Abstract
Rationale:
Gut microbes influence cardiovascular disease (CVD) and thrombosis risks through the production of trimethylamine N-oxide (TMAO). Microbiota-dependent generation of trimethylamine (TMA), the precursor to TMAO, is rate-limiting in the metaorganismal TMAO pathway in most humans, and is catalyzed by several distinct microbial choline TMA-lyases, including the proteins encoded by the choline-utilization C/D genes (cutC/D) in multiple human commensals.
Objective:
Direct demonstration that the gut microbial cutC gene is sufficient to transmit enhanced platelet reactivity and thrombosis potential in a host via TMA/TMAO generation has not yet been reported.
Methods and Results:
Herein, we use gnotobiotic mice and a series of microbial colonization studies to show that microbial cutC-dependent TMA/TMAO production is sufficient to transmit heightened platelet reactivity and thrombosis potential in a host. Specifically, we examine in vivo thrombosis potential employing germ-free mice colonized with either high TMA-producing stable human fecal polymcrobial communities, or a defined CutC deficient background microbial community coupled with a CutC-expressing human commensal ± genetic disruption of its cutC gene (i.e. Clostridium sporogenes ΔcutC).
Conclusions:
Collectively, these studies point to the microbial choline TMA-lyase pathway as a rational molecular target for the treatment of atherothrombotic heart disease.
Keywords: Gut microbiome, cutC, gnotobiotic mice, trimethylamine N-oxide, cardiovascular disease, thrombosis, TMAO, metabolism
Keywords: Animal Models of Human Disease, Platelets, Thrombosis
INTRODUCTION
Over the last decade a critical role for gut microbiota in both human health and disease has been established1–4. A growing number of mechanistic studies have shown that direct microbial transfer from donor to recipient can facilitate transmission of disease-associated phenotypes, susceptibility for development of diseases, and even responses to therapy5–12. Microbial transplantation studies allow for a clear demonstration of transmission of a host phenotype or disease susceptibility, providing strong evidence in support of a causal contribution of gut microbiota in host physiological processes and disease risks. Follow-up studies have therefore naturally begun to shift in focus toward understanding what microbial genes, gene products, and potential down-stream metabolic products derived from microorganisms are interacting with and impacting the host 7,13.
One gut microbiota-dependent metabolite, trimethylamine N-oxide (TMAO), has garnered significant attention because of its involvement in cardiovascular disease (CVD) development, enhanced platelet reactivity, and thrombosis potential7,14–16. Numerous animal model and clinical studies support a role for TMAO in CVD development. Plasma levels of TMAO in multiple large scale clinical studies are independently associated with incident CVD development and adverse event risks7,14–16. The metaorganismal pathway resulting in TMAO generation is initiated by ingestion of nutrients rich in a Western diet (phosphatidylcholine, choline, carnitine), and their subsequent use by gut microbes to produce the waste product trimethylamine (TMA). Following absorption from the gut into the portal circulation, TMA is rapidly delivered to the liver where it is oxidized to form TMAO by a cluster of hepatic enzymes, the flavin monooxygenases (FMOs)17. FMO3 serves as the major enzyme catalyzing the conversion of TMA into TMAO in both rodents and humans17. Animal studies employing genetic suppression of FMO3 demonstrate alterations in sterol, bile acid and metabolic phenotypes relevant to vascular disease18–20. Multiple recent meta-analyses have reviewed available clinical studies investigating TMAO and CVD risks, and concluded that elevated TMAO levels are dose-dependently associated with CVD and mortality risks21–23. Moreover, animal model studies using a non-lethal inhibitors of microbial TMA production from choline demonstrate inhibition in diet-dependent atherosclerosis24, and more recently, thrombosis susceptibility25. Thus, there is substantial interest in studies examining potential gut microbial participants in TMA and TMAO generation, and demonstration of their potential involvement in disease processes relevant to CVD.
Choline represents a common abundant nutrient precursor for gut microbiota-dependent TMA production in omnivores and vegans/vegetarians owing to the high concentration of phosphatidylcholine and choline in bile26. Balskus and colleagues first identified a bacterial gene cluster in human gut isolates, the choline utilization (cut) gene cluster, which promotes anaerobic choline metabolism via two cut gene products, catalytic and regulatory polypeptides encoded by the cutC and cutD genes, respectively27. Present in a phylogenetically diverse range of sequenced human gut bacteria, the presence of predicted cutC/D genes within human gut microorganisms is correlated with their ability to grow on choline, and promote the choline → TMA transformation (i.e. choline TMA-lyase activity)28–30. Use of synthetic microbial communities has demonstrated the presence or absence of choline TMA-lyase activity is sufficient to regulate microbial choline utilization and subsequent TMAO accumulation29,30. However, to date no studies have directly demonstrated that the presence of the microbial cutC gene can impact platelet function and thrombosis potential in the host, nor have human fecal transplantation studies been used to transmit choline TMA-lyase activity and thrombosis potential into a recipient. Herein we employ genetically defined synthetic microbial communities, as well as polymicrobial fecal communities of human origins, to demonstrate that functional CutC levels in the intestinal microbiota can dose-dependently transmit enhanced platelet reactivity and heightened thrombosis potential within a host. Our studies suggest that global functional inhibition of gut microbial CutC represents a potential therapeutic approach for the treatment or prevention of CVD.
METHODS
The authors declare that data and methods used in support of the findings presented in this study will be made available from the corresponding author upon reasonable request.
Sequencing data sets used for community composition analysis are publicly available through NCBI’s Sequence Read Archive (SRA) Database under BioProject PRJNA480401.
Human studies.
All subjects involved in studies presented gave informed consent with protocols approved by either the Cleveland Clinic Institutional Review Board, or the Institutional Review Board of the University of Wisconsin-Madison. Human fecal samples (and accompanying plasma samples) used for microbial transplantation studies in the main manuscript were collected as part of the Carnival study (NCT01731236 clinicaltrials.gov), where results were previously reported in aggregate (group wise) form7. Healthy donors were pre-screened and confirmed to have no preceding history of antibiotics or probiotics use within 3 months of study enrollment. Additional exclusionary criteria for this study included: 1) a significant chronic illness or end-organ dysfunction; 2) ingestion of yogurt within the past 7 days; 3) chronic gastrointestinal disorders; 4) having undergone bariatric procedures; or 5) pregnancy. Subjects (46±5 years of age, 40% male, nonsmokers without hypertension, diabetes mellitus, or cardiovascular disease) were given oral choline supplementation (choline bitartrate 500 mg twice daily, equivalent to 450mg total choline per day) for two months with monthly fasting blood and stool collections. From these studies, differences in diet was previously highlighted, and thus only omnivores are shown here. Of these, two subjects (corresponding to high and low plasma levels of TMAO) were selected to serve as donors for human fecal microbial transplantation studies. These human donors were approximately matched based on age (51 and 59yo), sex (female), and BMI (30.8 and 31.7).
In separate fecal microbial transplantation studies, human feces recovered from a “high” versus “low” TMAO producing community when transplanted into germ-free mice were provided from the University of Wisconsin-Madison31. These “validation” human fecal microbial transplant studies (reported in Online Figure I) followed the same experimental design and used frozen human donor fecal samples that were previously utilized as colonization material for mice in methods studies describing and characterizing a new fecal microbial collection and processing protocol31.
Human fecal microbial transplant studies.
Stool samples from human donors collected at the Cleveland Clinic were stored for future humanization studies as fecal slurries (0.15g/ml) in 10% glycerol at −80ºC. Germ-free C57BL/6J mice (8–10 week old, female, purchased from Jackson labs) were colonized with 200μl of a 5× dilution of the above stock by oral gavage. Mice were transferred to a chemically defined diet supplemented with 1% w/w choline (Envigo TD.09041) the morning of fecal transplantation. One week following colonization, blood was collected for use in in vitro aggregometry studies, or mice were utilized for in vivo thrombosis7. Upon completion of studies, tissues were collected and immediately frozen at −80ºC. Following colonization, the investigator was not blinded from treatment groups to allow testing first on the low TMA-producer colonized mice prior to testing on the high TMA-producer colonized mice to avoid cross contamination. Fecal samples for the validation studies were collected using the FAST method and stored at −80C until use31. Validation animals were colonized with 200μl freshly prepared fecal slurry using Mega Medium.
Construction of cutC directed insertional mutants that are enzymatically null.
Disruptions of the cutC gene in Clostridium sporogenes (CLOSPO_02864), was accomplished using the ClosTron mutagenesis procedure as previously reported33,34. The cutC targeting DNA sequence was cloned into the shuttle plasmid pMTL007C-E2 to make the retargeted vector pMTL007C-E2::Csp-cutC. The retargeted pMTL007C-E2::Csp-cutC plasmid was conjugated into C. sporogenes and plated onto TYG agar plates supplemented with D-cycloserine (250μg/ml) and thiamphenicol (15μg/ml)34. Antibiotic resistant colonies were verified by diagnostic PCR for harboring the plasmid of interest. PCR positive colonies were spread onto TYG agar plates supplemented with erythromycin (5μg/mL) prior to selection again on TYG plates supplemented with D-cycloserine and erythromycin. Screening for intron insertion was performed after bacterial genomic DNA was extracted using the Zymo Fungal/Bacterial DNA Extraction Kit. Extracted DNA was used for diagnostic PCR using the primer set (Fwd: 5’-CGTGTTCATAAGGAACTTGCAC-3’; Rev: 5’-GCTTTTCTCTCGATTATACC-3’), which results in a ~1000 bp product for the wild-type cutC DNA sequence and ~3200 bp product for the directed insertional mutant containing the intron. PCR verified directional insertion clones were confirmed to not produce TMA by stable isotope dilution LC/MS/MS analyses32.
Gnotobiotic mouse husbandry.
All experiments involving mice were performed using protocols approved by the Cleveland Clinic Animal Care and Use Committee. Gnotobiotic C57BL/6 mice aged 8–10 weeks were provided from the University of Wisconsin-Madison. Animals were shipped to the Cleveland Clinic Lerner Research Institute Gnotobiotic facility and upon arrival were maintained in sterile isolators and equilibrated onto the defined choline diet for two weeks prior to and following simple randomization and colonization where appropriate. In some mice, as indicated, TMAO was provided in filter sterilized drinking water (0.2% w/w TMAO). Food and water were provided ad libitum and lights maintained on a strict 12h light/dark cycle. Male mice were not utilized in these studies due to the known reduced hepatic expression of FMO enzymes in adult males, leading to a significant decrease in capacity to generate TMAO.
Gnotobiotic mouse colonization.
Microbial strains used to colonize mice were grown as monocultures on Mega Medium agar plates anaerobically for 48 to 72 h at 37°C. Single colonies were then inoculated into 3 ml of Mega Medium and grown anaerobically at 37°C until late log phase. Strains belonging to the same treatment group were combined in an equal volume ratio in a Hungate tube. Germ-free, C57Bl/6 8–10-week-old mice were inoculated by oral gavage with 200μl of mixed bacterial culture inside the gnotobiotic isolator or biological safety cabinet. At the time of sacrifice tissues were immediately collected, frozen, and stored at −80°C. Following colonization, the investigator was not blinded from treatment groups to avoid cross contamination.
In vitro aggregometry.
Mice were anesthetized and platelets were collected from whole blood as previously described7. Platelets were counted and concentrations adjusted to 2 × 108 cells/ml with platelet poor plasma as the diluent. CaCl2 and MgCl2 (both 1 mM final concentration) were added immediately before platelet aggregation studies. Platelet aggregation in response to 1 μM ADP was assessed in triplicate at 37oC in a dual channel Type 500 VS aggregometer (Chrono-log Corporation, Havertown, PA) with stirring at 1000 rpm. Sample sizes of animals are indicated in the figures. Instances of mismatched samples are due to inability to obtain a complete blood sample.
Choline TMA-lyase activity assay.
Choline TMA-lyase enzyme activity was determined by quantifying d9-TMA production from d9-choline substrate using stable isotope dilution LC/MS/MS analyses as previously described32. Activity is shown as pmol d9-TMA / mg tissue / hour. Sample sizes are indicated in the figures. Instances of mismatched samples are due to incomplete reaction. Samples were identified by code number only and investigators remained blinded to sample identity during laboratory analyses.
Hepatic FMO activity assay.
Total liver FMO activity was determined by quantifying flavin-dependent d9-TMAO production from d9-TMA substrate using stable isotope dilution LC/MS/MS analyses14 with minor modifications. Briefly, livers samples were homogenized in 10 mM HEPES, pH 7.4, containing protease inhibitor cocktail. Protein concentration was determined from supernatants by BCA protein assay. FMO activity was measured in liver homogenates (200 μg protein buffered with 10mM HEPES, pH7.4) in a 200 μl reaction mixture containing d9-TMA (100 μM final) in the presence vs. absence of freshly prepared NADPH (100 μM final) for 8 hours at 37 C. Reactions were stopped by adding 50μl 0.2N formic acid containing 10 μM [13C3]TMAO as internal standard. The concentration of d9-TMAO and [13C3]TMAO was quantified by stable isotope dilution LC/MS/MS using multiple reaction monitoring of parent and characteristic daughter ions: m/z 85 → 66 for d9-TMAO; and m/z 79 → 61 for [13C3]TMA. FMO activity is shown as nmol d9-TMAO / mg protein / hour. Sample sizes are indicated in the figures. Instances of mismatched samples are due to incomplete reaction. Samples were identified by code number only and investigators remained blinded to sample identity during laboratory analyses.
Carotid artery FeCl3 injury thrombosis assay.
Mice were then anesthetized and subjected to a common carotid artery injury by application of 10% FeCl3 for 1 min, as previously described7. Rhodamine 6G (100μl; 0.5 mg/ml) was injected directly into the right jugular vein to label platelets. Thrombus formation was observed in real time using intravital fluorescence microscopy equipped with video recording. Time to cessation of blood flow through clot formation for all studies was determined by visual inspection by two different investigators. End points were set as cessation of blood flow for 30 seconds to 30 minutes.
Microbial community analysis.
Microbial DNA from transplanted mice was extracted following MO BIO PowerSoil-htp 96 Well Soil DNA Isolation Kit procedure with an additional incubation at 65°C for 10 min prior to plate shaking. The 16S rRNA V4 hypervariable region was amplified with barcoded primers in triplicate using the 5 PRIME HotMasterMix (VWR)35. Products were quantified with Quant-iT™ PicoGreen dsDNA Assay Kit (Thermo Fisher) and samples were combined in equal amounts (~250ng per sample) to be purified with the UltraClean PCR® Clean-Up Kit (MO BIO). Pooled amplicons were sequenced on the Illumina HiSeq platform over two lanes to generate single-end reads.
Raw sequences were processed with the open source Quantitative Insights Into Microbial Ecology (QIIME) software package version 1.9.1 to produce de-multiplexed and quality controlled sequences36. Samples were binned into OTUs using the pick_closed_reference_otus.py script at 97% similarity using SUMACLUST against a Greengenes reference database37,38. Microbial community patterns were visualized with Principal Component Analysis (PCoA) using unweighted UniFrac distance measure on samples rarefied to 267,600 reads per sample in an effort to reduce the effect of unequal sequencing depth39. Treatment groups had similar multivariate homogeneity of dispersions and so were compared using the non-parametric PERMANOVA test with the adonis function. Hierarchical clustering by unweighted pair group method with arithmetic mean (UPGMA) was performed using the unweighted unifrac distance matrices in QIIME. Heatmap containing OTUs at 0.005% relative abundance by sample were organized by row using PCoA with unweighted UniFrac distance measure. All graphs and analyses were performed in R using the phyloseq package v 1.19.1 and the associated dependencies40. Comparison of specific microbial genera abundance with TMAO or aggregation levels were determined by unpaired t test with Welch’s correction. Heatmaps were generated with the R statistical package with DESeq, vegan, and vegan programs.
Bacterial communities resulting from inoculation of germ-free animals with synthetic communities were analyzed using previously reported methods30. Instances of mismatched samples are due to either animals who died prior to terminal measurement completion being sequenced or sacrificed animals failing to properly sequence.
Statistics.
Human data was determined to be normally distributed by the D’Agostino and Pearson omnibus normality test. Pearson correlation coefficient for normally distributed data, and Spearman correlations for non-normally distributed data were used to analyze associations between quantitative variables. Student T-tests and One-Way ANOVAs, followed by multiple comparison post-tests, were completed in Prism and are detailed in the respective figure legends. For all statistical tests, p < 0.05 was considered significant.
RESULTS
Transmission of both TMA/TMAO production and thrombotic risk phenotypes through human fecal transplant to a germ-free recipient.
We recently described the involvement of TMAO in promoting heightened thrombosis potential in vivo by demonstrating TMAO directly induces heightened responsiveness in isolated platelets to multiple agonists like ADP, thrombin and collagen7. In the present studies, we initially sought to test whether human fecal transplantation could transmit gut microbial choline TMA-lyase activity and thrombosis potential into a recipient. Thus, our experimental design involved use of germ free mice as recipients that were colonized by human fecal microbial transfer from subjects with high versus low TMAO levels, and then subsequent testing to determine if recipients showed heightened gut microbial (cecal) choline TMA-lyase activity, circulating TMA and TMAO levels, and correspondingly enhanced platelet reactivity (Figure 1A). For selection of a stable polymicrobial community to serve as donor, we utilized fecal samples recovered during the conduct of a recently reported human intervention study in which choline supplements (choline bitartrate, 500 mg orally twice daily) were shown to elevate TMAO and foster enhanced platelet reactivity as monitored by platelet aggregometry in subjects41 (Figure 1B). Subjects on an omnivorous diet showed significant variation in the extent of TMAO elevation. Plasma levels of all subjects at baseline versus 1 and 2 month time points are shown in Figure 1B, and the donors and time points selected to represent low versus high TMAO are indicated (fecal samples corresponding to plasma TMAO of 2.1 μM and 34.2 μM, respectively; Figure 1B)41. Choline TMA-lyase activity measured (from d9-choline substrate) in the human fecal donors selected was 0.4 ± 0.5 and 11.1 ± 2.2 pmol d9-TMA/mg tissue/h for the low and high donors, respectively. Feces were transplanted as a slurry via gastric gavage into germ-free mice on a choline diet as described under Methods. After transplantation, colonized mice were sacrificed, whole blood and tissues recovered, and multiple indices of the TMAO metaorganismal pathway examined, along with platelet aggregation responses in platelet rich plasma using a submaximal (1 μM) level of ADP (Figure 1C-F). Notably, significantly elevated levels cecal choline TMA-lyase activity (p=0.006), plasma TMAO levels (p=0.011), and platelet aggregation responses (p=0.0005) were observed in recipients of the high (versus low) TMAO donor feces. Examination of hepatic total FMO activity in both groups of recipients showed no significant difference (p=0.68; Figure 1D).
Figure 1. Transmission of thrombotic risk phenotypes through human fecal transplant to a germ-free recipient.
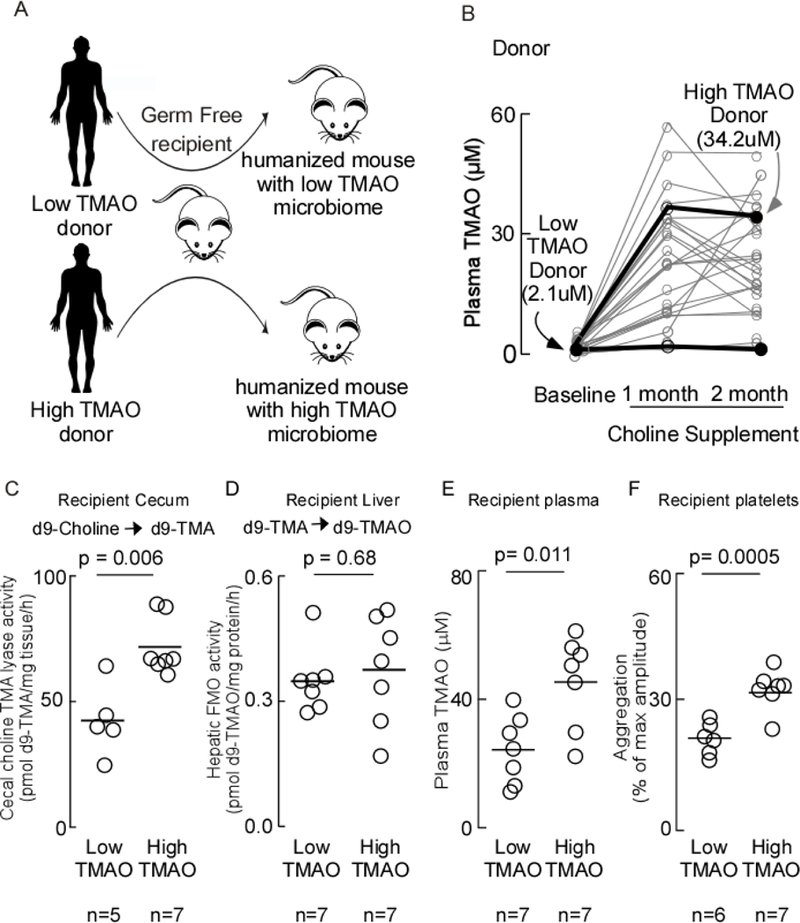
A) Scheme illustrating fecal microbial transplant study design. Germ-free C57Bl/6J mice were colonized by oral gavage with a fecal microbial community from B) human subjects with either high or low plasma TMAO levels (donors shown with dark symbols along with corresponding TMAO levels). Recipient mice were maintained on a choline diet. Five days post-transplant, blood was collected, tissues harvested, and C) Cecal choline TMA-lyase activity was measured; D) Hepatic FMO activity was measured; E) plasma TMAO was measured; and F) platelet function was assessed by ex vivo monitoring of aggregation response to sub-maximal ADP (1μM). P-values were determined by two-tailed unpaired t-test with Welch’s correction.
In an independent set of human fecal microbial transplantation studies, we sought to replicate these findings. Human fecal samples used for these studies were collected at the University of Wisconsin-Madison and were previously characterized for their TMA / TMAO producing capacity31,42. Direct measurement of the choline TMA-lyase enzymatic activity in these donor samples showed 20.3 ± 2.0 and 49.4 ± 3.2 pmol d9-TMA/mg tissue/h for the low and high donors, respectively; p<0.05). Following transplantation, the recipient mice (maintained on high choline diet) demonstrated significant differences in circulating TMAO levels with recipients of the “high” TMAO donor feces showing nearly double the TMAO level of that observed in recipients of the “low” TMAO donor feces (p<0.0001; Online Figure IA). Similarly, cecal microbial choline TMA-lyase activity measured in the murine recipients was significantly different (p=0.01; Online Figure IB). Faster clot formation was observed in the “high” TMAO recipients, and representative images of clot formation at different times following injury between the recipient groups are shown in Online Figure ID. The time to cessation of blood flow was also significantly decreased in mice colonized with the high TMAO donor (p < 0.0001) (Online Figure IC).
In a final series of analyses, we examined the dose-dependent relationship between plasma TMAO levels and indices of platelet function and thrombosis potential in each of the human fecal transplantation studies. Notably, platelet aggregation responses within the recipients in the first study showed a highly significant dose dependent positive association (r = 0.75, p=0.003; Figure 2A). Similarly, examination of the in vivo thrombosis potential in recipients of the second human fecal transplantation study, as monitored by time to cessation of blood flow in the carotid artery injury model, revealed a significant dose dependent relationship (r = −0.75, p=0.0009; Figure 2B).
Figure 2. Relationship between plasma TMAO levels and platelet aggregation.
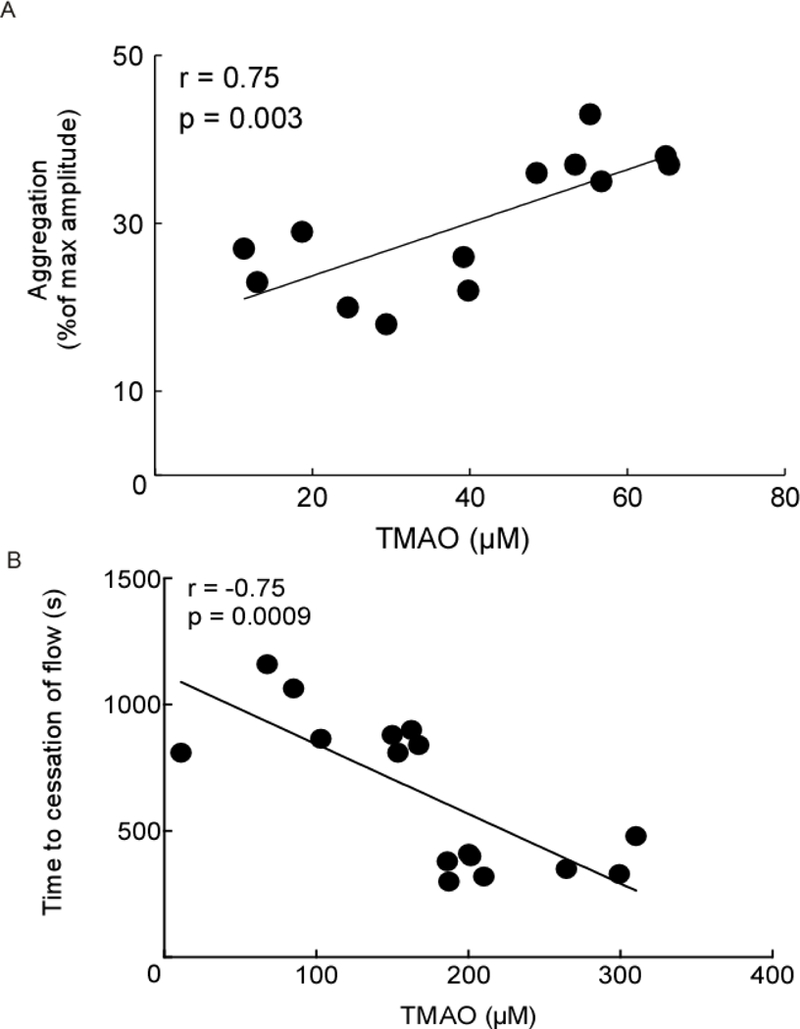
Relationship between A) plasma TMAO levels and in vitro aggregometry in platelet rich plasma recovered from recipient groups of mice of human fecal microbiota from the human TMAO high or low donors and B) plasma TMAO levels and time to cessation of blood flow in the carotid artery injury model. Spearman rank correlation A) R = 0.75, p = 0.003 and B) R = −0.75, p=0.0009.
Microbial composition analyses of human fecal transplantation studies.
We examined microbial community structure via 16S rRNA gene sequencing analyses of both human fecal donor and murine cecal recipient communities in the initial human fecal transplantation experiment, as described under Methods. Notably, principal component analysis revealed the microbial phylogeny of the high TMAO versus low TMAO transplantation resulted in significant differences in the murine recipient microbiome (Figure 3A). Hierarchical clustering analyses further established the defined separation of the recipient mice based on donor TMAO status (Figure 3B). These results provide evidence of transmission of multiple features of ‘high TMAO’ versus ‘low TMAO’ microbial communities. Microbial taxa whose proportions are characteristic of the high TMAO versus low TMAO communities (in recipients) were discerned through linear discriminant analysis (Figure 3C). A heatmap of relative proportions of taxa in recipients classify the colonized recipient mice based on donor microbiome (High vs. Low TMAO) (Online Figure II). The relative abundance of several characteristic (in LDA analyses) microbial genera in the high TMAO colonized mice were observed to be significantly associated with both high plasma TMAO levels and increased platelet aggregation responsiveness (Figure 3D-F).
Figure 3. Microbial composition of human fecal transplantation.
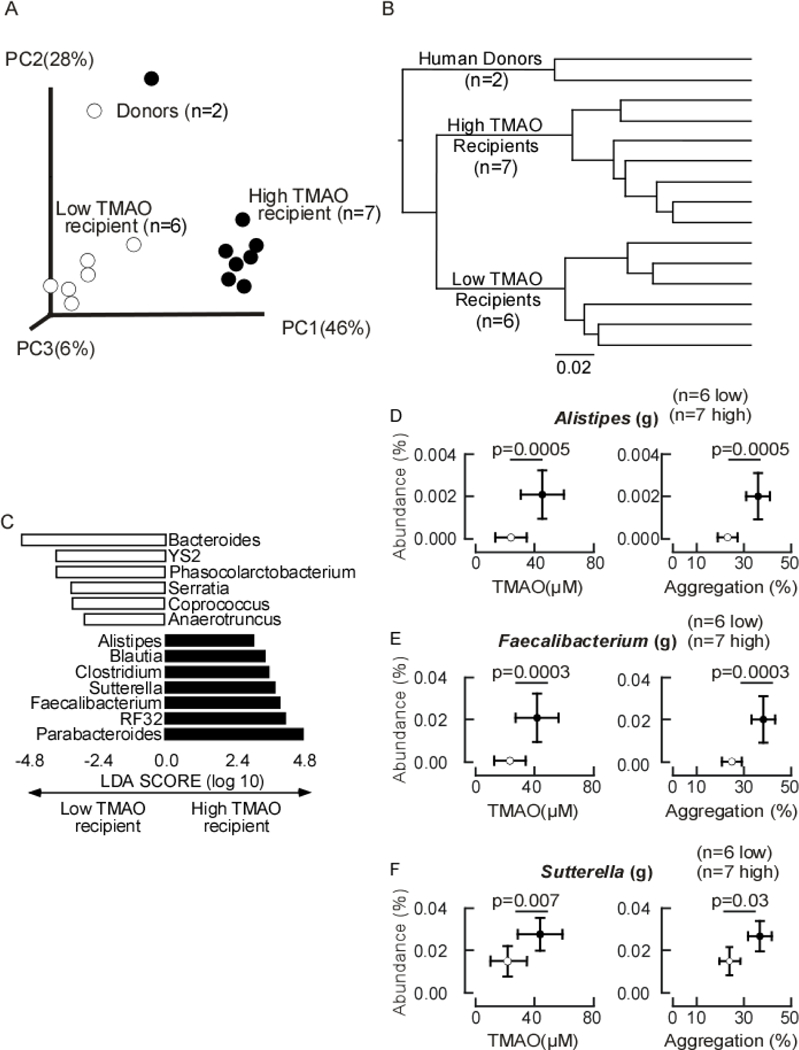
A) Unweighted Unifrac distances plotted in principle component analysis comparing the fecal (donors) or cecal (mouse) microbiota of donors and recipients. Each data point represents a sample from a distinct human donor or mouse recipient projected onto the first three principal coordinates (representing 89% of the total variance). B) Heirarchical clustering using unweighted pair group method with arithmetic mean (UPGMA) of human donor and recipient mice reveals a relationship among recipient mice in which the mice colonized with the high TMAO donor cluster together and distinctly from the mice colonized with the low TMAO donor. Branch lengths indicate distance of separation based on unweighted unifrac distance matrices. C) Linear discriminant analysis (LDA) effect size (LEfSe) identified cecal taxa from mouse characteristic in low TMAO recipient (empty bars) and high TMAO recipients (filled bars). D-F) The relationship of specific indicated genera abundance to plasma TMAO levels or aggregation values in low TMAO recipient mice (empty dots, n=6) and high TMAO recipient mice (filled dots, n=7). P-values were determined by unpaired t test with Welch’s correction.
Microbial colonization of a cutC containing human commensal within a defined (ΔcutC) microbial community transmits into recipients TMA/TMAO generation, heightened platelet responsiveness and enhanced thrombosis potential.
We next sought to test whether colonization of germ-free mice with a human commensal that possesses the cutC gene and can produce TMA from choline in vitro confers altered platelet responsiveness and in vivo thrombosis potential in the host. Previous published studies examined a large panel of culturable human gut isolates for the production of d9(trimethyl)-TMA from d9(trimethyl)-choline, and demonstrated that of those capable of producing TMA from choline in culture (n=8 of 79 screened), the majority (n=7 of 8) possessed components of the choline utilization gene cluster based on sequence homology29. Armed with this information, we utilized a core set of microorganisms (B. thetaiotaomicron, B. caccae, B. ovatus, C. aerofaciens, E. rectale) to serve as a synthetic “Core community” for transplantation studies because they had previously been shown to lack TMA production capacity from choline (TMA-null). Indeed, when cultured anaerobically, both individually and in combination in the presence of d9-choline, we confirmed no detectable d9-TMA was produced (Figure 4A). In some mice, a single microorganism, Clostridium sporogenes, which possesses a predicted cutC gene and was previously shown to be capable of producing d9-TMA from d9-choline under anaerobic culture, was chosen to be added to this synthetic TMA-null Core community to form a TMA-producing community (Figure 4A). We thus colonized all germ-free mice with the TMA-null Core community of microorganisms, and in the group indicated, also included C. sporogenes. In an additional group, germ-free mice colonized with the TMA-null Core community were provided TMAO in sterilized drinking water, as described under Methods. Following colonization, mice were maintained for two weeks on a high choline diet, and blood, tissues and cecum recovered for assessment of platelet function through aggregometry, and biochemical and microbiota composition analyses.
Figure 4. Microbial colonization of a cutC containing human commensal transmits TMA/TMAO generation and heightened platelet responsiveness.
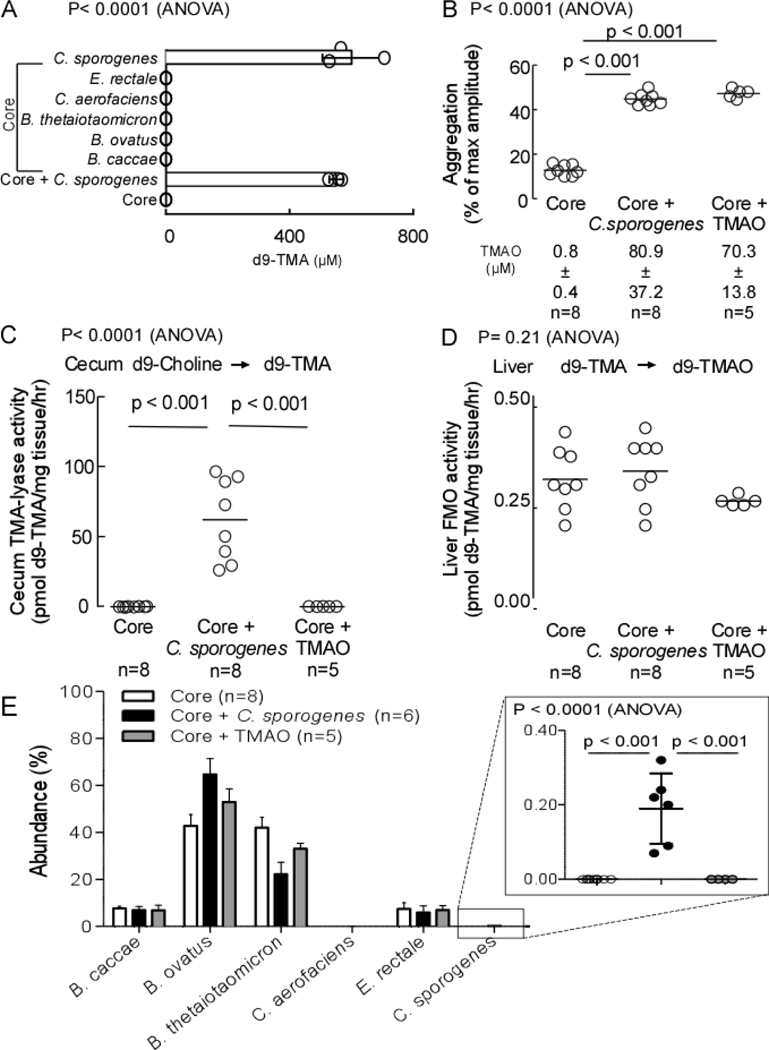
A) Capability to produce d9-TMA from d9-choline substrate in individual microbes or in combination was determined in vitro. B) C57Bl/6 germ-free mice colonized by gastric gavage with a core community +/− C. sporogenes (TMA producer). The non-TMA producing core microorganisms consist of B. caccae, B. ovatus, B. thetaiotaomicron, C aerofaciencs, E. rectale. Core + TMAO mice received TMAO in drinking water (0.2% w/w TMAO). All mice received a choline diet. Fourteen days post-transplant blood was collected, platelet rich plasma recovered and ex vivo platelet aggregation response to ADP (1μM) was measured. C) Cecal choline TMA-lyase activity; and D) Hepatic FMO enzyme activity were assessed as described under Methods. E) Cecal microbial community composition was determined sequencing of mouse cecum, as described in Methods. Two mice from the Core + C. sporogenes group and one mouse from the Core + TMAO group failed to sequence. All p-values shown were determined by One-Way ANOVA with Tukey’s multiple comparison post-test (B-E).
As expected, amongst the three groups, only mice co-colonized with C. sporogenes demonstrated detectable cecal choline TMA-lyase activity (Figure 4C). Upon further examination of plasma, virtually no detectable TMAO (submicromolar) was observed in the TMA-null Core community colonized mice, compared with approximately 100-fold higher levels of TMAO in the mice concomitantly colonized with C. sporogenes, or supplemented with TMAO via the drinking water (Figure 4B, bottom). Importantly, analyses of platelet aggregation results using sub-maximal (1μM) ADP to stimulate platelet-rich plasma recovered from each group demonstrated increased platelet responsiveness in the C. sporogenes co-colonized group similar to that observed in the TMAO supplemented group (Figure 4B). In additional control studies, liver tissue was recovered and total hepatic Flavin-dependent FMO activity was quantified. No significant differences were observed amongst all groups of mice (Figure 4D). These results support microbiota-dependent changes in the observed changes in TMAO levels and platelet function, and not the host hepatic (FMO-mediated) transformation of TMA → TMAO. In a final series of studies, microbial composition analyses were performed on cecal contents from each group of mice as described under Methods. Notably, C. sporogenes represented only ~0.2% of the total community (Figure 4E).
In a separate set of studies, we similarly colonized germ-free mice with only the TMA-null Core community alone, or in the presence of the TMA producing C. sporogenes, for the purpose of examining in vivo thrombosis potential. All mice were maintained in microisolators on high choline diets for two-weeks, and following FeCl3 injury of the carotid artery, the rate of thrombus formation in vivo, and the time to vessel occlusion, were assessed as described under Methods. Figure 5A shows representative images at the indicated times following FeCl3 – induced injury, as visualized using vital microscopy equipped with time lapse fluorescent camera to permit time-dependent quantification of fluorescently tagged platelets during clot formation, and determination of the time of vessel occlusion (cessation of blood flow). As before, only mice colonized with both the synthetic Core community and C. sporogenes demonstrated significant TMAO levels, with accompanying faster rate of clot formation, and substantially shorter time to vessel occlusion (illustrative example Figure 5A). Quantitative analyses of all mice and images revealed over 100-fold higher plasma TMAO levels (p<0.0001) and significantly shorter (p=0.0002) time to vessel occlusion in the mice colonized with both the TMA-null Core community and C. sporogenes (Figure 5B). Again, examination of choline TMA-lyase activity (d9-choline → d9-TMA) in cecum recovered post colonization showed significant activity only within cecum recovered from recipients of the TMA producing C. sporogenes (p<0.0001; Fig 5C). Moreover, quantification of total hepatic FMO activity (Flavin-dependent conversion of d9-TMA → d9-TMAO in liver homogenates) in both groups of mice showed no significant difference between treatment groups (p=0.06; Fig 5D). Microbial composition analyses of the recipient groups of mice post colonization again showed C. sporogenes represented only approximately 0.08% of the total community (Fig 5E). Thus, despite representing only a small fraction of the overall community inhabitants, the addition of C. sporogenes accounted for all measured functional differences observed (i.e. elevated choline TMA-lyase activity, elevated TMAO levels, heightened platelet responsiveness as monitored by platelet aggregometry, and heightened in vivo thrombosis potential as monitored by both thrombus rate of formation and time to vessel occlusion following injury).
Figure 5. Microbial colonization of a cutC containing human commensal transmits TMA/TMAO generation and heightens thrombosis potential.
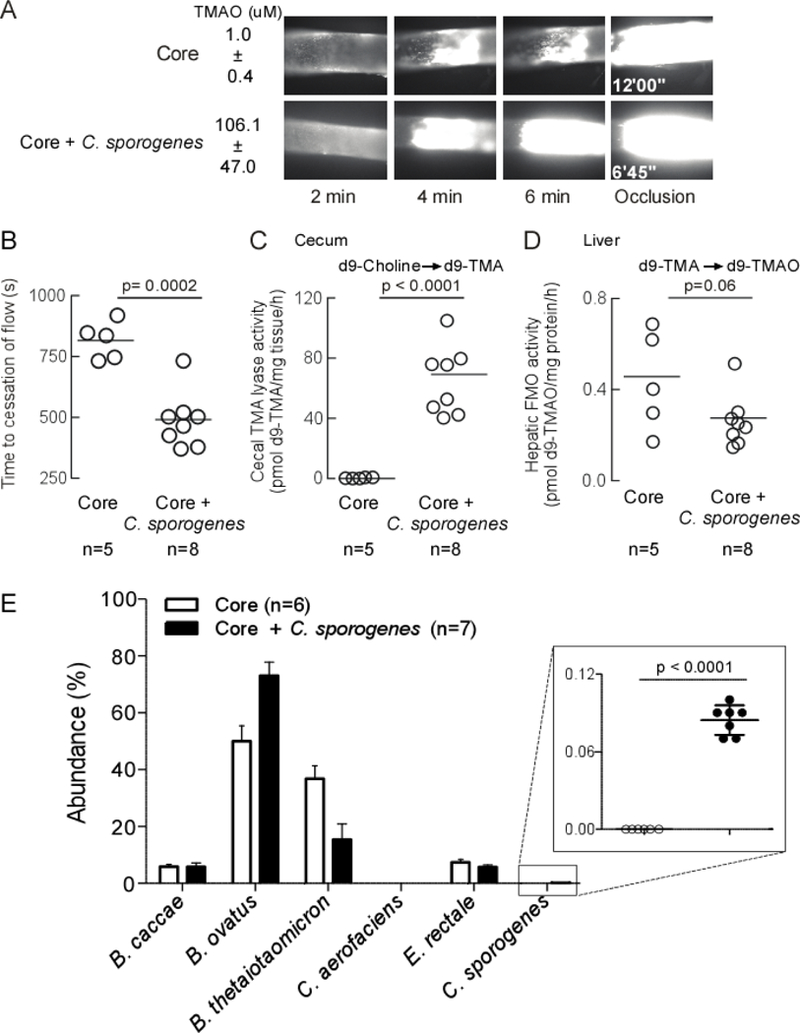
Germ-free C57Bl/6 mice were colonized by oral gavage. Core mice received non-TMA producing B. caccae, B.ovatus, B. thetaiotaomicron, C. aerofaciens, E. rectale. Core + C. sporogenes mice received the additional TMA producing microorganism. All mice maintained on a choline diet. A) Fourteen days after colonization, thrombosis potential (FeCl3 carotid injury model) and plasma TMAO levels were assessed. Example clot formation images are shown with average plasma TMAO ± SD values. B) Time to cessation of blood flow was determined as described in Methods. C) Cecal choline TMA-lyase activity; and D) Hepatic FMO enzyme activity were assessed as described under Methods. E) Cecal microbial community composition was determined by sequencing of mouse cecum, as described in Methods. One animal in the Core group died before terminal measurements could be completed was still used in sequencing analysis. P-values were determined by unpaired t test with Welch’s correction.
Mice colonized with a C. sporogenes ΔcutC strain do not accumulate TMAO and exhibit reduced thrombosis potential.
Beyond cutC, additional microbial genes with distinct catalytic mechanisms have been identified that can catalyze the transformation of choline or other TMA-containing nutrient precursors into TMA32,43. While the cut gene cluster appears to be the most abundant choline TMA-lyase known amongst sequenced human gut commensals, and genome screening of C. sporogenes reveals that the only predicted choline utilization gene it harbors is cutC, we thought it prudent to perform additional studies to ensure that CutC within C. sporogenes was responsible for the heightened TMAO and thrombosis related phenotypes (platelet aggregation responses, thrombus rates of formation) in the germ-free mice colonized with the microbe. Directed insertional disruption mutation of the cutC gene in C. sporogenes was therefore performed, as described under Methods, in order to enable studies to directly compare results observed between this human commensal in wild-type (WT) form, versus C. sporogenes lacking a functional CutC enzyme (ΔcutC). Examination of the growth rates of C. sporogenes (WT) versus C. sporogenes (ΔcutC) in anaerobic culture in choline containing media shows a modest but detectable reduction in growth rate (Figure 6A, left), and complete absence of capacity to generate d9-TMA from d9-choline (Figure 6A, right). After confirming that the C. sporogenes (ΔcutC) was viable yet lacked choline → TMA transforming activity, we performed additional microbial transplantation studies in germ-free mice as recipients. Three groups of mice were examined and maintained in isolators following colonization. All three groups received the synthetic TMA-null Core community. One group was co-colonized with C. sporogenes (WT) while the second group received the TMA-null C. sporogenes (ΔcutC). The third group of mice remained colonized with only the Core community but received TMAO in drinking water. Two weeks after colonization, the mice were examined for quantification of in vivo thrombus rate of formation and time to cessation of blood flow following FeCl3 induced common carotid artery injury. Upon sacrifice, blood and tissues were harvested for biochemical and gut microbe composition analyses. As seen in Figure 6B, visual inspection of images captured with the fluorescence camera on vital microscope platform shows faster rate of clot formation, and shortened time to vessel occlusion in the representative mouse colonized with Core + C. sporogenes (WT) and Core + TMAO (drinking water), relative to the Core + C. sporogenes (ΔcutC) group. Quantification of plasma TMAO levels amongst all mice within each group again showed elevated levels in both the mice colonized with the Core + C. sporogenes community, and the Core mice supplemented with TMAO in the drinking water. Importantly, the germ-free mice colonized by the TMA-null Core community + C. sporogenes (ΔcutC) showed negligible levels of TMAO. Moreover, quantification of the time to cessation of blood flow in these groups of mice revealed disruption of cutC both eliminated the heightened plasma TMAO levels otherwise observed in the other groups, and markedly reduced the in vivo rate of thrombosis (i.e. the time to cessation of blood flow, or the occlusion time, was substantially longer; p<0.001; Figure 6C). As expected, examination of cecal choline TMA-lyase enzyme activity in recovered tissues from the different mice groups showed significant and detectable levels only among the group colonized with C. sporogenes (WT), and not the cutC insertional mutant (C. sporogenes, ΔcutC), or the Core community only (+ TMAO in drinking water) (Figure 6D). No differences in hepatic total FMO activity were observed amongst the three groups (Figure 6E). Finally, examination of the cecal microbial community compositions amongst each group of colonized mice showed C. sporogenes (WT) and C. sporogenes (ΔcutC) represented similar proportions that again only represented quantitatively a very modest component of the entire community (approximately 0.1% of total) (Figure 6F, right).
Figure 6. Deletion of the cutC gene from C. sporogenes blocks generation of TMAO in the host reducing thrombosis potential.
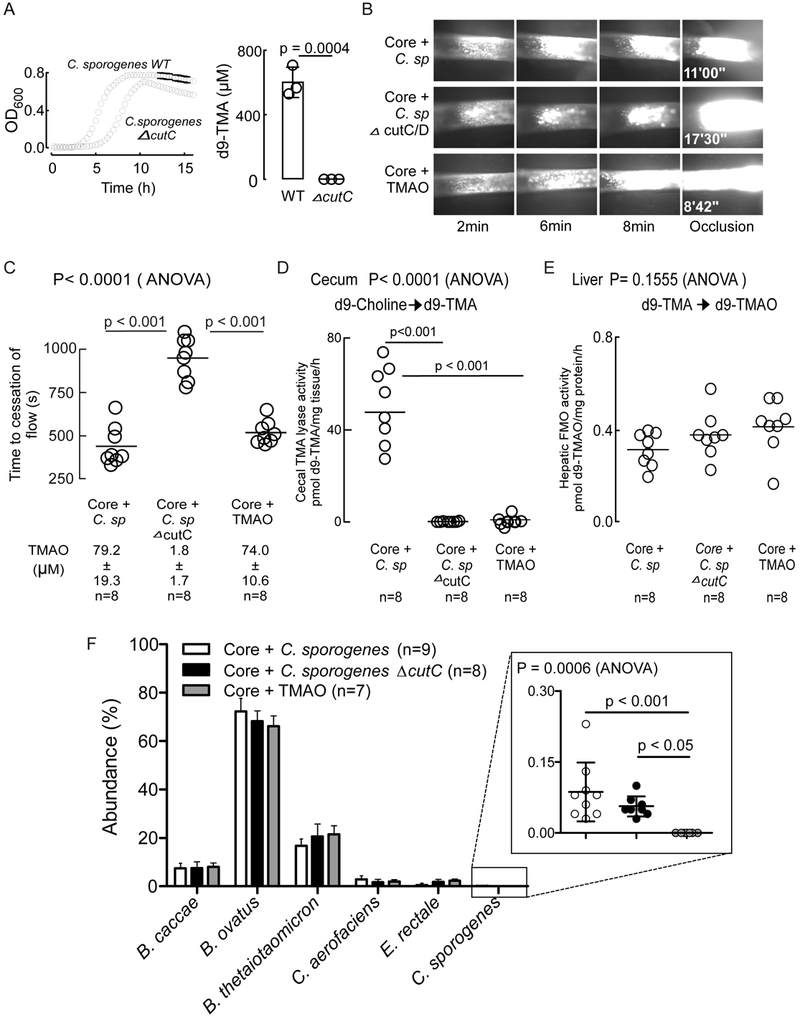
A) Growth curves of C. sporogenes WT or C. sporogenes ΔcutC strains in mega media supplemented with d9-Choline (1mM). LC/MS/MS quantification of d9-TMA produced by C. sporogenes WT or C. sporogenes ΔcutC strains in mega media containing d9-choline (1mM). All values are averages of three independent replicates ± SD. B) Germ-free C57Bl/6 mice were colonized by oral gavage with core (B. caccae, B. ovatus, B. thetaiotaomicron, C. aerofaciens, E. rectale) +/− TMA producing C. sporogenes or C. sporogenes ΔcutC. Core + TMAO mice also harbored the non-TMA producing Core and received TMAO in drinking water (0.2% w/w). All mice were maintained on a choline diet. After two weeks of colonization the FeCl3 carotid artery injury model was performed. Representative images are shown. C) Time to cessation of blood flow was determined during the FeCl3 carotid injury model as described under Methods. Respective plasma TMAO values are indicated as measured by LC/MS/MS. D) Cecal choline TMA-lyase activity; and E) Hepatic FMO enzyme activity were assessed as described under Methods. F) Microbial community composition was assessed via sequencing of mouse cecal material as described under Methods. One animal in the Core + C. sporogenes group died before terminal measurements could be completed was still used in sequencing analysis. One animal in the Core + TMAO group failed to sequence. All p-values shown were determined by One-Way ANOVA followed by Tukey’s multiple comparison post-test.
DISCUSSION
Despite the clinical importance of the microbiome in human physiology and potential diseases such as CVD, we are only just beginning to appreciate potential mechanism through which this occurs. To date, research has primarily focused on defining the composition of microorganisms present in the microbiome, though efforts to directly functionally interrogate the participants involved and elaborate the chemical mediators are of intense interest. To make matters more complex, it is also recognized that many of the vital functions of the gut microbiome are redundant, and such is likely also the case for undesirable functions of the microbiome. Herein we employ a systematic approach to study the potential role of a gut microbial enzyme impacting the host in a directed and organized fashion. Our studies provide direct experimental evidence for a link between CutC within gut microbiota and potential to alter platelet function and thrombosis potential in the host. Specifically, we demonstrate that transplantation of human fecal polymicrobial communities from subjects with substantial differences in specific activity of functional CutC levels can differentially transmit TMA and TMAO generation within the host, with accompanying heightened platelet aggregometry responsiveness (with higher choline→TMA and TMAO levels), and in vivo thrombosis potential, as monitored by the carotid artery FeCl3 injury model. Moreover, we show that gnotobiotic mice colonized with a defined synthetic microbial community comprised of human gut commensals lacking TMA generation (a.k.a. the TMA-null Core), in the absence versus presence of C. sporogenes, a TMA generating human gut commensal that contains CutC, can transmit TMA/TMAO generation, heightened platelet responsiveness to agonist, and both enhanced thrombus rate of formation and shortened vessel occlusion time in vivo. It is important to note that the proportion of C. sporogenes was repeatedly observed to occupy only a relatively small overall niche (~0.1%) within the gut microbial community, yet capable of driving these critical gain of function phenotypes. Finally, microbial transplantation studies employing C. sporogenes (ΔcutC) in which a group II intron was used to mutate the cutC gene, is shown to still result in comparable engraftment of C. sporogenes relative to the wild-type strain within the gut microbial community. Not only did engraftment of C.sporogenes ΔcutC functionally eliminate TMA and TMAO production within the host, but it also eliminated the otherwise observed choline diet dependent prothrombotic phenotype observed within germ-free mice recipients colonized with a TMA-null Core community + functional CutC containing C. sporogenes (WT). The presented studies thus confirm gut microbiota possessing CutC specifically, and choline TMA-lyase activity in general, can contribute to heightened TMAO levels, altered platelet function, and enhanced thrombosis potential within a host. The studies also suggest that functional CutC is not essential to microbial (C. sporogenes) fitness as the ΔcutC mutant shows comparable engraftment and overall community proportion relative to the wild-type strain.
The present study adds to the growing body of literature providing a strong argument for the targeting of the TMAO metaorganismal pathway. Further, the present studies suggest global functional inhibition of microbial CutC may serve as a therapeutic approach for favorably impacting risk for CVD and thrombosis potential. Although the present studies have been focused on TMAO and its effect on platelet responsiveness and thrombosis risk, the general design and approach taken in the present studies can be broadly used for other CVD and metabolic phenotypes associated with TMAO (e.g. adverse ventricular remodeling and heart failure44–46, renal functional decline and chronic kidney disease47,48). Indeed, the present approach may serve as a template to apply toward examination of other microbial or metaorganismal pathways implicated as a potential participant in physiological processes or disease-associated phenotypes.
Supplementary Material
NOVELTY AND SIGNIFICANCE
What Is Known?
Trimethylamine-N-oxide (TMAO) is a meta-organismal metabolite that is dose dependently associated with major adverse cardiovascular events.
Microbial production of trimethylamine (TMA) is required for significant (and sustained) TMAO accumulation.
What New Information Does This Article Contribute?
The presence of a functional gut microbial cutC gene is sufficient to impact platelet response and thrombosis potential in the host.
Functional CutC levels in the intestinal microbiota can be transmitted by fecal transplantation, thereby enhancing platelet reactivity and thrombosis potential within the recipient.
Inhibition of microbial CutC may serve as a promising therapeutic approach to favorably impact cardiovascular disease (CVD) pathogenesis and reduce thrombotic potential.
The gut microbiome is associated with an ever-increasing array of diseases. Before novel therapeutic strategies can leverage these discoveries, a mechanistic role of specific gut microbiota dependent pathways in both host physiology and disease pathogenesis must be understood. Numerous studies suggest the meta-organismal TMAO pathway is a promising target for such endeavors. In animal models, TMAO has been shown to promote platelet hyperresponsiveness. It is therefore important to establish which host phenotypes can be manipulated to alter gut microbial TMAO production – both at the microbiota gene and the enzyme activity level. TMAO synthesis is dependent on microbial formation of TMA, which is mediated in large part by the microbial enzyme choline TMA-lyase CutC. Herein, we establish the presence and expression of the gut microbial cutC gene may be transmitted from donor to recipient, transferring enhanced TMA and TMAO generation, platelet responsiveness and thrombosis potential. Collectively, these findings support targeting the microbial choline TMA-lyase pathway for the treatment of atherothrombotic heart disease.
Acknowledgments
SOURCES OF FUNDING
This research was supported by grants from the National Institutes of Health and the Office of Dietary Supplements (HL103866 (to SLH), HL126827 and DK106000 (to S.L.H. and W.H.W.T.)). F.E.R. was supported by DK108259 and by the National Institute of Food and Agriculture, U.S. Department of Agriculture under award number 2016–67017-24416. M.A.F. was supported in part by National Institutes of Health grants R01 DK101674, DP1 DK113598, and R01 DK110174, and an HHMI-Simons Faculty Scholars Award. A.J.L. was supported by HL30568. S.M.S. is partially supported by TRN512130. S.L.H., F.E.R., and M.A.F. are also partially supported by an award from the Leducq Foundation. Mass spectrometry studies were performed in a core facility partially supported by a Center of Innovations Award from Shimadzu Inc and by NIH shared instrumentation grant S10OD016346.
Nonstandard Abbreviations and Acronyms:
- TMAO
Trimethylamine N-oxide
- TMA
Trimethylamine
- CVD
Cardiovascular disease
- Cut
choline utilization genes
- FMO3
flavin monooxygenase 3
Footnotes
CONFLICT OF INTEREST
Z.W. and S.L.H. are named as co-inventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics and have the right to receive royalty payment for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab, Quest Diagnostics and Proctor & Gamble. S.L.H. also reports having been paid as a consultant from Proctor & Gamble and having received research funds from Proctor & Gamble and Roche.
REFERENCES
- 1.Jonsson AL, Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. 2017;14:79–87. [DOI] [PubMed] [Google Scholar]
- 2.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang WHW, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124:4204–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanton LV, Barratt MJ, Charbonneau MR, Ahmed T, Gordon JI. Childhood undernutrition, the gut microbiota, and microbiota-directed therapeutics. Science. 2016;352:1533. [DOI] [PubMed] [Google Scholar]
- 5.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nature Medicine. 2017;23:850–858. [DOI] [PubMed] [Google Scholar]
- 7.Zhu W, Gregory JC, Org E, et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, Wagner MA, Bennett BJ, Li L, DiDonato JA, Lusis AJ, Hazen SL. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647–5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tremaroli V, Karlsson F, Werling M, Ståhlman M, Kovatcheva-Datchary P, Olbers T, Fändriks L, le Roux CW, Nielsen J, Bäckhed F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metabolism. 2015;22:228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. [DOI] [PubMed] [Google Scholar]
- 12.Pluznicka JL, Protzkoa RJ, Gevorgyan H, et al. Olfactory receptor responding to gut microbiota- derived signals plays a role in renin secretion and blood pressure regulation. Proceedings of the National Academy of Sciences. 2013;110:4410–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devlin AS, Marcobal A, Dodd D, Nayfach S, Plummer N, Meyer T, Pollard KS, Sonnenburg JL, Fischbach MA. Modulation of a Circulating Uremic Solute via Rational Genetic Manipulation of the Gut Microbiota. Cell Host and Microbe. 2016;20:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N Engl J Med. 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature Medicine. 2013;19:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-N-Oxide, a Metabolite Associated with Atherosclerosis, Exhibits Complex Genetic and Dietary Regulation. Cell Metabolism. 2013;17:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao J, Ling AV, Manthena PV, Gearing ME, Graham MJ, Crooke RM, Croce KJ, Esquejo RM, Clish CB, Morbid Obesity Study Group, Vicent D, Biddinger SB. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nature Communications. 2015;6:6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schugar RC, Shih DM, Warrier M, et al. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017;19:2451–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, Qi H, Wu J, Pan C, Brown JM, Vallim T, Bennett BJ, Graham M, Hazen SL, Lusis AJ. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi J, You T, Li J, Pan T, Xiang L, Han Y, Zhu L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med. 2018;22:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J Am Heart Assoc. 2017;6:e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. European Heart Journal. 2017;38:2948–2956. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Roberts AB, Buffa JA, et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015;163:1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted non-lethal therapeutic to inhibit thrombosis potential. Nature Medicine. 2018;[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeisel SH. A brief history of choline. Ann Nutr Metab. 2012;61:254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci USA. 2012;109:21307–21312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez-del Campo A, Bodea S, Hamer HA, Marks JA, Haiser HJ, Turnbaugh PJ, Balskus EP. Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. mBio. 2015;6:e00042–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio. 2015;6:e02481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romano KA, Martínez-del Campo A, Kasahara K, Chittim CL, Vivas EI, Amador-Noguez D, Balskus EP, Rey FE. Metabolic, Epigenetic, and Transgenerational Effects of Gut Bacterial Choline Consumption. Cell Host and Microbe. 2017;22:279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romano KA, Dill-McFarland KA, Kasahara K, Kerby RL, Vivas EI, Amador-Noguez D, Herd P, Rey FE. Fecal Aliquot Straw Technique (FAST) allows for easy and reproducible subsampling: assessing interpersonal variation in trimethylamine-N-oxide (TMAO) accumulation. Microbiome. 2018;6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koeth RA, Levison BS, Culley MK, Buffa JA, Wang Z, Gregory JC, Org E, Wu Y, Li L, Smith JD, Tang WHW, DiDonato JA, Lusis AJ, Hazen SL. γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metabolism. 2014; 20:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams JA. Strain engineering: methods and protocols. Springer Protocols Humana Press; 2011;765:389–407. [Google Scholar]
- 34.Heap JT, Kuehne SA, Ehsaan M, Cartman ST, Cooksley CM, Scott JC, Minton NP. The ClosTron: Mutagenesis in Clostridium refined and streamlined. J Microbiol Methods. 2010;80:49–55. [DOI] [PubMed] [Google Scholar]
- 35.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kopylova E, Navas-Molina JA, Mercier C, Xu ZZ, Mahé F, He Y, Zhou H-W, Rognes T, Caporaso JG, Knight R. Open-Source Sequence Clustering Methods Improve the State Of the Art. mSystems. 2016;1:e00003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6:610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8:e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu W, Wang Z, Tang WHW, Hazen SL. Gut Microbe-Generated Trimethylamine N-Oxide From Dietary Choline Is Prothrombotic in Subjects. Circulation. 2017;135:1671–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herd P, Schaeffer NC, DiLoreto K, Jacques K, Stevenson J, Rey F, Roan C. The Influence of Social Conditions Across the Life Course on the Human Gut Microbiota: A Pilot Project With the Wisconsin Longitudinal Study. J Gerontol B Psychol Sci Soc Sci. 2017;73:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Y, Jameson E, Crosatti M, Schafer H, Rajakumar K, Bugg TDH, Chen Y. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proceedings of the National Academy of Sciences. 2014;111:4268–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang WHW, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, Klein AL, Hazen SL. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang WHW, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, Polhemus DJ, Tang WHW, Wu Y, Hazen SL, Lefer DJ. Choline Diet and Its Gut Microbe-Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload-Induced Heart Failure. Circ Heart Fail. 2016;9:e002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun G, Yin Z, Liu N, Bian X, Yu R, Su X, Zhang B, Wang Y. Gut microbial metabolite TMAO contributes to renal dysfunction in a mouse model of diet-induced obesity. Biochem Biophys Res Commun. 2017;493:964–970. [DOI] [PubMed] [Google Scholar]
- 48.Tang WHW, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circulation Research. 2015;116:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


