Abstract
Objective:
To identify risk factors for symptomatic radiation pneumonitis (RP) after stereotactic radiation therapy (SRT) for lung tumours.
Methods:
We retrospectively evaluated 68 lung tumours in 63 patients treated with SRT between 2011 and 2015. RP was graded according to the National Cancer Institute—Common Terminology Criteria for Adverse Events version 4.0. SRT was delivered at 7.0–12.0 Gy per each fraction, once daily, to a total of 48–64 Gy (median, 50 Gy). Univariate analysis was performed to assess patient- and treatment-related factors, including age, sex, smoking index (SI), pulmonary function, tumour location, serum Krebs von den Lungen-6 value (KL-6), dose-volume metrics (V5, V10, V20, V30, V40 and VS5), homogeneity index of the planning target volume (PTV), PTV dose, mean lung dose (MLD), contralateral MLD and V2, PTV volume, lung volume and the PTV/lung volume ratio (PTV/Lung). Performance of PTV/Lung in predicting symptomatic RP was also analysed using receiver operating characteristic (ROC) analysis.
Results:
The median follow-up period was 21 months. 10 of 63 patients (15.9%) developed symptomatic RP after SRT. On univariate analysis, V10, V20, PTV volume and PTV/Lung were significantly associated with occurrence of RP ≥Grade 2. ROC curves indicated that symptomatic RP could be predicted using PTV/Lung [area under curve (AUC): 0.88, confidence interval (CI: 0.78–0.95), cut-off value: 1.09, sensitivity: 90.0% and specificity: 72.4%].
Conclusion:
PTV/Lung is a good predictor of symptomatic RP after SRT.
Advances in knowledge:
The cases with high PTV/Lung should be carefully monitored with caution for the occurrence of RP after SRT.
Introduction
Stereotactic radiation therapy (SRT) is increasingly recognised as one of the standard treatments for early stage lung cancer and lung metastasis.1–4 Radiation pneumonitis (RP) is the most important adverse event associated with SRT for lung tumours, which occurs at a rate of 9–28%.5–11 The percentage of the total lung volume receiving more than or equal to 20 Gy (V20) is reported to be a useful predictor of RP during conventional fractionated radiation therapy.12 Even in SRT, it has been reported that the incidence of lung toxicity increases when a large volume of the lung parenchyma is irradiated.5 Although many recent reports have defined risk factors for RP following SRT, the results differed; thus, there is no consensus.7, 8,13,14 Therefore, it is clinically important to further explore other influential factors to predict the risk for RP to increase safety.
The tumour size and tumour/lung volume ratio reportedly play a role in the onset of RP after SRT.14 Hence, risk factors for RP were investigated using dosimetric parameters, including the planning target volume (PTV) to total lung volume ratio (PTV/Lung) and patient characteristics. Furthermore, the predictive ability of clinical and dosimetric parameters was evaluated using receiver operating characteristic (ROC) analyses.
Methods and MATERIALS
Patient selection
This study was approved by the institutional review board of our hospital, and the need for informed consent acquisition was waived. From January 2011 to August 2015, 73 lung tumours in 68 patients were treated with SRT at our institution. All patients enrolled in this study satisfied the following eligibility criteria: (1) solitary or double lung tumours, (2) tumour diameter <50 mm, (3) no clinical evidence of regional lymph node metastasis, (4) Eastern Cooperative Oncology Group performance status 0–2, (5) tumour not located adjacent to the major bronchus, oesophagus, spinal cord, or great vessels and (6) written informed consent obtained. Patients were eligible whether treated with or without concurrent or sequential chemotherapy. There were no patients who had active interstitial pneumonitis. Five patients were excluded because the follow-up period was less than 12 months. Of the remaining 68 tumours in 63 patients, 24 lesions were histologically diagnosed as primary lung cancer and 18 as metastatic tumours. Among these lesions, 26 were not histologically confirmed because of either failure to obtain an appropriate biopsy sample or patient refusal for histological inspection. Malignant tumours were clinically diagnosed based on CT findings of tumour growth on repeated scans, increased expression of tumour markers, or high uptake of [18F]-fluorodeoxyglucose (FDG) on positron emission tomography (PET). 24 patients underwent SRT for inoperable primary lung carcinoma because of coexisting diseases or patient refusal of surgery. The primary sites of metastases were the soft tissue in three cases; bone in two; anus in two; skin in two and the larynx, thyroid, lung, oesophagus, stomach, rectum, liver, renal pelvis and ureter in one case each. Five patients with two metachronous lesions were treated with SRT twice at different times. For patients treated a second time, these data were re-evaluated at the time of presentation for each treatment. Patient and tumour characteristics are summarised in Table 1.
Table 1.
Clinical and treatment characteristics
| Characteristic | Patients (n) | Proportion or median (range) | ||
| Age | 74 (29–90) years | |||
| Follow-up period | 21.1 (4.3–58.2) months | |||
| Sex | Male | 40 | 58.8% | |
| Female | 28 | 41.2% | ||
| Histology | Primary lung carcinoma | Adeno carcinoma | 13 | 19.1% |
| Squamous cell carcinoma | 9 | 13.2% | ||
| Small cell carcinoma | 1 | 1.4% | ||
| Adeno squamous carcinoma | 1 | 1.4% | ||
| Metastatic tumour | 18 | 26.5% | ||
| Unknown | 26 | 38.2% | ||
| Tumour location | Upper lobe | 39 | 57.4% | |
| Middle or lower lobe | 29 | 42.6% | ||
| Peripheral | 68 | 100.0% | ||
| Central (<2 cm from mediastinum) | 0 | 0.0% | ||
| History of previous smoking | 29 | 42.6% | ||
| GTV volume | 2.8 (0.2–41.0) cm3 | |||
| PTV volume | 27.5 (4.0–149.0) cm3 | |||
| Lung volume | 2593.4 (1408.9–5627.1) cm3 | |||
| Number of SRT | Once | 58 | 92.1% | |
| Twice | 5 | 7.9% | ||
| Prescription dose | 48 Gy/4 Fx | 33 | 48.5% | |
| 50 Gy/5 Fx | 19 | 27.9% | ||
| 54 Gy/6 Fx | 1 | 1.5% | ||
| 56 Gy7 Fx | 9 | 13.2% | ||
| 56 Gy/8 Fx | 5 | 7.4% | ||
| 60 Gy/12 Fx | 1 | 1.5% |
Fx, fractions; GTV, gross tumour volume; PTV, planning target volume; SRT, stereotactic radiation therapy.
Treatment and follow-up
The patients were kept in the right position with accuracy measured using a thermoplastic positioning system (HipFix®; CIVCO Medical Solutions, Kalona, IA). The slice thickness for CT planning was 2 mm and each slice was scanned for 4 s. This method is generally referred to as a slow CT scan and includes the whole phase of one respiratory cycle to recognise tumour motion affected by respiration.15 Also, a respiratory monitoring system (AZ-733V Anzai Medical, Tokyo, Japan) was used beginning in January 2011 and a real-time position management system (Varian Medical Systems, Palo Alto, CA) since February 2014. The CT data, including internal motion, were transferred to a radiation treatment planning system (EclipseTM, v. 8.9–11; Varian Medical Systems). All patients underwent SRT using a linear accelerator (Novalis TxTM, Varian Medical Systems) with a photon energy of 6 or 10 MV. The prescribed dose was calculated using an analytical anisotropic algorithm.
The gross tumour volume (GTV) was contoured to include the primary tumour on axial CT slices using lung windows. The clinical target volume was determined by adding a margin of 0.5 cm to the GTV. The PTV was defined as the clinical target volume plus a margin of 0.5–1.0 cm for set-up uncertainty and individual respiratory motion. In addition, a leaf margin of 0.5 cm was added to the PTV.
SRT was administered as a total dose of 48–60 Gy in 4–12 fractions (Table 1). Seven to 18 non-coplanar beams were stereotactically directed toward the tumour using three-dimensional (3D) planning. We did not use either staticintensity modulated radiotherapy or volumetric arc therapy (VMAT). Risk-adapted dose schemes were used, depending on tumour size and location. The dose was prescribed to the isocentre. Cone beam CT was performed to ensure a correct target position before every treatment.
After SRT, all patients were followed up at 1 month intervals for the first 3 months, then periodically every 3 months thereafter using chest X-ray, CT scans, FDG-PET, or tumour markers. We included diagnostic CT examinations not on a regular schedule when a patient showed new respiratory symptoms.
Evaluation of clinical outcome
RP was evaluated using the National Cancer Institute—Common Terminology Criteria for Adverse Events v. 4.0, as follows: Grade 1, asymptomatic with clinical or diagnostic observations only; Grade 2, symptomatic and medical intervention indicated; Grade 3, severe symptomatic with limiting activities of daily living; Grade 4, life-threatening respiratory compromise with urgent intervention indicated; and Grade 5, death. Symptomatic RP was defined as Grade 2 or worse.
Lung volume at risk was defined as the total lung volume minus the PTV. From the point of dose-volume metrics, the following parameters were evaluated: maximum PTV dose, minimum PTV dose, mean PTV dose, PTV homogeneity index (HI), mean lung dose (MLD), contralateral MLD, dose-volume metrics (V5, V10, V20, V30, V40 and VS5) of both lungs excluding PTV, V2 of the contralateral lung, PTV volume and PTV/Lung. HI was defined as (D2%–D98%)/D50% according to the International Commission on Radiation Units and Measurements, report 83.16 VS5 denotes the absolute percentage of lung spared from doses ≥ 5 Gy.
Furthermore, age, sex, smoking index (SI), serum Krebs von den Lungen-6 (KL-6) value before SRT, tumour location, pulmonary function and chronic obstructive pulmonary disease (COPD) stage were examined as individual patient factors. Also, vital capacity/predicted vital capacity (%VC), percent of forced expiratory volume in 1 s (FEV1%) and FEV1%/predicted FEV1% (%FEV1) were used as pulmonary functions. COPD stages were classified according to the global initiative for chronic obstructive lung disease criteria. SI, pretreatment KL-6 and pretreatment respiratory function were unavailable in 7, 15 and 32 patients, respectively.
Statistical analysis
The data were analysed using SPSS software v. 23 (IBM-SPSS, Inc., Chicago, IL). All tumour characteristics and dosimetric parameters were evaluated using univariate logistic regression analysis. Specifically, the Mann–Whitney U test was used for all data except for sex and tumour location, which were analysed using the Fisher’s exact test. Multivariate logistic regression analysis was not performed for the limited number of this study. Instead, we performed the bivariate analysis using Cox regression model to compare PTV/Lung and PTV volume with V20. Cumulative hazard ratio curves were drawn using the Kaplan–Meier method, and the significance of the difference between hazard ratios was tested using the log-rank test. Furthermore, ROC curve analysis was performed using variables with significant p values on univariate analysis to assess the sensitivity and specificity of significant factors for prediction of symptomatic RP. Optimal cut-off values of sensitivity and specificity were determined by Youden Index. The area under the ROC curve (AUC) values were compared between PTV/Lung, PTV volume and V20. A probability (p) value of <0.05 was considered significant for all analyses. Lastly, Spearman correlation was used to investigate the correlation between the severity and PTV/volume.
RESULTS
The median follow-up period was 21.1 months (range, 4.3–58.2 months). All patients were followed up for at least 12 months or until death. 10 of 63 patients (15.9%) developed symptomatic RP. None of the patients who underwent SRT twice suffered from RP. RP grades of 0–1, 2, 3, 4 and 5 occurred in 53 (84.1%), 7 (11.1%), 0 (0%), 0 (0%) and 3 (4.8%) of these patients, respectively. Three patients with RP Grade 5 were patients of advanced aged over 75 years. They died of infection, acute respiratory distress syndrome, or rapid tumour growth after steroid treatment. No patient experienced severe adverse effects other than SRT-associated RP. The other 53 patients were asymptomatic and did not require specific treatment. All patients with symptomatic RP had a continuous cough, fever, or dyspnoea and received oral steroid treatment. Oral steroids were started at a median time of 3.2 months after SRT (range, 1.9–6.8 months). Three of 10 patients died from fatal pneumonitis with severe infection or rapid growth of primary tumour. The symptoms of the remaining seven improved after treatment with oral steroids, resulting were in complete remission, thus steroid therapy was discontinued.
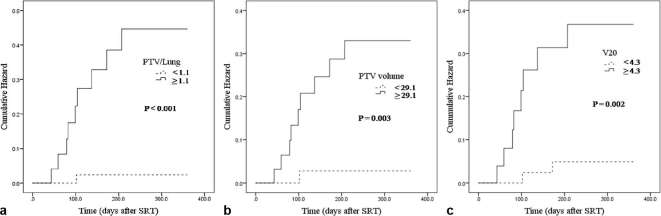
The outcomes of univariate logistic regression analysis are shown in Tables 2 and 3. Univariate analysis showed that V10 (p = 0.044), V20 (p = 0.027), PTV volume (p = 0.005) and PTV/Lung (p = 0.002) were associated with symptomatic RP. As a result of considering about the pvalue, odds ratio and 95% confidence interval (CI), PTV/Lung was the most significant. No other DVH parameters or patient or tumour characteristics were significantly correlated with symptomatic RP. Furthermore, in the bivariate analysis, PTV/Lung (p = 0.002) showed higher hazard ratio than V20 (Table 4). In a comparison of the PTV/Lung and PTV volume, there was also a strong parallel statistical correlation between the PTV/Lung and symptomatic RP (Table 4). Figure 1 showed the cumulative hazard of PTV/Lung, PTV volume and V20.
Table 2.
Univariate logistic analysis examining relationship between dose-volume histogram parameters and severe RP
| Grade 0–1 RP (n = 58) | Grade 2–5 RP (n = 10) | OR | 95% CI | p value | |
| PTV max (Gy) | 52.6 ± 3.9 | 51.6 ± 4.4 | 1.0 | 1.0–1.0 | 0.438 |
| PTV min (Gy) | 41.0 ± 4.9 | 40.5 ± 5.1 | 1.0 | 1.0–1.0 | 0.734 |
| PTV mean (Gy) | 48.6 ± 3.8 | 48.2 ± 3.9 | 1.0 | 1.0–1.0 | 0.755 |
| HI | 0.2 ± 0.1 | 0.2 ± 0 | 1.1 | 0.3–4.1 | 0.935 |
| MLD (Gy) | 3.4 ± 1.5 | 4.4 ± 1.8 | 1.0 | 1.0–1.0 | 0.060 |
| V5 (%) | 18.0 ± 8.0 | 22.3 ± 9.6 | 1.1 | 1.0–1.1 | 0.148 |
| V10 (%) | 10.7 ± 5.7 | 15.1 ± 7.0 | 1.1 | 1.0–1.2 | 0.044a |
| V20 (%) | 4.2 ± 2.7 | 6.5 ± 3.3 | 1.3 | 1.0–1.5 | 0.027a |
| V30 (%) | 2.1 ± 1.5 | 3.1 ± 1.9 | 1.4 | 1.0–2.0 | 0.075 |
| V40 (%) | 0.9 ± 0.8 | 1.3 ± 1.3 | 1.5 | 0.8–2.8 | 0.222 |
| VS5 (%) | 82.0 ± 8.0 | 78.7 ± 8.9 | 1.0 | 0.9–1.0 | 0.254 |
| V2 of contralateral lung (%) | 9.1 ± 7.0 | 9.5 ± 3.0 | 1.0 | 0.9–1.1 | 0.89 |
| MLD of contralateral lung (cGy) | 64.9 ± 56.5 | 66.1 ± 47.8 | 1.0 | 1.0–1.0 | 0.951 |
| PTV volume (cm3) | 28.3 ± 20.2 | 60.6 ± 38.9 | 1.0 | 1.0–1.1 | 0.005a |
| Lung volume (cm3) | 2778.3 ± 798.6 | 2581 ± 502.1 | 1.0 | 1.0–1.0 | 0.448 |
| PTV/Lung (%) | 0.9 ± 0.6 | 2.4 ± 1.5 | 5.4 | 1.9–15.8 | 0.002a |
CI, confidence interval; HI, homogeneity index; MLD, mean lung dose; OR, odds ratio; PTV, planning target volume; RP, radiation pneumonitis; SD, standard deviation; Vn, the percentage of the total lung volume receiving more than or equal to n Gy; VS5, the absolute percentage of lung spared from doses ≥ 5 Gy.
Data are presented as mean ± SD.
statistically significant.
Table 3.
Univariate analysis examining relationship between individual factors of patients and severe RP
| Grade 0–1 RP | Grade 2–5 RP | p value | |
| Age | 72.7 ± 12.4 (n = 58) | 74.9 ± 10.8 (n = 10) | 0.599d |
| Sex (M:F) | 32:26 (n = 58) | 8:2 (n = 10) | 0.179e |
| SIa | 677.0 ± 842 (n = 54) | 592.5 ± 599.9 (n = 7) | 0.783d |
| KL-6b | 381.4 ± 317.9 (n = 44) | 438.9 ± 277.9 (n = 9) | 0615a |
| Tumour location (upper:middle or lower) | 37:21 (n = 58) | 3:7 (n = 10) | 0.085e |
| %VCc | 82.4 ± 20.0 (n = 30) | 84.0 ± 18.0 (n = 6) | 0.856a |
| FEV1%c | 68.3 ± 17.5 (n = 30) | 63.4 ± 16.6 (n = 6) | 0.517d |
| %FEV1c | 77.0 ± 28.2 (n = 30) | 69.4 ± 24.0 (n = 6) | 0.573d |
| COPD stagesc | 1.7 ± 0.9 (n = 30) | 1.8 ± 0.8 (n = 6) | 0.810d |
%FEV1, FEV1%/predicted FEV1%; %VC, vital capacity/predicted vital capacity; COPD, chronic obstructive pulmonary disease; FEV1%, percent of forced expiratory volume in 1 s; KL-6, serum Krebs von den Lungen-6; PTV, planning target volume; RP, radiation pneumonitis; SD, standard deviation; SI, smoking index.
Data are presented as mean ±SD or ratio.
SI, unknown in seven patients.
KL-6, unmeasured in 13 patients.
Respiratory function test, unexamined in 32 patients.
Analysis by the Mann–Whitney U test.
Analysis by the Fisher’s exact test.
Table 4.
Bivariate Cox regression analysis
| Factor | Hazard ratio (95% CI) | p value |
| PTV/Lung (%)V20 (%) | 2.9 (1.5–5.7)1.0 (0.7–1.3) | 0.002a 0.786 |
| PTV volume (cm3) V20 (%) |
1.0 (1.0–1.0) 1.1 (0.9–1.3) |
0.011a 0.460 |
CI, confidence interval; PTV, planning target volume; V20, The percentage of the total lung volume receiving more than or equal to 20 Gy.
Statistically significant.
Figure 1.
Cumulative hazard curves. (a) Cumulative hazard of symptomatic RP for PTV/Lung. (b) Cumulative hazard of symptomatic RP for PTV volume. (c) Cumulative hazard of symptomatic RP for V20. PTV, planning target volume; RP, radiation pneumonitis; SRT, stereotactic radiation therapy.
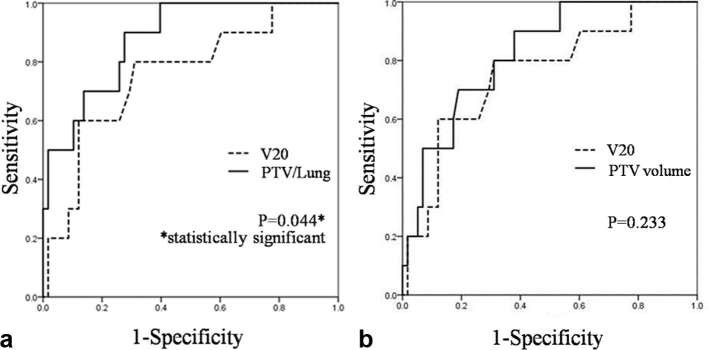
To further investigate the predictive utility of PTV/Lung, ROC curves were generated. The most appropriate cut-off value was 1.09, which had a sensitivity of 90.0% and a specificity of 72.4% (Table 5). The area under the ROC curve of PTV/Lung was 0.88 [95% CI (0.78–0.95)]. Comparisons of AUC values between PTV/Lung, PTV volume and V20 revealed that only PTV/Lung had significantly higher AUC areas than V20 (p = 0.044). ROC curves for PTV/Lung and PTV volume in comparison with that for V20 are shown in Figure 2.
Table 5.
Optimal cut-off values and crude rates of severe RP
| AUC (95% CI) | Cut-off values | Sensitivity | Specificity | p value | |
| PTV/Lung | 0.88 (0.78–0.95) | 1.1 | 90.0 | 72.4 | <0.001a |
| PTV volume | 0.82 (0.71–0.90) | 29.1 | 90.0 | 62.1 | <0.001a |
| V20 | 0.76 (0.64–0.85) | 4.3 | 80.0 | 69.0 | 0.003a |
| V10 | 0.72 (0.60–0.83) | 9.7 | 90.0 | 55.2 | 0.012a |
AUC, area under the receiver operating characteristic curve; CI, confidence interval; PTV, planning target volume; RP, radiation pneumonitis; Vn, the percentage of the total lung volume receiving more than or equal to n Gy.
Statistically significant.
Figure 2.
Comparison of the AUC for predicting the risk for symptomatic RP. (a) PTV/Lung (Solid Line) and V20 (Dash Line). (b) PTV volume (Solid Line) and V20 (Dash Line). AUC, area under the receiver operating characteristic curve, PTV, planning target volume.
As the result of Spearman correlation, PTV/Lung was significantly correlated with RP severity grade (ρ = 0.473, p < 0.001).
DISCUSSION
RP is the most severe adverse event after SRT for lung tumours. The reported incidence of symptomatic RP after SRT ranges from 9 to 29%.5–11 This might be due to variation in the timing of steroid therapy initiation, which is based on the judgement of each physician. In the present study, the incidence of symptomatic (≥Grade 2) RP was 15.9%. This is in agreement with that of previous reports, however, which is a little higher than other modern SRT cohorts.14 Our treatment protocol did not use the internal target volume to delineate PTV in each patient, which is recommended by the International Commission on Radiation Units and Measurements. This may partly explain increased rate of symptomatic RP in comparison with other modern SRT cohorts.
An unusually high rate of Grade 5 RP (4.8%) was noticed and we were searching for reasons to explain these results. The cause of these results might be their advanced age, but we could not conclude it. This is because the clinical data were collected retrospectively in this study and there is a lack of information of the detailed reports of the diagnosis, treatment and follow-up of individual patients after steroid therapy.
RP is less frequent in SRT than in conventional fractionated radiotherapy.17 Therefore, it is difficult to make firm conclusions on absolute levels for lung constraints.17 Graham et al reported that V20 was the single independent predictor of RP after 3D radiation therapy.12 Tsujino et al also concluded that V20 was the only factor associated with RP of Grade ≥2 in concurrent chemoradiation.18 The importance of V20 in predicting RP is widely accepted in conventional fractionated radiation therapy. Even in SRT, a constraint in V20 is prescribed in the Japan Clinical Oncology Group 0403 trial.3 Our univariate analysis revealed that V20 was a significant risk factor for symptomatic RP (Table 2), which is in line with previous publications.
Matsuo et al reported that a large PTV was a significant risk factor for symptomatic RP after SRT.13 The univariate analysis results of the present study also showed that the PTV volume was a significant factor, in accordance with the findings of previous reports that indicated that minimising the PTV volume was a viable option for reducing the risk for RP.13 On the other hand, Baker et al reported that the PTV/Lung was highly significant in SRT.14 In 3D-conformal radiotherapy, Dang et al also reported that PTV/Lung was a predictor of Grade >2 RP.19 Our univariate results showed that both PTV volume and PTV/Lung were significantly associated with developing symptomatic RP (Table 2). Furthermore, our bivariate analyses revealed that PTV/Lung but not PTV volume had higher hazard ratio than V20, suggesting that PTV/Lung is a better risk factor for symptomatic RP than V20 and PTV volume.
According to the ROC curve analyses, PTV/Lung showed the best value of AUC (Table 5), which is in accordance with the results of the univariate (Table 2) and bivariate (Table 4) analyses. ROC curve analyses also showed that PTV/Lung but not PTV volume had significantly higher value of AUC than V20. It suggests that PTV/Lung has the highest predictive accuracy. Nevertheless, the predictive performance of PTV/Lung with the optimal cut-off value determined in this study should be further tested on a validation cohort.
PTV/Lung is simple to measure and easy to put to practical use. There is a possibility that PTV/Lung have an influence on RP severity. However, it is inadequate to conclude it because the number of severe RP patients was too small.
There was no significant correlation between lung volume per se and the incidence of symptomatic RP (Table 2). Giaj et al showed the efficacy and safety of SRT in patients with a new primary lung cancer following previous pneumonectomy.20 Thus, it appears that patients with a small lung volume may be safely treated with SRT. Nevertheless, our data and those from Baker et al indicate that one must be aware of increased PTV/Lung in such cases with decreased lung volume. Minimising the PTV volume using a respiratory-gating system could help in reducing PTV/Lung and preventing RP.
Borst et al reported that the incidence of RP after SRT was 10.9% and there was a significant dose–response relationship between RP and MLD.7 The present study indicated that MLD was close to statistical significance (p = 0.06) in the univariate analysis. This may be because our data set was small. Guckenberger et al8reported an association between low-dose radiation distribution and the development of RP after SRT and suggested a low-dose radiation parameter of V2.5 as the best-fitting value for the development of RP. Neither high-dose nor low-dose radiation distribution (V40, V30, V5) was a useful risk factor for symptomatic RP (Table 2). Bongers et al21reported that contralateral MLD should be maintained at less than 3.6 Gy in VMAT). Ong et al reported that the relative volume of the contralateral lung receiving more than 5 Gy was the best predictor of RP with the use of VMAT in lung tumours > 80 cm3.9 The present study showed that V2 and MLD of the contralateral lung might not be significant risk factors for symptomatic RP and low-dose delivery to the contralateral lung is not so important if not using VMAT. This is presumably because the irradiated volume of the contralateral lung is very low in SRT, unlike in VMAT.
Haasbeek et al22showed that SRT was a safe treatment for stage I lung tumours arising after prior pneumonectomy, even in patients with severe COPD. Moreover, Ishijima et al reported that severe emphysema was associated with a low risk for RP following SRT.23 In the present study, none of the pulmonary function indices (%VC, FEV1%, %FEV1 and COPD stages) or SI were significantly correlated with symptomatic RP. Although there are a lot of missing parameters about respiratory function, it appears that SRT might be a good treatment option for patients with COPD. However, these toxicities may be critical for patients with severe COPD because they have little reserve pulmonary function. Therefore, further analysis of lung function might be needed to ascertain tolerability to SRT. There was a report that elderly patients had a higher risk for severe RP than younger patients in 3D-conformal radiotherapy.18 In our study, age was not clearly associated with the risk for RP after SRT. Zhao et al24reported a pooled analysis of 88 studies with 7752 patients about factors associated with lung toxicity after SRT. They showed that tumour size, V20, MLD and older patient age were risk factors of radiation-induced pneumonitis. Their data are highly reliable and our data in this study almost coincides with them except age. There is the possibility that this study is not sufficiently powerful to analyse about age.
Some researchers have showed the significant association between FDG-PET and RP.25–28 However, we could not analyse the relationship between FDG-PET and RP because the population who underwent FDG-PET before SRT in our hospital was too small (n = 3). Further investigation should be necessary to clarify whether the use of FDG-PET is superior to PTV/Lung for predicting RP.
Our study has several limitations. First, this study was based on a retrospective study. Second, the sample population was small and follow-up period was limited. Third, clinical factors like FDG-PET and consolidation chemotherapy were not taken into consideration. Longer follow-up and more experience are needed.
CONCLUSION
Our results suggest that PTV/Lung is important risk factor and reliable predictor for symptomatic RP after SRT for lung tumours. When creating SRT treatment plans for lung tumours, V10, V20, PTV volume and PTV/Lung should be comprehensively evaluated. Prospective studies and analyses of larger cohorts of patients treated at multiple institutions are needed to establish constraints of PTV/Lung.
Contributor Information
Tomoko Ueyama, Email: k9020916@m2.kufm.kagoshima-u.ac.jp.
Takeshi Arimura, Email: arimura-takeshi@medipolis.org.
Koji Takumi, Email: takumi@m2.kufm.kagoshima-u.ac.jp.
Fumihiko Nakamura, Email: fumibunn@m2.kufm.kagoshima-u.ac.jp.
Ryutaro Higashi, Email: h-ryu@m3.kufm.kagoshima-u.ac.jp.
Soichiro Ito, Email: tarotaro@m3.kufm.kagoshima-u.ac.jp.
Yoshihiko Fukukura, Email: fukukura@m.kufm.kagoshima-u.ac.jp.
Tomokazu Umanodan, Email: tomo1228@m.kufm.kagoshima-u.ac.jp.
Masanori Nakajo, Email: masanori@m3.kufm.kagoshima-u.ac.jp.
Chihaya Koriyama, Email: fiy@m.kufm.kagoshima-u.ac.jp.
Takashi Yoshiura, Email: yoshiura@m3.kufm.kagoshima-u.ac.jp.
REFERENCES
- 1.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303: 1070–6. doi: 10.1001/jama.2010.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takai Y, Mituya M, Nemoto K, Ogawa Y, Kakuto Y, Matusita H, et al. Simple method of stereotactic radiotherapy without stereotactic body frame for extracranial tumors. Nihon Igaku Hoshasen Gakkai Zasshi 2001; 61: 403–7. [PubMed] [Google Scholar]
- 3.Nagata Y, Hiraoka M, Shibata T, Onishi H, Kokubo M, Karasawa K, et al. Prospective trial of stereotactic body radiation therapy for both operable and Inoperable T1N0M0 non-small cell lung cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015; 93: 989–96. doi: 10.1016/j.ijrobp.2015.07.2278 [DOI] [PubMed] [Google Scholar]
- 4.Nanda RH, Liu Y, Gillespie TW, Mikell JL, Ramalingam SS, Fernandez FG, et al. Stereotactic body radiation therapy versus no treatment for early stage non-small cell lung cancer in medically inoperable elderly patients: a national cancer data base analysis. Cancer 2015; 121: 4222–30. doi: 10.1002/cncr.29640 [DOI] [PubMed] [Google Scholar]
- 5.Yamashita H, Nakagawa K, Nakamura N, Koyanagi H, Tago M, Igaki H, et al. Exceptionally high incidence of symptomatic grade 2-5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat Oncol 2007; 2: 21. doi: 10.1186/1748-717X-2-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricardi U, Filippi AR, Guarneri A, Giglioli FR, Mantovani C, Fiandra C, et al. Dosimetric predictors of radiation- induced lung injury in stereotactic body radiation therapy. Acta Oncol 2009; 48: 571–7. doi: 10.1080/02841860802520821 [DOI] [PubMed] [Google Scholar]
- 7.Borst GR, Ishikawa M, Nijkamp J, Hauptmann M, Shirato H, Onimaru R, et al. Radiation pneumonitis in patients treated for malignant pulmonary lesions with hypofractionated radiation therapy. Radiother Oncol 2009; 91: 307–13. doi: 10.1016/j.radonc.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 8.Guckenberger M, Baier K, Polat B, Richter A, Krieger T, Wilbert J, et al. Dose-response relationship for radiation-induced pneumonitis after pulmonary stereotactic body radiotherapy. Radiother Oncol 2010; 97: 65–70. doi: 10.1016/j.radonc.2010.04.027 [DOI] [PubMed] [Google Scholar]
- 9.Ong CL, Palma D, Verbakel WF, Slotman BJ, Senan S. Treatment of large stage I-II lung tumors using stereotactic body radiotherapy (SBRT): planning considerations and early toxicity. Radiother Oncol 2010; 97: 431–6. doi: 10.1016/j.radonc.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Barriger RB, Forquer JA, Brabham JG, Andolino DL, Shapiro RH, Henderson MA, et al. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2012; 82: 457–62. doi: 10.1016/j.ijrobp.2010.08.056 [DOI] [PubMed] [Google Scholar]
- 11.Stauder MC, Macdonald OK, Olivier KR, Call JA, Lafata K, Mayo CS, et al. Early pulmonary toxicity following lung stereotactic body radiation therapy delivered in consecutive daily fractions. Radiother Oncol 2011; 99: 166–71. doi: 10.1016/j.radonc.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 12.Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1999; 45: 323–9. doi: 10.1016/S0360-3016(99)00183-2 [DOI] [PubMed] [Google Scholar]
- 13.Matsuo Y, Shibuya K, Nakamura M, Narabayashi M, Sakanaka K, Ueki N, et al. Dose-volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys 2012; 83: e545–e549. doi: 10.1016/j.ijrobp.2012.01.018 [DOI] [PubMed] [Google Scholar]
- 14.Baker R, Han G, Sarangkasiri S, DeMarco M, Turke C, Stevens CW, et al. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int J Radiat Oncol Biol Phys 2013; 85: 190–5. doi: 10.1016/j.ijrobp.2012.03.041 [DOI] [PubMed] [Google Scholar]
- 15.Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, Jiang SB, et al. The management of respiratory motion in radiation oncology report of AAPM task group 76. Med Phys 2006; 33: 3874–900. doi: 10.1118/1.2349696 [DOI] [PubMed] [Google Scholar]
- 16. ICRU Report 83. 3. Special considerations regarding absorbed-dose and dose-volume prescribing and reporting in IMRT. J Icru 2010; 10: 27–40. [DOI] [PubMed] [Google Scholar]
- 17.Franks KN, Jain P, Snee MP. Stereotactic ablative body radiotherapy for lung cancer. Clin Oncol 2015; 27: 280–9. doi: 10.1016/j.clon.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 18.Tsujino K, Hirota S, Endo M, Obayashi K, Kotani Y, Satouchi M, et al. Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 2003; 55: 110–5. doi: 10.1016/S0360-3016(02)03807-5 [DOI] [PubMed] [Google Scholar]
- 19.Dang J, Li G, Ma L, Diao R, Zang S, Han C, et al. Predictors of grade ≥ 2 and grade ≥ 3 radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with three-dimensional conformal radiotherapy. Acta Oncol 2013; 52: 1175–80. doi: 10.3109/0284186X.2012.747696 [DOI] [PubMed] [Google Scholar]
- 20.Giaj Levra N, Filippi AR, Guarneri A, Badellino S, Mantovani C, Ruffini E, et al. Efficacy and safety of stereotactic ablative radiotherapy in patients with previous pneumonectomy. Tumori 2015; 101: 148–53. doi: 10.5301/tj.5000227 [DOI] [PubMed] [Google Scholar]
- 21.Bongers EM, Botticella A, Palma DA, Haasbeek CJ, Warner A, Verbakel WF, et al. Predictive parameters of symptomatic radiation pneumonitis following stereotactic or hypofractionated radiotherapy delivered using volumetric modulated arcs. Radiother Oncol 2013; 109: 95–9. doi: 10.1016/j.radonc.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 22.Haasbeek CJ, Lagerwaard FJ, de Jaeger K, Slotman BJ, Senan S. Outcomes of stereotactic radiotherapy for a new clinical stage I lung cancer arising postpneumonectomy. Cancer 2009; 115: 587–94. doi: 10.1002/cncr.24068 [DOI] [PubMed] [Google Scholar]
- 23.Ishijima M, Nakayama H, Itonaga T, Tajima Y, Shiraishi S, Okubo M, et al. Patients with severe emphysema have a low risk of radiation pneumonitis following stereotactic body radiotherapy. Br J Radiol 2015; 88: 20140596. doi: 10.1259/bjr.20140596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J, Yorke ED, Li L, Kavanagh BD, Li XA, Das S, et al. Simple factors associated with radiation-induced lung toxicity after stereotactic body radiation therapy of the thorax: a pooled analysis of 88 studies. Int J Radiat Oncol Biol Phys 2016; 95: 1357–66. doi: 10.1016/j.ijrobp.2016.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhuri AA, Binkley MS, Rigdon J, Carter JN, Aggarwal S, Dudley SA, et al. Pre-treatment non-target lung FDG-PET uptake predicts symptomatic radiation pneumonitis following Stereotactic Ablative Radiotherapy (SABR). Radiother Oncol 2016; 119: 454–60. doi: 10.1016/j.radonc.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 26.Petit SF, van Elmpt WJ, Oberije CJ, Vegt E, Dingemans AM, Lambin P, et al. [18F]fluorodeoxyglucose uptake patterns in lung before radiotherapy identify areas more susceptible to radiation-induced lung toxicity in non-small-cell lung cancer patients. Int J Radiat Oncol Biol Phys 2011; 81: 698–705. doi: 10.1016/j.ijrobp.2010.06.016 [DOI] [PubMed] [Google Scholar]
- 27.Castillo R, Pham N, Castillo E, Aso-Gonzalez S, Ansari S, Hobbs B, et al. Pre-radiation therapy fluorine 18 fluorodeoxyglucose PET helps identify patients with esophageal cancer at high risk for radiation pneumonitis. Radiology 2015; 275: 822–31. doi: 10.1148/radiol.14140457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang JY, Liu H, Balter P, Komaki R, Liao Z, Welsh J, et al. Clinical outcome and predictors of survival and pneumonitis after stereotactic ablative radiotherapy for stage I non-small cell lung cancer. Radiat Oncol 2012; 7: 152. doi: 10.1186/1748-717X-7-152 [DOI] [PMC free article] [PubMed] [Google Scholar]