Abstract
Context
Individuals with idiopathic hypogonadotropic hypogonadism (IHH), even those with evidence of some hypothalamic reproductive endocrine activity, fail to complete puberty and fail to respond to physiologic doses of kisspeptin.
Objective
This case series examined whether treatment with sex steroids could stimulate kisspeptin responsiveness in patients with IHH.
Design
This was a case series.
Setting
This study was conducted at an academic medical center.
Participants
Seven patients with IHH were studied.
Intervention(s)
Participants, both on and off sex steroid therapy, underwent frequent blood sampling to measure LH at baseline, in response to kisspeptin and GnRH.
Main Outcome Measure(s)
The main outcome measure was LH responses to kisspeptin on and off sex steroids.
Results
All participants responded to exogenous GnRH, but no participant responded to exogenous kisspeptin. Sex steroid treatment did not modify responsiveness to kisspeptin.
Conclusions
The functional impairment of the GnRH neuronal network in patients with IHH, as evidenced by their inability to respond to a physiologic dose of kisspeptin, is observed in both sex steroid– deficient and sex steroid–replete states. In this case series, a normalized sex steroid milieu does not appear capable of overcoming the kisspeptin resistance of these patients.
Keywords: hypogonadotropic hypogonadism, kisspeptin, sex steroids
Since mutations in KISS1R were first associated with impaired pubertal development, numerous investigations have explored kisspeptin’s role as a gatekeeper of sexual maturation. Most GnRH neurons express the kisspeptin receptor, Kiss1r [1, 2]. As such, even at extremely low concentrations, kisspeptin stimulates GnRH secretion in mammalian species [1–5]. GnRH neurons also become more sensitive to kisspeptin as sexual maturation progresses, suggesting that changes in hormonal milieu may influence kisspeptin responsiveness [6–8].
Because of kisspeptin’s stimulatory capability in several animal species, exogenous kisspeptin administration has been used as a probe of GnRH neuronal function in physiologic and pathophysiologic states in the human. Kisspeptin stimulates GnRH-induced LH secretion in healthy volunteers [9–11] and in patients with congenital hypogonadotropic hypogonadism who have undergone reversal (spontaneous recovery of the reproductive cascade) [12]. In addition, kisspeptin stimulates LH secretion in patients with acquired hypogonadotropism (hypothalamic amenorrhea) [13]. However, kisspeptin fails to stimulate LH release in patients with abiding hypogonadotropic hypogonadism (no reversal) [14–16]. The idiopathic hypogonadotropic hypogonadism (IHH) kisspeptin “nonresponders” represent a spectrum of neuroendocrine phenotypes ranging from a complete absence of LH secretion to measurable but apulsatile LH levels [16]. Different pathomechanisms may underlie these subphenotypes. One possible explanation for an absence of LH secretion is an absence of GnRH neurons; in that setting, an inability to respond to exogenous kisspeptin would be expected. However, the presence of detectable LH levels—even if apulsatile—implies the existence of a GnRH neuronal cohort. In that setting, the inability to respond to exogenous kisspeptin is unexpected. Explaining the underlying lack of kisspeptin responsiveness in both of these IHH subpopulations—those with no underlying LH and those with some LH—is difficult to reconcile.
Against this backdrop, the relationship between kisspeptin and sex steroid levels is now appreciated to be increasingly complex. Kisspeptin administration results in GnRH-induced LH secretion and, by extension, LH-induced sex steroid synthesis. However, the association between kisspeptin and sex steroid levels is more than a simple, unidirectional relationship. Sex steroids are capable of augmenting kisspeptin signaling, supporting a bidirectional relationship between these hormones. Support for this hypothesis comes from a variety of models, including primate species. In vitro studies demonstrate enhanced kisspeptin responsiveness with sex steroids [17]. In the rhesus monkey, kisspeptin responsiveness changes from a state of sex steroid independence to sex steroid dependence across pubertal development [8]. In healthy women, both those of reproductive age and those in menopause, estradiol appears to modulate the ability to respond to kisspeptin [10, 18, 19]. In adolescents, testosterone treatment of individuals with delayed puberty has been associated with earlier initiation of sexual maturation compared with children who do not receive testosterone therapy [20, 21]. Although the exact mechanism underlying this observation is unknown, responsiveness to endogenous kisspeptin is one possible factor.
Given the data suggesting sex steroids may have a positive impact on kisspeptin responsiveness in patients with IHH, this study examined patients with IHH on and off of sex steroid therapy.
1. Materials and Methods
A. Patients and Eligibility Criteria
All studies were approved by the Institutional Review Board of Massachusetts General Hospital (MGH)/Partners Healthcare or by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland. Seven patients with IHH were enrolled in this study. IHH was defined as hypogonadal sex steroid levels (estradiol <20 pg/mL in women, testosterone <100 ng/dL in men) in the setting of low or inappropriately normal gonadotropin levels at age ≥18 years and in the absence of any identifiable medical condition that could have caused hypogonadotropic hypogonadism [22]. In this study, only patients who had previously participated in research studies in the Reproductive Endocrine Unit were enrolled. Specifically, study patients were required to have prior evidence by history, examination, genetics, or laboratory studies that suggested the capability to respond to kisspeptin. All individuals with ANOS1 (formerly, KAL1) mutations or prior evidence of severe midline facial defects were excluded as they were less likely to have GnRH neurons in the hypothalamus. Likewise, all individuals with homozygous or compound heterozygous KISS1R, GNRH1, and GNRHR mutations were excluded as they lack a component of the signaling cascade needed for a kisspeptin response. Study patients were included with genotypes that are likely to have GnRH neurons in the hypothalamus based on animal models, such as FGFR1 [23]. A specific effort was made to recruit study patients with prior detailed neuroendocrine phenotyping demonstrating endogenous LH secretion or an endogenously primed pituitary.
B. Genetics
Patients’ DNA was screened for rare sequence variants in CHD7 (MIM 608892), FGF8 (MIM 600483), FGFR1 (MIM 136350), GNRH1 (MIM 152760), GNRHR (MIM 138850), HS6ST1 (MIM 604846), ANOS1 (previously called KAL1, MIM 300836), KISS1 (MIM 603286), KISS1R (MIM 604161), NSMF (previously called NELF, MIM 60813), PROK2 (MIM 607002), PROKR2 (MIM 607123), TAC3 (MIM 162330), and TACR3 (MIM 162332) by PCR amplification of exons followed by Sanger sequencing, as described previously [24]. Rare sequence variants were defined as having a minor allele frequency of <0.1% in the Genome Aggregation Database, a resource of >123,136 exome sequences and 15,496 whole-genome sequences from unrelated individuals [25]. Rare sequence variants were reported if they were predicted to be damaging by at least two of three in silico prediction programs: PolyPhen-2 [26], SIFT [27], and Mutation Taster [28].
C. Source of Peptides
Kisspeptin 112–121, the 10-amino-acid isoform of kisspeptin (corresponding to amino acids 112 to 121 of the preprohormone), and GnRH were synthesized using good manufacturing practices by NeoMPS (PolyPeptide Laboratories, San Diego, CA). NeoMPS provided kisspeptin 112–121 under contract to the National Institute of Child Health and Human Development.
D. Baseline Neuroendocrine Phenotyping
Five of the seven study patients participated in a detailed phenotyping study prior to participating in the kisspeptin study. These studies consisted of inpatient visits to the National Institutes of Health Clinical Research Center or to the Harvard Catalyst Clinical Research Center (CRC) at MGH for blood sampling every 10 minutes to evaluate LH secretion for 12 hours overnight.
E. Neuroendocrine Phenotyping in Response to Kisspeptin and Sex Steroids
This study consisted of two visits to the Harvard Catalyst CRC at MGH. For one visit, patients were studied while using their prescribed clinical sex steroid treatment [testosterone or oral contraceptive (OC)]. For the other visit, patients withdrew from sex steroid treatment and were studied in the hypogonadal state (patients 4 and 6 did not complete this visit). Given the different half-lives of sex steroid therapies, this “washout” period was 2 weeks for participants receiving topical testosterone treatment, 6 weeks for participants receiving testosterone cypionate and/or testosterone enanthate, and 8 weeks for participants receiving OC treatment.
For each visit, participants were admitted to the CRC for blood sampling every 10 minutes to evaluate LH secretion for 12 to 14 hours. During both visits, all patients received at least one bolus of kisspeptin-10 at 0.24 nmol/kg. This dose was chosen as prior studies demonstrated that this dose consistently produces a GnRH-induced LH pulse of physiologic amplitude in healthy men and healthy luteal-phase women [9, 10]. Following administration of kisspeptin, all patients were administered a bolus of 75 ng/kg GnRH to assess pituitary gonadotrope function [16].
All but one patient underwent pituitary “priming” prior to undergoing studies at the CRC to ensure that the pituitary gonadotropes were capable of producing an LH pulse in response to exogenous or endogenous GnRH [29]. These patients underwent pituitary “priming” via a Crono F portable infusion pump (Canè S.p.A, Rivoli, Italy) administering GnRH 25 ng/kg subcutaneously every 2 hours for 5 to 7 days. The final dose of GnRH via the infusion pump was given 48 hours prior to CRC admission. One patient (patient 2) was known to have an endogenously primed pituitary based on prior physiologic research studies (ability to respond to exogenous GnRH without priming); therefore, the patient did not undergo further priming.
F. Laboratory Assays and Pulse Analysis
For both National Institutes of Health and MGH studies, LH was measured at each time point and on an all-study quality control pool; FSH was measured on 2-hour study pools. LH and FSH samples were processed using an automated Abbott ARCHITECT system or its predecessor, AXM (Abbott Laboratories, Inc., Abbott Park, IL). For the kisspeptin studies on patients 1, 3, 4, and 7, LH and FSH were run at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core using Siemens IMMULITE 2000 (Siemens Healthcare Diagnostics, Inc., Tarrytown, NY) using previously described assays [12, 16]. All paired studies were assayed in the same laboratory. We further tested the concordance between the two laboratories in 132 other samples representing a range of physiologic and pathophysiologic states and found close correlation (r2 = 0.98) [12].
For the kisspeptin studies, estradiol and testosterone were measured on 2-hour study pools by LabCorp, using electrochemiluminescence immunoassay methods (Roche Diagnostics, Indianapolis, IN) [30, 31]. For the baseline studies and kisspeptin studies on patients 2, 5, and 6, estradiol and testosterone were measured on a total study pool using validated assays traceable to mass spectroscopy as previously described [32, 33].
LH pulses were identified with a validated modification of the Santen and Bardin method [34, 35]. Pulse amplitude was calculated as the difference between time 0 of kisspeptin administration and peak of the pulse. If LH decreased after kisspeptin, the pulse amplitude was assigned a value of 0. P values <0.05 were considered significant. Values are represented as mean ± SD unless noted otherwise.
2. Results
A. Baseline Characteristics
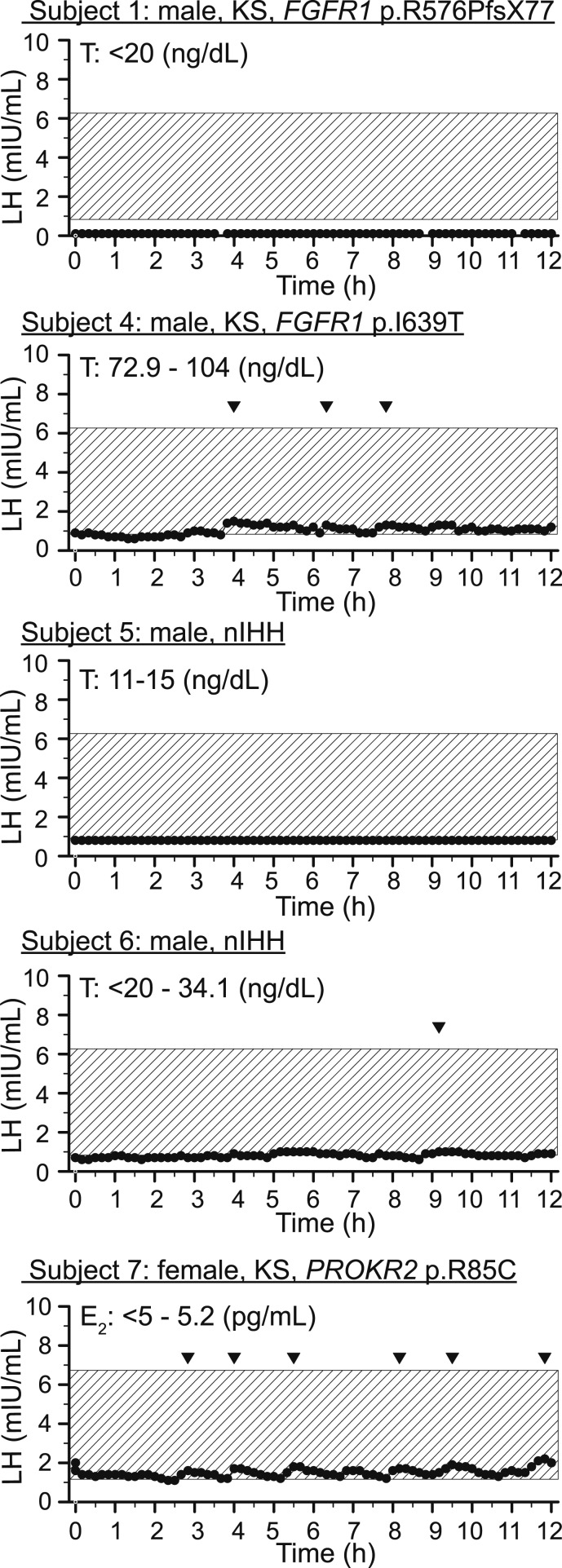
Six men and one woman with IHH receiving sex steroid therapy for clinical care participated in this study (Table 1). Prior reports suggest 41% of patients with IHH present with partial puberty development, and up to 22% of patients with IHH show some evidence of hypothalamic-pituitary-gonadal (HPG) axis activity in adulthood [36, 37]. In this case series, 85% of patients met one or both of these criteria demonstrating (1) spontaneous activation of the HPG cascade as evidenced by an increase in testicular volume, (2) LH pulses, (3) normal LH levels, or (4) all of the above (Table 2, Fig. 1). Three of seven patients demonstrated spontaneous LH pulses, and six of the seven demonstrated evidence for HPG activity in the absence of GnRH or gonadotropins therapy. The remaining patient with normosmic IHH was previously treated with GnRH pump therapy for fertility, so the natural history of his development could not be fully assessed. Consistent with the “reversal subphenotype” of IHH, one patient demonstrated testicular volume increases in the absence of gonadotropins after age 18 years, previously published in Lippincott et al. [12].
Table 1.
Clinical, Imaging, and Genetics of Study Patients
| ID |
Clinical Characteristics
|
Imaging
|
Genetics
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at Evaluation | Sex | Sense of Smell (by UPSIT) | LH, IU/L | FSH, IU/L | T (ng/dL) or E2 (pg/mL) | TV (mL) or Breast Development | Maximal TV, mL a | MRI of Olfactory Structures | Mutations in IHH Genes | MAF gnomAD, % | |
| 1 | 17 y 10 mo | M | Anosmic | <0.1 | 0.2 | <20 | -;2b | -;4b | Nl bulb and tract | FGFR1 p.R576PfsX77 | Novel |
| 2 | 21 y | M | Anosmic | 0.3 | 0.5 | 34 | 4;5 | 4;5 | No comment | FGFR1 p.G687R; | Novel |
| CHD7 p.K666T | Novel | ||||||||||
| 3 | 18 y | M | Anosmic | 1.0 | 1.0 | 20 | “Atrophic” | 10;8c | No comment | PROKR2 p.R85H | 0.07 |
| 4 | 18 y | M | Anosmic | 1.8 | 1.7 | 12 | 12;12 | 12;12 | Nl bulb and sulci | FGFR1 p.I639T | Novel |
| 5 | 27 y | M | Normosmic | <1.6 | <1.6 | 88 | 1;1 | 1;1 | Nl bulbs, nerves, and sulci | Exome negative | NA |
| 6 | 18y | M | Normosmic | Low | Low | Low | 3;3 | 20;25 | Nl bulb | Exome negative | NA |
| 7 | 22y | F | Anosmic | 5.0 | 5.0 | 17 | + thelarched | NA | Nl right bulb and nerve; left missing | PROKR2 p.R85C | 0.06 |
Abbreviations: E2, estradiol; F, female; gnomAD, Genome Aggregation Database; M, male; MAF, minor allele frequency; NA, not applicable; Nl, normal limit; T, testosterone; TV, testicular volume; UPSIT, University of Pennsylvania Smell Identification Test.
Maximal TV development achieved without gonadotropins or gonadotropin releasing hormone therapy.
Bilateral cryptorchidism treated with orchidopexy, at time of orchidopexy one testicular removed.
TV estimated due to unilateral varicocele.
Spontaneous thelarche in absence of gonadotropins.
Table 2.
Evidence for HPG Axis Activity
| ID | Developmental and Clinical Data |
|---|---|
| 1 | TV: 4 mL, intact olfactory structures |
| 2 | TV: 4–5 mL |
| 3 | TV: 8–10 mL |
| 4 | TV: 12 mL, LH pulses at baseline,a intact olfactory structures |
| 5 | LH within normal range, normosmic, intact olfactory structures |
| 6 | TV: 20–25 mL, normosmic, LH pulse at baselinea |
| 7 | Spontaneous thelarche, LH pulses at baselinea |
Abbreviation: TV, testicular volume.
See Fig. 1 for LH data.
Figure 1.
Neuroendocrine profiling of patients with IHH at baseline. Every 10-min blood sampling for LH overnight from 9 pm to 9 am. Shaded box represents normative LH data matched for sex [38, 39]. E2, estradiol; KS, Kallmann syndrome; nIHH, normosmic idiopathic hypogonadotropic hypogonadism; T, testosterone.
B. Genetics
All patients were screened for mutations in IHH genes to ensure that they did not have any genetic variants that would preclude a response to kisspeptin (Table 1). Five of the seven patients carried rare sequence variants in known IHH genes predicted to be deleterious. The other two study patients were negative on whole-exome sequencing. Patients 1, 2, and 4 were found to harbor rare sequence variants within FGFR1 (Table 1). Patient 2 harbored one rare variant in FGFR1 as well as an additional variant in CHD7, which is associated with CHARGE syndrome (a syndrome with multiple congenital anomalies including coloboma of the eye, choanal atresia, hearing loss, heart defects, and hypogonadism): CHD7 c.1997A>C p.K666T; FGFR1 c.2059G>A p.G687R. Despite the presence of this CHD7 variant, patient 3 did not have any of the signs or symptoms of CHARGE aside from IHH [40]. Patient 7 had a nonnovel (minor allele frequency 0.06% in non-Finnish Europeans) variant in PROKR2 c.253C>T p.R85C, which was predicted to be damaging in all prediction programs [25]. This variant displayed variable expressivity as it was also found in the patient’s father, who had severe hyposmia, and her brother, who had delayed puberty. Patient 3 had a nonnovel (minor allele frequency 0.1% in non-Finnish Europeans) PROKR2 variant c.254G>A p.R85H. In the entire cohort, no patients carried variants in KISS1R, GNRH1, or GNRHR, suggesting that all participants possessed the receptors and ligands needed to respond to exogenously administered kisspeptin and GnRH. In addition, no patient carried a variant in ANOS1/KAL1, which could result in failure of GnRH neurons to reach the hypothalamus [41].
C. Pituitary Function
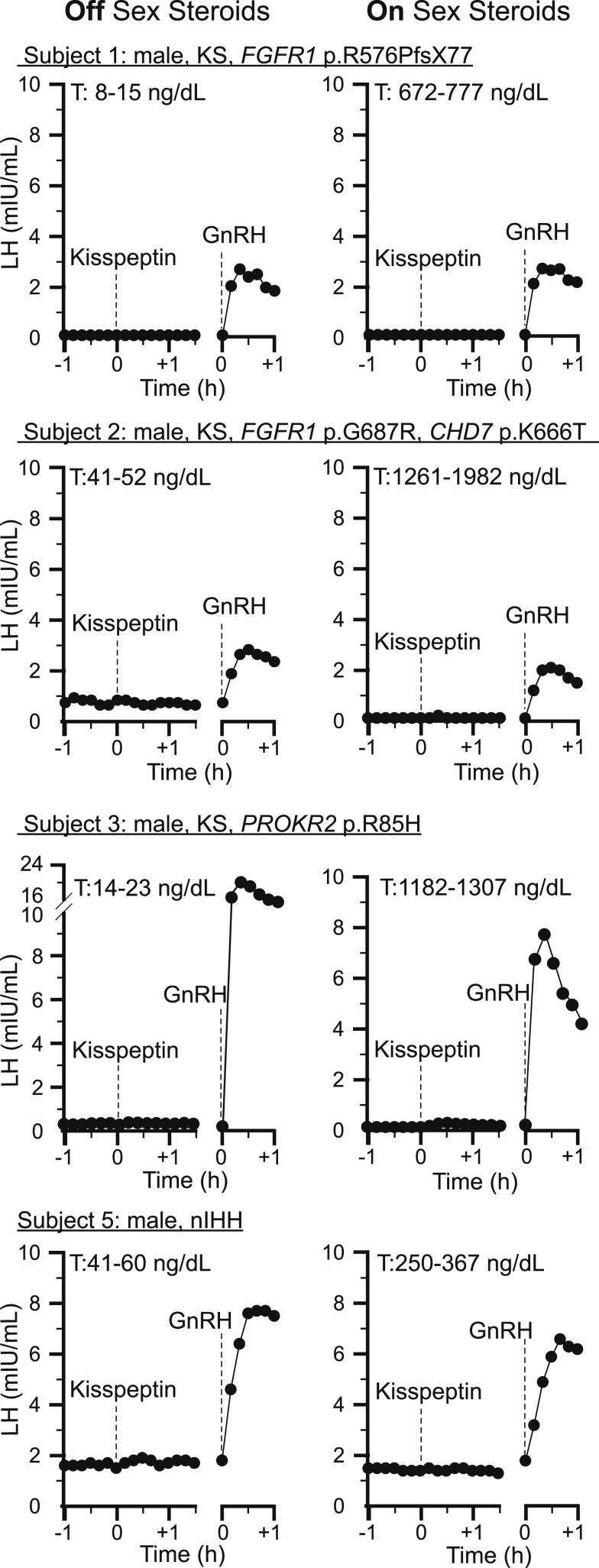
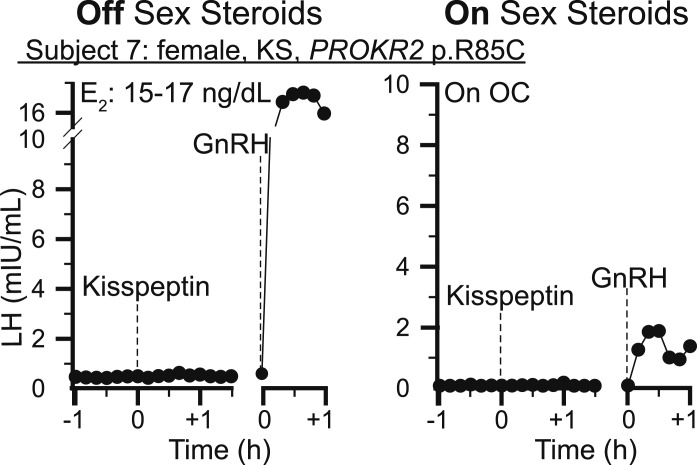
Because a lack of an LH response to kisspeptin could be due to failure to respond to kisspeptin or failure to respond to GnRH, testing was done to ensure the functional integrity of the pituitary gonadotropes. All patients underwent pituitary priming, except patient 2, who had prior evidence of endogenous GnRH secretion [16]. Exogenous pituitary priming was halted prior to study start to ensure baseline LH levels during the study represented endogenous activity. To confirm gonadotrope integrity during the study, a GnRH bolus was administered. All male patients demonstrated robust responses of a similar magnitude to exogenous GnRH off and on sex steroid therapy (Figs. 2 and 3: range LH amplitude; off testosterone: 2.2 to 23 mIU/mL; on testosterone: 2 to 13 mIU/mL). One female participant, patient 7, demonstrated an LH pulse in response to GnRH that was smaller on supraphysiologic sex steroids than off sex steroids due to estrogen-negative feedback at the level of the pituitary (Fig. 4: LH pulse amplitude; off OCs: 18.1 mIU/mL; on OCs: 1.8 mIU/mL).
Figure 2.
Neuroendocrine profiling in response to neuropeptide probes in men with IHH on and off sex steroids. Every 10-min blood sampling for LH. Kisspeptin-10 IVB 0.24 nmol/kg. GnRH IVB 75 ng/kg. KS, Kallmann syndrome; nIHH, normosmic idiopathic hypogonadotropic hypogonadism; T, testosterone.
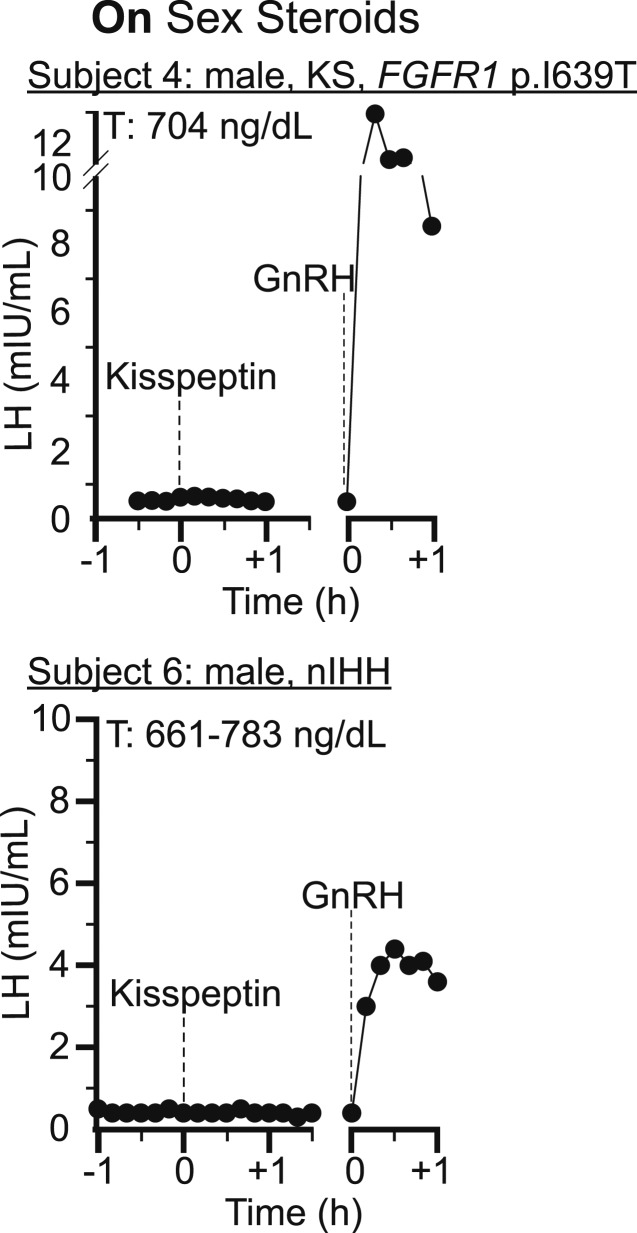
Figure 3.
Neuroendocrine profiling in response to neuropeptide probes in men with IHH on sex steroid therapy. Every 10-min blood sampling for LH. Kisspeptin-10 IVB 0.24 nmol/kg. GnRH IVB 75 ng/kg. KS, Kallmann syndrome; nIHH, normosmic idiopathic hypogonadotropic hypogonadism; T, testosterone.
Figure 4.
Neuroendocrine profiling in response to neuropeptide probes in a woman with KS. Kisspeptin-10 IVB 0.24 nmol/kg. GnRH IVB 75 ng/kg. E2, estradiol; KS, Kallmann syndrome.
D. Kisspeptin Responsiveness
When studied off sex steroid therapy, no patient responded to kisspeptin-10 0.24 nmol/kg, consistent with prior published data in individuals with IHH (Figs. 2–4; Table 3) [16]. This dose of kisspeptin-10 elicits a robust response in healthy men, luteal-phase women, and men whose IHH has reversed [9, 10, 12]. To determine whether sex steroid therapy could augment kisspeptin responsiveness, all study patients repeated the protocol on sex steroid therapy. Despite the restoration of the sex steroid milieu, no patient responded to kisspeptin-10 0.24 nmol/kg. Furthermore, there was no difference in the maximal increase in LH within 30 minutes after kisspeptin-10 administration whether on or off sex steroid therapy (LH increase off sex steroids range, 0.0 to 0.4 mIU/mL; LH increase on sex steroids range, 0.0 to 0.2 mIU/mL).
Table 3.
Neuroendocrine Phenotyping and LH Response to IVB Kisspeptin
| ID |
Off Sex Steroid Treatment
|
On Sex Steroid Treatment
|
||||||
|---|---|---|---|---|---|---|---|---|
| LH, IU/L | FSH, IU/L | T (ng/dL) or E2 (pg/mL) | LH Kisspeptin 10 Response a | LH, IU/L | FSH, IU/L | T (ng/dL) or E2 (pg/mL) | LH Kisspeptin 10 Response a | |
| 1 | 0.1 | 0.1 | 15 | 0.0 | 0.1 | 0.2 | 672 | 0.0 |
| 2 | 1.0 | 1.0 | 47 | 0.0 | 0.1 | 0.1 | 1982 | 0.1 |
| 3 | 0.2 | 0.7 | 19 | 0.1 | 0.1 | 0.3 | 1182 | 0.2 |
| 4 | 0.5 | 1.0 | 704 | 0.0 | ||||
| 5 | 1.6 | 0.5 | 60 | 0.4 | 1.5 | 0.5 | 358 | 0.1 |
| 6 | 0.6 | 0.9 | 688 | 0.0 | ||||
| 7 | 0.4 | 1.5 | <5.0 | 0.1 | 0.1 | 0.1 | OCP | 0.0 |
FSH, T, and E2 on 2-h am pools.
Abbreviations: E2, estradiol; OCP, OC pill; T, testosterone.
Peak LH within 30 min of kisspeptin administration subtracted from LH at time of kisspeptin administration. Negative values reported as zero.
3. Discussion
This study sought to test the hypothesis that sex steroids can modify kisspeptin responsiveness. Kisspeptin can stimulate GnRH-induced LH secretion in a variety of physiologic and pathophysiologic states. However, patients with IHH (with one exception) appear to be resistant to exogenous kisspeptin administration at the same doses that are able to bring about GnRH-induced LH release in other healthy populations [9–11, 16, 42].
The hypothesis that sex steroids might affect the ability of GnRH neurons to respond to kisspeptin has been suggested in a number of human models. Pediatric endocrinologists treat patients with constitutional delay of puberty (CDP) with sex steroids to correct their transient hypogonadotropic hypogonadism. Furthermore, some believe that sex steroid treatment may stimulate the HPG axis to accelerate the onset of “endogenous” puberty. For example, testosterone-treated boys with CDP had larger testicular size and endogenous testosterone levels 6 months after treatment compared with untreated boys with CDP, suggesting spontaneous activation of the HPG axis [21]. Although there have yet to be large-scale randomized controlled trials of the sex steroid treatment of patients with delayed puberty, based on this and other similar observations, many believe that sex steroids “jumpstart” sexual maturation. The mechanisms that underlie such an association remain unknown, but a change in the ability to respond to kisspeptin is one possibility.
In this case series, we selected patients with IHH to test the hypothesis that sex steroids can modify kisspeptin responsiveness. Because IHH is much rarer in women than in men, with an almost 4:1 male predominance, only one of the patients with IHH was female [43]. Unlike patients with classic complete IHH, participants in this study had subtleties within their presentations that suggested they might have a capacity to respond to kisspeptin. Eighty-five percent of patients had evidence for HPG axis activity by their history, physical examination, and laboratory studies, suggesting the existence of a functioning complement of GnRH neurons at least at an earlier timepoint. This HPG activity was manifested by increased testicular volume in three individuals; this testicular growth in the absence of fertility drugs is a common presentation of the reversal subphenotype in IHH [37]. In addition, five of seven patients had detectable LH secretion at the time of the every 10-minute blood sampling studies (baseline or kisspeptin study). Although clearly not pulsatile, these LH levels are evidence for some form of attenuated GnRH secretion. A complement of neurons capable of secreting GnRH, even at low levels, might be a complement capable of responding to exogenous kisspeptin.
In addition to their histories and neuroendocrine patterns, all seven patients with IHH had previously undergone genetic testing to look for rare variants in the genes that have been associated with IHH/Kallmann syndrome. None of the patients carried deleterious variants in genes that encode proteins involved in kisspeptin or GnRH signaling. In addition, none of the patients carried variants in ANOS1/KAL1, which has been associated with an absence of GnRH neurons in the brain [41]. Therefore, genetic testing did not reveal any mechanism that would have precluded the ability to respond to kisspeptin.
Despite all of these factors, these patients with IHH (six male and one female), once sex steroid replete, did not show a response to exogenous kisspeptin. Stated alternatively, a normalized sex steroid milieu was not capable of overcoming the kisspeptin resistance (simply meaning a failure to respond to exogenous administration of the peptide) of these patients. Because the administration of kisspeptin should bypass any abnormalities in the synthesis or secretion of kisspeptin itself or any factors upstream from kisspeptin, it is possible that the inability to respond to exogenous kisspeptin is due to problems further downstream. These include, but are not limited to, abnormalities in kisspeptin receptor synthesis, expression, trafficking, or downstream signaling. The latter could include abnormalities in second-messenger signaling systems within GnRH neurons related specifically to kisspeptin, abnormalities within GnRH neurons completely unrelated to kisspeptin signaling, or a problem related to other cofactors/neurotransmitters that affect GnRH neuronal synchronization.
Thus, despite the existing data suggesting sex steroids may have a positive impact on kisspeptin responsiveness, patients with congenital hypogonadotropism and evidence for functional GnRH neurons appear to be resistant to kisspeptin despite exogenous sex steroid treatment. Therefore, in this population, sex steroids do not appear to have the capability of augmenting kisspeptin signaling.
Acknowledgments
We thank the research patients, members of the MGH Reproductive Endocrine Unit for discussions and reading of the manuscript, staff of the Harvard Catalyst CRC for assistance with the frequent sampling studies, the MGH Investigational Drug Service, MGH Clinical Laboratory Research Core, and University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. We also thank physicians who referred research patients: Susan Kirsch and Barry Reiner.
Financial Support: This work was supported by grants R01 HD043341 (to S.B.S.) and P50 HD-28138 from the Eunice K. Shriver National Institute for Child Health and Human Development (NICHD) and the Harvard Catalyst | Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Awards UL1 RR 025758 and UL1 TR000170 and financial contributions from Harvard University and its affiliated academic health care centers). Support was also provided by a Doris Duke Clinical Scientist Development Award (grant 2013110; Y.-M.C.), the NICHD Intramural Research Program (to A.D.), a Catalyst Medical Research Investigator Training Award from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers (to M.F.L.), National Institutes of Health NICHD Grant F32 HD078083; to M.F.L., and Postdoctoral Fellowship Award for Clinical Research from the MGH Executive Committee on Research Fund for Medical Discovery (to M.F.L.). S.B.S. is a Robert and Laura Reynolds Research Scholar. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver NICHD/NIH (NCTRI) Grant P50-HD28934.
Clinical Trial Information: ClinicalTrials.gov nos. NCT00494169 (registered 29 June 2007) and NCT00914823 (registered 5 June 2009).
Disclosure Summary: The authors have nothing to declare.
Glossary
Abbreviations:
- CDP
constitutional delay of puberty
- CRC
Clinical Research Center
- HPG
hypothalamic-pituitary-gonadal
- IHH
idiopathic hypogonadotropic hypogonadism
- MGH
Massachusetts General Hospital
- OC
oral contraceptive
References and Notes
- 1. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2005;80(4):264–272. [DOI] [PubMed] [Google Scholar]
- 2. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102(5):1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–4077. [DOI] [PubMed] [Google Scholar]
- 4. Kadokawa H, Matsui M, Hayashi K, Matsunaga N, Kawashima C, Shimizu T, Kida K, Miyamoto A. Peripheral administration of kisspeptin-10 increases plasma concentrations of GH as well as LH in prepubertal Holstein heifers. J Endocrinol. 2008;196(2):331–334. [DOI] [PubMed] [Google Scholar]
- 5. Lents CA, Heidorn NL, Barb CR, Ford JJ. Central and peripheral administration of kisspeptin activates gonadotropin but not somatotropin secretion in prepubertal gilts. Reproduction. 2008;135(6):879–887. [DOI] [PubMed] [Google Scholar]
- 6. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guerriero KA, Keen KL, Millar RP, Terasawa E. Developmental changes in GnRH release in response to kisspeptin agonist and antagonist in female rhesus monkeys (Macaca mulatta): implication for the mechanism of puberty. Endocrinology. 2012;153(2):825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan YM, Butler JP, Pinnell NE, Pralong FP, Crowley WF Jr, Ren C, Chan KK, Seminara SB. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab. 2011;96(6):E908–E915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan YM, Butler JP, Sidhoum VF, Pinnell NE, Seminara SB. Kisspeptin administration to women: a window into endogenous kisspeptin secretion and GnRH responsiveness across the menstrual cycle. J Clin Endocrinol Metab. 2012;97(8):E1458–E1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jayasena CN, Nijher GM, Comninos AN, Abbara A, Januszewki A, Vaal ML, Sriskandarajah L, Murphy KG, Farzad Z, Ghatei MA, Bloom SR, Dhillo WS. The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. J Clin Endocrinol Metab. 2011;96(12):E1963–E1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lippincott MF, Chan YM, Delaney A, Rivera-Morales D, Butler JP, Seminara SB. Kisspeptin responsiveness signals emergence of reproductive endocrine activity: implications for human puberty. J Clin Endocrinol Metab. 2016;101(8):3061–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jayasena CN, Nijher GM, Abbara A, Murphy KG, Lim A, Patel D, Mehta A, Todd C, Donaldson M, Trew GH, Ghatei MA, Bloom SR, Dhillo WS. Twice-weekly administration of kisspeptin-54 for 8 weeks stimulates release of reproductive hormones in women with hypothalamic amenorrhea. Clin Pharmacol Ther. 2010;88(6):840–847. [DOI] [PubMed] [Google Scholar]
- 14. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 16. Chan YM, Lippincott MF, Butler JP, Sidhoum VF, Li CX, Plummer L, Seminara SB. Exogenous kisspeptin administration as a probe of GnRH neuronal function in patients with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2014;99(12):E2762–E2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149(4):1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Narayanaswamy S, Jayasena CN, Ng N, Ratnasabapathy R, Prague JK, Papadopoulou D, Abbara A, Comninos AN, Bassett P, Bloom SR, Veldhuis JD, Dhillo WS. Subcutaneous infusion of kisspeptin-54 stimulates gonadotrophin release in women and the response correlates with basal oestradiol levels. Clin Endocrinol (Oxf). 2015;84(6):939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lippincott MF, Chan YM, Rivera Morales D, Seminara SB. Continuous kisspeptin administration in postmenopausal women: impact of estradiol on luteinizing hormone secretion. J Clin Endocrinol Metab. 2017;102(6):2091–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lawaetz JG, Hagen CP, Mieritz MG, Blomberg Jensen M, Petersen JH, Juul A. Evaluation of 451 Danish boys with delayed puberty: diagnostic use of a new puberty nomogram and effects of oral testosterone therapy. J Clin Endocrinol Metab. 2015;100(4):1376–1385. [DOI] [PubMed] [Google Scholar]
- 21. Soliman AT, Khadir MM, Asfour M. Testosterone treatment in adolescent boys with constitutional delay of growth and development. Metabolism. 1995;44(8):1013–1015. [DOI] [PubMed] [Google Scholar]
- 22. Hoffman AR, Crowley WF Jr. Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med. 1982;307(20):1237–1241. [DOI] [PubMed] [Google Scholar]
- 23. Chung WC, Tsai PS. Role of fibroblast growth factor signaling in gonadotropin-releasing hormone neuronal system development. Front Horm Res. 2010;39:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sykiotis GP, Plummer L, Hughes VA, Au M, Durrani S, Nayak-Young S, Dwyer AA, Quinton R, Hall JE, Gusella JF, Seminara SB, Crowley WF Jr, Pitteloud N. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci USA. 2010;107(34):15140–15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lek M, Karczewski K, Minikel E, Samocha K, Banks E, Fennell T, O’Donnell-Luria A, Ware J, Hill A, Cummings B, Tukiainen T, Birnbaum D, Kosmicki J, Duncan L, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Cooper D, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki M, Levy Moonshine A, Natarajan P, Orozco L, Peloso G, Poplin R, Rivas M, Ruano-Rubio V, Ruderfer D, Shakir K, Stenson P, Stevens C, Thomas B, Tiao G, Tusie-Luna M, Weisburd B, Won H-H, Yu D, Altshuler D, Ardissino D, Boehnke M, Danesh J, Roberto E, Florez J, Gabriel S, Getz G, Hultman C, Kathiresan S, Laakso M, McCarroll S, McCarthy M, McGovern D, McPherson R, Neale B, Palotie A, Purcell S, Saleheen D, Scharf J, Sklar P, Patrick S, Tuomilehto J, Watkins H, Wilson J, Daly M, MacArthur D. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31(13):3812–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7(8):575–576. [DOI] [PubMed] [Google Scholar]
- 29. Crowley WF., Jr An overview of LHRH and its analogues: clinical uses. Ups J Med Sci. 1984;89(1):3–12. [DOI] [PubMed] [Google Scholar]
- 30.Roche Diagnostics. Elecsys Estradiol III Assay. 2016. http://www.rochecanada.com/content/dam/roche_canada/en_CA/documents/package_inserts/ESTRADIOL%20III%20_06656021190_CAN_V4_EN-final.pdf. Accessed 20 November 2017.
- 31. Oldekamp JSW, Roth HJ, Gassner D. 2009 Elecsys Testosterone II: New Immunoassay With Improved Performance for the Measurement of Testosterone in Women. Innsbruck, Austria: Euromedlab; 2009. [Google Scholar]
- 32. Sluss PM, Hayes FJ, Adams JM, Barnes W, Williams G, Frost S, Ramp J, Pacenti D, Lehotay DC, George S, Ramsay C, Doss RC, Crowley WF Jr. Mass spectrometric and physiological validation of a sensitive, automated, direct immunoassay for serum estradiol using the Architect. Clin Chim Acta. 2008;388(1–2):99–105. [DOI] [PubMed] [Google Scholar]
- 33. Radicioni A, Lenzi A, Spaziani M, Anzuini A, Ruga G, Papi G, Raimondo M, Foresta C. A multicenter evaluation of immunoassays for follicle-stimulating hormone, luteinizing hormone and testosterone: concordance, imprecision and reference values. J Endocrinol Invest. 2014;36(9):739–744. [DOI] [PubMed] [Google Scholar]
- 34. Santen RJ, Bardin CW. Episodic luteinizing hormone secretion in man: pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest. 1973;52(10):2617–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hayes FJ, McNicholl DJ, Schoenfeld D, Marsh EE, Hall JE. Free alpha-subunit is superior to luteinizing hormone as a marker of gonadotropin-releasing hormone despite desensitization at fast pulse frequencies. J Clin Endocrinol Metab. 1999;84(3):1028–1036. [DOI] [PubMed] [Google Scholar]
- 36. Sidhoum VF, Chan YM, Lippincott MF, Balasubramanian R, Quinton R, Plummer L, Dwyer A, Pitteloud N, Hayes FJ, Hall JE, Martin KA, Boepple PA, Seminara SB. Reversal and relapse of hypogonadotropic hypogonadism: resilience and fragility of the reproductive neuroendocrine system. J Clin Endocrinol Metab. 2014;99(3):861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pitteloud N, Hayes FJ, Boepple PA, DeCruz S, Seminara SB, MacLaughlin DT, Crowley WF Jr. The role of prior pubertal development, biochemical markers of testicular maturation, and genetics in elucidating the phenotypic heterogeneity of idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87(1):152–160. [DOI] [PubMed] [Google Scholar]
- 38. Crowley WF Jr, Filicori M, Spratt DI, Santoro NF. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res. 1985;41:473–531. [DOI] [PubMed] [Google Scholar]
- 39. Hall JE, Schoenfeld DA, Martin KA, Crowley WF Jr. Hypothalamic gonadotropin-releasing hormone secretion and follicle-stimulating hormone dynamics during the luteal-follicular transition. J Clin Endocrinol Metab. 1992;74(3):600–607. [DOI] [PubMed] [Google Scholar]
- 40. Verloes A. Updated diagnostic criteria for CHARGE syndrome: a proposal. Am J Med Genet A. 2005;133A(3):306–308. [DOI] [PubMed] [Google Scholar]
- 41. Schwanzel-Fukuda M, Bick D, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH)–expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res. 1989;6(4):311–326. [DOI] [PubMed] [Google Scholar]
- 42. Young J, George JT, Tello JA, Francou B, Bouligand J, Guiochon-Mantel A, Brailly-Tabard S, Anderson RA, Millar RP. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological, pathophysiological and therapeutic implications. Neuroendocrinology. 2013;97(2):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laitinen EM, Vaaralahti K, Tommiska J, Eklund E, Tervaniemi M, Valanne L, Raivio T. Incidence, phenotypic features and molecular genetics of Kallmann syndrome in Finland. Orphanet J Rare Dis. 2011;6(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]