20, 595–631. ( 10.1146/annurev.neuro.20.1.595) [DOI] [PubMed] [Google Scholar]
- 6.Eishten HL. 2002. Why are olfactory systems of different animals so similar? Brain Behav. Evol. 59, 273–293. ( 10.1159/000063564) [DOI] [PubMed] [Google Scholar]
- 7.Derby CD, Kozma MT, Senatore A, Schmidt M. 2016. Molecular mechanisms of reception and perireception in crustacean chemoreception: a comparative review. Chem. Senses 41, 381–398. ( 10.1093/chemse/bjw057) [DOI] [PubMed] [Google Scholar]
- 8.Silbering AF, Benton R. 2010. Ionotropic and metabotropic mechanisms in chemoreception: ‘chance or design’? EMBO Rep. 11, 173–179. ( 10.1038/embor.2010.8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benton R. 2015. Multigene family evolution: perspectives from insect chemoreceptors. Trends Ecol. Evol. 30, 590–600. ( 10.1016/j.tree.2015.07.009) [DOI] [PubMed] [Google Scholar]
- 10.Derby CD. 2000. Learning from spiny lobsters about chemosensory coding of mixtures. Physiol. Behav. 69, 203–209. ( 10.1016/S0031-9384(00)00202-X) [DOI] [PubMed] [Google Scholar]
- 11.Derby CD, Sorensen PW. 2008. Neural processing, perception, and behavioral responses to natural chemical stimuli by fish and crustaceans. J. Chem. Ecol. 34, 898–914. ( 10.1007/s10886-008-9489-0) [DOI] [PubMed] [Google Scholar]
- 12.Wertz A, Rössler W, Obermayer M, Bickmeyer U. 2006. Functional neuroanatomy of the rhinophore of Aplysia punctata. Front. Zool. 3, 6 ( 10.1186/1742-9994-3-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummins SF, Erpenbeck D, Zou Z, Claudianos C, Moroz LL, Nagle GT, Degnan BM. 2009. Candidate chemoreceptor subfamilies differentially expressed in the chemosensory organs of the mollusc Aplysia. BMC Biol. 7, 28 ( 10.1186/1741-7007-7-28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsay SM. 2009. Ecology and biology of chemoreception in polychaetes. Zoosymposia 2, 339–367. (doi:10.11646/zoosymposia.2.1.24) [Google Scholar]
- 15.Horridge GA. 1963. Proprioceptors, bristle receptors, efferent sensory impulses, neurofibrils and number of axons in the parapodial nerve of the polychaete Harmothoe. Proc. R. Lond. Soc. B 157, 199–222. ( 10.1098/rspb.1963.0005) [DOI] [Google Scholar]
- 16.Dorsett DA. 1964. The sensory and motor innervation of Nereis. Proc. R. Soc. Lond. B 159, 652–667. ( 10.1098/rspb.1964.0024) [DOI] [Google Scholar]
- 17.Boilly-Marer Y, Lassalle B. 1980. Electrophysiological responses of the central nervous system in the presence of homospecific and heterospecific sex pheromones in nereids (Annelida polychaeta). J. Exp. Zool. 213, 33–39. ( 10.1002/jez.1402130106) [DOI] [Google Scholar]
- 18.Ram JL, Müller CT, Beckmann M, Hardege JD. 1999. The spawning pheromone cysteine-glutathione disulfide (nereithione) arouses a multicomponent nuptialbehavior and electrophysiological activity in Nereis succinea males. FASEB J. 13, 945–952. ( 10.1096/fasebj.13.8.945) [DOI] [PubMed] [Google Scholar]
- 19.Gross AO. 1921. The feeding habits and chemical sense of Nereis virens, sars. J. Exp. Zool. 32, 427–442. ( 10.1002/jez.1400320304) [DOI] [Google Scholar]
- 20.Clark RB, Evans SM. 1961. The effect of delayed brain extirpation and replacement on caudal regeneration in Nereis diversicolor. Development 9, 97–105. [PubMed] [Google Scholar]
- 21.Evans SM. 1969. Habituation of the withdrawal response in nereid polychaetes: 2. Rates of habituation in intact and decerebrate worms. Biol. Bull. 137, 105–117. ( 10.2307/1539934) [DOI] [Google Scholar]
- 22.Riordan J, Lindsay SM. 2002. Feeding responses to particle-bound cues by a deposit-feeding spionidpolychaete, Dipolydora quadrilobata (Jacobi 1883). J. Exp. Mar. Biol. Ecol. 277, 79–95. ( 10.1016/S0022-0981(02)00292-7) [DOI] [Google Scholar]
- 23.Retzius G. 1892. Das sensible Nervensystem der Polychäten. Biol. Untersuchungen Neue Folge IV.
- 24.Racovitza EG. 1896. Le lobe céphalique et l'encéphale des annélides polychètes. (Anatomie, morphologie, histologie.). Arch. de Zool. Expérimentale, Série S, Tome 4, No. 1,2., 133, 143.
- 25.Hamaker JI. 1898. The nervous system of Nereis virens Sars. A study of comparative neurology. Bull. Mus. Comp. Zool. Harv. 32, 89–124. [Google Scholar]
- 26.Langdon F. 1900. The sense-organs of Nereis virens, sars. J. Comp. Neurol. 10, 1–77. ( 10.1002/cne.910100102) [DOI] [Google Scholar]
- 27.Holmgren N. 1916. Zur vergleichenden Anatomie des Gehirns Polychaeten, Onychophoren, Xiphosuren, Arachniden, Crustaceen, Myriapoden, und Insekten. K. Sven. Vetenskapsakademiens Handl. 56, 1–103. [Google Scholar]
- 28.Smith JE. 1957. The nervous anatomy of the body segments of nereid polychaetes. Phil. Ttrans. R. Lond. Soc. B 240, 135–196. ( 10.1098/rstb.1957.0001) [DOI] [Google Scholar]
- 29.Gilpin-Brown JB. 1958. The development and structure of the cephalic nerve in Nereis. J. Comp. Neurol. 109, 317–348. ( 10.1002/cne.901090302) [DOI] [PubMed] [Google Scholar]
- 30.Boilly-Marer Y. 1966. Contribution à l’étude des cirres parapodiaux dorsaux des formes épitoques chez Nereis pelagica L. (Annélide Polychète). Comptes rendus Hebd. des séances de l'Académie des Sci. Série Sci. Nat. 262, 2052–2054. [Google Scholar]
- 31.Dorsett DA, Hyde R. 1969. The fine structure of the compound sense organs on the cirri of Nereis diversicolor. Z. für Zellforsch. und Mikrosk. Anat. 97, 512–527. ( 10.1007/BF00332800) [DOI] [PubMed] [Google Scholar]
- 32.Rytz R, Croset V, Benton R. 2013. Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem. Mol. Biol. 43, 888–897. ( 10.1016/j.ibmb.2013.02.007) [DOI] [PubMed] [Google Scholar]
- 33.Saina M, Busengdal H, Sinigaglia C, Petrone L, Oliveri P, Rentzsch F, Benton R. 2015. A cnidarian homologue of an insect gustatory receptor functions in developmental body patterning. Nat. Commun. 6, 6243 ( 10.1038/ncomms7243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chartier TF. 2018. Chemosensation in the marine annelid Platynereis dumerilii: anatomy, physiology, behaviour. PhD thesis, University of Heidelberg, Germany. [Google Scholar]
- 35.Orrhage L, Müller M. 2005. Morphology of the nervous system of Polychaeta (Annelida). Hydrobiologia 535/536, 79–111. ( 10.1007/s10750-004-4375-4) [DOI] [Google Scholar]
- 36.Purschke G. 2005. Sense organs in polychaetes (Annelida). Hydrobiologia 535/536, 53–78. ( 10.1007/1-4020-3240-4_5) [DOI] [Google Scholar]
- 37.Dauer DM. 1991. Functional morphology and feeding behavior of Polydora commensalis (Polychaeta: Spionidae). Ophelia 5, 607–614. [Google Scholar]
- 38.Lindsay SM, Riordan TJ, Forest D. 2004. Identification and activity-dependent labeling of peripheral sensory structures on a spionid polychaete. Biol. Bull. 206, 65–77. ( 10.2307/1543537) [DOI] [PubMed] [Google Scholar]
- 39.Schulte E, Riehl R. 1976. Elektronenmikroskopische Untersuchungen an den Tentakeln von Lanice conchilega (Polychaeta, Sedentaria). Helgoländer Wiss. Meeresunters. 28, 191–205. ( 10.1007/BF01610353) [DOI] [Google Scholar]
- 40.Bullock TH, Horridge GA. 1965. Structure and function of the nervous system in invertebrates. San Francisco: Freeman. [Google Scholar]
- 41.Vergara HM, Bertucci PY, Hantz P, Tosches MA, Achim K, Vopalensky P, Arendt D. 2017. Whole-organism cellular gene-expression atlas reveals conserved cell types in the ventral nerve cord of Platynereis dumerilii. Proc. Natl Acad. Sci. USA 114, 5878–5885. ( 10.1073/pnas.1610602114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jékely G, Colombelli J, Hausen H, Guy K, Stelzer E, Nédélec F, Arendt D. 2008. Mechanism of phototaxis in marine zooplankton. Nature 456, 395–399. ( 10.1038/nature07590) [DOI] [PubMed] [Google Scholar]
- 43.Randel N, Asadulina A, Bezares-Calderón LA, Verasztó C, Williams EA, Conzelmann M, Shahidi R, Jékely G. 2014. Neuronal connectome of a sensory-motorcircuit for visual navigation. eLife 3, e02730 ( 10.7554/elife.02730.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verasztó C, Ueda N, Bezares-Calderón LA, Panzera A, Williams EA, Shahidi R, Jékely G. 2017. Ciliomotor circuitry underlying whole-body coordination of ciliary activity in the Platynereis larva. eLife 6, e26000( 10.7554/eLife.26000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Case J. 1962. Responses of Nereis virens to alcohols. Comp. Biochem. Physiol. 6, 47–56. ( 10.1016/0010-406X(62)90042-7) [DOI] [PubMed] [Google Scholar]
- 46.Alexander J, Audesirk TE, Audesirk GJ. 1984. One-trial reward learning in the snail Lymnea stagnalis. J. Neurobiol. 15, 67–72. ( 10.1002/neu.480150107) [DOI] [PubMed] [Google Scholar]
- 47.Hara TJ. 1973. Olfactory responses to amino acids in rainbow trout, Salmo gairdneri. Comp. Biochem. Physiol. A: Physiol. 44, 407–416. ( 10.1016/0300-9629(73)90493-3) [DOI] [PubMed] [Google Scholar]
- 48.Idler DR, Fagerlund UHM, Mayoh H. 1956. Olfactory perception in migrating salmon. J. Gen. Physiol. 39, 889–892. ( 10.1085/jgp.39.6.889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kidawa A. 2005. The role of amino acids in phagostimulation in the shallow-water omnivorous Antarctic sea star Odontaster validus. Polar Biol. 28, 147–155. ( 10.1007/s00300-004-0664-7) [DOI] [Google Scholar]
- 50.Johnson BR, Ache BW. 1978. Antennular chemosensitivity in the spiny lobster, Panulirus argus: amino acids as feeding stimuli. Mar. Freshw. Behav. Physiol. 5, 145–157. ( 10.1080/10236247809378530) [DOI] [Google Scholar]
- 51.Gambi MC, Zupo V, Buia MC, Mazzella L. 2000. Feeding ecology of Platynereis dumerilii (Audouin & Milne-Edwards) in the seagrass Posidonia oceanica system: the role of the epiphytic flora (Polychaeta, Nereididae). Ophelia 53, 189–202. ( 10.1080/00785326.2000.10409449) [DOI] [Google Scholar]
- 52.Ramanathan N, Simakov O, Merten CA, Arendt D. 2015. Quantifying preferences and responsiveness of marine zooplankton to changing environmental conditions using microfluidics. PLoS ONE 10, e0140553 ( 10.1371/journal.pone.0140553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heuer CM, Müller CH, Todt C, Loesel R. 2010. Comparative neuroanatomy suggests repeated reduction of neuroarchitectural complexity in Annelida. Front. Zool. 7, 13 ( 10.1186/1742-9994-7-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hadfield MG, Meleshkevitch EA, Boudko DY. 2000. The apical sensory organ of a gastropod veliger is a receptor for settlement cues. Biol. Bull. 198, 67–76. ( 10.2307/1542804) [DOI] [PubMed] [Google Scholar]
- 55.Conzelmann M, Williams EA, Tunaru S, Randel N, Shahidi R, Asadulina A, Berger J, Offermanns S, Jekely G. 2013. Conserved MIP receptor-ligand pair regulates Platynereis larval settlement. Proc. Natl Acad. Sci. USA 110, 8224–8229. ( 10.1073/pnas.1220285110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marlow H, Tosches MA, Tomer R, Steinmetz PR, Lauri A, Larsson T, Arendt D. 2014. Larval body patterning and apical organs are conserved in animal evolution. BMC Biol. 12, 7 ( 10.1186/1741-7007-12-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams EA, Verasztó C, Jasek S, Conzelmann M, Shahidi R, Bauknecht P, Jékely G. 2017. Synaptic and peptidergic connectome of a neurosecretory centre in the annelid brain. eLife 6, e26349 ( 10.7554/eLife.26349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tessmar-Raible K, Raible F, Christodoulou F, Guy K, Rembold M, Hausen H, Arendt D. 2007. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell 129, 1389–1400. ( 10.1016/j.cell.2007.04.041) [DOI] [PubMed] [Google Scholar]
- 59.Purschke G. 1997. Ultrastructure of nuchal organs in polychaetes (Annelida)—new results and review. Acta Zool. 78, 123–143. ( 10.1111/j.1463-6395.1997.tb01133.x) [DOI] [Google Scholar]
- 60.Schmidtberg H, Dorresteijn A. 2010. Ultrastructure of the nuchal organs in the polychaete Platynereis dumerilii (Annelida, Nereididae). Invertebr. Biol. 129, 252–265. ( 10.1111/j.1744-7410.2010.00201.x) [DOI] [Google Scholar]
- 61.Shahidi R, Williams EA, Conzelmann M, Asadulina A, Verasztó C, Jasek S, Bezares-Calderón LA, Jékely G. 2015. A serial multiplex immunogold labeling method for identifying peptidergic neurons in connectomes. eLife 4, e11147 ( 10.7554/elife.11147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsie MS, Rawson PD, Lindsay SM. 2008. Immunolocalization of a Gupalphaq protein to the chemosensory organs of Dipolydora quadrilobata (Polychaeta: Spionidae). Cell Tissue Res. 333, 469–480. ( 10.1007/s00441-008-0660-2) [DOI] [PubMed] [Google Scholar]
- 63.Mollo E, Fontana A, Roussis V, Polese G, Amodeo P, Ghiselin MT. 2014. Sensing marine biomolecules: smell, taste, and the evolutionary transition from aquatic to terrestrial life. Front. Chem. 2, 92 ( 10.3389/fchem.2014.00092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giordano G, et al. 2017. Volatile secondary metabolites as aposematic olfactory signals and defensive weapons in aquatic environments. Proc. Natl Acad. Sci. USA 114, 3451–3456. ( 10.1073/pnas.1614655114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ayers T, Tsukamoto H, Gühmann M, Rajan VBV, Tessmar-Raible K. 2018. A Go-type opsin mediates the shadow reflex in the annelid Platynereis dumerilii. BMC Biol. 16, 41 ( 10.1186/s12915-018-0505-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carr WE, Netherton I, Gleeson RA, Derby CD. 1996. Stimulants of feeding behavior in fish: analyses of tissues of diverse marine organisms. Biol. Bull. 190, 149–160. ( 10.2307/1542535) [DOI] [PubMed] [Google Scholar]
- 67.Derby CD, Steullet P, Horner AJ, Cate HS. 2002. The sensory basis of feeding behaviour in the Caribbean spiny lobster, Panulirus argus. Mar. Freshw. Res. 52, 1339–1350. ( 10.1071/MF01099) [DOI] [Google Scholar]
- 68.Hara TJ. 2006. Gustation. In Fish physiology, vol. 25: Sensory systems neuroscience (eds Hara TJ, Zielinski BS), pp. 45–96. San Diego, CA: Academic Press. [Google Scholar]
- 69.Schmidt M., Mellon D. 2010. Neuronal processing of chemical information in crustaceans. In Chemical communication in crustaceans (eds Breithaupt T, Thiel M), pp. 123–147, New York, NY: Springer. [Google Scholar]
- 70.Rouse GW, Fauchald K. 1997. Cladistics and polychaetes. Zool. Scr. 26, 139–204. ( 10.1111/j.1463-6409.1997.tb00412.x) [DOI] [Google Scholar]
- 71.Schmidt H. 1922. Untersuchungen über den chemischen Sinn einiger Polychaeten. Biol. Zentralblatt. 42, 193–200. [Google Scholar]
- 72.Jouin C, Tchernigovtzeff C, Baucher MF, Toulmond A. 1985. Fine structure of probable mechano- and chemoreceptors in the caudal epidermis of the lugworm Arenicola marina (Annelida, Polychaeta). Zoomorphology 105, 76–82. ( 10.1007/BF00312141) [DOI] [Google Scholar]
- 73.Heuer CM, Loesel R. 2008. Immunofluorescence analysis of the internal brain anatomy of Nereis diversicolor (Polychaeta, Annelida). Cell Tissue Res. 331, 713–724. ( 10.1007/s00441-007-0535-y) [DOI] [PubMed] [Google Scholar]
- 74.Wolff GH, Strausfeld NJ. 2015. Genealogical correspondence of mushroom bodies across invertebrate phyla. Curr. Biol. 25, 38–44. ( 10.1016/j.cub.2014.10.049) [DOI] [PubMed] [Google Scholar]
- 75.Heisenberg M, Borst A, Wagner S, Byers D. 1985. Drosophila mushroom body mutants are deficient in olfactory learning. J. Neurogenet. 2, 1–30. ( 10.3109/01677068509100140) [DOI] [PubMed] [Google Scholar]
- 76.De Belle JS, Heisenberg M. 1994. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science 263, 692–694. ( 10.1126/science.8303280) [DOI] [PubMed] [Google Scholar]
- 77.Akalal DBG, Wilson CF, Zong L, Tanaka NK, Ito K, Davis RL. 2006. Roles for Drosophila mushroom body neurons in olfactory learning and memory. Learn. Mem. 13, 659–668. ( 10.1101/lm.221206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eichenbaum H. 1998. Using olfaction to study memory. Ann. New York Acad. Sci. 855, 657–669. ( 10.1111/j.1749-6632.1998.tb10642.x) [DOI] [PubMed] [Google Scholar]
- 79.Alvarez P, Lipton PA, Melrose R, Eichenbaum H. 2001. Differential effects of damage within the hippocampal region on memory for a natural, nonspatial odor–odor association. Learn. Mem. 8, 79–86. ( 10.1101/lm.38201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frankland PW, Bontempi B. 2005. The organization of recent and remote memories. Nat. Rev. Neurosci. 6, 119–130. ( 10.1038/nrn1607) [DOI] [PubMed] [Google Scholar]
- 81.Tomer R, Denes AS, Tessmar-Raible K, Arendt D. 2010. Profiling by image registration reveals common origin of annelid mushroom bodies and vertebrate pallium. Cell 142, 800–809. ( 10.1016/j.cell.2010.07.043) [DOI] [PubMed] [Google Scholar]
- 82.Hanström B. 1927. Das zentrale und periphere Nervensystem des Kopflappens einiger Polychäten. Z. für Morphol. und Ökologie. 7, 543–596. ( 10.1007/BF00540532) [DOI] [Google Scholar]
- 83.Schlawny A, Griinig C, Pfannenstiel H-D. 1991. Sensory and secretory cells of Ophryotrocha puerilis (Polychaeta). Zoomorphology 110, 209–215. ( 10.1007/BF01633005) [DOI] [Google Scholar]
- 84.Purschke G. 1993. Structure of the prostomial appendages and the central nervous system in the Protodrilida (Polychaeta). Zoomorphology 113, 1–20. ( 10.1007/BF00430973) [DOI] [Google Scholar]
- 85.Whittle AC, Zahid ZR. 1974. Fine structure of nuchal organs in some errant polychaetous annelids. J. Morphol. 144, 167–184. ( 10.1002/jmor.1051440204) [DOI] [PubMed] [Google Scholar]
- 86.Schlötzer-Schrehardt U. 1987. Ultrastructural investigation of the nuchal organs of Pygospio elegans (Polychaeta). II. Adult nuchal and dorsal organs. Zoomorphology 107, 169–179. ( 10.1007/BF00312310) [DOI] [Google Scholar]
- 87.Wu Y, Wawrzusin P, Senseney J, Fischer RS, Christensen R, Santella A, Colón-Ramos DA. 2013. Spatially isotropic four-dimensional imaging with dual-view plane illumination microscopy. Nat. Biotechnol. 31, 1032 ( 10.1038/nbt.2713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Toepke MW, Beebe DJ. 2006. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip. 6, 1484–1486. ( 10.1039/B612140C) [DOI] [PubMed] [Google Scholar]
- 89.Chen T, Gomez-Escoda B, Munoz-Garcia J, Babic J, Griscom L, Wu PYJ, Coudreuse D. 2016. A drug-compatible and temperature-controlled microfluidic device for live-cell imaging. Open Biol. 6, 160156 ( 10.1098/rsob.160156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lachaux J, et al. 2017. Thermoplastic elastomer with advanced hydrophilization and bonding performances for rapid (30 s) and easy molding of microfluidic devices. Lab Chip. 17, 2581–2594. ( 10.1039/C7LC00488E) [DOI] [PubMed] [Google Scholar]
- 91.Candelier R, Murmu MS, Romano SA, Jouary A, Debrégeas G, Sumbre G. 2015. A microfluidic device to study neuronal and motor responses to acute chemical stimuli in zebrafish. Sci. Rep. 5, 12196 ( 10.1038/srep12196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Achim K, Pettit JB, Saraiva LR, Gavriouchkina D, Larsson T, Arendt D, Marioni JC. 2015. High-throughput spatial mapping of single-cell RNA-seq data to tissue of origin. Nat. Biotechnol. 33, 503–509. ( 10.1038/nbt.3209) [DOI] [PubMed] [Google Scholar]
- 93.Achim K, et al. 2018. Whole-body single-cell sequencing reveals transcriptional domains in the annelid larval body. Mol. Biol. Evol. 35, 1047–1062. ( 10.1093/molbev/msx336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gühmann M, Jia H, Randel N, Verasztó C, Bezares-Calderón LA, Michiels NK, Yokoyama S, Jékely G. 2015. Spectral tuning of phototaxis by a Go-Opsin in the rhabdomeric eyes of Platynereis. Curr. Biol. 25, 2265–2271. ( 10.1016/j.cub.2015.07.017) [DOI] [PubMed] [Google Scholar]
- 95.Bauknecht P, Jékely G. 2015. Large-scale combinatorial deorphanization of Platynereis neuropeptide GPCRs. Cell Rep. 12, 684–693. ( 10.1016/j.celrep.2015.06.052) [DOI] [PubMed] [Google Scholar]
- 96.Xia Y, Whitesides GM. 1998. Soft lithography. Angew. Chem. Int. Ed. 37, 550–575. ( 10.1002/(SICI)1521-3773(19980316)37:5%3C550::AID-ANIE550%3E3.0.CO;2-G) [DOI] [PubMed] [Google Scholar]
- 97.Edelstein A, Amodaj N, Hoover K, Vale R, Stuurman N. 2010. Computer control of microscopes using µManager. Curr. Protoc. Mol. Biol. 92, 1–17. ( 10.1002/0471142727.mb1420s92) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hauenschild C, Fischer A. 1969. Mikroskopische Anatomie, Fortpflanzung, Entwicklung von Platynereis dumerilii. Stuttgart: Gustav Fischer. [Google Scholar]
- 99.Chen T-W, et al. 2013. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. ( 10.1038/nature12354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thevenaz P, Ruttimann UE, Unser M. 1998. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27–41. ( 10.1109/83.650848) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article, as well as videos showing examples of calcium activity, have been provided as the electronic supplementary material. Raw calcium recordings are available on demand.