Abstract
Introduction
Diabetes is an emerging epidemic in the developing world and represents a major risk factor for cardiovascular disease. Among other issues, patients with diabetes suffer from diminished endothelial cell (EC) function, which contributes to impaired vasculogenesis and recovery from ischemic insult. The formation of cells into three-dimensional spheroids promotes cell survival and activates key signaling pathways through the upregulation of cell–cell contacts, providing an opportunity to overcome shortcomings associated with individual autologous cells.
Methods
We hypothesized that forming human microvascular endothelial cells (HMVECs) from diabetic patients into spheroids would restore their vasculogenic potential following upregulation of these cell–cell interactions. HMVEC spheroids were formed and suspended in fibrin gels to quantify vasculogenic potential.
Results
Individual HMVECs from diabetic patients exhibited similar proliferative and chemotactic potential to cells from healthy donors but reduced tubulogenesis. HMVEC spheroids formed from diabetic donors formed more sprouts than spheroids from healthy donors, and more sprouts than individual cells from either population. Compared to cells from healthy donors, sprout formation was more efficiently abrogated in HMVECs from diabetic patients by blocking matrix metalloproteinase activity.
Conclusions
This study demonstrates a promising approach for restoring the diminished vasculogenic potential of endothelial cells in diabetic patients.
Keywords: Spheroid, Diabetes, Ischemia, Tissue engineering, Angiogenesis
Introduction
Diabetes mellitus is an increasing clinical concern. In 2015, 415 million people were diagnosed with diabetes, a number projected to rise to 642 million by 2040 and currently requiring 12% of global expenditure to treat diabetes and related complications.44 These metabolic abnormalities contribute to the development of endothelial cell dysfunction and present further risk factors for cardiovascular disease and chronic limb ischemia (CLI).41 In turn, the threat of compromised tissue repair and wound healing due to insufficient blood supply constitutes a key clinical challenge.
Current treatments for chronic implications of diabetes such as peripheral vascular disease and diabetic foot ulcers often include surgical intervention or amputation.3 There is a tremendous clinical need to rapidly and effectively restore vascularization in ischemic tissues to provide necessary oxygen and nutrients for wound healing. Natural polymeric matrix wound dressings have been developed to provide scaffolding to help support infiltrating cells,1,11,42 but these do not stimulate neovascularization. Others have delivered potent angiogenic factors to catalyze blood vessel formation including vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF).3,26 However, the mode of delivery remains a limitation, as these factors undergo rapid degradation and potential proteolysis before target tissue recognition, thus requiring supraphysiological concentrations with increased cost and potential for undesirable effects.4,18 As an alternative to growth factor-based strategies, cell-based approaches utilize cell populations that respond and function under local mechanical and chemical cues, thus more closely mimicking the natural environment to support wound healing. Recent cell-based strategies to treat CLI involve transplantation of autologous cell populations to provide cells that can form new vascular structures in situ. Several endothelial cell populations have been evaluated including human microvascular endothelial cells (HMVECs) harvested from tissue biopsies and endothelial progenitor cells isolated from peripheral blood.19 Although direct implantation of endothelial cells offers appropriate signals for vasculogenesis, cell survival upon transplantation remains a limitation.10,20 This is especially true for diabetic patients, as the diabetic environment depletes vasculogenic subpopulations of cells essential for wound healing.37 Thus, there is a substantial need to enhance the efficacy of autologous endothelial cells for use in therapies for tissue vascularization.
Cell aggregates, known as spheroids, provide a promising alternative to individual cells, as they mimic the cell–cell contacts necessary for angiogenesis.9,34 Unlike individual cells that are separated from their endogenous extracellular matrix (ECM) following trypsinization, spheroids retain this instructive ECM during culture and transplantation. Spheroids formed of human mesenchymal stem cells (MSCs) exhibit enhanced therapeutic potential with greater resistance to apoptosis compared to individual MSCs.15,29,31 This improved function is modulated by spheroid size and packing density to ultimately dictate nutrient transport.30 Furthermore, spheroid formation with endothelial cell (EC) populations to promote microvessel formation has been established in both in vitro and in vivo models.22 In light of improved survival and function of cells when formed into spheroids, we hypothesized that spheroid formation of diabetic HMVECs would improve network formation and enhance vasculogenic potential in vitro compared to individual HMVECs. To explore this hypothesis, we examined the vasculogenic potential of HMVECs from diabetic and healthy donors in vitro. Furthermore, we explored the mechanism by which spheroid formation enhanced diabetic HMVEC vasculogenic potential. The results of these studies offer enhanced translational relevance for using cell-based therapies for treating ischemia in diabetic patients.
Materials and Methods
Cell Culture
Human dermal-derived microvascular endothelial cells (HMVECs) (Lonza, Walkersville, MD) from two non-diabetic (healthy) and two type II diabetic donors (diabetic) were expanded in endothelial cell growth medium (EGM-2 MV, Lonza) under standard conditions (37 °C, 5% CO2, 21% O2) until use at passage 5–8. Media changes were performed every 2 days. For each experiment, aliquots were derived from the same batch of serum to ensure serum consistency. GF-deficient EGM-2 MV medium (GF-Def, Lonza) was prepared with serum-containing EGM-2 MV but lacking VEGF, IGF (insulin-like growth factor) and FGF (fibroblast growth factor), as they are key factors for mitogenic activity.23
Assessment of HMVEC Vasculogenic Potential
We investigated the performance of individual HMVEC populations in key angiogenic stages: proliferation, migration, and network formation. The mitogenic potential of HMVECs from healthy and diabetic donors was determined by testing their growth in culture. Cells were plated at 5000 cells/cm2 on 12-well tissue culture dishes in GF-Def media. The cells were allowed to attach for 24 h, and the media was then replaced with complete or GF-Def media. After 72 h, all cells were removed with a solution of 0.25% trypsin/2.21 mM EDTA (Corning), and the number of cells was quantified using a Countess cell counter (Invitrogen, Carlsbad, CA).
HMVEC chemotaxis was quantified as previously described.8 Briefly, 24-well FluoroBlock™ transwell inserts (3 µm pore size, BD Biosciences, San Jose, CA) were coated with a thin layer of gelatin solution (0.1% fish oil gelatin, Sigma, St. Louis, MO). HMVECs (1 × 105 cells/well) were seeded on the top of the transwell inserts in 300 µL GF-Def EGM-2 MV. Transwell inserts were then placed over 1 mL EGM-2 MV to create a positive chemotactic gradient. The no-gradient control consisted of 1 mL GF-Def EGM-2 MV. Plates were then incubated for 20 h. Cells that migrated through the transwell insert were stained via calcein AM (3 µg/mL in PBS) for 30 min, and fluorescence was quantitated using a microplate reader (Synergy HTTR, Wisnooski, VT) at 485/530 nm.
The potential of HMVECs to form networks was determined as described.31 Briefly, 100 µL of Growth Factor Reduced Matrigel (Corning) was pipetted into 48-well plates and allowed to gel at 37 °C for 1 h. HMVECs were seeded on Matrigel at 30,000 cells/cm2 in EGM-2 MV or GF-Def EGM-2 MV. Cells were cultured for 8 h, and images of network formation were captured by fluorescence microscopy. Sprout length and number of branch points were measured from images using fluorescence microscopy with Nikon Eclipse TE2000U software. Sprouts were defined as branches longer than 20 µm, while cell clusters that were thicker than 100 µm and did not form a continuous branch were not counted as sprouts. Average coverage area was quantified by converting fluorescent images to binary and rendering pixel counts at bins 0 and 255 using NIH ImageJ.
Spheroid Formation
HMVECs were formed into spheroids by seeding cells in nonadhesive wells and applying a high-throughput forced gravitational method.40 HMVECs were seeded onto agarose molds with 300 pre-defined wells at 3 × 105 cells/mL in a 24-well plate and centrifuged at 163×g for 8 min to form spheroids containing 1000 cells/spheroid. Aggregates were incubated at 37 °C, and media was changed after 48 h. Spheroids were allowed to form for 3 days after initial seeding to ensure complete formation.
Fibrin gel Sprouting Assay
Spheroids were collected from agarose molds and suspended in fibrinogen dissolved in EGM-2 MV at 2 mg/mL. The spheroid/fibrinogen suspension (0.5 mL; 75 spheroids per gel) was added to a 24-well plate with 0.625 µL thrombin (0.625 U/mL). After 1 h, fresh EGM-2 MV was added to each well. DNA content from agarose molds was measured to ensure all cells were collected and to confirm equal number of cells in each group (data not shown). After 24 h, cells were stained with calcein AM (3 µg/mL in PBS) for 30 min, and images were captured by fluorescence microscopy. Quantification was performed with Nikon Eclipse TE2000U (Nikon, Melville, NY). Equal numbers of individual HMVECs in GF-Def EGM-2 MV served as the negative control, while individual cells in EGM-2 MV served as the positive control. For spheroid groups, the number of sprouts was defined as the number of any protrusions from the initial diameter of the spheroid, and all sprouts contributed to the average length. The resulting values of average number of sprouts and sprout length were derived from 30 spheroids per condition. For individual cell groups, the number of sprouts was defined as the number of segments that contributed to closed networks, and average length was quantified by any length ≥ 50 µm in the field of view.16 The resulting values of average number of sprouts and sprout length were derived from 3 wells per condition.
Western Blot
Individual cell samples were seeded at 5000 cells/cm2 in 6-well plates and collected at 80% confluency to mimic standard culture procedures. Spheroids were collected after 3 days of formation, as previously described. Spheroid and individual cell samples, one donor per cell type, were lysed and homogenized with a 30-gauge needle in RIPA buffer (Thermo Fisher Scientific, Waltham, MA), and all lysates were cleared by centrifugation. Protein concentration was determined with a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Human endothelial cell lysate (BD Biosciences) and human umbilical vein endothelial cell lysate (Abnova, Walnut, CA) were used as positive controls for vascular endothelial (VE)-cadherin and VEGFR2, respectively. HeLa cell lysate (BD Biosciences, Sparks, MD) was used as a negative control. Equal amounts of protein were loaded onto a 10% Nu-PAGE Bis-Tris Gel (Invitrogen) and resolved by gel electrophoresis. Proteins were transferred using the iBlot system (Invitrogen). Membranes were blocked with blocking buffer (2.5% nonfat dry milk in TBS, Tween-20, and ultrapure H2O). Primary VE-cadherin rabbit monoclonal antibody (mAB) (1:1000, #2500; Cell Signaling Technology, Danvers, MA), Primary VEGFR2 rabbit mAB (1:1000, #9698; Cell Signaling Technology) and GAPDH rabbit mAB (1:1000, #5174; Cell Signaling Technology) were added in blocking buffer at 1:1000 dilution as recommended by the manufacturer. Membranes were washed with 10× TBS, Tween-20, and ultrapure H2O. Anti-rabbit IgG HRP-linked antibody (1:1000, #7074; Cell Signaling) was added in blocking buffer. Membranes were washed, and detection was performed using the ChemiDoc MP Imaging System (BioRad, Hercules, CA). Densitometry was performed using NIH ImageJ software to quantify band intensities. Degradation products as indicated by the manufacturers were not included for quantification.
Quantification of Gene Expression
Total RNA was collected and isolated in TRIzol (Thermo Fisher Scientific) and 800 ng of total RNA was reverse-transcribed with the QuantiTect Reverse Transcription Kit (Qiagen). qPCR was performed using TaqMan1 Universal PCR Master Mix (Applied Biosystems). Primers and probes consisted of MMP2 (Hs01548727_m1), MMP9 (Hs00957562_m1), KDR (Hs00911700_m1), CDH5 (Hs00901465_m1), OCLN (Hs05465837_g1), GJA1 (Hs00748445_s1), ITGA2 (Hs00158127_m1), ITGA5 (Hs01547673_m1), PLAT (Hs00263492_m1), and PLAU (Hs01547054_m1) (all from Thermo Fisher Scientific). Amplification conditions were 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Quantitative PCR results were normalized to RPL13 (Hs00204173_m1) transcript level to yield ΔCt. Values are represented as 2−ΔCt.
Interrogation of Matrix Metalloproteinase on HMVEC Sprouting
HMVEC spheroids from diabetic and healthy donors, one donor per cell type, were suspended in fibrin gels as previously described. N-[(1,1′-biphenyl)-4-ylsulfonyl]-d-phenylalanine, an inhibitor of matrix metalloproteinase (MMP)-2 and MMP-9, was reconstituted according to the manufacturer’s instructions (Abcam, Cambridge, MA). Inhibitor was added to fresh media at 10X the measured IC50 value of the compound27 and used for both fibrin gel fabrication and media addition.
Statistical Analysis
Unless otherwise stated, data are presented as mean ± standard deviation from at least three independent experiments and two biological donors from each population. Statistical significance was assessed by either Student’s t test or ordinary one-way ANOVA with Tukey’s multiple comparisons test, and p-values < 0.05 were considered statistically significant. Statistical analysis was performed using GraphPad Prism® 7 analysis software (GraphPad Software, La Jolla, CA).
Results
HMVECs from Diabetic Donors have Reduced Vasculogenic Potential
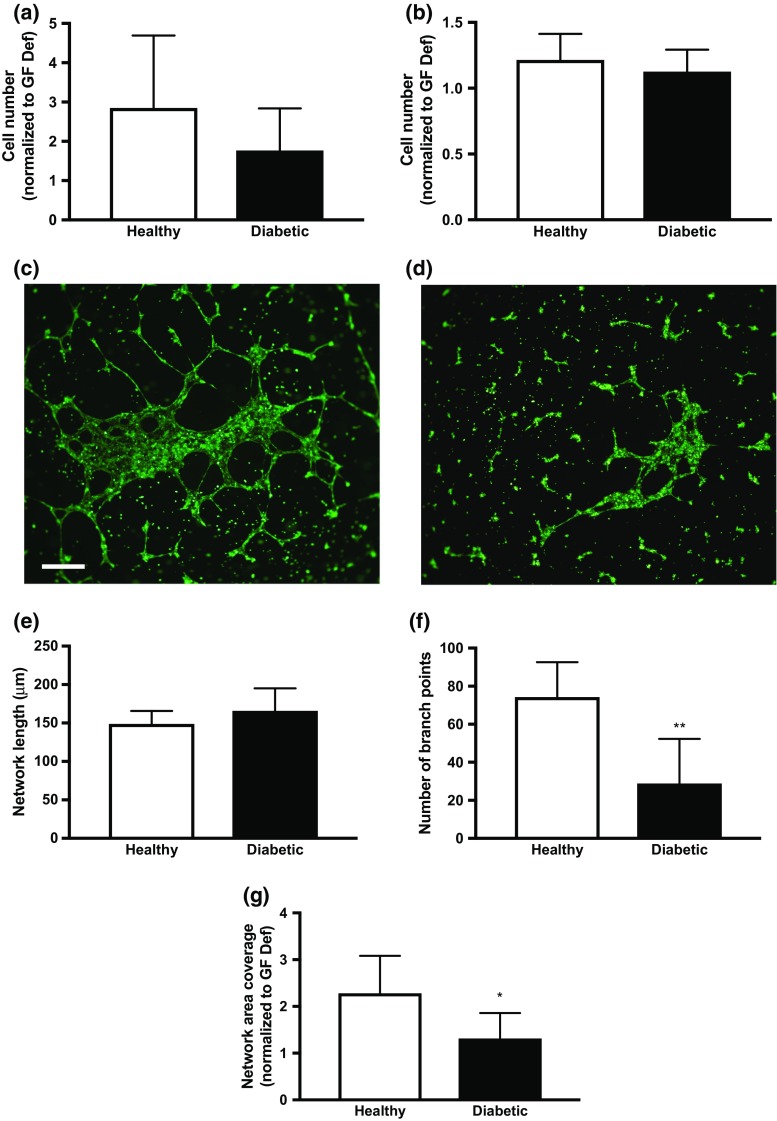
When cultured in complete media containing proangiogenic factors, we did not detect differences in proliferation (Fig. 1a) or migration (Fig. 1b) between healthy and diabetic populations. Upon gross examination, differences in network formation were apparent between HMVECs from healthy and diabetic donors (Figs. 1c and 1d). Individual HMVECs from diabetic donors formed networks with morphology resembling that of cells cultured in GF-Def conditions (data not shown), while HMVECs from healthy donors formed a robust network. HMVECs from healthy and diabetic donors exhibited similar network lengths when cultured on Matrigel (Fig. 1e). However, we observed that networks formed of HMVECs from diabetic donors formed structures with significantly fewer branch points and reduced coverage area compared to healthy donors (Figs. 1f and 1g), suggesting reduced complexity of the network structure.
Figure 1.
Diabetic HMVECs have diminished tubule formation capacity. (a) Mitogenic potential of HMVECs from healthy and diabetic donors. (b) Chemotactic response of HMVECs from healthy and diabetic donors. Representative image of network formation of individual (c) healthy and (d) diabetic HMVECs in complete media. (e) Average length of HMVEC networks on Matrigel. (f) Average number of branch points of HMVEC networks on Matrigel. (g) Average network coverage area. Scale bars represent 500 μm at ×4 magnification. Chart values represent mean ± standard deviation (n = 6; *p < 0.05; **p < 0.01).
HMVEC Spheroids Possess Enhanced Vasculogenic Potential
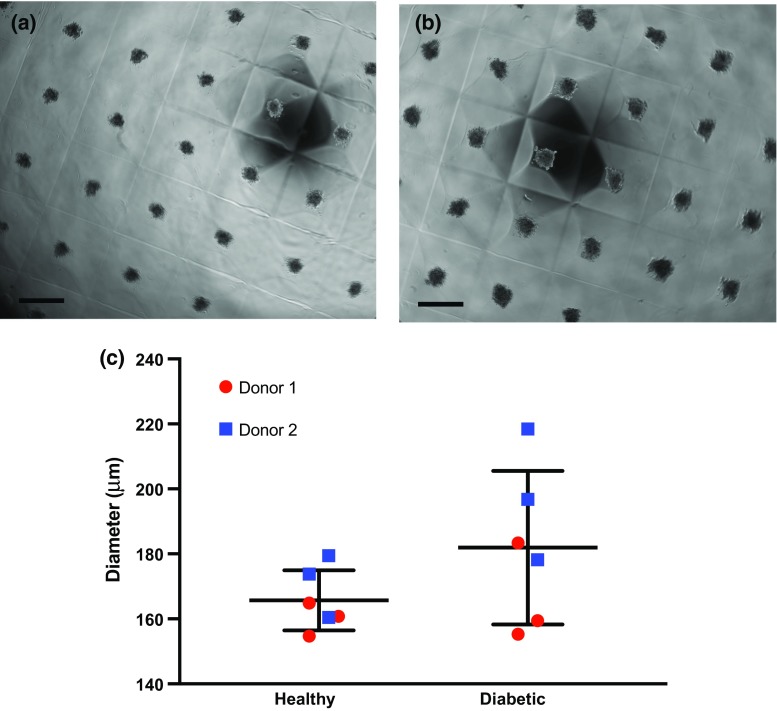
HMVECs derived from healthy or diabetic donors formed spheroids over the 3-day period (Figs. 2a and 2b). Spheroids derived from HMVECs of healthy donors were visibly more compact and slightly smaller, on average, while spheroids from diabetic HMVECs were more variable in diameter (Fig. 2c). Spheroids were then suspended in fibrin gels for 24 h in complete media to evaluate their vasculogenic potential and compared to equal densities of individual cells from the same donor. Fluorescence microscopy of individual HMVECs (Figs. 3a and 3b) and HMVEC spheroids (Figs. 3c and 3d) revealed morphological differences between cells from healthy and diabetic donors. Diabetic HMVEC spheroids exhibited more sprouts (Fig. 3e) yet similar sprout length (Fig. 3f) compared to spheroids from healthy HMVECs. Although healthy individual HMVECs exhibited significantly increased sprout length compared to both diabetic and healthy HMVEC spheroids, the morphology of sprouts derived from individual cells is not compatible with full, closed sprout networks apparent in their spheroid counterparts.
Figure 2.
HMVECs form spheroids over 3 days in culture. Brightfield images of spheroids formed from (a) healthy and (b) diabetic HMVECs at Day 3. (c) Quantification of HMVEC spheroid diameters. Scale bars represent 500 μm at ×4 magnification. Chart values represent mean ± standard deviation (n = 6).
Figure 3.
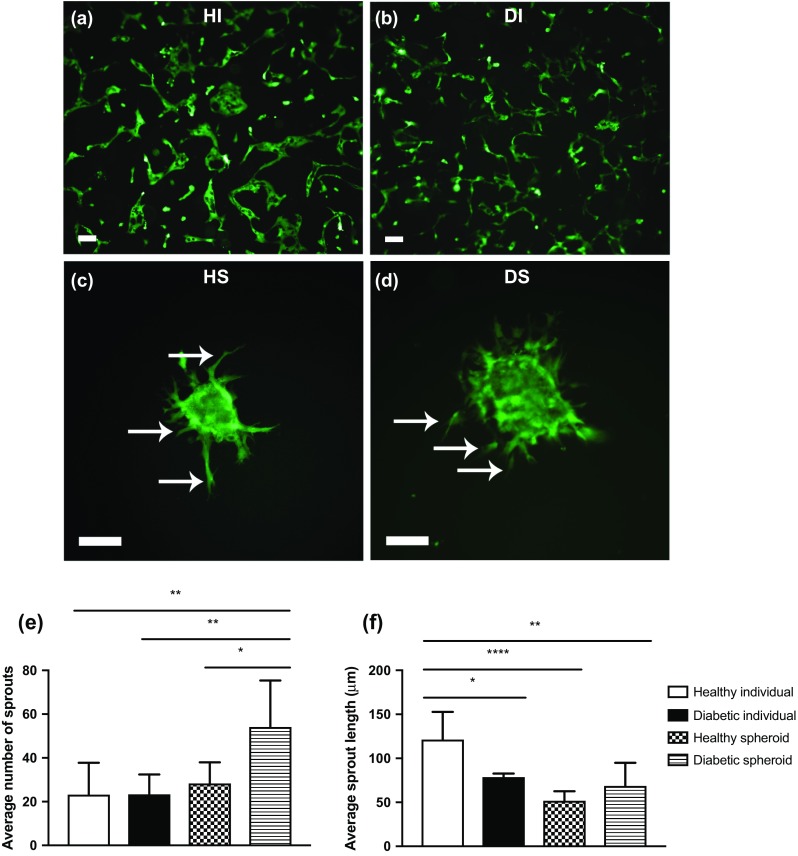
HMVEC spheroids exhibit increased sprouting compared to individual HMVECs when entrapped in fibrin gels. Representative fluorescence imaging of individual (a) healthy and (b) diabetic HMVECs entrapped in fibrin gel for 24 h. Representative fluorescence imaging of (c) healthy and (d) diabetic HMVEC spheroids entrapped in fibrin gel for 24 h. Scale bars represent 100 μm at a ×10 magnification, and sprouts are denoted by white arrows. (e) Quantification of sprouts from HMVEC spheroids. (f) Quantification of average sprout length of HMVEC spheroids. Chart values represent mean ± standard deviation (n = 6; *p < 0.05; **p < 0.01; ****p < 0.0001). HI healthy individual, DI diabetic individual, HS healthy spheroid, DS diabetic spheroid.
Spheroid Formation Restores VE-Cadherin Expression in HMVECs from Diabetic Donors
Individual cells from healthy donors expressed significantly more VE-cadherin compared to individual diabetic cells (Figs. 4a and 4b; 1.43 ± 0.29 vs. 0.59 ± 0.17, respectively, p < 0.05). Upon spheroid formation, we detected similar VE-cadherin levels between healthy and diabetic spheroid groups (1.57 ± 0.08 and 1.59 ± 0.44, respectively). Spheroid formation of HMVECs from healthy donors did not affect VE-cadherin expression. In contrast, diabetic HMVECs formed into spheroids exhibited a 2.7-fold increase in VE-cadherin compared to individual HMVECs (Figs. 4a and 4b). Furthermore, VEGFR2 expression increased in diabetic HMVEC spheroids compared to individual cells (4.00 ± 0.99 vs. 1.97 ± 0.33, respectively, p < 0.05). Similar to VE-cadherin, no differences were detected between individual and spheroid groups in healthy HMVEC populations (Figs. 4c and 4d).
Figure 4.
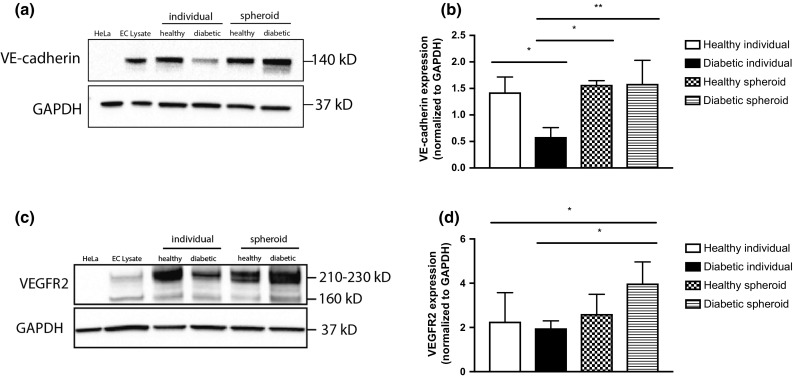
VE-cadherin and VEGFR2 expression are increased in diabetic HMVEC spheroids. (a) Western Blot of VE-cadherin expression in HMVECs as individual cells or spheroids after formation. (b) Quantification of VE-cadherin densitometry. Chart values represent mean ± standard deviation (n = 3; *p < 0.05; **p < 0.01). (c) Western Blot of VEGFR2 expression in HMVECs as individual cells or spheroids. (d) Quantification of VEGFR2 densitometry. Chart values represent mean ± standard deviation (n = 5; *p < 0.05). HeLa HeLa cells, EC lysate endothelial cell lysate.
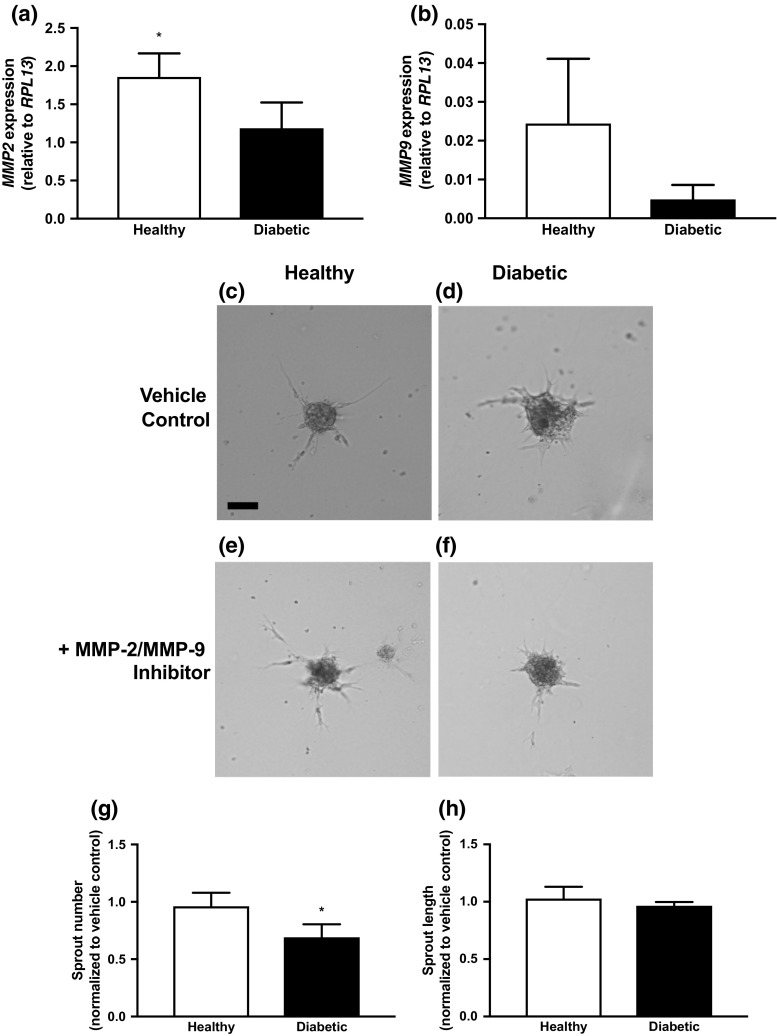
MMP-2/9 is a Key Modulator of Sprouting from HMVEC Spheroids
We assessed gene expression of key factors involved in matrix remodeling and cell adhesion between healthy and diabetic HMVEC spheroids after 3 days of formation. We detected significant differences in MMP2 expression, while a similar yet not significant trend was observed for MMP9 expression (Figs. 5a and 5b). We did not detect differences in the expression of KDR, CDH5, OCLN, GJA1, ITGA2, ITGA5, PLAT, and PLAU (data not shown). To interrogate the interplay of MMPs and HMVEC spheroid sprouting, we quantified the number and length of sprouts from spheroids that were cultured in the presence of a soluble MMP-2/9 antagonist. While spheroids formed from either HMVEC population exhibited impaired sprouting in the presence of the MMP antagonist (Figs. 5c, 5d, 5e and 5f), the addition of the MMP-2/9 antagonist diminished the number of sprouts from diabetic HMVEC spheroids significantly more compared to healthy HMVEC spheroids (Fig. 5g; 0.7 ± 0.1 vs. 1.0 ± 0.1, respectively, p < 0.05). MMP abrogation did not result in differences in sprout length for either group (Fig. 5h).
Figure 5.
Sprouting from HMVEC spheroids is dependent upon MMP-2/-9 activity. (a) Expression of MMP2 and (b) MMP9 in HMVEC spheroids after 3 days of formation. (c–f) Sprouting of healthy and diabetic spheroids in the absence or presence of an MMP-2/-9 inhibitor. Scale bars represent 100 μm at ×10 magnification. (g) Quantification of sprout number of healthy and diabetic spheroids when normalized to non-inhibited control. (h) Quantification of sprout length of healthy and diabetic spheroids when normalized to non-inhibited control. Chart values represent mean ± standard deviation (n = 3; *p < 0.05).
Discussion
Vascular complications from diabetes necessitate effective strategies for therapeutic angiogenesis. Patients with chronic limb ischemia or compromised vascularization suffer from necrotic wounds, such as the diabetic foot ulcer, which often need amputation. As an alternative to wound dressings or growth factor-based approaches, current cell-based strategies commonly involve transplantation of vessel-forming endothelial cells or cell populations that support tissue vascularization via paracrine-acting biological cues. However, these cells are rapidly lost upon transplantation, likely due to drastic changes in oxygen tension between the culture environment and implantation site or separation from endogenous extracellular matrix accumulated during culture. While it is unclear whether reductions in available oxygen or loss of contact with the ECM are the limiting factors, alternative strategies to enhance cell survival and function to promote vascular repair in ischemic tissues are necessary. The formation and transplantation of spheroids represent a novel approach to cell-based therapy, as spheroids formed from MSCs or ECs exhibit increased cell survival compared to individual cells.2,24,36 However, previous data have established the therapeutic benefits of spheroid formation using only cells from healthy donors. The therapeutic potential of spheroid formation using HMVECs from diabetic donors has not been explored. The results of these studies confirm improved vasculogenic potential in spheroids formed of HMVECs from both healthy and diabetic donors. Compared to individual cells, HMVECs from diabetic donors exhibited significant improvement in sprouting. These data suggest that spheroid formation can enhance the vasculogenic potential of HMVECs, providing a new approach for using autologous cells for use in treating ischemia in diabetic patients.
We first assessed the performance of individual HMVECs from healthy and diabetic donors in key stages of angiogenesis: proliferation, migration, and tubule formation. Both healthy and diabetic populations showed little difference in proliferation and migration when stimulated by proangiogenic cues. However, we observed marked differences in terms of tubule formation. Diabetic HMVECs formed fewer branch points when cultured on Matrigel compared to healthy populations, translating to reduced capacity to form closed networks. These data confirm previous reports of diminished network formation in diabetic endothelial progenitor cells in vitro.14 The diminished network-forming potential of diabetic EC populations suggests that restoring the potential for this stage in angiogenesis is key to restore vascularization potential in diabetes.
Spheroids offer numerous benefits compared to transplanting individual cell populations. Compared to cells in monolayer culture, spheroids do not require harsh physical or enzymatic detachment methods for collection. This allows for maintenance of cell–cell contacts, supporting increased cellular interactions with the endogenous ECM deposited during culture. The transplantation of mesenchymal stem cells (MSCs) on endogenous cell-secreted ECM resulted in improved cell survival and tissue formation in vivo,13,17 demonstrating the value of retaining cell-ECM contacts. Similar increases in cell survival were observed with cells transplanted as sheets for treatment of myocardial infarction.39 Recent studies have transplanted spheroids formed of human umbilical vein endothelial cells (HUVECs) and MSCs from healthy donors to accelerate vascularization in ischemic tissue in vivo8,35 or incorporation into prevascularized scaffolds for subsequent implantation.28,43 In these studies, diabetic HMVEC spheroids exhibited increased numbers of sprouts compared to individual diabetic HMVECs. Furthermore, sprout lengths were comparable to those measured from healthy HMVEC spheroids, suggesting that diabetic HMVEC spheroids have potential in vivo for treating ischemic tissues.
Cell–cell adhesion in endothelial cells is largely mediated by VE-cadherin, a transmembrane protein that allows endothelial cells to regulate barrier function essential for angiogenesis.6 To confirm the role of cell–cell contacts for improved vascular potential, we measured VE-cadherin expression in individual HMVECs and HMVEC spheroids from both healthy and diabetic donors. Compared to individual cells in monolayer culture, VE-cadherin expression in HMVECs from diabetic donors demonstrated a 2.7-fold increase as spheroids, similar to levels in cells from healthy donors. This may suggest that spheroid formation enhances cell–cell communication needed for diabetic HMVECs to develop a robust microvasculature. Further investigation of the influence of VE-cadherin via steric hindrance may confirm a connection with cell–cell contact with sprouting, but VE-cadherin expression is complex, and internalization is transient through angiogenic processes.33 Future study of these cell–cell contacts is necessary to understand not only expression profiles but also localization at the cell surface. The difference in VE-cadherin expression between diabetic spheroids, healthy spheroids, and the positive control was not significant, providing evidence of enhanced vasculogenic potential by spheroid formation. We also investigated the expression of vascular endothelial growth factor receptor-2 (VEGFR2) in spheroids, as this receptor is known to form a complex with VE-cadherin at cell–cell junctions for survival and angiogenesis.5 Similar to VE-cadherin expression, VEGFR2 expression increased in spheroids formed from diabetic HMVECs compared to individual HMVECs. However, we did not detect differences in VEGFR2 expression in healthy HMVECs, either as individual cells or spheroids. These findings align with our observations of reduced network formation by individual diabetic HMVECs, as their response to angiogenic cues such as VEGF-A was poor when seeded on Matrigel. Deletion of VE-cadherin can abolish transmission of key remodeling and maturation signals via VEGFR2.5 VE-cadherin and VEGFR2 association has also been suggested as an essential mechanosensor in response to shear flow.7 Although our experiments were performed under static culture, our findings offer a simple method to upregulate expression of these key signaling pathways to ultimately improve survival and vessel maturation.
Endothelial cell sprouting and tubule formation depend upon the ability of cells to remodel their environment, often by secretion of endogenous enzymes. Vascular disease states can impact EC transformation and further vessel stabilization.38 Thus, we interrogated the necessity of a key matrix metalloproteinases, MMP-2 and MMP-9, on sprouting from spheroids formed of HMVECs from healthy and diabetic donors. Abnormal expression of MMP-9, either excessive or insufficient concentrations, is an indicator of poor wound healing in lower extremities in diabetic patients.25 The inhibition of both MMPs and serine proteases was required to completely block HUVEC capillary formation when in fibrin gels.12 MMPs play a complex role in pro- and anti-angiogenic processes, contributing to the balance of EC function during angiogenesis. Furthermore, hyperglycemic environments in diabetic retinopathy increase vascular permeability associated with increased MMP expression and degradation of VE-cadherin, where inhibition of MMPs prevents the loss of VE-cadherin.32 In agreement with these data, our results revealed that HMVEC sprouting from spheroids is sensitive to MMP-2/9 abrogation. These data suggest that metalloproteinases are key participants in sprouting. However, the interplay between spheroid formation, cell–cell adhesion, cell–matrix adhesion, and secretion of other remodeling enzymes such as serine proteases that modulate ECM remodeling by endothelial cells merit further investigation. Within the natural wound healing environment, there is great complexity beyond homotypic cell populations, as stromal cell populations offer enhanced stability and improved capillary networks in co-culture with endothelial cells.21
Overall, these findings suggest a method to boost cell–cell interactions and ultimately increase endothelial cell capacity to promote vascularization using HMVECs from diabetic patients. The interplay between VE-cadherin and VEGFR2 expression during spheroid formation indicates that increasing initial cell–cell contacts may enhance the responsiveness of HMVEC spheroids to proangiogenic cues delivered as recombinant growth factors or in conjunction with other cells secreting paracrine factors. As cell viability and function remain unresolved clinical challenges when using cell-based therapies, this work provides insight into alternative implantation strategies to harness the full regenerative efficiency and therapeutic potential of autologous cell populations.
Acknowledgments
Research reported in this publication was supported by National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number R01DE025475 (JKL). KM was supported by American Heart Association Western States Affiliate Predoctoral Fellowship (15PRE21920010). CEV was supported by the National Heart, Lung & Blood Institute T32 Training Program in Basic and Translational Cardiovascular Science (T32HL086350). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This study was funded by National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number R01DE025475 (JKL). KM was supported by American Heart Association Western States Affiliate Predoctoral Fellowship (15PRE21920010). CEV was supported by the National Heart, Lung & Blood Institute T32 Training Program in Basic and Translational Cardiovascular Science (T32HL086350).
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Abbreviations
- EC
Endothelial cell
- HMVEC
Human microvascular endothelial cell
- CLI
Chronic limb ischemia
- VEGF
Vascular endothelial growth factor
- PDGF
Platelet derived growth factor
- MSC
Mesenchymal stem cell
- EGM-2 MV
Endothelial cell growth medium
- GF-def
Growth factor-deficient EGM-2 MV medium
- IGF
Insulin-like growth factor
- FGF
Fibroblast growth factor
- VEGFR2
Vascular endothelial growth factor receptor 2
- VE-cadherin
Vascular endothelial cadherin
- MMP
Matrix metalloproteinase
- ECM
Extracellular matrix
- HUVEC
Human umbilical vein endothelial cell
References
- 1.American Diabetes Classification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care in Diabetes–2016. Diabetes Care 2016;39(Suppl. 1):S13–S22. Diabetes Care. 2016;39:1653. doi: 10.2337/dc16-er09. [DOI] [PubMed] [Google Scholar]
- 2.Bhang SH, et al. Three-dimensional cell grafting enhances the angiogenic efficacy of human umbilical vein endothelial cells. Tissue Eng Pt A. 2012;18:310–319. doi: 10.1089/ten.tea.2011.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briquez PS, Clegg LE, Martino MM, Mac Gabhann F, Hubbell JA. Design principles for therapeutic angiogenic materials. Nat Rev Mater. 2016;1:15006. doi: 10.1038/natrevmats.2015.6. [DOI] [Google Scholar]
- 5.Carmeliet P, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/S0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 6.Chen DY, et al. Three-dimensional cell aggregates composed of HUVECs and CBMSCs for therapeutic neovascularization in a mouse model of hindlimb ischemia. Biomaterials. 2013;34:1995–2004. doi: 10.1016/j.biomaterials.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 7.Coon BG, et al. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J Cell Biol. 2015;208:975–986. doi: 10.1083/jcb.201408103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decaris ML, Lee CI, Yoder MC, Tarantal AF, Leach JK. Influence of the oxygen microenvironment on the proangiogenic potential of human endothelial colony forming cells. Angiogenesis. 2009;12:303–311. doi: 10.1007/s10456-009-9152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Bio. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 10.Eppler SM, et al. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin Pharmacol Ther. 2002;72:20–32. doi: 10.1067/mcp.2002.126179. [DOI] [PubMed] [Google Scholar]
- 11.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 12.Ghajar CM, George SC, Putnam AJ. Matrix metalloproteinase control of capillary morphogenesis. Crit Rev Eukaryot Gene Expr. 2008;18:251–278. doi: 10.1615/CritRevEukarGeneExpr.v18.i3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvestine JN, et al. Extracellular matrix-coated composite scaffolds promote mesenchymal stem cell persistence and osteogenesis. Biomacromolecules. 2016;17:3524–3531. doi: 10.1021/acs.biomac.6b01005. [DOI] [PubMed] [Google Scholar]
- 14.Hill JM, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. New Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 15.Ho SS, Murphy KC, Binder BYK, Vissers CB, Leach JK. Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Transl Med. 2016;5:773–781. doi: 10.5966/sctm.2015-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoch AI, Binder BY, Genetos DC, Leach JK. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS ONE. 2012;7:e35579. doi: 10.1371/journal.pone.0035579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoch AI, et al. Cell-secreted matrices perpetuate the bone-forming phenotype of differentiated mesenchymal stem cells. Biomaterials. 2016;74:178–187. doi: 10.1016/j.biomaterials.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes C, Wrobel JS, Maceachern MP, Boles BR. Collagen-based wound dressings for the treatment of diabetes-related foot ulcers: a systematic review. Diabetes Metab Syndr Obes. 2013;6:17–29. doi: 10.2147/DMSO.S36024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou L, Kim JJ, Woo YJ, Huang NF. Stem cell-based therapies to promote angiogenesis in ischemic cardiovascular disease. Am J Physiol Heart Circ Physiol. 2016;310:H455–H465. doi: 10.1152/ajpheart.00726.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kannan RY, Salacinski HJ, Sales K, Butler P, Seifalian AM. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a review. Biomaterials. 2005;26:1857–1875. doi: 10.1016/j.biomaterials.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Kolbe M, et al. Paracrine effects influenced by cell culture medium and consequences on microvessel-like structures in cocultures of mesenchymal stem cells and outgrowth endothelial cells. Tissue Eng Pt A. 2011;17:2199–2212. doi: 10.1089/ten.tea.2010.0474. [DOI] [PubMed] [Google Scholar]
- 22.Laib AM, et al. Spheroid-based human endothelial cell microvessel formation in vivo. Nature Protocols. 2009;4:1202–1215. doi: 10.1038/nprot.2009.96. [DOI] [PubMed] [Google Scholar]
- 23.Leach JK, Kaigler D, Wang Z, Krebsbach PH, Mooney DJ. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27:3249–3255. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev. 2008;14:61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Guo S, Yao F, Zhang Y, Li T. Increased ratio of serum matrix metalloproteinase-9 against TIMP-1 predicts poor wound healing in diabetic foot ulcers. J Diabetes Complicat. 2013;27:380–382. doi: 10.1016/j.jdiacomp.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Li XD, Xu GL, Chen JQ. Tissue engineered skin for diabetic foot ulcers: a meta-analysis. Int J Clin Exp Med. 2015;8:18191–18196. [PMC free article] [PubMed] [Google Scholar]
- 27.Mastyugin V, McWhinnie E, Labow M, Buxton F. A quantitative high-throughput endothelial cell migration assay. J Biomol Screen. 2004;9:712–718. doi: 10.1177/1087057104269495. [DOI] [PubMed] [Google Scholar]
- 28.Mishra R, et al. Effect of prevascularization on in vivo vascularization of poly(propylene fumarate)/fibrin scaffolds. Biomaterials. 2016;77:255–266. doi: 10.1016/j.biomaterials.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy KC, Fang SY, Leach JK. Human mesenchymal stem cell spheroids in fibrin hydrogels exhibit improved cell survival and potential for bone healing. Cell Tissue Res. 2014;357:91–99. doi: 10.1007/s00441-014-1830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy KC, et al. Measurement of oxygen tension within mesenchymal stem cell spheroids. J R Soc Interface. 2017 doi: 10.1098/rsif.2016.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy KC, et al. Multifactorial experimental design to optimize the anti-inflammatory and proangiogenic potential of mesenchymal stem cell spheroids. Stem Cells. 2017;35:1493–1504. doi: 10.1002/stem.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navaratna D, McGuire PG, Menicucci G, Das A. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes. 2007;56:2380–2387. doi: 10.2337/db06-1694. [DOI] [PubMed] [Google Scholar]
- 33.Orsenigo F, et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pampaloni F, Reynaud EG, Stelzer EHK. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Bio. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 35.Pan B, et al. Diabetic HDL is dysfunctional in stimulating endothelial cell migration and proliferation due to down regulation of sr-bi expression. PLoS ONE. 2012 doi: 10.1371/journal.pone.0048530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park IS, Chung PS, Ahn JC. Enhancement of ischemic wound healing by spheroid grafting of human adipose-derived stem cells treated with low-level light irradiation. PLoS ONE. 2015;10:e0122776. doi: 10.1371/journal.pone.0122776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rennert RC, et al. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res Ther. 2014;5:79. doi: 10.1186/scrt468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz MA, Vestweber D, Simons M. A unifying concept in vascular health and disease. Science. 2018;360:270–271. doi: 10.1126/science.aat3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sekine H, et al. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng Part A. 2011;17:2973–2980. doi: 10.1089/ten.tea.2010.0659. [DOI] [PubMed] [Google Scholar]
- 40.Vorwald CE, Ho SS, Whitehead J, Leach JK. High-throughput formation of mesenchymal stem cell spheroids and entrapment in alginate hydrogels. Methods Mol Biol. 2018;1758:139–149. doi: 10.1007/978-1-4939-7741-3_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wils J, Favre J, Bellien J. Modulating putative endothelial progenitor cells for the treatment of endothelial dysfunction and cardiovascular complications in diabetes. Pharmacol Ther. 2017;170:98–115. doi: 10.1016/j.pharmthera.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Xiao Y, Ahadian S, Radisic M. Biochemical and biophysical cues in matrix design for chronic and diabetic wound treatment. Tissue Eng Part B Rev. 2017;23:9–26. doi: 10.1089/ten.teb.2016.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan J, et al. Recovery from hind limb ischemia is less effective in type 2 than in type 1 diabetic mice: roles of endothelial nitric oxide synthase and endothelial progenitor cells. J Vasc Surg. 2009;50:1412–1422. doi: 10.1016/j.jvs.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmet P, Alberti KG, Magliano DJ, Bennett PH. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol. 2016;12:616–622. doi: 10.1038/nrendo.2016.105. [DOI] [PubMed] [Google Scholar]