Abstract
Aim
In recent years, we have seen a considerable increase in the relevance of nanostructures for the safe delivery of therapeutic agents and their capacity as an immunomodulatory tool.
Materials and methods
Potential clinical applications related to their unique structural properties have been described in the evolving landscape of immunotherapy.
Results
This review briefly summarizes the evidence for the role of nanoparticles in regulating the immune response.
Conclusions
Their main features to highlight how to provide an innovative means of biomedical application to oncology research.
Keywords: Nanotechnology, Homing, Gold nanoparticles, Liposomes, Dendrimers, Carbon nanocarriers
1. Background
Despite the great progress made over the past years with advances in surgical resection, radiotherapy and chemotherapy, cancer is still the second leading cause of death in Europe and the United States.1 Many forms of the disease remain fundamentally difficult to manage mainly due to the wide heterogeneity of tumors.2 Nowadays, in the post-genomic era, the tendency is to find more personalized treatments with the use of targeted therapies, immunotherapies or many combinatorial approaches changing the way that physicians attack neoplasms.3 In particular, immunotherapy has emerged as a powerful new strategy and holds tremendous promise for improving cancer treatments but, unfortunately, response rates for this strategy remain generally low, and it has become quite clear that there is no “cure-all wonder drug” to be discovered.4
For this reason, in these coming years there will remain three urgent unmet medical needs: to identify novel methods to enhance the treatment response to immunotherapy, to improve the efficacy of the “traditional” treatments, and also to reduce the side effects of these treatments in many instances. A fascinating approach which has recently been demonstrated to potentially include most of these hallmarks, is the medical application of nanotechnology, summarized as nanoparticles (NPs).
NPs, defined as synthetic particles with a diameter of less than 100 nm5 and generally derived from polymers, lipids, or metals, such as gold, have been found to be highly useful in several medical applications, from diagnostics to cancer therapy. The size of these NPs are very similar to the majority of biological structures and molecules; thus conferring functional properties for both in vitro and in vivo cancer research.6 Such NPs, if accompanied by biodegradable carriers, can be safely loaded with therapeutic compounds, to achieve concentrated local drug delivery with potential for sustained release.7 Thanks to this, they can enter into the body cavities and the blood circulation for treatment with minimal invasion and improved bioavailability.8
In addition, NPs have a larger surface-to-volume ratio than that of micro and macro sized particles, which enables them to be covered with various ligands at once (leading to superior drug loading) and can facilitate interaction with a number of molecules, such as receptors present on the surface of target cells.9
Immunogenicity is the ability of different substances to trigger an adaptive immune response of cellular and humoral type that in the long term constitutes immunological memory. Immunotoxicity is damage to the immune system caused by exposure to chemicals. The analysis of immunotoxicity is a standard part of the development of substances as possible new drugs.
Their applications as nanocarriers have grown immensely over the last ten years, now we can find several publications describing their many functions, namely: (1) to concentrate anti-cancer drugs in the tumor microenvironment with a superior therapeutic efficacy10; (2) to deliver cancer antigens to immune cells, or to directly stimulate T cells as an artificial antigen presenting cell (APC)11; (3) and also to induce and enhance the abscopal effect (a phenomenon in which local tumor treatments produce a systemic regression of distant lesions) by capturing the tumor-derived protein antigens (TDPAs) released by radiation therapy.12
Cytotoxicity of NPs can be affected by size, concentration and surface functionalization. Even though NPs are inert and biocompatible, conflicting results have been reported regarding their toxicity to cells (Table 1). NPs’ cytotoxicity may be due to the tiny size which makes them have a larger reactive surface area relative to the volume ratio for extracellular or intracellular interactions25 involved in oxidative stress production.26 On the other hand, studies (Table 1) have shown a very low cytotoxicity for different sizes of NPs on T cells and DCs, regardless of surface functionalization and concentrations,27 which is important for their application in immunotherapy development.
Table 1.
Examples of common therapeutic nanoparticles conjugated with different types of drugs in pre-clinical models.
| Nanoparticle category | Diameter (nm) | Binding molecule | In favor | In detriment | Indication |
|---|---|---|---|---|---|
| Gold | 12 | TKIs and FLT3 Inhibitors | Inhibition of BCR-ABL and FLT3 pathways | Resistance to chemotherapy, ↑ risk for relapse | AML10 |
| 50 | Doxorubicin | ↑ Cellular uptake, ↑ cytotoxicity vs multi-drug resistance, blood-brain barrier pass | Cardiotoxicity, haematological toxicity | Breast cancer13 | |
| 50 | Oxaliplatin | ↑ Cytotoxicity and uptake | Undiscriminated cytotoxicity | Colorectal cancer14 | |
| 50 | Cisplatin | ↑ cytotoxicity, free active form of the drug | Renal toxicity and irreversible neuropathy | Gynaecological cancer15 | |
| Liposomes | 80–100 | Doxorubicin, EGFR, Epirubicin, Vinorelbine | ↑ antitumor effect | ↓ tumor internalization rate | EGFR+ tumors16 |
| 90–100 | Anti-HER2 fragments, Doxorubicin | ↑ antitumor effect and drug accumulation in tumor cells | Toxicity profile and efficacy to be determined | Breast cancer17 | |
| ≈100 | Folate, Doxorubicin | ↑ citotoxicity | Folate efficacy was inversely proportional with liposome uptake | Lung cancer18 | |
| <200 | Thiolated Anti-HER2, Paclitaxel | ↑cellular uptake and antitumor effects | ↑ adverse events | Breast cancer19 | |
| Dendrimers | 17 | Paclitaxel | Stop mitosis in a dividing cell | ↑ side effects | Ovarian cancer20 |
| ≈30 | Trastuzumab + Docetaxel | ↑ apoptosis and cellular uptake | ↑ hemolysis | Breast cancer21 | |
| 50–100 | Doxorubicin | ↑ anticancer activity and tumor site infiltration | ↑ adverse events and out of target accumulation of dendrimers | ALL22 | |
| Carbon nanotubes | 2–3 | Doxorubicin | ↑ blood circulation, ↓ toxicity and ↑ tumor uptake | ↑ side effect of free Doxorubicin and out of target accumulation | Non-Hodgkin lymphoma23 |
| 200 | Paclitaxel | ↑cell penetration and cytotoxic effect | Accumulation in internal organs and ↑ side effects | Not a specific tumor line24 | |
nm: nanometers; TKIs: tyrosine-kinase inhibitors; AML: acute myeloid leukemia; siRNA: short interfering RNA; EGFR: epidermal growth factor receptor; ALL: acute lymphoblastic leukemia.
2. Delivery systems used in cancer research
Recent advances in chemistry and synthetic biology have encouraged the development of nanoscale devices with controllable structures. Despite this, the large-scale manufacturing of these materials remains challenging because of the difficulty to produce structurally homogeneous populations of particles.28
On the other hand, nanomaterials based on viruses allow production on living cells of millions of identical nanoparticles. Viruses are an excellent starting point because they have evolved to interact with specific cellular proteins, delivering their nucleic acid cargo and taking charge of the intracellular machinery to develop the components of progeny. Therefore, they can be programmed for the delivery of other subsets of molecules, such as drugs.
3. Viral delivery systems
Virus-based nanoparticles (VNPs) own regular layers of coat proteins with a highly defined three-dimensional structure, providing an engineering scaffold which is superior to that of synthetic particles. The structural characteristics of viruses allow for the resistance to physical and chemical degradation and the protection of viral genome from nucleases. It is an advantage for the development of VNPs because it means that they have a long lifespan and can withstand the chemical treatments necessary to conjugate them with ligands or to load with payloads.29 The structure of VNPs can be changed by modifying the genetic information that codes for viral proteins prior to synthesis. VNPs can also be altered by chemical modification adding conjugates to specific side chains of amino acids. These characteristics have led to the production of VNPs based on mammalian viruses for their use in gene therapy; nevertheless, it is difficult to dismiss pathogenic effects resulting from natural virus–host interactions.30, 31 However, VNPs based on bacteriophages and plant viruses have shown to be safe, because they cannot infect humans.
VNPs can be crafted to target particular cells, including specific cells of the immune system or cancer cells. Targeting can be achieved by the genetic or chemical addition of ligands which bind to overexpressed receptors on distinctive cells. For example, VNPs have been targeted through the use of folic acid,32 epidermal growth factor33 or transferrin.34 Tissue specificity can also be swayed by the size, shape and aspect ratio of the VNPs. Therefore, these are properties that can be considered at the design state. As an example, filamentous or tubular VNPs can have better properties in vivo than spherical VNPs, like enhanced flow and margination towards vessel walls, and reduce the clearance by the mononuclear phagocytic system, which means increased tumor homing.35
4. Gold nanoparticles
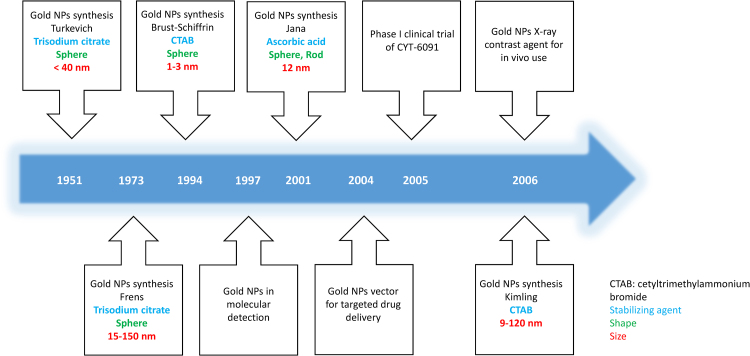
NPs can be developed using various types of materials, such as metal oxides (e.g. iron oxide, titanium oxide), organic biodegradable (e.g. polysaccharide, lipid, polymeric matrix), inorganic biocompatible polymers (e.g. polystyrene), metalloids (e.g. crystalline or amorphous silica) or inorganic metals (e.g. gold, silver and carbon).36 Gold NPs are adaptable in their shape, surface, functionality and size, which provides advantageous properties for immunotherapies. It is therefore necessary to determine a suitable and appropriate method for gold NP synthesis. The most popular mechanism of synthesizing gold NPs is through citrate reduction of hydrogen tetrachloroaurate in boiling water. This route was first proposed in the early 1950s by Turkevich et al.,37 and later modified by Frens in 1973, improving the regulation of particle size resulting in gold NPs with diameters ranging from 15 to 150 nm.38 The Turkevich and Frens methods were used for about three decades until they were further improved by Kimling et al.39 who reported that the final particle size is independent of the absolute concentration of gold precursor. Many years of research have left us with several gold NPs techniques that have been explored and studied to produce well defined gold NPs. Currently, the most applied method is called the seed growth method, developed by Richard Zsigmondy, that synthesizes gold NPs in two separate nucleation processes followed by successive growth steps.40 The initial production of small-sized gold NPs called seeds, were enlarged with the addition of growth solution, thus reducing and stabilizing agents in the second step. This technique achieves large size gold NPs with a more defined shape and more varied forms due to the first nucleation process. The current trend in gold NPs synthesis method is the use of biological compounds (e.g. star anise, pomegranate) rather than chemicals (e.g. citrate) that have been shown to be toxic at the cellular level. Therefore, this new trend is more environmentally friendly and also reduces the cellular toxicity of gold NPs which is crucial for future implementation41 (Fig. 1).
Fig. 1.
Timeline of Gold NPs development and methods of synthesis.
As stated above, the customizable shape, size and properties of gold NPs have an important role in their interaction with APCs or other targeted cells (e.g. dendritic cells), and interaction capacity with these cells, such as the internalization process. The role of gold NPs for immunomodulation has been harnessed to deliver therapeutic compounds in diverse diseases, but the best effort is focused on cancer nanomedicine. Targeted delivery in immunotherapies for cancer and other immune diseases (e.g. rheumatoid arthritis) is important in terms of reducing systemic toxicity and achieving optimal therapeutic effects. The characteristics of the above mentioned gold NPs for surface association with drugs or other molecules to target immune cells turn these NPs into a promising therapeutic tool.42, 43
This targeted method could be a different option to the conventional chemotherapy, with a reduction of related side effects. As an example, a new cancer chemotherapy strategy focuses on the association of gold NPs with chemotherapy drugs, showing a significantly lower cellular proliferation of hepatocellular carcinoma-derived cells than those exposed to chemotherapy alone.44
Similarly, several approaches have been adopted to develop cancer immunotherapies using gold NPs, and some of them are in their clinical trial phase (see Table 2). One of them is CYT-6091, a gold NP conjugated with PEG and recombinant human tumor necrosis factor (rhTNF), which was clinically tested in patients with advanced-stage of solid tumors. Results obtained from this study showed that the combination of rhTNF with gold NPs allowed to reach concentrated doses in tumor cells that had previously been toxic.45
Table 2.
Examples of on-going clinical trial with novel therapeutic nanoparticles conjugated with different types of drugs.
| Nanoparticle Category | NCT | Status | Clinical phase | Binding molecule | Challenges | Questions |
|---|---|---|---|---|---|---|
| Gold | NCT03020017 | Recruiting | Early Phase I | siRNA (Spherical Nucleic Acid) | Targeting BCL2L12 gene in glioblastoma | Safety to be determined |
| NCT01420588 | Recruiting | n/a | Na-Nose (chemical nanosensors) | Discrimination between gastric lesions | Effectiveness to be demonstrated | |
| Liposomal | NCT01455389 | Recruiting | Phase I–II | FUS-1 and Erlotinib | Transfer FUS-1 gene into lung cancer cells | Efficacy and dosing to be determined |
| NCT00734682 | Completed | Phase I | Liposomal Irinotecan | To treat patients with recurrent high-grade gliomas | Safety and dosing to be defined | |
| (SMARTICLES©) | NCT02716012 | Recruiting | Phase I | MTL-CEBPA (double stranded RNA) | To activate the CEBPA gene in advanced liver cancer | Toxicity profile and dose for phase II to be determined |
| NCT02631733 | Recruiting | Phase I | Liposomal Irinotecan plus Veliparib | To test this combination in solid tumors | Safety and dosing to be determined | |
| Dendrimers | NCT03255343 | Recruiting | Phase I | ImDendrim o [188Re] rhenium complex | To evaluate responder in Stage IV liver cancer | Non-removal nanodevice |
| Carbon nanotubes | NCT02123407 | Unknown | Phase III | Harvesting lymph nodes | Guidance to lymph node dissection in gastric cancer surgery | Insufficient evidence to date |
| NCT03350945 | Not yet recruiting | n/a | None | To better localize tumor and lymph nodes mapping in the colorectal surgery | Safety, efficacy and economy efficiency To be determined | |
| Other NPs categories | ||||||
| Sugar molecule (CRLX101) | NCT02769962 | Recruiting | Phase I–II | Camptothecin plus olaparib | To test in small cell lung cancer | Toxicity profile to be determined |
| Magnetic NPs | NCT02033447 | Completed | Early Phase I | None | To treat prostate cancer with thermoablation | Retention and/or maintenance in the prostate |
| Polysiloxane Gd-Chelates | NCT03308604 | Not yet recruiting | Phase I | With chemo-radiation and brachytherapy | To escalate dose in cervical cancer | Dosing to be determined |
| Radio-enhancer (NBTXR3) | NCT02805894 | Recruiting | Phase I–II | Activated by radiotherapy | To treat locally-advanced prostate cancer | Efficacy to be demonstrated |
NCT: number of clinical trial (reference of clinicaltrials.gov); siRNA: short interfering RNA; n/a: not applicable; NPs: nanoparticles.
Due to the ability and main roles of NPs in antigen presentation and T cell activation, there is great interest in manipulation of DC functions helped by gold NPs for induction of immune responses in immunotherapy development. This strategy is conducted to manage several challenges of immunotherapies, such as the lack of vaccine effectiveness,46 persistent viral infection, immune escape in cancer and the adverse effects of therapies on healthy cells.47
One of the current strategies in DCs immunotherapies is to use materials to trigger immune specific response and also modulate immune-cell functions. As previously mentioned, gold NPs have plenty of characteristics that make them a potential tool to target DCs. To modulate the immune function of DCs, gold NPs are an instrument utilized to resolve several points in DCs immunomodulation such as to penetrate the mucosal barriers and tissues, enhance antigen uptake and to improve intracellular targeting.48 Conducting studies on interactions and effects of gold NPs and human DCs is crucial due to the main roles of DCs in the activation of immune response, either adaptive or innate. Studies and applications of gold NPs often require that nanoparticles be introduced into biological systems, such as into the bloodstream of an organism, cell of interest or growth medium. Thus, when exposed to biological systems, consisting of a complex mixture containing proteins, nutrients and metabolites, gold NPs would interact with them and change their physicochemical composition in terms of surface charge, surface chemistry, aggregation and size. Gold NPs would embed proteins of the medium, building a protein shell named “protein corona” which alters the effective surface charge of gold NPs.49 On this basis, altered surface charge would later mediate the cellular uptake of gold NPs throughout several mechanisms including receptor-mediated endocytosis, phagocytosis and macropynocitosis, depending on their physiochemical properties of sizes and surface modifications.
In terms of size, some studies have shown a better cellular uptake of smaller size gold NPs (1–2 nm) by DCs rather than larger gold NPs (12 nm). These results were attributed to a higher diffusion capacity of smaller particles compared to a larger equivalent, in addition to a greater cellular uptake interaction.27 These results were contradictory to a previous study in which 50 nm gold NPs were more efficiently internalized by DCs than 10 nm gold NPs.50 This difference may be attributed to the minimum wrapping time required for 50 nm gold NPs, compared to smaller counterparts, which results in a faster and higher accumulation into DCs. Wrapping time is the ratio between the free energy required to introduce particles in a cell and the diffusion kinetics of the receptor, and depends on the size of the particles.51
Surface chemistry used to functionalize gold NPs is another critical factor that allows cellular uptake and internalization into DCs. In addition, surface modification of gold NPs protects them from aggregation and protein surface coating formation that leads to reduced cellular uptake in different lines.49, 52
Cellular uptake and antigen internalization by DCs would activate their maturation and starts a T cell response based on antigen presented. Nevertheless, internalization and cellular uptake of gold NPs does not always result in enhanced DCs maturation and T cell response activation. Several factors such as size and type of ligands on the surface of gold NPs would affect DCs maturation, T cell responses and cytokines secretion in many ways.27 Therefore, careful selection and further experimentation with the size of gold NPs and their protein corona will be critical to achieve the effects obtained when future research is done.
5. Dendrimers
Dendrimers are well-defined polymeric systems, which have the ability to interact with various forms of nucleic acids to form complexes that protect the nucleic acid from degradation. They are the key focus of biomedical research due to their precise molecular weight, biocompatibility, high water solubility and polyvalence.
The extremely branched structure of dendrimers allows them to integrate an extensive diversity of therapeutic (hydrophilic or hydrophobic) molecules. We can appreciate differences in the structure of the dendrimers with respect to other polymeric delivery systems that allow them to obtain more favorable physical–chemical properties and higher biocompatibility.53 Their structure consists of a core, dendrons, and surface active groups. The electrostatic interaction between the dendrimers and the nucleic acids, where the cationic dendrimer condenses the anionic nucleic acids, the selection of a core and the surface functional groups, determines their cytotoxicity and biological activity. For example, the presence of several surface functional groups enables a concurrent interaction with numerous receptors. The drug properties are a fundamental factor in choosing the immobilization method. Encapsulation is chosen when the active compounds are virtually insoluble, labile or toxic, whereas chemical attachment offers the possibility to control the number of drugs on the dendrimer surface by modifying the number of covalent bonds.54
Commercially available dendrimers, such as Poly (amido amide) (PAMAM), are frequently used in biomedical applications and have been intensively investigated (Table 2), their structure is composed of a diamine core reacted with methyl acrylate, followed by another diamine, which results in regular radially concentric layers or ‘generations’ that give rise to successively larger dendrimers.55
One of the most helpful features of dendrimers is that they can modulate the release of cytokines and chemokines; in contrast, they can also produce severe secondary effects.56 The PAMAM creation of 3,5-glucosamine dendrimers stimulates an important immunomodulatory effect generating the production of pro-inflammatory chemokines, such as MIP-1a, IL-8, MIP-1 beta, and cytokines TNF-α, IL-6 IL-1beta in human dendritic cells and macrophages. Chauhan et al. studied in vivo a PAMAM dendrimer toxicity profile in mice, elaborating a study of the dendrimer surface chemistry and different dosage levels and concluding that dendrimer toxicity is essentially a function of its outer surface layer, and can be controlled judiciously by pacifying the surface layer. The researchers assessed that PAMAM dendrimers can be used safely by correct choice of concentrations, surface groups and additives.57
Dendrimer glucosamine inhibited Toll-like Receptor 4-mediated inflammatory responses, allowing upregulation of the costimulatory molecules CD25, CD80, CD83 and CD86.58 The dynamic behavior and the structural characteristics of this moderately glycosylated dendrimer revealed that its flexibility and polarity modified with the addition of glucosamine molecules. Remarkably, these surface glucosamine molecules continued to be available for interaction with the biological target.59 Using this approach, it could be useful to design additional macromolecular dendrimers as novel antagonists of biologically important Toll like receptor–ligand interactions for treating a variety of malignant and inflammatory diseases.
A complete loss of TLR8-stimulatory activity with selective retention of the TLR7-agonistic activity have also been studied using a dendrimer synthesized from the TLR7 and TLR8 agonist imidazoquinoline.60 Even though the mechanisms by which Toll-like receptors and dendrimers connect innate immune responses with adaptive immunity are not clear, it offers an opportunity that can be exploited to modulate the innate immune response for therapeutic use.
6. Solid lipids nanoparticles, liposomes and micelles
There are certain differences between these compounds, although all can be included within the category of Lipid-based nanotechnology. Solid lipids nanoparticles are characterized by being solid at room temperature, while micelles and liposomes are liquid at these temperatures.
Liposomes were the first colloidal drug carriers investigated in gene therapy and have been extensively studied in various drug delivery applications. They consist of spherical lipid vesicles with phospholipid bilayers with a hydrophilic centre enclosing an aqueous core in the size range of 80–300 nm.61 Because of their high versatility of surface chemical modication, specific targeting using selected ligands62 and potential of encapsulating both the hydrophilic and hydrophobic drugs, liposomes are attractive for drug designing, enable ease of surface manipulation and have been extensively studied in multiple clinical trials increasing the solubility of drugs, improving their pharmacokinetic properties and diminishing the side effects of chemotherapy or antimicrobial therapies.63, 64
The release of a drug from liposomes depends on the liposome structure, biocompatibility, and the surrounding environment,65 among others. However, liposomes have several unresolved problems, such as their accumulation in cells (liver macrophages) outside the target tissues, low encapsulation efficiency and short release time.66
Data on the use of liposomes and micelles as carriers to achieve immunosuppression are available, including the use of bisphosphonates for liposomes67 and camptothecin for micelles.68 The ability of liposomes to target specific cells is exemplified by the selective delivery of small interfering RNAs (siRNAs) to DCs using CD40 siRNAs containing liposomes with a monoclonal antibody that is specific to DCs69 (Table 2).
7. Carbon nanocarriers
Among the most widely studied nanomaterials are carbon nanocarriers, differentiated into nanotubes (CNTs) and nanohorns (CNH). CNTs belong to the fullerene family of carbon allotropes and are formed by rolling of single (SWNCTs – single walled carbon nanotubes) or multi (MWCNTs – multi walled carbon nanotubes) layers of graphene sheets with a cylindrical nanostructure and an excellent electronic and thermal conductivity.70 CNH exhibit similar properties to CNTs.71
The physicochemical and biological characteristics of CNTs make them a promising carrier for drug delivery, these nanoparticles are able to immobilize therapeutic agents, diminishing the cytotoxicity for healthy tissues. On the other hand, CNTs have several disadvantages, such as poor water solubility, low degradation rate and toxicity72; however, they have the ability to be surface functionalized using various methods such as absorption and electrostatic and covalent interaction, so that the release of drugs from carbon nanotubes can be electrically or chemically controlled.
8. Conclusions
Nanotechnology is a field that is currently being used to engineer specific immune responses for prophylactic and therapeutic effects. In the near future, the application of nanoparticles that have distinctive immunological properties determined by the type of polymer, its charge, size and the route of administration could offer tremendous potential to produce an appropriate immune response for elimination and prevention of future recurrence of cancer. Nanoparticle engineering for this purpose will remain an exciting opportunity for ongoing and future studies and an increasing focus of clinical trials and cancer treatments.
Conflict of interest
None declared.
Financial disclosure
None declared.
Footnotes
Article from the Special Issue on Nanoparticle and Immunotherapy.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer Statistics 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C.E., Lin C.C., Mariotto A.B. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 3.Al-Lazikani B., Banerji U., Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat Biotechnol. 2012;30(7):679. doi: 10.1038/nbt.2284. [DOI] [PubMed] [Google Scholar]
- 4.Strebhardt K., Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8(6):473. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 5.Horikoshi S., Serpone N. Introduction to nanoparticles. In: Horikoshi S., Serpone N., editors. Microwaves in nanoparticle synthesis: fundamentals and applications. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2013. pp. 1–24. [Google Scholar]
- 6.Almeida J.P., Lin A.Y., Langsner R.J. In vivo immune cell distribution of gold nanoparticles in naive and tumor bearing mice. Small. 2014;10:812–819. doi: 10.1002/smll.201301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow E.K., Ho D. Cancer nanomedicine: from drug delivery to imaging. Sci Transl Med. 2013;5:216rv214. doi: 10.1126/scitranslmed.3005872. [DOI] [PubMed] [Google Scholar]
- 8.Baetke S.C., Lammers T., Kiessling F. Applications of nanoparticles for diagnosis and therapy of cancer. Br J Radiol. 2015;88(1054):20150207. doi: 10.1259/bjr.20150207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thanh N.T.K., Green L.A.W. Functionalisation of nanoparticles for biomedical applications. Nano Today. 2010;5:213–230. [Google Scholar]
- 10.Petrushev B., Boca S., Simon T. Gold nanoparticles enhance the effect of tyrosine kinase inhibitors in acute myeloid leukemia therapy. Int J Nanomedicine. 2016;11:641–660. doi: 10.2147/IJN.S94064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grippin A.J., Sayoura E.J., Mitchella D.A. Translational nanoparticle engineering for cancer vaccines. Oncoimmunology. 2017;6(10):e1290036. doi: 10.1080/2162402X.2017.1290036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Min Y., Roche K.C., Tian S. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12(9):877–882. doi: 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dreaden E.C., Austin L.A., Mackey M.A., El-Sayed M.A. Size matters: gold nanoparticles in targeted cancer drug delivery. Ther Deliv. 2012;3(4):457–478. doi: 10.4155/tde.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tummala S., Kumar M.N., Pindiprolu S.K. Improved anti-tumor activity of oxaliplatin by encapsulating in anti-DR5 targeted gold nanoparticles. Drug Deliv. 2016;23(9):3505–3519. doi: 10.1080/10717544.2016.1199606. [DOI] [PubMed] [Google Scholar]
- 15.Johnstone T.C., Suntharalingam K., Lippard S.J. The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem Rev. 2016;116(5):3436–3486. doi: 10.1021/acs.chemrev.5b00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mamot C., Drummond D.C., Noble C.O. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65(24):11631–11638. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- 17.Kirpotin D.B., Drummond D.C., Shao Y. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66(13):6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 18.Yamada A., Taniguchi Y., Kawano K., Honda T., Hattori Y., Maitani Y. Design of folate-linked liposomal doxorubicin to its antitumor effect in mice. Clin Cancer Res. 2008;14(24):8161–8168. doi: 10.1158/1078-0432.CCR-08-0159. [DOI] [PubMed] [Google Scholar]
- 19.Yang T., Choi M.K., Cui F.D. Antitumor effect of paclitaxel-loaded PEGylated immunoliposomes against human breast cancer cells. Pharm Res. 2007;24(12):2402–2411. doi: 10.1007/s11095-007-9425-y. [DOI] [PubMed] [Google Scholar]
- 20.Cline E.N., Li M.H., Choi S.K. Paclitaxel-conjugated PAMAM dendrimers adversely affect microtubule structure through two independent modes of action. Biomacromolecules. 2013;14(3):654–664. doi: 10.1021/bm301719b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulhari H., Pooja D., Shrivastava S. Trastuzumab-grafted PAMAM dendrimers for the selective delivery of anticancer drugs to HER2-positive breast cancer. Sci Rep. 2016;6:23179. doi: 10.1038/srep23179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S.J., Jeong Y.I., Park H.K. Enzyme-responsive doxorubicin release from dendrimer nanoparticles for anticancer drug delivery. Int J Nanomedicine. 2015;10:5489–5503. doi: 10.2147/IJN.S87145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z., Fan A.C., Rakhra K. Supramolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapy. Angew Chem Int Ed Engl. 2009;48(41):7668–7672. doi: 10.1002/anie.200902612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobhani Z., Dinarvand R., Atyabi F., Ghahremani M., Adeli M. Increased paclitaxel cytotoxicity against cancer cell lines using a novel functionalized carbon nanotube. Int J Nanomedicine. 2011;6:705–719. doi: 10.2147/IJN.S17336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jahnen-Dechent W., Simon U. Function follows form: shape complementarity and nanoparticle toxicity. Nanomedicine (Lond) 2008;3:601–603. doi: 10.2217/17435889.3.5.601. [DOI] [PubMed] [Google Scholar]
- 26.Akhtar M.J., Ahamed M., Kumar S., Khan M.M., Ahmad J., Alrokayan S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int J Nanomed. 2012;7:845–857. doi: 10.2147/IJN.S29129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez T.D., Pearson J.R., Leal M.P., Torres M.J., Blanca M., Mayorga C., Le Guevel X. Intracellular accumulation and immunological properties of fluorescent gold nanoclusters in human dendritic cells. Biomaterials. 2015;43:1–12. doi: 10.1016/j.biomaterials.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 28.Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14:282–295. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 30.Guenther C.M., Kuypers B.E., Lam M.T., Robinson T.M., Zhao J., Suh J. Synthetic virology: engineering viruses for gene delivery. WIRES Nanomed Nanobiotechnol. 2014;6:548–558. doi: 10.1002/wnan.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirth T., Parker N., Ylä-Herttuala S. History of gene therapy. Gene. 2013;525:162–169. doi: 10.1016/j.gene.2013.03.137. [DOI] [PubMed] [Google Scholar]
- 32.Zeng Q., Wen H., Wen Q. Cucumber mosaic virus as drug delivery vehicle for doxorubicin. Biomaterials. 2013;34:4632–4642. doi: 10.1016/j.biomaterials.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Pokorski J.K., Hovlid M.L., Finn M.G. Cell targeting with hybrid Qβ virus-like particles displaying epidermal growth factor. ChemBioChem. 2011;12:2441–2447. doi: 10.1002/cbic.201100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galaway F.A., Stockley P.G. MS2 viruslike particles: a robust, semisynthetic targeted drug delivery platform. Mol Pharm. 2013;10:59–68. doi: 10.1021/mp3003368. [DOI] [PubMed] [Google Scholar]
- 35.Shukla S., Ablack A.L., Wen A.M., Lee K.L., Lewis J.D., Steinmetz N.F. Increased tumor homing and tissue penetration of the filamentous plant viral nanoparticle Potato Virus X. Mol Pharm. 2013;10:33–42. doi: 10.1021/mp300240m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohamud R., Xiang S.D., Selomulya C. The effects of engineered nanoparticles on pulmonary immune homeostasis. Drug Metab Rev. 2014;46:176–190. doi: 10.3109/03602532.2013.859688. [DOI] [PubMed] [Google Scholar]
- 37.Turkevich J., Stevenson P.C., Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75. [Google Scholar]
- 38.Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci. 1973;241:20–22. [Google Scholar]
- 39.Kimling J., Maier M., Okenve B., Kotaidis V., Ballot H., Plech A. Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B. 2006;110:15700–15707. doi: 10.1021/jp061667w. [DOI] [PubMed] [Google Scholar]
- 40.Jana N.R., Gearheart L., Murphy C.J. Seeding growth for size control of 5–40 nm diameter gold nanoparticles. Langmuir. 2001;17:6782–6786. [Google Scholar]
- 41.Lokina S., Suresh R., Giribabu K., Stephen A., Lakshmi Sundaram R., Narayanan V. Spectroscopic investigations, antimicrobial, and cytotoxic activity of green synthesized gold nanoparticles. Spectrochim Acta A: Mol Biomol Spectrosc. 2014;129:484–490. doi: 10.1016/j.saa.2014.03.100. [DOI] [PubMed] [Google Scholar]
- 42.Niikura K., Matsunaga T., Suzuki T. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7:3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 43.Paul A.M., Shi Y., Acharya D. Delivery of antiviral small interfering RNA with gold nanoparticles inhibits dengue virus infection in vitro. J Gen Virol. 2014;95:1712–1722. doi: 10.1099/vir.0.066084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomuleasa C., Soritau O., Orza A. Gold nanoparticles conjugated with cisplatin/doxorubicin/capecitabine lower the chemoresistance of hepatocellular carcinoma-derived cancer cells. J Gastrointest Liver Dis. 2012;21:187–196. [PubMed] [Google Scholar]
- 45.Libutti S.K., Paciotti G.F., Byrnes A.A. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohn L., Delamarre L. Dendritic cell-targeted vaccines. Front Immunol. 2014;5:255. doi: 10.3389/fimmu.2014.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kreutz M., Tacken P.J., Figdor C.G. Targeting dendritic cells-why bother? Blood. 2013;121:2836–2844. doi: 10.1182/blood-2012-09-452078. [DOI] [PubMed] [Google Scholar]
- 48.Hubbell J.A., Thomas S.N., Swartz M.A. Materials engineering for immunomodulation. Nature. 2009;462:449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 49.Alkilany A.M., Murphy C.J. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J Nanopart Res. 2010;12:2313–2333. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomic S., Ethokic J., Vasilijic S. Size-dependent effects of gold nanoparticles uptake on maturation and antitumor functions of human dendritic cells in vitro. PLoS ONE. 2014;9:e96584. doi: 10.1371/journal.pone.0096584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chithrani B.D., Chan W.C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7:1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 52.Cheng X., Tian X., Wu A. Protein Corona influences cellular uptake of gold nanoparticles by phagocytic and nonphagocytic cells in a size-dependent manner. ACS Appl Mater Interfaces. 2015;7:20568–20575. doi: 10.1021/acsami.5b04290. [DOI] [PubMed] [Google Scholar]
- 53.Caminade A.M., Laurent R., Majoral J.P. Characterization of dendrimers. Adv Drug Deliv Rev. 2005;57:2130–2146. doi: 10.1016/j.addr.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Singh P., Gupta U., Asthana A., Jain N.K. Folate and Folate-PEG-PAMAM dendrimers: synthesis, characterization, and targeted anticancer drug delivery potential in tumor bearing mice. Bioconjugate Chem. 2008;19:2239–2252. doi: 10.1021/bc800125u. [DOI] [PubMed] [Google Scholar]
- 55.Smith D.M., Simon J.K., Baker J.R., Jr. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13(8):592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duncan R., Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57:2215–2237. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 57.Chauhan A.S., Jain N.K., Diwan P.V. Pre-clinical and behavioural toxicity profile of PAMAM dendrimers in mice. Proc R Soc A. 2010;466:1535–1550. [Google Scholar]
- 58.Shaunak S., Thomas S., Gianasi E. Polyvalent dendrimer glucosamine conjugates prevent scar tissue formation. Nat Biotechnol. 2004;22:977–984. doi: 10.1038/nbt995. [DOI] [PubMed] [Google Scholar]
- 59.Barata T.S., Teo I., Brocchini S., Zloh M., Shaunak S. Partially glycosylated dendrimers block MD-2 and prevent TLR4-MD-2-LPS complex mediated cytokine responses. PLoS Comput Biol. 2011;7:e1002095. doi: 10.1371/journal.pcbi.1002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shukla N.M., Salunke D.B., Balakrishna R., Mutz C.A., Malladi S.S., David S.A. Potent adjuvanticity of a pure TLR7-agonistic imidazoquinoline dendrimer. PLoS ONE. 2012;7:e43612. doi: 10.1371/journal.pone.0043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sunderland C.J., Steiert M., Talmadge J.E., Derfus A.M., Barry S.E. Targeted nanoparticles for detecting and treating cancer. Drug Dev Res. 2006;67:70–93. [Google Scholar]
- 62.Musacchio T., Torchilin V.P. Recent developments in lipid-based pharmaceutical nanocarriers. Front Biosci. 2011;16:1388–1412. doi: 10.2741/3795. [DOI] [PubMed] [Google Scholar]
- 63.Kleinstreuer C., Childress E., Kennedy A. Targeted drug delivery: multifunctional nanoparticles and direct micro-drug delivery to tumors. In: Becker S.M., Kuznetsov A.V., editors. Transport in biological media. Elsevier; Boston: 2013. pp. 391–416. [chapter 10] [Google Scholar]
- 64.Rohl ng S., Aurich M., Schöning T., Ho A.D., Witzens-Harig M. Nonpegylated liposomal doxorubicin as a component of R-CHOP is an effective and safe alternative to conventional doxorubicin in the treatment of patients with diffuse large B-cell lymphoma and preexisting cardiac diseases. Clin Lymphoma Myeloma Leuk. 2015;15(8):458–463. doi: 10.1016/j.clml.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 65.Dos Santos Giuberti C., de Oliveira Reis E.C., Ribeiro Rocha T.G., Leite E.A., Lacerda R.G., Ramaldes G.A., de Oliveira M.C. Study of the pilot production process of long-circulating and pH-sensitive liposomes containing cisplatin. J Liposome Res. 2011;21:60–69. doi: 10.3109/08982101003754377. [DOI] [PubMed] [Google Scholar]
- 66.Daemen T., Hofstede G., Ten Kate M.T., Bakker-Woudenberg I.A., Scherphof G.L. Liposomal doxorubicin-induced toxicity: depletion and impairment of phagocytic activity of liver macrophages. Int J Cancer. 1995;61:666–671. doi: 10.1002/ijc.2910610520. [DOI] [PubMed] [Google Scholar]
- 67.Gaca J.G., Palestrant D., Lukes D.J., Olausson M., Parker W., Davis R.D., Jr. Prevention of acute lung injury in swine: depletion of pulmonary intravascular macrophages using liposomal clodronate. J Surg Res. 2003;112:19–25. doi: 10.1016/s0022-4804(03)00142-2. [DOI] [PubMed] [Google Scholar]
- 68.Koo O.M., Rubinstein I., Onyuksel H. Actively targeted low-dose camptothecin as a safe, long-acting, disease-modifying nanomedicine for rheumatoid arthritis. Pharm Res. 2011;28:776–787. doi: 10.1007/s11095-010-0330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng X., Vladau C., Zhang X. A novel in vivo siRNA delivery system specifically targeting dendritic cells and silencing CD40 genes for immunomodulation. Blood. 2009;113:2646–2654. doi: 10.1182/blood-2008-04-151191. [DOI] [PubMed] [Google Scholar]
- 70.Beg S., Rizwan M., Sheikh A.M., Hasnain M.S., Anwer K., Kohli K. Advancement in carbon nanotubes: basics, biomedical applications and toxicity. J Pharm Pharmacol. 2011;63:141–163. doi: 10.1111/j.2042-7158.2010.01167.x. [DOI] [PubMed] [Google Scholar]
- 71.Shiba K., Yudasaka M., Iijima S. Carbon nanohorns as a novel drug carrier. Nihon Rinsho. 2006;64:239–246. [PubMed] [Google Scholar]
- 72.Gherman C., Tudor M.C., Constantin B. Pharmacokinetics evaluation of carbon nanotubes using FTIR analysis and histological analysis. J Nanosci Nanotechnol. 2015;15(4):2865–2869. doi: 10.1166/jnn.2015.9845. [DOI] [PubMed] [Google Scholar]