Abstract
The specific objective of this study was to test the clinically derived hypothesis associating a high prevalence of depression in young men with nonclassical hypogonadism. We studied the entire population of men aged 18 to 40 years who had an outpatient visit at an academic health system in the years 2013 to 2015. The study group comprised 186 patients with a diagnosis of eugonadotropic hypogonadism and a testosterone value below 10.4 nmol/L with no apparent cause. We compared their demographic factors, other diagnoses, and treatments with those of (i) the entire population, (ii) a matched population of 930 controls, and (iii) 404 controls with normal testosterone determinations, and no hypogonadism diagnosis. Depression, defined as either an International Classification of Diseases, Ninth Revision (ICD-9) diagnosis or treatment with an antidepressant medication, was found in 22.6% of cases vs 6.6% of population controls [P < 0.001; OR: 1.13 (1.09 to 1.17); 95% CI]. Obesity was also higher in the cases (P < 0.001). The matched controls had a depression rate of 13.4% compared with the case rate of 22.6% [P < 0.002; OR 1.14 (1.08 to 1.17)]. Controls with normal testosterone determinations had a depression rate of 16.8% [P = 0.121; OR: 1.04 (0.96 to 1.12)], suggesting that clinicians may have ordered a testosterone determination because of symptoms consistent with both depression and hypogonadism. The high incidence of depression in nonclassical hypogonadism in young men, although only associative, supports a depression evaluation and treatment as appropriate as well as investigation of the proximate causes of this form of hypogonadism.
Keywords: depression, hypogonadism, non-classical, young men
Clinicians regularly face the diagnostic dilemma of fully mature adult men presenting with recent onset of fatigue, sleep disturbance, loss of libido, and exercise intolerance. These symptoms are consistent with depression and hypogonadism, precipitating testosterone and gonadotropin measurements. The diagnosis of hypogonadism requires a low total, free, or bioavailable testosterone level. When associated with normal gonadotropins, it is termed “central” hypogonadism. The completion of puberty and moderate hormonal declines tend to rule out genetic hypothalamic hypogonadism. Hypogonadism of this type often has a presumed etiology, including traumatic brain injury, brain or pituitary tumors, congenital anomalies, active HIV infection, active neoplasm and chemotherapy, or chronic opioid administration, or follows a period of anabolic steroid use [1]. However, many symptomatic men, especially those under the age of 40 years, do not have an apparent cause.
Conflicting evidence exists on whether patients with hypogonadism have increased depression, whether patients with depression have a high prevalence of hypogonadism, and whether treatment with testosterone ameliorates depression. Both the Massachusetts Male Aging Study [2] and the Veterans Experience Study [3] demonstrated minimal correlation between testosterone and depression. However, patients with hypogonadism who are referred to andrology experts may be high in depression [4, 5]. These correlative studies included mainly older individuals with multiple cofactors that could interfere with gonadal function and did not relate the findings to a representative population. The prevalence of hypogonadism in depressed populations was variable as reviewed in two studies [2, 3]. Many men with depression with and without hypogonadism received treatment with testosterone based on the assumption that the hypogonadism was causative, with variable results as elaborated in the following sections.
We hypothesized that a population of men 18 to 40 years of age and carrying a diagnosis of eugonadotropic hypogonadism with no obvious etiology would have a significantly greater rate of depression than controls. If true, that would raise the question of a mechanistic relationship between depression and hypogonadism analogous to that found in hypothalamic amenorrhea in women [4] and would emphasize the importance of diagnosing and treating depression in the hypogonadal population.
1. Methods
A. Study Setting and Data Sources
We conducted a retrospective medical record review of the clinical database of a large urban academic health system. The system includes physicians’ offices as well as hospitals across the metropolitan area connected by an integrated electronic medical record platform. The system accepts patients from a wide variety of insurance carriers as well as specific referrals for specialized care. The study included de-identified data derived from patient records from January 2013 through December 2015. The institutional review board agreed that this anonymized retrospective review did not require its approval.
We based the diagnoses of hypogonadism and depression on International Classification of Diseases, Ninth Revision (ICD-9) codes, which represented the operative diagnostic coding system in the United States and elsewhere in the years 2013 to 2015. Patient cases were required to have a documented total testosterone level below 10.4 nmol/L (300 ng/dL) with FSH levels below 12.4 mIU/mL and LH levels below 8.6 mIU/mL, if recorded, to rule out primary testicular failure. The testosterone values were from two commercial sources: Esoterix, normal range 12.1 to 41.5 nmol/L (348 to 1197 ng/dL), and Quest, testosterone normal range 8.7 to 38.2 nmol/L (251 to 1102 ng/dL) and free testosterone by radioimmunoassay at 1.2 to 5.4 nmol/L (35 to 155 ng/dL). This was before harmonization of testosterone values [5], so we used the range of 10.4 to 38.2 nmol/L (300 to 1100 ng/dL). The bioavailable testosterone values were all from Esoterix: normal range 4.4 to 15 nmol/L (128 to 430 ng/dL). Controls included all ambulatory male patients aged 18 to 40 years without a diagnosis of hypogonadism. Patients were excluded from the study as both cases and controls if they had ICD-9 comorbidities in their problem lists that could affect gonadal function, including endocrine disorders, kidney disease, malignancy, and a transplant, as well as hospitalization during the study interval.
The variables obtained from the medical records included the encounter diagnosis, age, weight, body mass index (BMI), race, ethnicity, tobacco use status, alcohol use status, opiate use, comorbidities, medications, and laboratory values including testosterone and gonadotropin levels. The definition of depression included either a diagnosis based on a set of depression ICD-9 codes or a record of antidepressant medication prescription. It is possible that some of the patients were taking these drugs for a diagnosis other than depression; more likely, however, some patients had asked that the stigma of depression not be entered in the medical record, and some physicians did not identify depression as a reason for the encounter. Missing observations were not imputed.
B. Statistical Methods
This retrospective cohort study used collated, anonymized clinical data. We ascertained antidepressant use from the current medication list in comparison with a list of antidepressant medications. Tobacco use was formatted as current, former, never, and unknown. We formatted alcohol use as current user, not current user, and unknown. We obtained opiate use from the medication list. BMI conversion into weight status categories followed Centers for Disease Control and Prevention recommendations [6].
We compared three nonhypogonadal cohorts with the cases: (i) all ambulatory patients without a diagnosis of hypogonadism or a low testosterone level meeting the remaining inclusion and exclusion criteria; (ii) a matched cohort for comparison of outcomes and accounting for unbalanced covariates (variables included in the matching were age, BMI, race, ethnicity, alcohol use, opiate use, and tobacco history, and the matching algorithm was a 1-to-5 propensity score model with nearest neighbor matching); (iii) the set of patients with a documented normal testosterone level during the study period and no diagnosis of hypogonadism.
We summarized data as mean with SD for quantitative measures or number with percentage for categorical measures. Univariate analysis used a t test or χ2 test as appropriate. ANOVA compared total testosterone levels across BMI subgroups. We constructed multivariable logistic regression models to identify predictors of the outcome of depression for each of the case/control comparison cohorts. For each model, we included the effect of hypogonadism while adjusting for confounders used in the matching algorithm. For each categorical variable with missing data, missingness was coded as unknown and was included as a category in the multiple regression model. All statistical tests were two-sided, and a P value below 0.05 indicated statistical significance. We analyzed the data using the R Statistical Computing Environment (R Core Team, Vienna, Austria).
2. Results
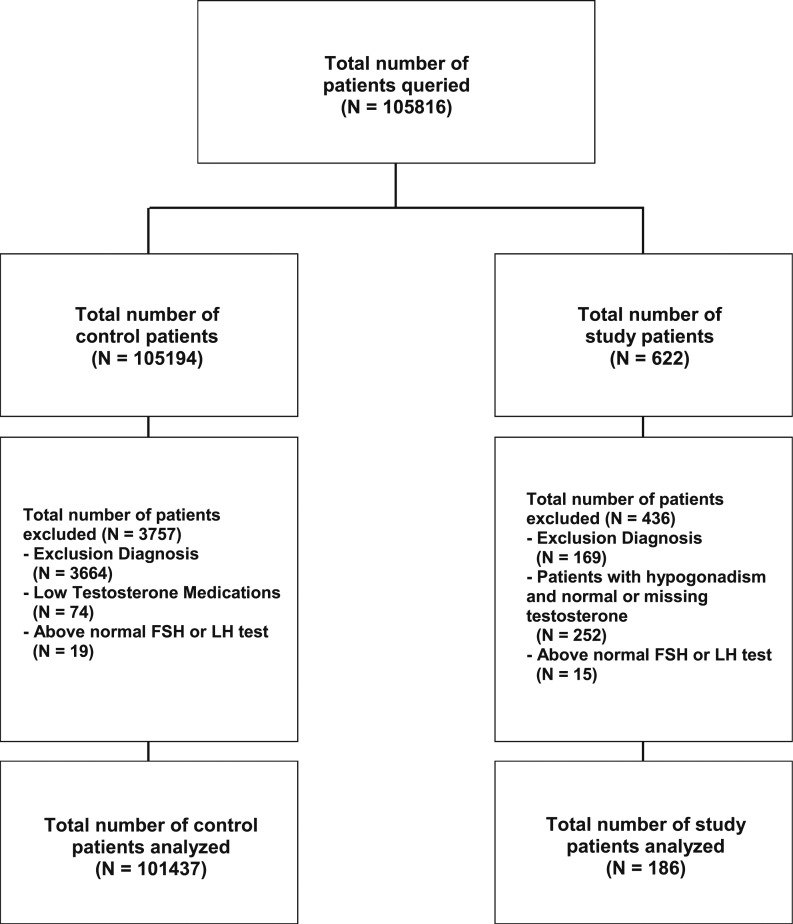
The initial population consisted of 105,816 males from 18 to 40 years of age seen in an ambulatory setting (Fig. 1). Six hundred twenty-two patients were eligible to be cases because they had a diagnosis of hypogonadism. Of these, 436 patients had to be eliminated, including 169 patients with exclusion diagnoses, 252 patients who never had a low testosterone level because they were already receiving therapy when first seen or had no recorded testosterone value in the system, and 15 patients with a high FSH or LH value, leaving 186 cases. Of the 105,194 original control patients (no diagnosis of hypogonadism), 3757 were excluded, including 3664 with exclusionary diagnoses, 74 who were taking testosterone medication, and 19 with a high LH or FSH level, leaving 101,437. Thus, control groups included (1) 101,437 patients for the entire nonhypogonadal population, (2) 930 members of the control population matched by age, BMI, race, ethnicity, and alcohol, opiate, and tobacco consumption to the hypogonadal cases, and (3) 404 members of the control group with no hypogonadism diagnosis and with reported testosterone values that were consistently normal.
Figure 1.
Allocation of controls and patients with hypogonadism on the basis of a recorded total testosterone value <10.4 nmol/L or a diagnosis of hypogonadism. Excluded from each group were patients with exclusion diagnoses or with elevated FSH or LH levels; excluded from the study group were those without a recorded low testosterone value.
A. Study Population Compared With the Overall Control Population
Compared to the study population, the population of ambulatory control patients were younger, less obese, and more likely to be a current tobacco user (Table 1). There were no observed differences in race, ethnicity, or history of alcohol or opiate use. The depression rate was 6.6% in the controls vs 22.6% in the cases. After adjustments for clinical and demographic factors, the OR for the presence of a depression diagnosis or antidepressant medication use in the medical record was 95% CI: 1.13 (CI: 1.09 to 1.17; P < 0.001) in patients with hypogonadism compared with the overall control population. When corrected for age alone (not shown), the difference between the controls and cases remained the same [OR = 1.13 (1.07 to 1.18); P < 0.001; 95% CI].
Table 1.
Comparisons of Hypogonadal Cases With Three Control Groups
| Variable | Case (n = 186) | Unmatched Control (n = 101,437) | Unmatched P Value | Matched Control (n = 930) | Matched P Value | Known Testosterone Control (n = 404) | Known Testosterone Control P Value |
|---|---|---|---|---|---|---|---|
| Age, y, mean (SD) | 35.1 (5.1) | 29.6 (6.6) | <0.001 | 35.1 (5.2) | 0.925 | 32.6 (5.8) | <0.001 |
| BMI | <0.001 | 0.993 | <0.001 | ||||
| Obese | 76 (42.5%) | 7858 (15.6%) | 373 (41.8%) | 44 (12.4%) | |||
| Overweight | 57 (31.8%) | 18,987 (37.6%) | 287 (32.1%) | 141 (39.7%) | |||
| Normal | 43 (24.0%) | 22,928 (45.4%) | 220 (24.6%) | 166 (46.8%) | |||
| Underweight | 3 (1.7%) | 755 (1.5%) | 13 (1.5%) | 4 (1.1%) | |||
| Unknown | 7 | 50,909 | 37 | 49 | |||
| Race | 0.337 | 0.607 | 0.500 | ||||
| White | 89 (64.5%) | 42,830 (63.9%) | 453 (66.0%) | 199 (66.1%) | |||
| Black | 5 (3.6%) | 3643 (5.4%) | 26 (3.8%) | 8 (2.7%) | |||
| Asian | 20 (14.5%) | 7070 (10.5%) | 73 (10.6%) | 31 (10.3%) | |||
| Other | 24 (17.4%) | 13,475 (20.1%) | 134 (19.5%) | 63 (20.9%) | |||
| Unknown | 48 | 34,419 | 244 | 103 | |||
| Ethnicity | 1 | 1 | 0.133 | ||||
| Hispanic | 21 (15.2%) | 9875 (15.4%) | 101 (14.9%) | 28 (9.7%) | |||
| Not Hispanic | 117 (84.8%) | 54,411 (84.6%) | 576 (85.1%) | 260 (90.3%) | |||
| Unknown | 48 | 37,151 | 253 | 116 | |||
| Tobacco Status | 0.007 | 0.273 | 0.778 | ||||
| Current | 16 (8.8%) | 9702 (15.0%) | 52 (5.7%) | 40 (10.8%) | |||
| Quit | 33 (18.2%) | 7886 (12.2%) | 170 (18.6%) | 65 (17.5%) | |||
| Never | 132 (72.9%) | 47,222 (72.9%) | 693 (75.7%) | 267 (71.8%) | |||
| Unknown | 5 | 36,627 | 15 | 32 | |||
| History of alcohol use | 90 (63.4%) | 32,466 (68.9%) | 0.187 | 461 (64.0%) | 0.959 | 218 (80.4%) | <0.001 |
| Unknown | 44 | 54,296 | 210 | 133 | |||
| Opiate prescription | 21 (14.5%) | 15,767 (15.5%) | 0.341 | 120 (12.9%) | 0.635 | 77 (19.1%) | 0.219 |
| Minimum within-patient testosterone (total), mean (SD) | 7.3 (2.3) | 16.9 (5.8) | <0.001 | 13.6 (4.8) | <0.001 | 18.0 (5.2) | <0.001 |
| Antidepressant | 35 (18.8%) | 5264 (5.2%) | <0.001 | 105 (11.3%) | 0.007 | 55 (13.6%) | 0.131 |
| Depression diagnosis | 23 (12.4%) | 3123 (3.1%) | <0.001 | 55 (5.9%) | 0.003 | 44 (10.9%) | 0.700 |
| Antidepressant or depression diagnosis | 42 (22.6%) | 6666 (6.6%) | <0.001 | 125 (13.4%) | 0.002 | 68 (16.8%) | 0.121 |
The number and percentage of cases or controls in whom a variable was obtained are given. Each P value compares a rate in a control with the rate in the cases.
B. Study Population Compared With the Matched Control Patients
Propensity scores matching patients with hypogonadism to controls showed good balance in the covariates (Table 1). Differences in age, BMI categories, tobacco status, and opiate use were closely aligned after matching. The incidence of depression was 13.4% in the matched controls vs 22.6% in the cases [OR: 1.14 (1.08 to 1.19); P < 0.002; 95% CI]. These data suggest that hypogonadism relates more closely to depression than to obesity.
C. Study Population Compared With the Known Testosterone Controls
The control patients with a known testosterone value were slightly younger, were less obese, and had a higher incidence of alcohol use than the cases (Table 1). The mean (SD) testosterone value in the control patients was 18 (5.2) nmol/L [518.5 (149.2) ng/dL] compared with 7.25 (2.34) nmol/L [209.2 (67.6) ng/dL] in patients with hypogonadism (P < 0.001). On univariate analysis, the depression incidence between the known testosterone controls and the cases was 16.8% and 22.6%, respectively (P = 0.121). The relationship between cases and controls was not significant on multivariable analysis [OR: 1.06 (0.98 to 1.15); 95% CI; P = 0.158]. The encounter diagnoses of these controls more closely resembled those of the cases than those of the remainder of the controls, reinforcing the concept that many symptoms of depression resemble those of hypogonadism and may lead to a testosterone measurement that is normal by definition in these controls.
D. Obesity
A question arises regarding the extent to which obesity influenced testosterone values in this population. The mean total testosterone level in male populations declines with obesity partly because of a reduction in the level of sex hormone‒binding globulin. In the cases (Table 2), the testosterone levels were quite similar in all three categories of BMI. The free testosterone values increased significantly with degree of obesity, whereas the few bioavailable testosterones measured did not differ by BMI. As expected, the total testosterone levels in the controls fell with degree of obesity. Very few free or bioavailable testosterone values were drawn in the controls.
Table 2.
Testosterone Values in the Cases and Controls in Relation to Obesity
| Testosterone Value (nmol/L) | N | Obese | N | Overweight | N | Normal Weight | P Value |
|---|---|---|---|---|---|---|---|
| Total | 76 | 7.62 (SD = 1.93) | 57 | 7.28 (SD = 2.18) | 43 | 7.2 (SD = 2.52) | 0.51 |
| Bioavailable | 17 | 4.82 (SD = 1.83) | 17 | 4.41 (SD = 2.57) | 6 | 4.7 (SD = 1.93) | 0.864 |
| Free | 57 | 1.67 (SD = 0.6) | 40 | 1.46 (SD = 0.51) | 36 | 1.33 (SD = 0.71) | 0.026 |
| Control Patients Total | 58 | 14.59 (SD = 4.77) | 166 | 16.82 (SD = 6.03) | 172 | 18.25 (SD = 5.72) | <0.001 |
Obesity is also related to depression. In this population, 12% of men with obesity had depression compared with 9% of the overweight and normal weight men and 11% of underweight men. These data and the results with control group 2, which was BMI matched, attest to a limited influence of obesity on the relationship of depression to hypogonadism.
3. Discussion
The vast majority of men over the age of 50 years with hypogonadism have normal LH levels, with a mean similar to that for eugonadal men, suggesting that the condition is central and not primarily due to testicular failure [7]. This study addressed the relationship of depression to hypogonadism in an unselected patient population and excluded patients with classical hypogonadism and those with a coexisting condition that might produce hypogonadism. By focusing on men aged 18 to 40 years, it also reduced the probability of many chronic conditions that could lead to hypogonadism. Studies by Burris et al. [8], Wang et al. [9], Wang et al. [10], and Lašaite et al. [11], which reported on mood in younger men with hypogonadism, enrolled mainly young men with classical hypogonadism whose androgen treatment led to physical development, sexual maturation, and psychological improvement. In a cohort of older men selected for depression, Giltay et al. [12] found significantly lower testosterone levels within the normal range in individuals with major depressive disorder as distinguished from those who also had anxiety. Westley et al. [13] reported that a cohort of patients who were referred to an andrology center for borderline testosterone values had a nearly 60% incidence of concurrent depression (a Patient Health Questionnaire-9 score >10 or taking an antidepressant), including a group from 20 to 39 years of age. They emphasized the similarity of the symptoms of hypogonadism and depression. Recognition of this relationship and the assumption that patients’ hypogonadism was primary led investigators to use testosterone to treat both eugonadal and hypogonadal men with depression. Testosterone therapy [3, 14–18] sometimes (see comprehensive review [19]) had positive effects on amelioration of some depressive symptoms in older patients, generally, with coexisting conditions. The best studies showed improvement in sexual function but little evidence of androgen effectiveness in restoring the emotional state [14–17]. Results from “The Testosterone Trials,” the best performed and analyzed study of 790 men using carefully selected cohorts of patients older than 65 years with specific symptoms for each subgroup, showed a mean Patient Health Questionnaire-9 value of 6.6, indicating substantial depression in the group. In the “vitality” substudy in which fatigue was a major factor, testosterone therapy had very limited effectiveness [18].
It is noteworthy that exploring for depression is barely mentioned in two leading endocrine clinical practice guidelines regarding male hypogonadism [20, 21]; further, only 6.3% of veterans of all ages receiving a prescription for testosterone had classical hypogonadism, whereas the rest were eugonadotropic [16, 20].
This epidemiologic report on the associations of hypogonadism was limited to men 40 years and younger and excluded conditions known to result in hypogonadism. The younger men identified had much lower rates of chronic conditions than other reported populations. Less than 1% of the 105,816 outpatients reviewed had symptoms precipitating a testosterone measurement, of which approximately half were normal (control 3) and half were low (cases). The salient clinical finding associated with testosterone measurement was depression, identified via an ICD-9 diagnosis or by antidepressant therapy in 22.4% of the men with hypogonadism. However, a substantial number of the patients whose symptoms generated a testosterone measurement had a normal value at the time, further emphasizing the similarity of hypogonadism and depressive symptoms. The only other characteristic of the group with hypogonadism was a significant increase in obesity.
Associations do not imply causality, but they do raise the possibility of a causal relationship, especially in the presence of a feasible physiological basis. Although hypogonadism until now has been considered etiologic, perhaps the underlying problem in this association in many cases is depression, and hypogonadism is a consequence. We could not find a study of testosterone levels in depressed patients with hypogonadism after successful treatment of the depression. Other findings support the concept of depression or stress-induced hypogonadism as a possible explanation for these results. Stress-induced hypogonadism occurs in patients with depression [20]. Reduced reactivity of the hypothalamic-pituitary-gonadal axis in depression [21] and a marked reduction in GnRH impulse strength due to fasting [22] are attributed to activation of the hypothalamic-pituitary-adrenal axis, which inhibits GnRH secretion.
A. Study Limitations
Because this was a post hoc observational study, there are limitations to the quality and quantity of the data. There was no systematic effort to diagnose depression, and we believe that depression was underdiagnosed in the entire population. The carefully determined annual major depression rate in both sexes in similar age groups in US-born whites is ~26% and in Mexican Americans is ~19%, supporting this concept of an undercount [23]. The visits were for a variety of reasons, took place in many clinics, and involved many physicians in the health care system, so there was limited uniformity and completeness in the assessments. On the other hand, we did not have the selection bias of referral to experts. We could not address the textual part of the record, so we could not explore treatment and response. The testosterone assays were from commercial laboratories, Esoterix and Quest, before harmonization, and the patients were young, so we used 10.4 nmol/L as the lower limit of normal. Another limitation of the study was that the population, although ethnically and racially representative of the region, consisted almost exclusively of insured patients.
Low testosterone syndrome in men under the age of 40 years is uncommon but not rare. Now that we are measuring androgens in more men, we frequently find low values in symptomatic individuals. Because the symptoms of hypogonadism coincide with the principal symptoms of depression in men, it is impossible to separate them. We must assess both conditions and treat both as indicated. This takes on increased importance when considering the findings of Weinberger et al. [24] that the prevalence of depression is increasing in the United States in men and women and that the increases are greatest in the young, in non-Hispanic whites, in the lowest income groups, and in the highest educational and income groups. The results of this study mandate investigation of endogenous testosterone levels in men with nonclassical hypogonadism before and after aggressive treatment of depression and determination of the mechanisms of GnRH suppression in men with nonclassical hypogonadism.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health/National Center for Advancing Translational Sciences Grant UL1TR001881 (to S.G.K., J.F.G., D.S.B., D.A.E).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- ICD-9
International Classification of Diseases, Ninth Revision
References and Notes
- 1. Zarotsky V, Huang MY, Carman W, Morgentaler A, Singhal PK, Coffin D, Jones TH. Systematic literature review of the risk factors, comorbidities, and consequences of hypogonadism in men. Andrology. 2014;2(6):819–834. [DOI] [PubMed] [Google Scholar]
- 2. Araujo AB, O’Donnell AB, Brambilla DJ, Simpson WB, Longcope C, Matsumoto AM, McKinlay JB. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2004;89(12):5920–5926. [DOI] [PubMed] [Google Scholar]
- 3. Mazur A. Biosocial models of deviant behavior among male army veterans. Biol Psychol. 1995;41(3):271–293. [DOI] [PubMed] [Google Scholar]
- 4. Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, Murad MH, Santoro NF, Warren MP. Functional hypothalamic amenorrhea: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(5):1413–1439. [DOI] [PubMed] [Google Scholar]
- 5. Travison TG, Vesper HW, Orwoll E, Wu F, Kaufman JM, Wang Y, Lapauw B, Fiers T, Matsumoto AM, Bhasin S. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102(4):1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuczmarski MF, Kuczmarski RJ, Najjar M. Descriptive anthropometric reference data for older Americans. J Am Diet Assoc. 2000;100(1):59–66. [DOI] [PubMed] [Google Scholar]
- 7. Korenman SG, Morley JE, Mooradian AD, Davis SS, Kaiser FE, Silver AJ, Viosca SP, Garza D. Secondary hypogonadism in older men: its relation to impotence. J Clin Endocrinol Metab. 1990;71(4):963–969. [DOI] [PubMed] [Google Scholar]
- 8. Burris AS, Banks SM, Carter CS, Davidson JM, Sherins RJ. A long-term, prospective study of the physiologic and behavioral effects of hormone replacement in untreated hypogonadal men. J Androl. 1992;13(4):297–304. [PubMed] [Google Scholar]
- 9. Wang C, Alexander G, Berman N, Salehian B, Davidson T, McDonald V, Steiner B, Hull L, Callegari C, Swerdloff RS. Testosterone replacement therapy improves mood in hypogonadal men: a clinical research center study. J Clin Endocrinol Metab. 1996;81(10):3578–3583. [DOI] [PubMed] [Google Scholar]
- 10. Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Berman N, Hull L, Swerdloff RS. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89(5):2085–2098. [DOI] [PubMed] [Google Scholar]
- 11. Lašaite L, Čeponis J, Preikša RT, Žilaitiene B. Effects of two-year testosterone replacement therapy on cognition, emotions and quality of life in young and middle-aged hypogonadal men. Andrologia. 2017;49(3):e12633. [DOI] [PubMed] [Google Scholar]
- 12. Giltay EJ, van der Mast RC, Lauwen E, Heijboer AC, de Waal MWM, Comijs HC. Plasma testosterone and the course of major depressive disorder in older men and women. Am J Geriatr Psychiatry. 2017;25(4):425–437. [DOI] [PubMed] [Google Scholar]
- 13. Westley CJ, Amdur RL, Irwig MS. High rates of depression and depressive symptoms among men referred for borderline testosterone levels. J Sex Med. 2015;12(8):1753–1760. [DOI] [PubMed] [Google Scholar]
- 14. Zarrouf FA, Artz S, Griffith J, Sirbu C, Kommor M. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract. 2009;15(4):289–305. [DOI] [PubMed] [Google Scholar]
- 15. Seidman SN, Miyazaki M, Roose SP. Intramuscular testosterone supplementation to selective serotonin reuptake inhibitor in treatment-resistant depressed men: randomized placebo-controlled clinical trial. J Clin Psychopharmacol. 2005;25(6):584–588. [DOI] [PubMed] [Google Scholar]
- 16. Cunningham GR, Stephens-Shields AJ, Rosen RC, Wang C, Bhasin S, Matsumoto AM, Parsons JK, Gill TM, Molitch ME, Farrar JT, Cella D, Barrett-Connor E, Cauley JA, Cifelli D, Crandall JP, Ensrud KE, Gallagher L, Zeldow B, Lewis CE, Pahor M, Swerdloff RS, Hou X, Anton S, Basaria S, Diem SJ, Tabatabaie V, Ellenberg SS, Snyder PJ. Testosterone treatment and sexual function in older men with low testosterone levels. J Clin Endocrinol Metab. 2016;101(8):3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P; BLAST Study Group . Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med. 2014;11(3):840–856. [DOI] [PubMed] [Google Scholar]
- 18. Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM, Barrett-Connor E, Swerdloff RS, Wang C, Ensrud KE, Lewis CE, Farrar JT, Cella D, Rosen RC, Pahor M, Crandall JP, Molitch ME, Cifelli D, Dougar D, Fluharty L, Resnick SM, Storer TW, Anton S, Basaria S, Diem SJ, Hou X, Mohler ER III, Parsons JK, Wenger NK, Zeldow B, Landis JR, Ellenberg SS; Testosterone Trials Investigators . Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huo S, Scialli AR, McGarvey S, Hill E, Tügertimur B, Hogenmiller A, Hirsch AI, Fugh-Berman A. Treatment of men for “low testosterone”: a systematic review. PLoS One. 2016;11(9):e0162480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schweiger U, Deuschle M, Weber B, Körner A, Lammers CH, Schmider J, Gotthardt U, Heuser I. Testosterone, gonadotropin, and cortisol secretion in male patients with major depression. Psychosom Med. 1999;61(3):292–296. [DOI] [PubMed] [Google Scholar]
- 21. Rupprecht R, Rupprecht C, Rupprecht M, Noder M, Schwarz W. Different reactivity of the hypothalamo-pituitary-gonadal-axis in depression and normal controls. Pharmacopsychiatry. 1988;21(6):438–439. [DOI] [PubMed] [Google Scholar]
- 22. Veldhuis JD, Iranmanesh A, Evans WS, Lizarralde G, Thorner MO, Vance ML. Amplitude suppression of the pulsatile mode of immunoradiometric luteinizing hormone release in fasting-induced hypoandrogenemia in normal men. J Clin Endocrinol Metab. 1993;76(3):587–593. [DOI] [PubMed] [Google Scholar]
- 23. González HM, Tarraf W, Whitfield KE, Vega WA. The epidemiology of major depression and ethnicity in the United States. J Psychiatr Res. 2010;44(15):1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weinberger AH, Gbedemah M, Martinez AM, Nash D, Galea S, Goodwin RD. Trends in depression prevalence in the USA from 2005 to 2015: widening disparities in vulnerable groups. Psychol Med. 2018;48(8):1308–1315. [DOI] [PubMed] [Google Scholar]