Abstract
The implications of the neurosteroid 3α-hydroxy-5α-pregnan-20-one (allopregnanolone, Allo) in neuropsychiatric disorders has been highlighted in several recent clinical investigations. For instance, Allo levels are decreased in the cerebrospinal fluid (CSF) of patients with PTSD and major unipolar depression. Neurosteroidogenic antidepressants [i.e., selective brain steroidogenic stimulants (SBSSs)], including fluoxetine and analogs, correct this decrease in a manner that correlates with improved depressive symptoms. Allo positively and allosterically modulates GABA action at post- and extra-synaptic GABAA receptors. It is synthesized both in human and rodent brain cortex by principal glutamatergic pyramidal neurons from progesterone by the sequential action of 5α-reductase type I (5α-RI), which is the rate-limiting step enzyme in Allo biosynthesis, and 3α-hydroxysteroid dehydrogenase (3α-HSD), which converts 5α-dehydroprogesterone into Allo. We thus hypothesized that decreased CSF levels of Allo in depressed patients could reflect a brain dysfunction of 5α-RI. In a pilot study of samples from 6 patients per group (6 depressed patients and 6 non psychiatric subjects), we studied the expression of 5α-RI mRNA in prefrontal cortex BA9 and cerebellum from depressed patients obtained from the Maryland Brain Collection at the Maryland Psychiatric Research Center (Baltimore, MD) that were age-matched with non-psychiatric subjects (NPS). The levels of 5α-RI mRNA were decreased from 25 ± 5.8 in NPS to 9.1 ± 3.1 fmol/pmol NSE (t1,10 = 2.7, P = 0.02) in depressed patients. These differences are absent in the cerebellum of the same patients. The levels of neurosteroids were determined in the prefrontal cortex BA9 of depressed patients obtained from the Stanley Foundation Brain Bank Neuropathology Consortium, Bethesda (MD). The BA9 levels of Allo in male depressed patients failed to reach statistical difference from the levels of NPS (1.63± 1.01 pg/mg, n=8, in NPS and 0.82±0.33 pg/mg, n=5, in non-treated depressed patients). However, depressed patients who had received antidepressant treatment (3 patients SSRI and 1 TCA) exhibited increased BA9 Allo levels (6.16±2.5 pg/mg, n=4, t1,9 = 2.4, P = 0.047) when compared with non-treated depressed patients. Although in a small number of patients, this finding is in line with previous reports in the field that have observed an increase of Allo levels in CSF and plasma of depressed patients following antidepressant treatment. Hence, the molecular mechanisms underlying major depression may include a GABAergic neurotransmission deficit caused by a brain Allo biosynthesis downregulation, which can be normalized by SBSSs.
Keywords: Allopregnanolone, selective brain steroidogenic stimulants, 5α-reductase type I, major unipolar depression, GABAA receptor
Introduction
Depression and anxiety spectrum disorders, which include generalized anxiety, panic, and post-traumatic stress disorder (PTSD) could reflect a brain perturbation of GABAergic neurotransmission as the result of a reduction of the GABAA receptor-active neurosteroid, 3α-hydroxy-5α-pregnan-20-one (allopregnanolone, Allo) levels. Indeed, Allo levels are decreased in the cerebrospinal fluid (CSF) and plasma of patients with PTSD and major unipolar depression in a manner that correlates with severity of symptoms (Rasmusson et al. 2006; Uzunova et al. 1998; Romeo et al., 1998). Remarkably, neurosteroidogenic antidepressants [i.e., selective brain steroidogenic stimulants (SBSSs)] (Pinna et al. 2004; Pinna et al. 2006a; Pinna et al. 2009), including fluoxetine and analogs, correct this Allo level deficit and improve depression (Uzunova et al. 1998; Romeo et al., 1998).
Allo acts as an endogenous potent positive allosteric modulator of the action of GABA at post- and extra-synaptic GABAA receptors (Gunn et al. 2011; Herd et al. 2007; Belelli and Lambert, 2005; Puia et al., 1991). In both human and rodent brain, Allo is synthesized from progesterone by the sequential action of two enzymes: 5α-reductase type I (5α-RI), which is the rate-limiting step enzyme in Allo biosynthesis, and 3α-hydroxysteroid dehydroxygenase (3α-HSD), the enzyme that converts 5α-dihydro-progesterone (5α-DHP) into Allo (Dong et al., 2001; Herd et al. 2007; Pinna et al. 2008). In mice, these enzymes co-localize and are highly expressed in glutamatergic corticolimbic neurons, including pyramidal neurons of the cortex, pyramidal neurons and granular cells of the hippocampus, and pyramidal-like neurons of the amygdala but fail to be expressed by GABAergic interneurons (Agis-Balboa et al. 2006; 2007). Upon secretion from glutamatergic neurons, Allo may act in a paracrine fashion at GABAA receptors located on cell bodies or dendrites of pyramidal neurons, or in an autocrine manner at GABAA receptors located on glutamatergic neurons dendrites or cell bodies, or may access and act at the intracellular sites of GABAA receptors (Agis-Balboa et al. 2007; Agis-Balboa et al. 2006; Akk et al. 2005). The finding that Allo in nmolar concentrations facilitates the efficacy of GABAA receptor agonists and allosteric modulators substantiates its endogenous physiological relevance (Pinna et al. 2000; Guidotti et al. 2001). In experiments in which Allo biosynthesis was decreased by inhibiting 5α-RI with the potent competitive 5α-RI inhibitor, SKF 105,111, we have demonstrated a reduced pharmacological response to GABAA receptor agonists and allosteric modulators (Pinna et al., 2000).
Acute and protracted stress induce an opposite effect on neurosteroid biosynthesis in opposite directions; acute stress both in humans or experimental animals increases the brain and plasma levels of Allo (Bali and Jaggi, 2013; Barbaccia et al., 1996; Purdy et al., 1991; Vallée et al., 2000) on the other hand, environmental stress for prolonged periods of time is associated with a decline of neurosteroid biosynthesis and involves changes in the expression of 5α-RI (Agís-Balboa et al., 2007; Bortolato et al., 2011; Dong et al., 2001; Matsumoto et al., 1999; Pibiri et al., 2008; Pinna et al., 2003; Pinna and Rasmusson, 2012; Serra et al., 2000). In the socially isolated (SI) mouse, the mRNA and protein expression of 5α-RI was halved when compared with group-housed counterparts in frontal cortex, hippocampus, and basolateral amygdala pyramidal neurons and unchanged in spiny-medium neurons of the striatum and Purkinje cells of the cerebellum (Agis-Balboa et al., 2007). We observed that pharmacological interventions using SKF 105,111 or decreasing Allo biosynthesis by a protracted social isolation stress results in a GABAergic neurotransmission dysfunction characterized by deficits in emotional behavior such as anxiety-like behavior, aggression, and exaggerated contextual fear conditioning responses (Agis-Balboa et al. 2007; Pibiri et al. 2008; Pinna et al. 2003; 2008). Furthermore, the social isolation-induced corticolimbic decrease of Allo levels and behavioral deficits has been associated with the decreased levels of brain derived neurotropic factor (BDNF) in SI mice (Nin et al., 2011). This decrease of BDNF expression was reversed following treatment with neurosteroidogenic antidepressants at doses too low to affect serotonergic mechanisms (Nin et al., 2011). It is of note that a decrease of BDNF levels has been implicated in depression and in the impairment of cognitive function in psychiatric disorders (Sen et al., 2008) and that depressed patients express both a decrease in the levels of Allo in the CSF and plasma and of BDNF in the hippocampus and plasma (Uzunova et al., 1998; Romeo et al., 1998; Sen et al., 2008). In addition, social stress, including social isolation decreases neurogenesis in the hippocampus (Dranovsky et al., 2011). Accordingly, rodent models of stress-induced depression show impaired neurogenesis (Kempermann et al., 2003). Neurosteroidogenic antidepressants, including fluoxetine, are able to increase the number, differentiation, and survival of newborn hippocampal neurons (Dranovsky and Hen, 2006). On the other hand, inhibiting 5α-RI activity with finasteride resulted in decrease of brain Allo levels and decreased hippocampal neurogenesis, which has been reported to contribute to the pathophysiology of depression (Römer 2010a,b; Melcangi et al, 2013; Anacker, 2014). These findings are consistent with reports suggesting that Allo and other neurosteroids may contribute to neuroprotection by inducing neurite outgrowth, dendritic spine formation, and synaptogenesis (Borowicz et al., 2011; Tsutsui, 2012).
Only limited knowledge is available on the expression, regulation, as well as the neuronal localization of 5α-RI in human brain and how this is affected during depression and related psychiatric disorders. Because CSF Allo levels are decreased in patients with unipolar major depression (Uzunova et al., 1998), we hypothesized that this neurosteroid decrease could reflect an altered brain expression of 5α-RI. In a pilot study, we investigated the expression of 5α-RI mRNA in prefrontal cortex BA9 and cerebellum from a small group of depressed patients and non-psychiatric controls (NPS) obtained from the Maryland Brain Collection at the Maryland Psychiatric Research Center, Baltimore, MD (Table 1). The Allo levels were determined in samples obtained from the Stanley Foundation Brain Bank Neuropathology Consortium, Bethesda (MD) that were age-matched with NPS. This report suggests that in depressed patients, the decreased expression of neurosteroidogenic enzymes that are directly involved in Allo biosynthesis, i.e., 5α-RI might be responsible for the CSF decline of Allo levels observed in major unipolar depression and in PTSD patients. Steroidogenic antidepressants, including SBSSs correct this Allo levels downregulation. These results are discussed in terms of possible epigenetic mechanisms operative in these affective disorders and possible new treatments for these debilitating conditions.
Table 1.
Demographic, clinical, and treatment history of depressed patients (D) and non-psychiatric subjects (NPS) of the Maryland Brain Collection at the Maryland Psychiatric Research Center (Baltimore, MD)
| Diagnosis | Gender | Age (y) |
PMI | Cause of Death |
Medications | pH | Abuse or dependence |
|---|---|---|---|---|---|---|---|
| NPS | M | 43 | 17 | 6.46 | |||
| NPS | M | 48 | 26 | 6.10 | |||
| NPS | M | 33 | 16 | 6.35 | |||
| NPS | M | 35 | 24 | 6.20 | |||
| NPS | M | 37 | 24 | 6.42 | |||
| NPS | M | 47 | - | 6.38 | |||
| D | M | 24 | 7 | Gun shot | 6.55 | Ethanol | |
| D | M | 53 | 23 | Hung | Xanax Lidocaine | 6.18 | |
| D | F | 46 | 21 | Jumping | TCA | 5.97 | Ethanol |
| D | M | 43 | - | Hung | TCA | 6.51 | |
| D | M | 23 | 19 | Cardiov. | Fluoxetine | 6.48 | Ethanol |
| D | M | 63 | 15 | Overdose | Fluoxetine Meperidine | 6.20 | Ethanol |
PMI= post-mortem interval
TCA= tricyclic antidepressants
M= male
F= female
Methods
Sample collection
Frozen Specimens from human brains [Brodmann’s area 9 (BA9), and cerebellum] used to determine the expression levels of 5α-RI were obtained blind through the Maryland Brain Collection at the Maryland Psychiatric Research Center, Baltimore, (MD) (https://www.mprc.umaryland.edu/mbc.asp). Demographic, clinic, and treatment history of depressed patients and control subjects are reported in Table 1.
The sample utilized to determine the levels of neurosteroids were from the superior prefrontal cortex gyrus, BA9 specimen from subjects obtained blind from the Stanley Foundation Brain Bank Neuropathology Consortium, Bethesda (MD). These samples consist of 9 subjects with depression and 8 control brains free of depressive and other psychiatric disorders before or at the time of death, and herein defined as non-psychiatric subjects (NPS). Demographic, clinic, and treatment history of these groups of depressed patients and NPS are reported in Table 2.
Table 2.
Demographic, clinical, and treatment history of depressed patients (D) and non-psychiatric subjects (NPS) obtained from the Stanley Foundation Brain Bank Neuropathology Consortium, Bethesda (MD).
| Diagnosis | Gender | Age (y) |
PMI | Cause of Death |
Medications | pH | Abuse or dependence |
|---|---|---|---|---|---|---|---|
| NPS | M | 52 | 28 | Cardiov. | None | 6.40 | Ethanol Abstinent 8 y |
| NPS | M | 59 | 26 | Cardiov. | None | 6.4 | None |
| NPS | M | 52 | 8 | Cardiov. | None | 6.3 | None |
| NPS | M | 52 | 22 | Cardiov | None | 5.9 | None |
| NPS | M | 53 | 28 | Cardiov. | None | 6.2 | Eth. in his 20s, abstinent for 30y |
| NPS | M | 44 | 10 | Cardiov. | None | 6.5 | None |
| NPS | M | 41 | 11 | Pulmonary embolus | None | 6.3 | Sporadically marihuana in college |
| NPS | M | 42 | 27 | Cardiov. | None | 6.6 | None |
| NPS | M | 58 | 27 | Cardiov. | None | 6.3 | None |
| D1 | M | 39 | 23 | Suicide, Carbon monoxide | Never treated | 5.9 | Ethanol and Amphetamine |
| D1 | M | 42 | 7 | Hung | Temazepam, off for more than 2 w | 6.3 | Marihuana when young |
| D1 | M | 65 | 19 | Cardiov. | Phenytoin for 1 seizure, no meds for 5 y | 5.9 | Light Ethanol |
| D1 | M | 52 | 12 | Cardiov. | No meds for 6 y | 5.8 | 1–2 beers/day |
| D1 | M | 46 | 26 | Suicide, Carbon monoxide | Diphenhydram. Clonazepam | 6.3 | None |
| D2 | M | 51 | 26 | Suicide, Gunshot | Nefazadone | 6.6 | Ethanol |
| D2 | M | 56 | 23 | Cardiov. | Sertraline | 6.3 | None |
| D2 | M | 43 | 43 | Cardiov. | Trimipramine | 6.3 | Ethanol, Amphetamines, Benzodiazepine |
| D2 | M | 47 | 28 | Cardiov. | Fluoxetine, Nefazadone | 6.1 | Light Ethanol |
Depressed patients who were not receiving an antidepressant treatment.
Depressed patients who were receiving antidepressant treatment with tricyclic antidepressants (TCA) or SSRIs.
In Situ Hybridization and immunocytochemistry
To visualize 5α-RI mRNA, BA9 specimens were fixed in 4% formaldehyde. Free-floating 40-µm sections were incubated for 40–48 h with a mixture of 50 pmol/ml of two antisense oligonucleotide probes complementary to bases 1627–1650 (P1) and 4801–4824 (P2) of the human 5α-RI cDNA (GenBank accession no. NM_001047). These nucleotides failed to match 5α-RI sequences or any other known mRNA sequence and were selected according to the criteria reported by Agis-Balboa et al. (2006). The oligonucleotide 3’ terminals were labeled with digoxigenin by using the Oligonucleotide Digoxigenin Tailing Kit (Roche Diagnostics). Double in situ hybridization (ie, 5α-RI mRNA) and immunohistochemistry (ie, VGLUT2 protein) were carried out as described (Agis-Balboa et al. 2007; Agis-Balboa et al. 2006).
Confocal Fluorescence Microscopy
After 5α-RI mRNA in situ hybridization was completed, the sections were processed for immunohistochemistry with antibodies directed against VGLUT-2 (1:500; Synaptic Systems, Germany). The sections were incubated with the antibody for 48 h at 4°C and 2h at room temperature. The slices were then incubated with Cy-5-conjugated goat anti-rabbit IgG to label the antibodies that reacted with VGLUT-2. The reactions were carried out in 1% normal goat serum, 1% BSA, and 1% normal rabbit serum in PBS for 1h.
RNA extraction
RNA was extracted from tissue blocks using TRIZOL® reagent according the manufacturer’s specifications.
Reverse transcription-polymerase chain reaction (RT-PCR)
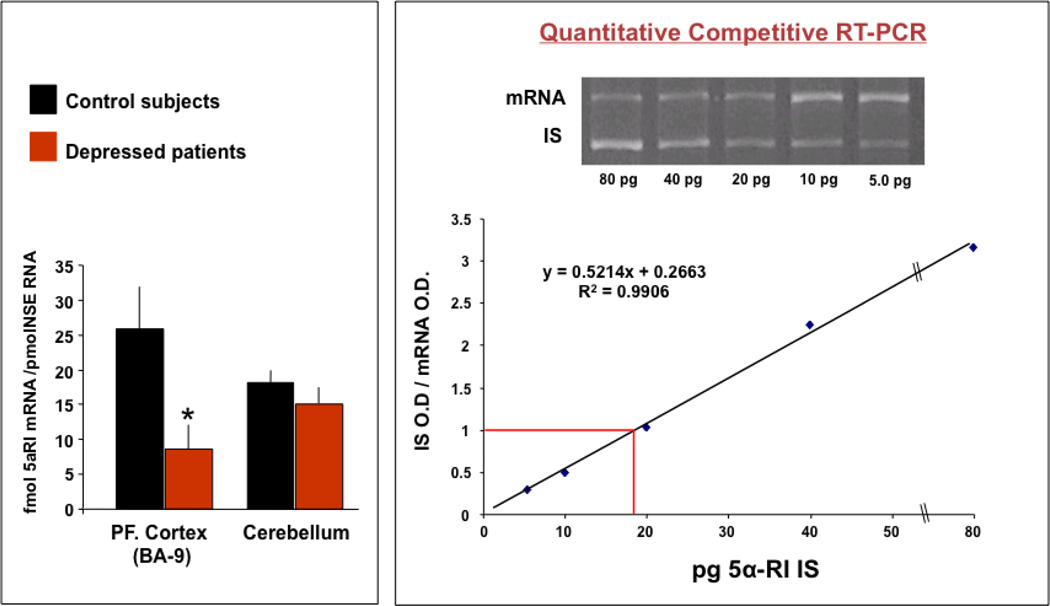
Reactions were carried out using the following conditions and primers designed to anneal to the following sites: human 5α-RI: forward 309–333, reverse 721–745. PCR cycle conditions were 94°C for five minutes followed by 30 cycles of 94°C for 30 seconds, 65°C for 30 seconds, and 72°C for 30 seconds, followed by 72°C for seven minutes. Neuronal Specific Enolase (NSE): forward 382–405, reverse 769–792. NSE PCR cycling conditions were the same as for 5α-RI. For competitive RT-PCR experiments, the internal standards (IS) were generated introducing a deletion at the center of each IS.
Digital Photomicrography
Confocal immunofluorescence was captured by using a Leica Confocal Microscope (Leica Microsystems). The final composites were processed by using Photoshop (Adobe Systems) and PowerPoint (Microsoft).
Measurement of neurosteroids
Extraction, derivatization, and GC-MS analyses of progesterone, 5α-DHP, and Allo were performed with minor modifications as described (Uzunov et al., 1996; Pinna et al 2000; 2003). (i) The tissues of interest were homogenized in 10 volumes of distilled water containing 2–5 fmol/ml [3H]-hormone (New England Nuclear) to monitor the HPLC retention profile and deuterium-labeled progesterone, 5α-DHP, or Allo (Steraloids) were used as an internal standard. The supernatants were extracted with ethyl acetate and after lyophilization, were purified with HPLC as described (Pinna et al., 2000). (ii) The HPLC fractions containing progesterone and Allo were derivatized with heptafluorobutyric acid anhydride (HFBA) (Supelco) and subjected to GC-mass fragmentography analysis. 5α-DHP was derivatized with N-Methyl-N-(trimethylsilyl)trifluoroacetamide(MSTFA)/ammonium iodide (NH4I)/1,4-Dithioerythritol(DTE)/Acetonitrile(CH3CN) (Sigma-Aldrich) in a ratio of 1000/2/5/1000. Mass fragmentography analysis of derivatized progesterone, 5α-DHP, and Allo was performed in the standard electron impact (EI) mode. The detection limit was ≈10 fmol. For quantification, the m/z ion-monitoring mode was 510 and 496 for HFBA-progesterone and Allo, 518 and 500 for HFBA-D-progesterone and Allo, respectively. The m/z ion-monitoring mode was 445 for 5α-DHP and 449 for D-5α-DHP.
Statistical Analysis
The data were analyzed by unpaired Student’s t-test or one way Anova followed by Bonferroni comparisons. Errors are displayed as mean ± standard error of the mean (SEM). The criterion for significance was P < 0.05.
Results
Neurosteroid biosynthesis in brain of depressed patients
a) Neuronal localization of 5α-RI in the human prefrontal cortex
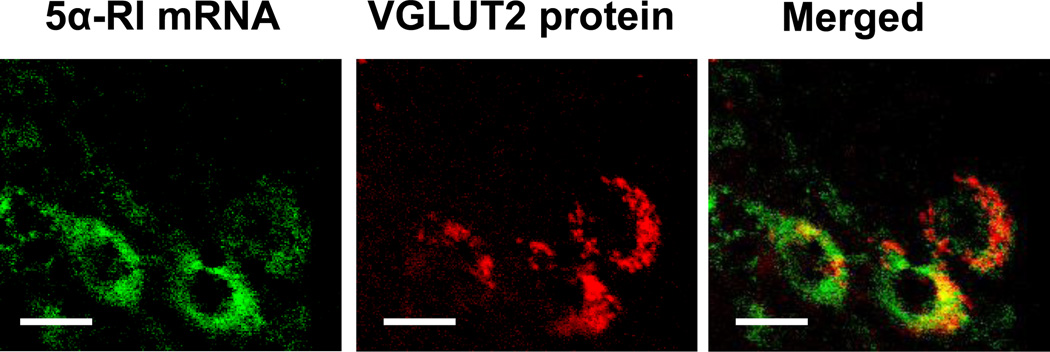
In previous studies in mouse brain, we showed a co-localization of 5α-RI with 3α-HSD mRNA and that these neurosteroidogenic enzymes are co-expressed in pyramidal neurons of cortical layers II-V (Agis-Balboa et al., 2006; 2007). Using a similar technique to that of our preclinical studies (in situ hybridization combined with immunostaining and confocal microscopy), we demonstrate that 5α-RI mRNA co-localizes with vesicular glutamate transporter 2 (VGLUT2) protein, a marker for glutamatergic neurons, and is expressed in human cortical pyramidal neurons of postmortem brain (Figure 1).
Figure 1.
Confocal fluorescence images showing that 5α-RI mRNA is expressed in pyramidal neurons of human cortex and colocalizes with vesicular glutamate transporter 2 (VGLUT2) protein. 5α-RI mRNA, green. VGLUT2 protein, red. Merge of A1 and A2, yellow. Scale bar = 20 µm.
b) 5α-RI mRNA expression in the prefrontal cortex/Brodmann’s area 9 (BA9) of depressed patients
To demonstrate that the decrease of Allo levels in CSF reflects a decrease of brain Allo content, we compared the expression of the rate-limiting step enzyme in Allo biosynthesis, 5α-RI mRNA in samples of BA9 from depressed patients that were age-matched with NPS (n=6, Table 1). The levels of 5α-RI mRNA were decreased from 25 ± 5.8 in NPS to 9.1 ± 3.1 fmol/pmol NSE (t1,10 = 2.7, P = 0.02) in depressed patients (see Figure 2). These differences are not influenced by age or pH and are absent in the cerebellum of the same patients. Due to the small number of samples and the incomplete demographic information, we cannot conclude that gender, PMI, or drug treatment affect the levels of 5α-RI expression in the major depression group.
Figure 2.
5α-RI mRNA expression is downregulated in Brodmann’s area 9 (BA9) pyramidal neurons of depressed patients. Left panel: Levels of 5α-RI mRNA were 25±5.8 in NPS to 9.1±3.1 fmol/pmol NSE (t1,12 = 2.7, P= 0.02) in depressed patients. Right panel: Internal standard.
c) Neurosteroid levels in post-mortem brain of depressed patients
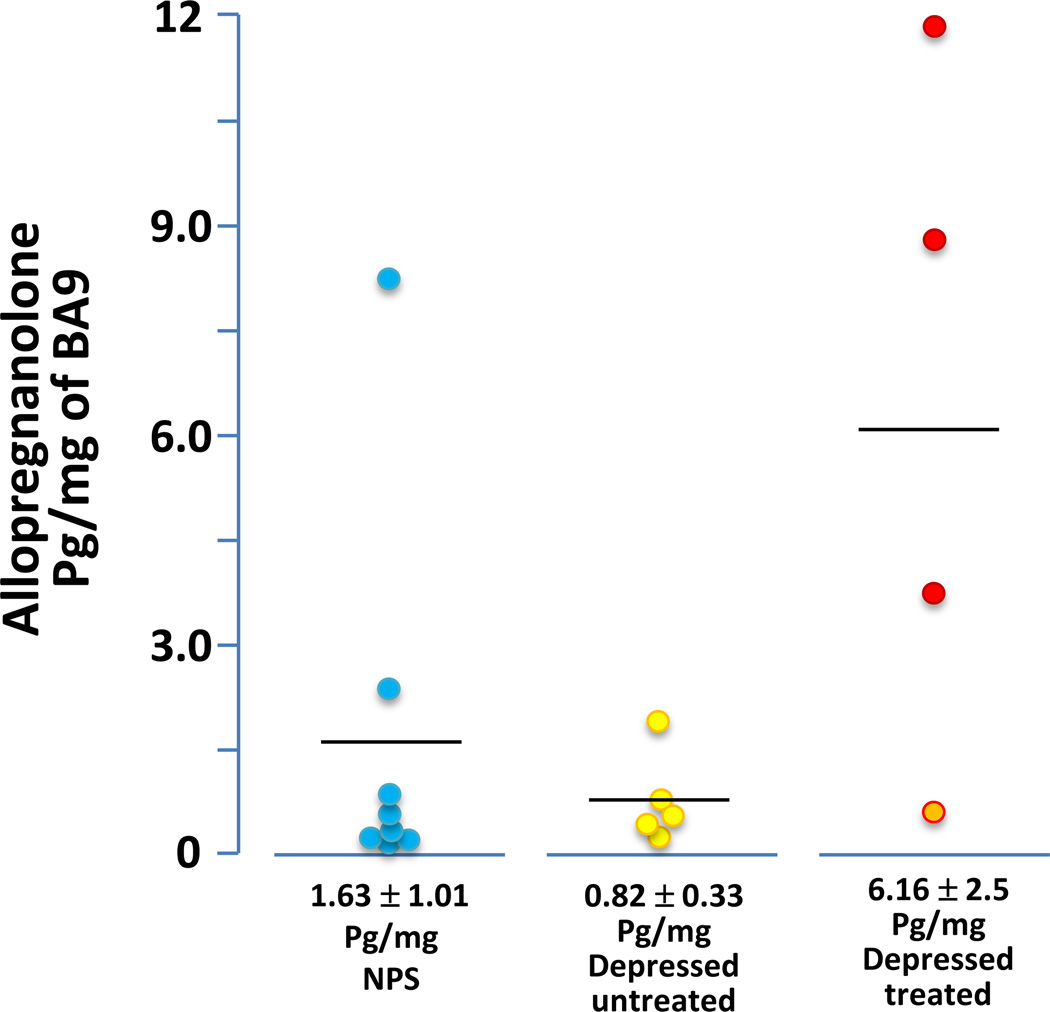
The levels of progesterone, 5α-DHP, and Allo were measured in post-mortem BA9 samples of depressed patients and NPS (Figure 3) obtained from the Stanley Foundation (Table 2). We used this cohort of patients because because the samples of the Maryland Brain Collection were not sufficient for the analyses. Neurosteroid levels were limited to male patients and NPS only in order to avoid gender variations due to the oscillation of progesterone associated with the menstrual cycle. The BA9 levels of Allo in male depressed patients failed to reach statistically different from those of NPS (1.63± 1.01 pg/mg, n=8, in NPS and 0.82±0.33 pg/mg, n=5, in non-treated depressed patients) (see Figure 3). The levels of progesterone (2.66±1.46 pg/mg, n=9 in NPS and 1.24±1.02 pg/mg, n=5 in depressed non-treated patients), and 5a-DHP (7.74±1.45 pg/mg, n=9 in NPS and 7.96±2.99 pg/mg, n=5 in depressed non-treated patients) also failed to change.
Figure 3.
Allo levels in BA9 of NPS, untreated depressed patients (yellow circles), and depressed patients who received an antidepressant treatment with tricyclic antidepressants (TCA) (orange circle), or steroidogenic antidepressants (red circles).
The small group of male depressed patients that were taking antidepressants exhibited elevated BA9 Allo levels when compared with depressed non-treated patients (6.16±2.5 pg/mg, n=4, t1,9 = 2.4, P = 0.047) (for details see Figure 3). However, the levels of progesterone (6.21±2.81 pg/mg, n=4, P=0.09), and 5a-DHP (17.53±5.39 pg/mg, n=4, P=0.14), in the depressed antidepressant-treated male patients failed to change.
Discussion
The results of our pilot study on a cohort of 6 depressed patients and 6 non psychiatric subjects show that the expression levels of 5α-RI mRNA are significantly decreased in prefrontal cortex of depressed patients (Figure 2). These differences are absent in the cerebellum of the same patients. Unfortunately, the small number of samples and the paucity of the tissue material available did not allow the determination of progesterone, 5α-DHP, and Allo in this cohort of patients. Despite the lack of Allo level downregulation in depressed subjects obtained from the Stanley Foundation Brain Bank Neuropathology Consortium, the finding of decreased 5α-RI mRNA expression in the Maryland Brain Collection and the tendency towards decreased Allo levels (1.63 versus 0.82 pg/mg), can be explained by the relatively small group of patients analyzed or by the fact that the elevated PMI of these samples may have affected possible differences in the neurosteroid levels (Table 2). Importantly, depressed patients under antidepressant treatment showed a significant increase of BA9 Allo levels in absence of changes of progesterone and 5a-DHP levels. Collectively, these findings obtained in pilot studies encourage further investigation in a larger cohort of treated and non-treated depressed patients and further suggest that the molecular mechanisms underlying major depression symptomatology may include a downregulation of brain Allo biosynthesis, which leads to a deficit of GABAergic neurotransmission. This deficit may be corrected by steroidogenic antidepressants, including fluoxetine and congeners as it has also been suggested by other clinical reports in which Allo levels were determined in the CSF and plasma (Uzunova et al., 1998; Romeo et al., 1998; reviewed in Pinna et al. 2006; Pinna and Rasmusson, 2012; Schule et al., 2011; 2014).
5α-RI expression in human brain
5α-reductase catalyzes the conversion of progesterone, testosterone and 11-deoxycorticosterone into 5α-DHP, 5α-dihydrotestosterone and 5α-dihydrodeoxycorticosterone, respectively, which are then further converted by 3α-HSD into their GABAA receptor-active metabolites, including Allo, which is probably the most prominent and potent regulator of emotional behavior. Two 5α-reductase isozymes encoded by two distinct genes, designated type I and type II, have been identified in rodents (Berman and Russell, 1993, Mahendroo et al., 1996 and Normington and Russell, 1992), monkey (Levy et al., 1995) and humans (Andersson et al., 1991, Labrie et al., 1992 and Russell and Wilson, 1994). 5α-RI is the most abundant isoform in the rodent and human brain (Melcangi et al., 1998, Russell and Wilson, 1994, Steckelbroeck et al., 2001, Stoffel-Wagner, 2003, Thigpen et al., 1993 and Torres and Ortega, 2003). Our results depicted in Figure 1 show that 5α-RI is highly expressed in cortical pyramidal neurons, which is in agreement with our previous work conducted in mouse brain where 5α-RI mRNA and immunoreactivity were detected in cortical pyramidal neurons, but not in GABAergic interneurons (Agis-Balboa et al., 2006; 2007). Other studies performed in the human brain, observed that 5α-RI mRNA has been detected and is unevenly expressed in the temporal cortex and hippocampal tissue obtained from patients with chronic temporal lobe epilepsy (Stoffel-Wagner et al., 1998; 2000). These studies showed that 5α-RI mRNA is expressed in cortex and hippocampi of both children and adults, whereas 5α-RII mRNA is predominantly expressed in peripheral tissue but not expressed in the brain. Furthermore, the expression of 5α-RI mRNA was not influenced by gender, however, the temporal lobe expression of 5α-RI was significantly higher in specimens of adults than in those of children. Another group demonstrated the expression of 5α-RI in the cerebellum, hypothalamus and pons from post-partum brains (Thigpen et al., 1993).
In accord with the findings showing the expression of 5α-RI mRNA and 5α-RI immunoreactivity, 5α-reductase activity has been determined in the human brain (Celotti et al., 1986, Steckelbroeck et al., 2001 and Stoffel-Wagner et al., 2000). Cerebral neocortex and subcortical white matter specimens neurosurgically removed from patients suffering from epilepsy, revealed the exclusive activity of the 5α-RI isoform. Furthermore, in contrast to liver, only 3α-HSD type II messenger RNA is expressed in the brain further suggesting the ability of neurons to convert progesterone, testosterone, and 11-deoxycorticosterone into their GABAA receptor-active metabolites.
Mouse models of depression/anxiety associated with a downregulation of neurosteroid biosynthesis
Preclinical studies using rodent stress models of anxiety and depression are a suitable basic research translational approach in support of the data presented in this report. In the past decade, using protracted social isolation stress, our group and other colleagues have shown that in rodents this form of stress is associated with a robust down-regulation of neurosteroid biosynthesis (Matsumoto et al., 1999; Serra et al., 2000; Dong et al., 2001; Bortolato et al., 2011). The expression of the rate-limiting step enzyme for Allo biosynthesis, 5α-RI was found decreased in several corticolimbic areas, namely frontal cortex, hippocampus, and basolateral amygdala (Dong et al., 2001; Agis-Balboa et al., 2007; Pinna et al., 2008). Immuniohistochemical studies have confirmed that this Allo level downregulation was caused by a decreased expression of 5α-RI specifically expressed in cortical and hippocampal pyramidal neurons and in basolateral amygdala pyramidal-like neurons (Agis-Balboa et al., 2007). These observations have since then been confirmed by other research groups (Bortolato et al. 2011; Serra et al. 2000; 2004). In socially isolated mice, the decrease of corticolimbic Allo levels has been associated with several emotional behavioral deficits such as aggressive behavior, anxiety-like behavior, and enhanced contextual fear conditioning responses (Matsumoto 1999); 2007; Pinna et al., 2003; 2008; Pibiri et al., 2008). Accordingly, using the bulbectomized rat as a model of depression, Uzunova and collaborators (Uzunova et al. 2003; Uzunova et al. 2006; Uzunova et al. 2004) have observed a similar corticolimbic Allo level downregulation, which was associated with a depressive-like phenotype. The administration of SBSSs in bulbectomized rodents was highly successful in normalizing both the brain Allo downregulation and the associated behavioral dysfunctions (Uzunova et al. 2006; Uzunova et al. 2004).
In experiments performed in our laboratory, SI mice that have been exposed to the acute stress of an electroshock as part of the fear conditioning paradigm, exhibit exaggerated conditioning fear responses and impaired fear extinction memory (Pibiri et al., 2008; Pinna et al., 2008; Pinna and Rasmusson, 2012). SI mice develop behavioral deficits similar to those found in PTSD patients. Interestingly, both PTSD patients and SI mice fail to respond to benzodiazepines and show decreased fronto-cortical benzodiazepine binding sites (Gelpin et al. 1996; Viola et al. 1997; Bremner et al. 2000; Geuze et al. 2008) and changes in the expression of subunit composition of GABAA receptors (SI mice) (Pinna et al., 2006). It is interesting that Allo levels have been associated with changes in the GABAA receptor subunit expression in a model of progesterone withdrawal or for the changes in neurosteroid levels following parturition, which are considered models of premenstrual dysphoria and post-partum depression, respectively (Concas et al. 1998; Smith et al. 1998). Notwithstanding, the neurochemical mechanisms involved in the neurosteroid biosynthesis downregulation and the changes in GABAA receptor subunits, these observations are relevant for the design of therapeutic strategies to overcome behavioral dysfunctions resulting from GABAA receptor signal transduction deficits.
Possible epigenetic mechanisms involved in the decline of neurosteroid biosynthesis in depressed patients
Gene–environment interactions in predisposed individuals have been linked with the susceptibility to a number of neuropsychiatric disorders. Ten years ago, the contribution of Caspi and collaborators (Caspi et al. 2003) to the epigenetic hypothesis of depression, demonstrated that abuse and maltreatment during childhood and later stressful life events predicted the onset of adult major depression. Since then, preclinical and clinical epigenetic studies have demonstrated that the post-translational modifications of histones as well as the methylated levels of cytosine of various promoters in response to stress, including BDNF, play a pivotal role in the pathophysiology of depression and anxiety disorders (Nestler, 2014). Recently, it has been shown that the 5’ upstream region of the rat 5α-RI includes all the features of CpG islands and contains several potential binding sites for the transcription factor Sp1, which has been implicated in the activation of a large number of genes. In the mouse, the expression of 5α-RI could also be regulated through an epigenetic regulation of promoter hypermethylation that may trigger 5α-RI mRNA downregulation after long-term social isolation.
Importantly, other studies have shown that antidepressants reverse the epigenetic changes due to defeat stress in mice by increasing histone acetylation mediated by a selective histone deacetylase (HDAC) downregulation (Tsankova et al. 2006). Hence, suggesting that antidepressant drugs among other mechanism may inhibit specific HDACs. The anticonvulsant and mood stabilizer, valproic acid, a pluripotent HDAC inhibitor (HDACi) offers a powerful pharmacological tool to reverse the adverse behavioral effects of protracted social isolation stress in mice (Tremolizzo et al. 2005). Also, chronic administration of the HDACi sodium butyrate induced antidepressant effects in a mouse model of depression (Schroeder et al. 2007). Accordingly, microinfusion of the HDACis, MS-275 or SAHA in mice that underwent defeat stress resulted in antidepressant effect of comparable efficacy to those elicited by fluoxetine and targeted the expression of genes that were also previously been reported to be influenced by fluoxetine (Covington et al. 2009). This study raised also the interesting question of whether fluoxetine itself may directly modify epigenetic mechanisms.
Hence, HDACi (e.g., valproate or MS-275) could be tested to reverse the 5α-RI expression downregulation. The characterization of these drug mechanisms using our model of protracted social isolation stress would be advantageous to gauge evidence for future treatments of stress-induced psychiatric disorders such as anxiety (e.g., PTSD, panic) or depression. As suggested by the present report and other studies in the field, in these psychiatric disorders, Allo levels are downregulated (Rasmusson et al. 2006; Romeo et al., 1998; Uzunova et al. 1998), probably because 5α-RI and/or 3α-HSD expression are epigenetically downregulated.
Therapeutic strategies to overcome the Allo level down-regulation in depressed patients
In 1996, Uzunov and collaborators observed for the first time that SSRI antidepressants’ pharmacological action could include the ability of these drugs to increase the levels of Allo. In 1998, Uzunova and collaborators and Romeo and collaborators suggested that the mechanism of action through which the beneficial effects of SSRIs, including fluoxetine and fluvoxamine, in the treatment of major unipolar depression, may be achieved through increasing the CSF (Guidotti and Costa 1998; Uzunova et al. 1998) and plasma (Romeo et al. 1998) levels of Allo. This SSRI-induced neurosteroidogenic effect correlated with improved depressive symptomatology (Uzunova et al., 1998) and was confirmed by several other reports in the field (Eser et al. 2006; Longone et al 2008; 2011). Notably, these former studies additionally reported an increase during depression in the levels of the Allo stereoisomer, isopregnanolone (Romeo et al., 1998), which acts as an antagonist for GABAergic steroids during depression (Rupprecht and Holsboer, 1999). Importantly, and in contrast with preclinical reports, these clinical studies observed an increase of plasma allopregnanolone levels following tricyclic antidepressants (Romeo et al., 1998), in addition to SSRIs and mirtazapine (Romeo et al., 1998; Uzunova et al., 1998; Schule et al., 2006). The group of Rupprecht and colleagues thoroughly investigated also the effects of mood stabilizers, including lithium or carbamazepine on neuroactive steroid levels in depressed patients (Schule et al., 2009) with or without therapy with mirtazapine and concluded that these mood stabilizers fail per se to enhance plasma allopregnanolone levels and even reverse the mirtazapine-induced increase in plasma levels of allopregnanolone (Schule et al., 2009). Furthermore, depressed patients treated with non-pharmacological therapies, including transcranial magnetic stimulation (Padberg et al., 2002), sleep deprivation (Schule et al., 2003), or electroconvulsive therapy (Baghai et al., 2005) failed to exhibit increased in plasma levels of neuroactive steroids suggesting that the effect of non-pharmacological therapies of depression may act downstream of Allo biosynthesis.
The role of Allo in the antidepressant-like effects of steroidogenic antidepressants was confirmed in experiments in which fluoxetine’s ability to induce neurosteroidogenesis in several corticolimbic structures was tested in mouse models of psychiatric disorders such as the socially isolated mouse (Pinna et al. 2004; Pinna et al. 2003). Interestingly, in these studies, fluoxetine’s action as a steroidogenic stimulant appeared to be the primary mechanism of SSRIs. The drug concentration that increased brain Allo levels was below and independent from the mechanism involving selective serotonin reuptake inhibitor, which justified a new name to better define the “SSRI” mechanism of action: selective brain steroidogenic stimulants or SBSS (Pinna et al. 2006b; 2009). This novel mechanism of action of SSRI has stimulated drug design to focus in the development of new more effective therapies for anxiety disorders by targeting the (18 kDa) translocase protein (TSPO), which is an important starting point and a rate-limiting step in neurosteroidogenesis (Costa et al., 1994). TSPO regulates neurosteroid biosynthesis from cholesterol in the inner mitochondrial membrane of glial cells (Costa et al., 1991; 1994; Rupprecht et al. 2009). These drugs are able to exert important anxiolytic effects but are devoid of the unwanted side effects associated with benzodiazepines, including over-sedation, tolerance, and withdrawal symptoms (Rupprecht et al. 2010; Rupprecht et al. 2009). In summary, these drugs, which fulfill the requirement as SBSS molecules may be a promising new class of drugs for the future treatment of PTSD and depression. Consistently, TSPO ligands have recently showed promising therapeutic effects in clinical studies (Rupprecht et al. 2010) (Schule et al. 2011).
In patients who cannot adequately synthesize Allo and in whom administration of an SBSS is ineffective, the administration of an Allo analog, such as ganaxolone that directly activates GABAA receptors may offer an important therapeutic alternative (Gulinello et al. 2003) (Kaminski et al. 2004). Preclinical studies have recently observed that ganaxolone is highly potent in decreasing aggression, anxiety-like behavior and restoring normal contextual fear responses (Pinna et al., in press). Notably, a multisite Phase II trial of the efficacy and safety of ganaxolone in PTSD is currently in progress.
Ideally, the new SBSS ligands will selectively induce anxiolytic, anti-PTSD, and antidepressant effects by reactivating a likely epigenetically obliterated neurosteroidogenesis cascade downstream, possibly stimulating Allo content at the level of 5α-RI or 3α-HSD. Indeed, reports in the field have showed that fluoxetine and congeners studied in in vitro experiments increased the formation of Allo by enhancing the activity of the cytosolic 3α-HSD toward the reductive direction (Griffin and Mellon, 1999). Importantly, another more recent in vitro investigation demonstrated a dose-dependent inhibition of mirtazapine on the activity of the microsomal 3α-HSD in the oxidative direction, a mechanism that also results in accumulation of Allo (reviewed in Schule et al., 2014). These studies are pivotal because they shed light into mechanisms of action instrumental in drug development, which is well needed given the implication of neurosteroids as possible contributors not only in the pathology of a number of affective disorders (Uzunova et al., 1998; Romeo et al., 1998; Rasmusson et al., 2006; Nothdurfter et al., 2012; Schule et al., 2014; Backstrom et al., 2003; Bloch et al., 2000; Marx et al., 2009; 2011), such as depression, anxiety, PTSD, premenstrual dysphoria, post-partum depression, and even schizophrenia but also in several neurological disorders (Herzog, 1995; Reddy, 2011; Luchetti et al., 2011; Follesa et al., 2006), including epilepsy, Alzheimer disease, and alcohol withdrawal.
Acknowledgement
This work was supported by NIMH grant MH085999 to Pinna G.
References
- Agis-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci U S A. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agis-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci U S A. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S. Neurosteroid access to the GABAA receptor. J Neurosci. 2005;25:11605–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C. Adult Hippocampal Neurogenesis in Depression: Behavioral Implications and Regulation by the Stress System. Curr Top Behav Neurosci. 2014 doi: 10.1007/7854_2014_275. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Andersson S, Berman DM, Jenkins EP, Russell DW. Deletion of steroid 5 alpha-reductase 2 gene in male pseudohermaphroditism. Nature. 1991;354:159–161. doi: 10.1038/354159a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom T, Andersson A, Andree L, Birzniece V, Bixo M, Bjorn I, Haage D, Isaksson M, Johansson IM, Lindblad C, Lundgren P, Nyberg S, Odmark IS, Stromberg J, Sundstrom-Poromaa I, Turkmen S, Wahlstrom G, Wang M, Wihlback AC, Zhu D, Zingmark E. Pathogenesis in menstrual cycle-linked CNS disorders. Ann. N.Y. Acad. Sci. 2003;1007:42–53. doi: 10.1196/annals.1286.005. [DOI] [PubMed] [Google Scholar]
- Bali A, Jaggi AS. Multifunctional aspects of allopregnanolone in stress and related disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;48C:64–78. doi: 10.1016/j.pnpbp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Baghai TC, di Michele F, Schule C, Eser D, Zwanzger P, Pasini A, Romeo E, Rupprecht R. Plasma concentrations of neuroactive steroids before and after electroconvulsive therapy in major depression. Neuropsychopharmacology. 2005;30:1181–1186. doi: 10.1038/sj.npp.1300684. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63(2):166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Berman DM, Russell DW. Cell-type-specific expression of rat steroid 5 alpha-reductase isozymes. Proc Natl Acad Sci U S A. 1993;90(20):9359–9363. doi: 10.1073/pnas.90.20.9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am. J. Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Borowicz KK, Piskorska B, Banach M, Czuczwar SJ. Neuroprotective actions of neurosteroids. Front Endocrinol (Lausanne) 2011;2:50. doi: 10.3389/fendo.2011.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Devoto P, Roncada P, Frau R, Flore G, Saba P, Pistritto G, Soggiu A, Pisanu S, Zappala A, Ristaldi MS, Tattoli M, Cuomo V, Marrosu F, Barbaccia ML. Isolation rearing-induced reduction of brain 5alpha-reductase expression: relevance to dopaminergic impairments. Neuropharmacology. 2011;60:1301–1308. doi: 10.1016/j.neuropharm.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Southwick SM, Staib L, Zoghbi S, Charney DS. Decreased benzodiazepine receptor binding in prefrontal cortex in combat-related posttraumatic stress disorder. Am J Psychiatry. 2000;157:1120–1126. doi: 10.1176/appi.ajp.157.7.1120. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Celotti F, Melcangi RC, Negri-Cesi P. A comparative study of the metabolism of testosterone in the neuroendocrine structures of several animal species. Neuroendocrinology Letters. 1986;8:227–236. [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci U S A. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E, Auta J, Guidotti A, Korneyev A, Romeo E. The pharmacology of neurosteroidogenesis. The Journal of Steroid Biochemistry and Molecular Biology. 1994;49(4–6):385–389. doi: 10.1016/0960-0760(94)90284-4. [DOI] [PubMed] [Google Scholar]
- Costa E, Guidotti A. Diazepam binding inhibitor (DBI): a peptide with multiple biological actions. Life Sci. 1991;49(5):325–344. doi: 10.1016/0024-3205(91)90440-m. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, 3rd, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Costa E, Guidotti A. Brain 5a-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci USA. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranovsky A, Picchini AM, Moadel T, Sisti AC, Yamada A, Kimura S, Leonardo ED, Hen R. Experience dictates stem cell fate in the adult hippocampus. Neuron. 2011;70:908–923. doi: 10.1016/j.neuron.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eser D, Schüle C, Baghai TC, Romeo E, Rupprecht R. Neuroactive steroids in depression and anxiety disorders: clinical studies. Neuroendocrinology. 2006;84(4):244–254. doi: 10.1159/000097879. [DOI] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Talani G, Murru L, Serra M, Sanna E, Biggio G. Neurosteroids, GABAA receptors, and ethanol dependence. Psychopharmacology (Berlin) 2006;186:267–280. doi: 10.1007/s00213-005-0126-0. [DOI] [PubMed] [Google Scholar]
- Gelpin E, Bonne O, Peri T, Brandes D, Shalev AY. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996;57:390–394. [PubMed] [Google Scholar]
- Geuze E, van Berckel BN, Lammertsma AA, Boellaard R, de Kloet CS, Vermetten E, Westenberg HG. Reduced GABAA benzodiazepine receptor binding in veterans with post-traumatic stress disorder. Mol Psychiatry. 2008;13:74–83. doi: 10.1038/sj.mp.4002054. 3. [DOI] [PubMed] [Google Scholar]
- Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc. Natl Acad. Sci. USA. 1999;96:13512–13517. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Costa E. Can the antidysphoric and anxiolytic profiles of selective serotonin reuptake inhibitors be related to their ability to increase brain 3 alpha, 5 alpha-tetrahydroprogesterone (allopregnanolone) availability? Biol Psychiatry. 1998;44:865–873. doi: 10.1016/s0006-3223(98)00070-5. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, Costa E. The socially-isolated mouse: a model to study the putative role of allopregnanolone and 5alpha-dihydroprogesterone in psychiatric disorders. Brain Res Brain Res Rev. 2001;37:110–115. doi: 10.1016/s0165-0173(01)00129-1. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Smith SS. Progesterone withdrawal increases the anxiolytic actions of gaboxadol: role of alpha4betadelta GABA(A) receptors. Neuroreport. 2003;14:43–46. doi: 10.1097/01.wnr.0000050303.92401.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn BG, Brown AR, Lambert JJ, Belelli D. Neurosteroids and GABA(A) Receptor Interactions: A Focus on Stress. Front Neurosci. 2011;5:131. doi: 10.3389/fnins.2011.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors. Pharmacol Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology. 1995;45:1660–1662. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Livingood MR, Rogawski MA. Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia. 2004;45:864–867. doi: 10.1111/j.0013-9580.2004.04504.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kronenberg G. Depressed new neurons – adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry. 200354:499.104–503.104. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- Labrie F, Sugimoto Y, Luu-The V, Simard J, Lachance Y, Bachvarov D, et al. Structure of human type II 5 alpha-reductase gene. Endocrinology. 1992;131:1571–1573. doi: 10.1210/endo.131.3.1505484. [DOI] [PubMed] [Google Scholar]
- Levy MA, Brandt M, Sheedy KM, Holt DA, Heaslip JI, Trill JJ, et al. Cloning, expression and functional characterization of type 1 and type 2 steroid 5 alpha-reductases from cynomolgus monkey: comparisons with human and rat isoenzymes. The Journal of Steroid Biochemistry and Molecular Biology. 1995;52:307–319. doi: 10.1016/0960-0760(94)00183-m. [DOI] [PubMed] [Google Scholar]
- Longone P, Rupprecht R, Manieri GA, Bernardi G, Romeo E, Pasini A. The complex roles of neurosteroids in depression and anxiety disorders. Neurochem Int. 2008;52(4–5):596–601. doi: 10.1016/j.neuint.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Longone P, di Michele F, D'Agati E, Romeo E, Pasini A, Rupprecht R. Neurosteroids as neuromodulators in the treatment of anxiety disorders. Front Endocrinol (Lausanne) 2011;2:55. doi: 10.3389/fendo.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchetti S, Huitinga I, Swaab DF. Neurosteroid and GABA-A receptor alterations in Alzheimer's disease, Parkinson's disease and multiple sclerosis. Neuroscience. 2011;191:6–21. doi: 10.1016/j.neuroscience.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Cala KM, Russell DW. 5 alpha-reduced androgens play a key role in murine parturition. Mol Endocrinol. 1996;10(4):380–392. doi: 10.1210/mend.10.4.8721983. [DOI] [PubMed] [Google Scholar]
- Marx CE, Keefe RS, Buchanan RW, Hamer RM, Kilts JD, Bradford DW, Strauss JL, Naylor JC, Payne VM, Lieberman JA, Savitz AJ, Leimone LA, Dunn L, Porcu P, Morrow AL, Shampine LJ. Proof-of-concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology. 2009;34:1885–1903. doi: 10.1038/npp.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Bradford DW, Hamer RM, Naylor JC, Allen TB, Lieberman JA, Strauss JL, Kilts JD. Pregnenolone as a novel therapeutic candidate in schizophrenia: emerging preclinical and clinical evidence. Neuroscience. 2011;191:78–90. doi: 10.1016/j.neuroscience.2011.06.076. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Uzunova V, Pinna G, Taki K, Uzunov DP, Watanabe H, Mienvielle JM, Guidotti A, Costa E. Permissive role of brain allopregnanolone content in the regulation of pentobarbital-induced righting reflex loss. Neuropharmacology. 1999;38:955–963. doi: 10.1016/s0028-3908(99)00018-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Puia G, Dong E, Pinna G. GABA(A) receptor neurotransmission dysfunction in a mouse model of social isolation-induced stress: possible insights into a non-serotonergic mechanism of action of SSRIs in mood and anxiety disorders. Stress. 2007;10(1):3–12. doi: 10.1080/10253890701200997. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Poletti A, Cavarretta I, Celotti F, Colciago A, Magnaghi V, et al. The 5alpha-reductase in the central nervous system: expression and modes of control. The Journal of Steroid Biochemistry and Molecular Biology. 1998;65:295–299. doi: 10.1016/s0960-0760(98)00030-2. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Caruso D, Abbiati F, Giatti S, Calabrese D, Piazza F, Cavaletti G. Neuroactive Steroid Levels are Modified in Cerebrospinal Fluid and Plasma of Post-Finasteride Patients Showing Persistent Sexual Side Effects and Anxious/Depressive Symptomatology. J Sex Med. 2013;10(10):2598–2603. doi: 10.1111/jsm.12269. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Epigenetic Mechanisms of Depression. JAMA Psychiatry. 2014 Feb 5; doi: 10.1001/jamapsychiatry.2013.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nin MS, Martinez LA, Pibiri F, Nelson M, Pinna G. Neurosteroids reduce social isolation-induced behavioral deficits: a proposed link with neurosteroid-mediated upregulation of BDNF expression. Front Endocrinol. 2011a;2:73. doi: 10.3389/fendo.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nin Schuler M, Martinez LA, Thomas R, Nelson M, Pinna G. Allopregnanolone and S-norfluoxetine decrease anxiety-like behavior in a mouse model of anxiety/depression. Trabajos del Instituto Cajal. 2011b;83:215–216. [Google Scholar]
- Normington K, Russell DW. Tissue distribution and kinetic characteristics of rat steroid 5 alpha-reductase isozymes. Evidence for distinct physiological functions. The Journal of Biological Chemistry. 1992;267:19548–19554. [PubMed] [Google Scholar]
- Nothdurfter C, Rupprecht R, Rammes G. Recent developments in potential anxiolytic agents targeting GABAA/BzR complex or the translocator protein (18 kDa) (TSPO) Curr. Top. Med. Chem. 2012;12:360–370. doi: 10.2174/156802612799078748. [DOI] [PubMed] [Google Scholar]
- Padberg F, di Michele F, Zwanzger P, Romeo E, Bernardi G, Schule C, Baghai TC, Ella R, Pasini A, Rupprecht R. Plasma concentrations of neuroactive steroids before and after repetitive transcranial magnetic stimulation (rTMS) in major depression. Neuropsychopharmacology. 2002;27:874–878. doi: 10.1016/S0893-133X(02)00355-X. [DOI] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2008;105:5567–5572. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Agis-Balboa RC, Doueiri MS, Guidotti A, Costa E. Brain neurosteroids in gender-related aggression induced by social isolation. Crit Rev Neurobiol. 2004;16:75–82. doi: 10.1615/critrevneurobiol.v16.i12.80. [DOI] [PubMed] [Google Scholar]
- Pinna G, Agis-Balboa RC, Pibiri F, Nelson M, Guidotti A, Costa E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem Res. 2008;33:1990–2007. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- Pinna G, Agis-Balboa RC, Zhubi A, Matsumoto K, Grayson DR, Costa E, Guidotti A. Imidazenil and diazepam increase locomotor activity in mice exposed to protracted social isolation. Proc Natl Acad Sci U S A. 2006a;103:4275–4280. doi: 10.1073/pnas.0600329103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology (Berl) 2006b;186:362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr Opin Pharmacol. 2009;9:24–30. doi: 10.1016/j.coph.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci U S A. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Uzunova V, Matsumoto K, Puia G, Mienville JM, Costa E, Guidotti A. Brain allopregnanolone regulates the potency of the GABA(A) receptor agonist muscimol. Neuropharmacology. 2000;39:440–448. doi: 10.1016/s0028-3908(99)00149-5. [DOI] [PubMed] [Google Scholar]
- Pinna G, Rasmusson AM. Up-regulation of neurosteroid biosynthesis as a pharmacological strategy to improve behavioural deficits in a putative mouse model of post-traumatic stress disorder. J Neuroendocrinol. 2012;24(1):102–116. doi: 10.1111/j.1365-2826.2011.02234.x. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G, Vicini S, Seeburg PH, Costa E. Influence of recombinant gamma-aminobutyric acid – a receptor subunit composition on the action of allosteric modulators of gammaaminobutyricacid-gated Cl- currents. Mol Pharmacol. 1991;39:691–696. [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc . Natl. Acad. Sci. USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60:704–713. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Front. Endocrinol. 2011;2 doi: 10.3389/fendo.2011.00038. article 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo E, Strohle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Römer B, Gass P. Finasteride-induced depression: new insights into possible pathomechanisms. J Cosmet Dermatol. 2010a;9:331–332. doi: 10.1111/j.1473-2165.2010.00533.x. [DOI] [PubMed] [Google Scholar]
- Römer B, Pfeiffer N, Lewicka S, Ben-Abdallah N, Vogt MA, Deuschle M, Vollmayr B, Gass P. Finasteride treatment inhibits adult hippocampal neurogenesis in male mice. Pharmacopsychiatry. 2010b;43:174–178. doi: 10.1055/s-0030-1249095. 2010. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9:971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schule C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F, Uzunov V, McAllister KH, Bertaina-Anglade V, La Rochelle CD, Tuerck D, Floesser A, Kiese B, Schumacher M, Landgraf R, Holsboer F, Kucher K. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325:490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annual Review of Biochemistry. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Schule C, di Michele F, Baghai T, Romeo E, Bernardi G, Zwanzger P, Padberg F, Pasini A, Rupprecht R. Influence of sleep deprivation on neuroactive steroids in major depression. Neuropsychopharmacology. 2003;28:577–581. doi: 10.1038/sj.npp.1300084. [DOI] [PubMed] [Google Scholar]
- Schule C, Baghai TC, Eser D, Zwanzger P, Jordan M, Buechs R, Rupprecht R. Time course of hypothalamic-pituitary-adrenocortical axis activity during treatment with reboxetine and mirtazapine in depressed patients. Psychopharmacology (Berlin) 2006;186:601–611. doi: 10.1007/s00213-006-0382-7. [DOI] [PubMed] [Google Scholar]
- Schule C, Baghai TC, Eser D, Nothdurfter C, Rupprecht R. Lithium but not carbamazepine augments antidepressant efficacy of mirtazapine in unipolar depression: an open-label study. World J. Biol. Psychiatry. 2009;10:390–399. doi: 10.1080/15622970701849978. [DOI] [PubMed] [Google Scholar]
- Schule C, Eser D, Baghai TC, Nothdurfter C, Kessler JS, Rupprecht R. Neuroactive steroids in affective disorders: target for novel antidepressant or anxiolytic drugs? Neuroscience. 2011;191:55–77. doi: 10.1016/j.neuroscience.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Schüle C, Nothdurfter C, Rupprecht R. The role of allopregnanolone in depression and anxiety. Prog Neurobiol. 2014;113:79–87. doi: 10.1016/j.pneurobio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy RH, Biggio G. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Floris I, Floris S, Cannas E, Mossa A, Trapani G, Latrofa A, Purdy RH, Biggio G. Social isolation increases the response of peripheral benzodiazepine receptors in the rat. Neurochem Int. 2004;45(1):141–148. doi: 10.1016/j.neuint.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3alpha-OH-5alpha-pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor alpha4 subunit in association with increased anxiety. J Neurosci. 1998;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckelbroeck S, Watzka M, Stoffel-Wagner B, Hans VH, Redel L, Clusmann H, et al. Expression of the 17beta-hydroxysteroid dehydrogenase type 5 mRNA in the human brain. Molecular and Cellular Endocrinology. 2001;171:165–168. doi: 10.1016/s0303-7207(00)00432-9. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Watzka M, Steckelbroeck S, Wickert L, Schramm J, Romalo G, Klingmüller D, Schweikert HU. Expression of 5alpha-reductase in the human temporal lobe of children and adults. J Clin Endocrinol Metab. 1998;83(10):3636–3642. doi: 10.1210/jcem.83.10.5157. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Beyenburg S, Watzka M, Blumcke I, Bauer J, Schramm J, et al. Expression of 5alpha-reductase and 3alpha-hydroxisteroid oxidoreductase in the hippocampus of patients with chronic temporal lobe epilepsy. Epilepsia. 2000;41:140–147. doi: 10.1111/j.1528-1157.2000.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B. Neurosteroid biosynthesis in the human brain and its clinical implications. Annals of the New York Academy of Sciences. 2003;1007:64–78. doi: 10.1196/annals.1286.007. [DOI] [PubMed] [Google Scholar]
- Thigpen AE, Silver RI, Guileyardo JM, Casey ML, McConnell JD, Russell DW. Tissue distribution and ontogeny of steroid 5 alpha-reductase isozyme expression. The Journal of Clinical Investigation. 1993;92:903–910. doi: 10.1172/JCI116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Differential regulation of steroid 5alpha-reductase isozymes expression by androgens in the adult rat brain. The FASEB Journal. 2003;17:1428–1433. doi: 10.1096/fj.02-1119com. [DOI] [PubMed] [Google Scholar]
- Tremolizzo L, Doueiri MS, Dong E, Grayson DR, Davis J, Pinna G, Tueting P, Rodriguez-Menendez V, Costa E, Guidotti A. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol Psychiatry. 2005;57:500–509. doi: 10.1016/j.biopsych.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsutsui K. Neurosteroid biosynthesis and action during cerebellar development. Cerebellum. 2012;11(2):414–415. doi: 10.1007/s12311-011-0341-7. [DOI] [PubMed] [Google Scholar]
- Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci U S A. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Ceci M, Kohler C, Uzunov DP, Wrynn AS. Region-specific dysregulation of allopregnanolone brain content in the olfactory bulbectomized rat model of depression. Brain Res. 2003;976:1–8. doi: 10.1016/s0006-8993(03)02577-0. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sampson L, Uzunov DP. Relevance of endogenous 3alpha-reduced neurosteroids to depression and antidepressant action. Psychopharmacology (Berl) 2006;186:351–361. doi: 10.1007/s00213-005-0201-6. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Wrynn AS, Kinnunen A, Ceci M, Kohler C, Uzunov DP. Chronic antidepressants reverse cerebrocortical allopregnanolone decline in the olfactory-bulbectomized rat. Eur J Pharmacol. 2004;486:31–34. doi: 10.1016/j.ejphar.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Vallée M, Rivera JD, Koob GF, Purdy RH, Fitzgerald RL. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Anal Biochem. 2000;287(1):153–166. doi: 10.1006/abio.2000.4841. 2000. [DOI] [PubMed] [Google Scholar]
- Viola J, Ditzler T, Batzer W, Harazin J, Adams D, Lettich L, Berigan T. Pharmacological management of post-traumatic stress disorder: clinical summary of a five-year retrospective study, 1990–1995. Mil Med. 1997;162:616–619. [PubMed] [Google Scholar]