Abstract
2-deoxy-2-(18Fluorine)-fluoro-D-glucose (FDG) PET/CT is an integral part of lung carcinoma staging and frequently used in the assessment of solitary pulmonary nodules. However, a limitation of conventional three-dimensional PET/CT when imaging the thorax is its susceptibility to motion artefact, which blurs the signal from the lesion resulting in inaccurate representation of size and metabolic activity. Respiratory gated (four-dimensional) PET/CT aims to negate the effects of motion artefact and provide a more accurate interpretation of pulmonary nodules and lymphadenopathy. There have been recent advances in technology and a shift from traditional hardware to more streamlined software methods for respiratory gating which should allow more widespread use of respiratory-gating in the future. The purpose of this article is to review the evidence surrounding four-dimensional PET/CT in pulmonary lesion characterisation.
INTRODUCTION
2-deoxy-2-(18Fluorine)-fluoro-D-glucose (FDG) PET/CT provides functional and anatomical information for characterisation of pulmonary nodules and staging of patients with lung carcinoma.1–3 The major incremental value of FDG PET/CT is in stratifying optimal patient management by detecting occult metastatic disease.4 Furthermore, FDG PET/CT has a role in the assessment of solid solitary pulmonary nodules with high reported sensitivity (95%) and specificity (82%).1 This is reflected in the latest British Thoracic Society pulmonary nodule guidelines, which stratify patients with a >10% risk of malignancy to further assessment with FDG PET/CT.1, 5 Use of a 4-point qualitative scale of nodule FDG activity, compared to physiological mediastinal blood pool (MBP) uptake is advocated, with post-test risk stratification incorporating this information using the Herder model.6 Optimal patient management is then defined; with a risk of malignancy >70% proceeding to treatment, 10–70% having a biopsy and <10% having CT surveillance.2
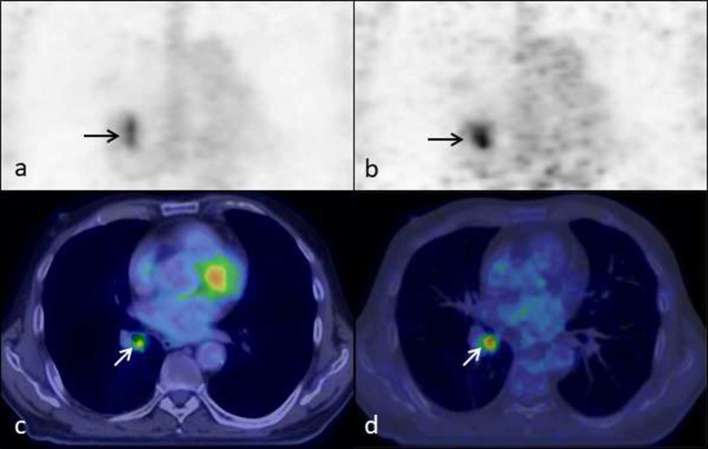
PET imaging is time-averaged covering many breathing cycles, whereas CT imaging represents a snapshot of part of one breathing cycle. Therefore, PET imaging is susceptible to movement and attenuation correction artefacts, particularly in thoracic or upper abdominal imaging due to respiratory motion. This can cause blurring of areas of interest resulting in inaccuracies in the assessment of standardised uptake value (SUV), which in turn can affect lesion characterisation and interpretation of staging. These effects can be confounded by the inherent spatial resolution of PET/CT, with smaller lesions not being as accurately assessed. The use of respiratory gated (four-dimensional, 4D) PET/CT aims to negate the effects of motion artefact and allow more accurate interpretation of pulmonary nodules and lymphadenopathy. An example of this is illustrated in Figure 1; a right lower lobe bronchus lesion is more accurately depicted on the gated study compared to the non-gated study. 4D PET/CT has the potential to aid in the detectability and quantification of upper abdominal lesions in the liver and pancreas as they are also susceptible to breathing artefact.7–14
Figure 1.
Ungated (a) and gated coronal PET images (b) and ungated (c) and gated (d) coronal fused PET/CT images of the thorax demonstrating a lesion in the right lower lobe bronchus (arrows). The lesion in the ungated images (a,c) is elongated in the superior/inferior plane when compared to the gated images (b, d). The tracer activity within the lesion is more avid on the gated study compared to the non-gated study, the SUVmax being 15.3 and 4.8 respectively. SUV, standardised uptake value.
The aim of this article is to review the literature surrounding the use of respiratory-gated 4D PET/CT in pulmonary lesion characterisation.
Respiratory-gated PET/CT
Respiratory-gating has traditionally been achieved by defining a patient’s respiratory cycle and reconstructing the image data into subdivisions or “bins” of the range of amplitude (amplitude-based gating) or the breathing cycle duration (phase-based gating)(Figure 2).15 The gated PET bins are amplitude-matched or phase-matched to the appropriate gated CT bins for attenuation correction. If the gated PET and CT data are not precisely matched, the quantitative accuracy of the PET reconstruction can be adversely affected.
Figure 2.
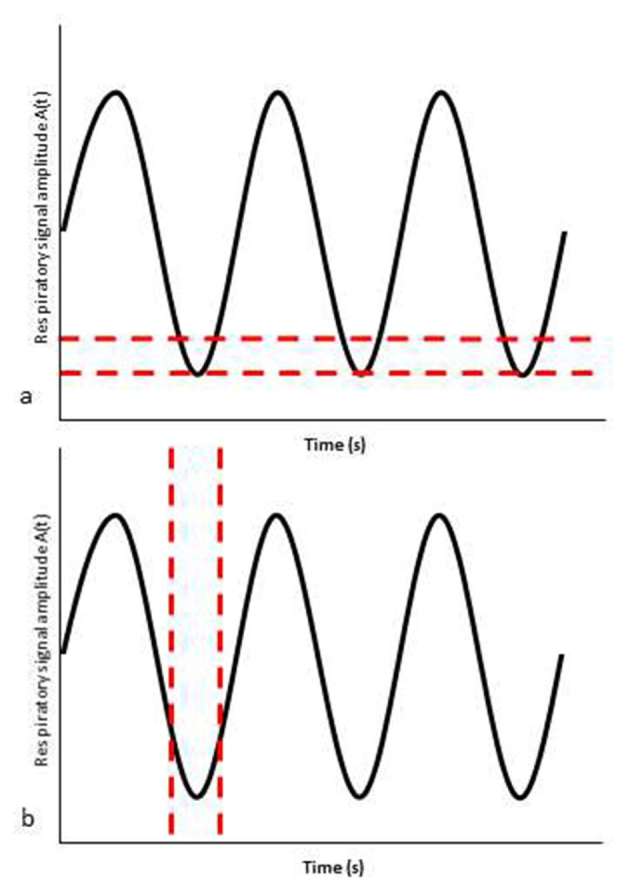
Representation of amplitude-based gating (a) and phase-based gating (b). The dotted lines demonstrate an example data “bin” incorporating the end expiratory phase of the respiratory cycle.
Established hardware-based respiratory gating techniques typically track a patient’s breathing cycle using external sensors.16 These hardware gating techniques include: an elastic belt with associated pressure monitoring which fits over the chest; measurement of displacement of infrared markers placed on patient’s chest wall; real time spirometry recording and monitoring of temperature change within the airways.16
When reconstructing data to create the gated study, a decision on how much data is included must be made. By reconstructing data using a small percentage of the respiratory cycle, fewer counts are sampled and as such, there is potential to either miss lesions or require a longer scan time. However, sampling too large a portion of the respiratory cycle potentially incorporates motion artefact into the imaging negating the effects of respiratory-gating. Phantom studies have reported variations in lesion volumes depending on the number of data bins or amount of data used.17 One of the limitations of the established gated reconstructions is its ability to handle irregular breathing patterns. Amplitude-gating has been demonstrated to be superior to phase-based gating in these patients.18 In addition, coaching patients before/whilst they are on the scanner can improve the regularity of their breathing cycle.19 Breath-hold techniques can be employed as a way of reconstructing data from the expiratory phase of the respiratory cycle however this is limited by patient co-operation. Software developments, such as Q.Freeze (GE Healthcare), have been introduced to overcome the issue of low counts in individual 4D gated bins.20–22 Q.Freeze applies Optical Flow techniques to merge and register count data from all the acquired gated bins to recover a motion-corrected lesion image with good count statistics. A further development has been data-driven gating or software gating which aims to eliminate the need for hardware tracking of external respiratory motion. Software gating involves direct mathematical modelling of the motion of tissues or lesions based on the PET list mode data.23 Various software techniques have been investigated, including centre of mass analysis, spectral analysis and principal component analysis.
Quantification & assessment of gated imaging: established techniques
Quantification of metabolic activity within pulmonary lesions using SUV can help determine the probability of malignancy.2 There is increasing interest in personalizing radiotherapy for individual patients with image-guided treatment planning and the potential for dose painting, which involves boosting radiotherapy doses to lung tumour subvolumes which are more metabolically active.24, 25 Recent technological advances in radiotherapy delivery with the use of volumetric modulated arc therapy have facilitated this. Consequently, the use of respiratory-gated PET/CT may be beneficial for more accurate representation of the metabolic activity within lung tumours by retrieving signal lost by movement. At present, there is a lack of evidence to support the routine use of 4D PET/CT for radiotherapy planning of thoracic and upper abdominal tumours due to the largely retrospective, single-centre nature of published data and absence of outcome data. A more detailed description of the use 4D PET/CT for radiotherapy planning in lung cancer is beyond the scope of this paper but this has been reviewed elsewhere.26 The potential use has also been demonstrated in radiotherapy planning of liver tumours.27
Multiple studies have demonstrated higher SUVmax of pulmonary lesions on 4D PET/CT compared to non-gated (3D) PET/CT (Table 1), the majority demonstrating statistical significance. Grootjans et al studied the use of optimal respiratory-gating (ORG) 4D PET/CT compared to 3D PET/CT in 83 lung lesions.31 ORG is an amplitude-based method which can be configured with different amounts of data included in the reconstruction with dedicated software determining the required amplitude to achieve this. The authors used this method to investigate different percentages of data or duty cycles (20, 35 and 50%) when compared to 3D PET/CT and the effect this had on SUV of pulmonary lesions. They reported a significant increase in mean SUVmax at 4D PET/CT compared to the non-gated study: 6.2 ± 12.2, 7.4 ± 13.3 and 9.2 ± 14% for the 20, 35 and 50% duty cycles respectively.31 Lesions were split into three categories: mediastinum and hila, upper lobe and middle and lower lobes. The largest difference in mean SUVmax was in the middle and lower group, however, all categories demonstrated significantly increased mean SUV. In mediastinal and hila lesions, there was a significant increase in mean SUV for all cycles, whereas in the upper lobe only the 20% cycle had a significant increase in mean SUV. This demonstrates the effect of tumour motion on perceived SUV.
Table 1.
Percentage difference of SUVmax at 3D PET/CT compared to 4D PET/CT reported by different studies
| Authors | Number of lesions | Type of gating | Pre/post treatment | Difference in SUVmax (4D vs 3D) | p-value |
| Aristophanous et al38 | 16 | Phase | Pre | 25% (median) | <0.0001 |
| Post | 18% (median) | 0.003 | |||
| Chang et al29 | 21 | Amplitude | Pre | 27% | <0.05 |
| Farid et al30 | 32 | Phase | Pre | 38% | <0.0001 |
| Grootjans et al31 | 83 | Amplitude 20% | Pre | 9.2% | <0.0001 |
| 35% | 7.4% | <0.0001 | |||
| 50% | 6.2% | <0.0001 | |||
| Guerra et al32 | 206 | Phase | Pre | 30.8% | <0.0001 |
| Huang et al20 | 6 | MF | Pre | 24.7% | 0.04 |
| Nehmeh et al33 | 5 | Phase | Pre | 62.9% | Not reported |
| Suzawa et al34 | 50 | Phase | Pre | 14.8% | <0.001 |
| Van Elmpt et al35 | 26 | Phase | Pre | 4.9% | <0.001 |
| Amplitude | Pre | 6.9% | <0.001 | ||
| Vicente et al36 | 42 | Phase | Pre | 83.3% | <0.05 |
| Vicente et al37 | 57 | Phase (average) | Pre | 48.8% | Not reported |
| Phase (best) | Pre | 71.9% | Not reported | ||
| Werner et al38 | 23 | Phase | Pre | 22.38% | <0.001 |
3D, three-dimensional; 4D, four-dimensional; MF, motion freeze; PET, positron emission tomography; SUV, standardised update value.
A larger study by Salavati et al involving 106 lung lesions in 55 patients used a phase-based reconstruction.39 They divided the respiratory cycle into four different phases and compared these with a non-gated study. They found that there was an increase in SUVmax in the 4D reconstructions compared to 3D PET/CT but this was not significant.39 They also reported no significant difference between SUVmax when comparing between the 4 different data bins of the respiratory cycle. However, if a partial volume algorithm was applied to the lesions the SUVmean was significantly increased when compared to studies without the algorithm. They also found that there was a more of a significant difference in lesions smaller than 3 cm.39 This highlights the idea that partial voluming may play a greater role in loss of detected activity than motion artefact when imaging small lesions.
When comparing amplitude-based ORG reconstruction with phase-gated reconstruction in 26 patients, Van Elmpt et al showed significantly higher average SUVmax with both methods compared to 3D PET/CT.35 There was no significant difference in SUVmax when comparing phase-gated and optimal-gated 4D PET/CT, however average SUV at optimal-gated 4D PET was significantly higher when >2.5 SUV was used as the threshold for tumour delineation. There was no significant difference in average SUV when a 40% SUV threshold was used to delineate lesions. They evaluated the level of noise present on studies by defining a region of interest in the contralateral lung and calculated standard deviations of SUVs within this area. This demonstrated that image noise was significantly greater on gated compared to non-gated studies, which is expected as the reconstructions are formed from fewer counts. Optimal-gated reconstruction was significantly less noisy than phased-based reconstruction which may be because ORG was performed using 35% of source data compared to 12.5% used in the phased-based study (the respiratory cycle was split into 8 separate data bins).
Lesion characterisation
As discussed, respiratory-gating has been shown to increase SUV of lesions when compared to 3D PET/CT. However, SUV is susceptible to multiple other factors including the phase of respiration during which attenuation correction CT is performed,40, 41 the time interval between injection of tracer and image acquisition,42 scanner make and model and patient’s blood glucose and body weight.43, 44 Variability in SUV can also be introduced into 4D PET/CT by using a non-gated CT for attenuation correction.45 Consequently, quantitative assessment using SUV at 4D PET/CT may not necessarily correlate with a greater accuracy in pulmonary nodule characterisation. The idea that SUVmax by itself cannot predict malignancy was demonstrated by Farid et al who studied classification of 32 pulmonary nodules, <2 cm in size, with 4D and 3D PET/CT using a >2.5 SUV threshold for diagnosing malignancy.30 The study demonstrated that whilst there was a significant difference in SUVmax between 3D and 4D PET/CT, there was no significant difference between SUVmax of benign and malignant lesions. Malignant lesions had a mean SUVmax of 3.8 measured on 4D PET/CT, whereas benign nodules had a SUVmax of 3.2. They concluded that SUV could not reliably distinguish between benign and malignant aetiology on 4D PET/CT.
Four further studies have examined the role of respiratory-gated PET/CT in characterisation of pulmonary nodules and lymph nodes. Guerra et al evaluated 206 lung lesions using both 3D and 4D PET/CT.32 They reported a mean SUVmax of 5.2 ± 5.1 at 3D PET/CT and 6.8 ± 6.1 at 4D PET/CT (p < 0.0001), with an average SUV increase of 30.8%. Lesions were defined as either positive, negative or equivocal based on both gated and non-gated PET/CT by comparing lesion activity with physiological MBP activity. Nodules were considered positive, if lesion uptake was visually significantly higher than MBP activity, negative if there was no significant visible uptake and equivocal if neither positive or negative criteria were met. There were 70 negative lesions at 3D PET/CT; after review of the 4D PET/CT, 3 were reclassified as positive and 2 were considered equivocal. 30/50 equivocal lesions at 3D PET/CT became positive at 4D PET/CT and 14/50 were considered negative. Histological data was available for 154 lesions; this led to the sensitivity, specificity and accuracy (with equivocal lesions removed) for 3D PET as 96.6, 71.6 and 85.7%, and 4D PET as 98.8, 90.8 and 95.3%, respectively. This study illustrates the potential for qualitative assessment at 4D PET/CT to improve characterisation of lung lesions.
Two studies by Vicente et al also looked at sensitivity of 4D PET/CT for determining malignancy in lung nodules compared to 3D PET/CT. The first studied characteristics of 57 pulmonary lesions in 37 patients with both 3D and 4D PET/CT.37 29 patients had a history of previous malignancy. 4D PET/CT data was divided into equal 2-min bins with lesions interpreted on a “best-bin”, where the highest SUVmax was demonstrated and an average SUVmax taken from all bins (average-bin). Their results demonstrated an increase in SUVmax on 4D PET/CT with the greatest percentage difference demonstrated in the best-bin 4D PET/CT. They reported no significant difference in SUVmax compared to lesion location. Histological correlation was available in 19 lesions, the remaining lesions were defined as malignant or benign based on radiological/clinical follow up. 4D PET/CT was associated with more false-positives than 3D PET/CT (six for best-bin, five for average-bin 4D PET/CT vs one from the 3D PET/CT study). The sensitivity, specificity, positive-predictive value and negative-predictive value were 37.8, 95, 93, and 45% respectively for 3D PET/CT and 70.3, 70, 81.2, and 56% respectively for 4D best-bin PET/CT and 51.3, 75, 79.2 and 45.4% respectively for average-bin 4D PET/CT. However, diagnostic accuracy of 3D and 4D PET/CT (either best-bin or average-gated), was not significantly different when using receiver operating curve analysis.
The second study by Vicente et al reviewed 42 lesions in 28 patients with minimal uptake (<2.5 SUV) not regarded as malignant on standard PET/CT and assessed 4D PET/CT (best-bin) characterisation of these lesions.36 Mean SUVmax % difference between 3D and 4D studies was 83.3% with significantly higher SUVmax changes in smaller lesions compared to larger lesions. This lead to 17/42 lesions being recharacterized as malignant on 4D PET/CT, of which 12 (71%) were true-positive and 5 (29%) were false-positive. False-negatives were reduced from 23 on the 3D study to 11 on the 4D study. Overall, the sensitivity, specificity, positive-predictive value, negative-predictive value and accuracy for lesions on 4D PET/CT which were not avid on 3D PET/CT was reported as 52, 74, 70, 56 and 62% respectively. As with other studies, this indicates that 4D PET/CT may improve the accuracy of lung nodule characterisation.
Callahan et al studied the impact of 4D PET/CT on classification of solitary lung nodules, using a 5-point classification, in 20 patients.46 They reported no change in characterisation of lesions initially classified as benign or malignant but demonstrated a slight increase in sensitivity (73–75%), specificity (56–63%) and accuracy (65–70%) of 4D PET/CT compared to 3D PET/CT for pulmonary lesions initially deemed indeterminate (2–3 times reference lung tissue but less than blood pool, SUV 1.5–2).46 These findings did not reach statistical significance and they concluded that use of 4D PET/CT may aid in the measurement of SUV and visualisation of lesions but did not improve differentiation between inflammatory processes and malignancy.
Impact on staging
Grootjans et al compared the ability of 4D and 3D PET/CT to accurately Stage 55 histologically proven lung carcinomas.47 PET/CT studies were blinded and read by two nuclear medicine physicians independently and staged using the tumour, node, metastasis (TMN) classification. Respiratory-gating resulted in five and eight more lesions being detected, depending on the reader, but these did not affect the T or M staging. A change in N stage was observed in four and seven patients, between readers 1 and 2, this was supported histologically in one case, histologically and radiologically in two cases and radiologically in one case. The follow-up data for the remaining patients was not available. These changes to staging did not alter the decision between radical or palliative treatment, but in three cases where positive lymph nodes were identified neoadjuvant chemotherapy was given prior to surgery. This relatively small study suggests that 4D PET/CT may be of value when staging malignancy compared to 3D PET/CT. However, evidence is limited and it is not possible to draw a definite conclusion. A comparative study of endobronchial ultrasound-guided biopsy vs 4D PET/CT would be useful in assessing its benefits.
The clinical benefit of respiratory gated imaging depends on the size, location, avidity and mobility of lesions. Also, efficacy is undermined because most PET/CT systems only have an option for phase-gated imaging, which has been shown to be less robust than amplitude gating, and fails to track shallow or irregular breathing appropriately.48 However, the multicentre studies with larger patient numbers e.g. Guerra et al, which best represent the range of lung lesions encountered and have protocol standardisation, show 4D has improved lesion detection accuracy and image quantification relative to 3D imaging.32 A recent multicentre study investigating liver lesions has found similar results, indicating that 4D gated imaging is broadly applicable to lesions affected by respiratory motion.49 The benefits may be increased and better demonstrated with improved patient selection, protocol standardisation and larger scale multicentre trials.
Quantification & assessment of gated imaging: novel techniques
Q.Freeze
Bouyeure-Petit et al21 and Minamimoto et al22 have recently investigated the current commercial version of Q.Freeze in phantom and patient studies. Initial results show that in phantom studies Q.Freeze is effective in restoring sphere object quantification (e.g. max SUV) in moving objects irrespective of breathing parameters. However, a minimum scan time of 4 min per PET bed position is required to control noise and static objects may be oversmoothed. The results from patient studies were less encouraging, as no significant differences in quantitative parameters were observed between Q.Freeze and ungated images. Further investigation is warranted, as results may have been impaired by limited patient numbers and lesion selection.
Software gating
Although there have been a number of proof-of-concept studies investigating software gating,22 only two notable studies have compared the performance of software and hardware gating in relatively large clinical patient groups.50, 51 Buther et al compared belt-gated FDG-PET/CT scans against a range of software gating solutions in a group of 48 patients with abdominal or thoracic lesions.50 Hardware and software gated images were superior to non-gated images and equivalent to each other in terms of lesion respiratory shifts, increase in SUV and reduction in lesion volume (compared to ungated images) and visual assessment of clinical reporters. Kesner et al reported a similar result in a group of 116 patients with pulmonary nodules using a previously reported software gating technique;51 software gating was preferred to hardware gating by clinical reporters in most cases.52
A problem with hardware gating is the overhead of extra acquisition and reconstruction time and equipment set up, coupled with often unpredictable and variable clinical value depending on factors such as lesion location and mobility. Recent studies indicate software solutions are capable of performing to a similar standard as established hardware solutions, but with the benefit of eliminating the overhead (excepting some additional image reconstruction time). This would allow gating to be applied routinely with minimal effect on established imaging workflows, allowing improved lesion detection and quantification, particularly in the subset of patients with small and mobile lesions near the diaphragm where there is likely to be the greatest clinical value.
However, quantitative challenges remain for software gating solutions related to matching PET and CT imaging (for purposes of attenuation corrections and localisation). A recent article by Cuplov et al highlights significant changes in lung volume and density during the breathing cycle related to variable composition of air and fluids.53 In a group of six lung cancer patients, volume/density correction techniques, such as air fraction correction, were shown to explain and correct for observed variation in activity concentration between PET respiratory gates.
Bayesian penalized likelihood (BPL) PET reconstruction (Q.Clear, GE Healthcare)
BPL PET reconstructions have been introduced into clinical practice, which offer the advantages of time-of-flight reconstruction, iterative convergence, image noise control and spatial resolution recovery. Recent studies investigating BPL reconstructions in lung lesions have found improved visual lesion conspicuity and significantly increases in signal-to-noise ratio and maximum SUV.54–56 Only one study, Vallot et al, incorporated a correction for respiratory motion; however it is unclear whether this provides additional clinical benefit.56 Further studies with gated BPL reconstructions are warranted.
Texture analysis
The ability to assess heterogeneity or “texture” of lung tumours may add key information aiding both lesion characterisation and treatment planning and is a current area of active investigation. Textural analysis of PET data involves mapping of tracer activity within different voxels. This data can be analysed using statistical methods, model-based methods or by transform-based methods. Each method can lead to the derivation of multiple different orders of parameters.57 The purest form of textural analysis is based on the statistical method with histogram analysis of SUV leading to parameters such as the mean, kurtosis, skewness, energy, entropy and standard deviation.57, 58 These are known as first order parameters, from which a multitude of higher-order parameters can be derived.
Grootjans et al studied 60 lung cancer patients, with lesions greater than 3 cm3, using 3D PET/CT (using 35% of the data) and amplitude-based 4D PET/CT.59 They analysed lesion texture by studying four parameters: entropy and dissimilarity, which represent variations in intensity or disorganisation within lesions, and zone percentage and high-intensity emphasis, which describe heterogeneity. When the cohort was analysed, there was no significant difference in textural analysis between non-gated and 4D studies. When dividing the lesions into three geographical zones, middle- and lower-zone lesions demonstrated a significant difference between gated and non-gated studies in all textural parameters apart from entropy. There was no significant difference in upper lobe lesions, likely due to them being less susceptible to motion artefact, and between different histological subtypes of tumour. None of the four textural parameters were, however, significant in the prediction of overall survival, this was attributed to the relatively small number of patients.
Yip et al also studied the heterogeneity of 35 lesions with both non-gated and respiratory-gated PET/CT in five different phase bins.60 They reported that 4D PET/CT significantly increased the long run low grey-level emphasis (LRLG) (51–2%, p = 0.02) and busyness (57–19%, p = 0.01), and decreased maximal correlation coefficient (MCC) (51–2%, p = 7.561023) and coarseness (55–10%, p = 0.05) compared to 3D-PET. LRLG measures the joint probability of long runs and low grey values, busyness is the comparison of single voxels and their surroundings, MCC is a measure of statistical relationship between voxels, and coarseness is a measure of uniformity within the defined area with coarse images having larger areas of uniformed intensity. Their data suggest that 4D PET/CT may allow superior delineation of intralesional heterogeneity, whereas 3D PET/CT is more susceptible to motion-related blurring. When comparing separate phase bins there was little difference in coarseness, MCC and LRLG between the data sets. There was variation with busyness put down to its predisposition to be affected by tumour motion.
Oliver et al expanded on the theme of heterogeneity in PET/CT by studying 56 different imaging features, consisting of shape and textural descriptors, in 23 patients with lung carcinoma using 3D and 4D PET/CT.61 They reported that features associated with the greatest difference (>50%) between gated and non-gated studies were minimum intensity, mean intensity, range of intensity, long run low level grey-level emphasis, shape of the distribution of intensity and total summed intensity, whereas features least affected by movement artefact (<5%) were those concerned with how spherical the signal was, how disorganized the data is, the short and long run distribution and run percentage disruption. They also demonstrated a difference in range of intensities with no significant difference in entropy.61
These studies include relatively small numbers of patients and, therefore, no definitive conclusions can be drawn regarding 4D PET/CT textural analysis. However, there appears to be significant differences in textural features between 3D and 4D PET/CT. A study comparing 4D PET/CT textural features of lung lesions with histological classification has not yet been reported. Another potential use of textural analysis would include dose painting, but this would require a study to create treatment planning protocols for lesions and develop a measurement of follow up and response.
Future Perspectives
The recent advances in data driven gating permit a fully automated, operator independent process, which requires minimal changes to current clinical image acquisition procedures. This shows great promise for translation into routine clinical imaging. However, these newer techniques need to be standardized and validated within a multicentre trial before the likely clinical benefits in thoracic (and upper abdominal) lesion characterisation can be fully realized.
CONCLUSION
Established hardware PET/CT gating has a role in lesion detection and characterisation, but it is underutilized due to practical limitations and an unclear patient management pathway. However, newer developments such as software gating, BPL reconstructions and texture analysis show promise to overcome practical limitations allowing wider accessibility and give improved image quantification and higher image quality.
Contributor Information
Russell Frood, Email: russellfrood@nhs.net.
Garry McDermott, Email: garry.mcdermott@nhs.net.
Andrew Scarsbrook, Email: a.scarsbrook@nhs.net.
REFERENCES
- 1.Cronin P, Dwamena BA, Kelly AM, Carlos RC. Solitary pulmonary nodules: meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy. Radiology 2008; 246: 772–82. doi: 10.1148/radiol.2463062148 [DOI] [PubMed] [Google Scholar]
- 2.Callister ME, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015; 70(Suppl 2): ii1–ii54. doi: 10.1136/thoraxjnl-2015-207168 [DOI] [PubMed] [Google Scholar]
- 3.NICE. Lung cancer : diagnosis and management. Clin guideline. 2011. Available from: http://nice.org.uk/guidance/cg121
- 4.van Tinteren H, Hoekstra OS, Smit EF, van den Bergh JH, Schreurs AJ, Stallaert RA, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 2002; 359: 1388–92. doi: 10.1016/S0140-6736(02)08352-6 [DOI] [PubMed] [Google Scholar]
- 5.Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 2001; 285: 914–24. doi: 10.1001/jama.285.7.914 [DOI] [PubMed] [Google Scholar]
- 6.Herder GJ, van Tinteren H, Golding RP, Kostense PJ, Comans EF, Smit EF, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest 2005; 128: 2490–6. doi: 10.1378/chest.128.4.2490 [DOI] [PubMed] [Google Scholar]
- 7.Van Der Gucht A, Serrano B, Hugonnet F, Paulmier B, Garnier N, Faraggi M. Impact of a new respiratory amplitude-based gating technique in evaluation of upper abdominal PET lesions. Eur J Radiol 2014; 83: 509–15. doi: 10.1016/j.ejrad.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 8.Suenaga Y, Kitajima K, Aoki H, Okunaga T, Kono A, Matsumoto I, et al. Respiratory-gated ¹⁸F-FDG PET/CT for the diagnosis of liver metastasis. Eur J Radiol 2013; 82: 1696–701. doi: 10.1016/j.ejrad.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 9.Revheim ME, Haugvik SP, Johnsrud K, Mathisen Ø, Fjeld JG, Skretting A. Respiratory gated and prolonged acquisition 18F-FDG PET improve preoperative assessment of colorectal liver metastases. Acta Radiol 2015; 56: 397–403. doi: 10.1177/0284185114529563 [DOI] [PubMed] [Google Scholar]
- 10.Fin L, Daouk J, Bailly P, Slama J, Morvan J, El Esper I, et al. Improved imaging of intrahepatic colorectal metastases with 18F-fluorodeoxyglucose respiratory-gated positron emission tomography. Nucl Med Commun 2012; 33: 656–62. doi: 10.1097/MNM.0b013e328351fce8 [DOI] [PubMed] [Google Scholar]
- 11.Schulz A, Godt JC, Dormagen JB, Holtedahl JE, Bogsrud TV, Labori KJ, et al. Respiratory gated PET/CT of the liver: a novel method and its impact on the detection of colorectal liver metastases. Eur J Radiol 2015; 84: 1424–31. doi: 10.1016/j.ejrad.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 12.Kishi T, Matsuo Y, Nakamura A, Nakamoto Y, Itasaka S, Mizowaki T, et al. Comparative evaluation of respiratory-gated and ungated FDG-PET for target volume definition in radiotherapy treatment planning for pancreatic cancer. Radiother Oncol 2016; 120: 217–21. doi: 10.1016/j.radonc.2016.07.012 [DOI] [PubMed] [Google Scholar]
- 13.Kasuya T, Tateishi U, Suzuki K, Daisaki H, Nishiyama Y, Hata M, et al. Role of respiratory-gated PET/CT for pancreatic tumors: a preliminary result. Eur J Radiol 2013; 82: 69–74. doi: 10.1016/j.ejrad.2012.05.037 [DOI] [PubMed] [Google Scholar]
- 14.Yukutake M, Sasaki T, Serikawa M, Minami T, Okazaki A, Ishigaki T, et al. The effect of respiratory-gated positron emission tomography/computed tomography in patients with pancreatic cancer. Hell J Nucl Med 2014; 17: 31–6. [PubMed] [Google Scholar]
- 15.Tsutsui Y, Kidera D, Taniguchi T, Akamatsu G, Komiya I, Umezu Y, et al. Accuracy of amplitude-based respiratory gating for PET/CT in irregular respirations. Ann Nucl Med 2014; 28: 770–9. doi: 10.1007/s12149-014-0870-5 [DOI] [PubMed] [Google Scholar]
- 16.Pépin A, Daouk J, Bailly P, Hapdey S, Meyer ME. Management of respiratory motion in PET/computed tomography: the state of the art. Nucl Med Commun 2014; 35: 113–22. doi: 10.1097/MNM.0000000000000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SJ, Ionascu D, Killoran J, Mamede M, Gerbaudo VH, Chin L, et al. Evaluation of the combined effects of target size, respiratory motion and background activity on 3D and 4D PET/CT images. Phys Med Biol 2008; 53: 3661–79. doi: 10.1088/0031-9155/53/13/018 [DOI] [PubMed] [Google Scholar]
- 18.Son HJ, Jeong YJ, Yoon HJ, Park JH, Kang DY. Visual and quantitative analysis methods of respiratory patterns for respiratory gated PET/CT. Biomed Res Int 2016; 2016: 1–11. doi: 10.1155/2016/7862539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mageras GS, Yorke E, Rosenzweig K, Braban L, Keatley E, Ford E, et al. Fluoroscopic evaluation of diaphragmatic motion reduction with a respiratory gated radiotherapy system. J Appl Clin Med Phys 2001; 2: 191–200. doi: 10.1120/jacmp.v2i4.2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang TC, Chou KT, Wang YC, Zhang G. Motion freeze for respiration motion correction in PET/CT: a preliminary investigation with lung cancer patient data. Biomed Res Int 2014; 2014: 1–7. doi: 10.1155/2014/167491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouyeure-Petit AC, Chastan M, Edet-Sanson A, Becker S, Thureau S, Houivet E, et al. Clinical respiratory motion correction software (reconstruct, register and averaged-RRA), for 18F-FDG-PET-CT: phantom validation, practical implications and patient evaluation. Br J Radiol 2017; 90: 20160549: 20160549. doi: 10.1259/bjr.20160549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minamimoto R, Mitsumoto T, Miyata Y, Sunaoka F, Morooka M, Okasaki M, et al. Evaluation of a new motion correction algorithm in PET/CT: combining the entire acquired PET data to create a single three-dimensional motion-corrected PET/CT image. Nucl Med Commun 2016; 37: 162–70. doi: 10.1097/MNM.0000000000000423 [DOI] [PubMed] [Google Scholar]
- 23.Kesner AL, Schleyer PJ, Büther F, Walter MA, Schäfers KP, Koo PJ. On transcending the impasse of respiratory motion correction applications in routine clinical imaging - a consideration of a fully automated data driven motion control framework. EJNMMI Phys 2014; 1: 8. doi: 10.1186/2197-7364-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Even AJ, van der Stoep J, Zegers CM, Reymen B, Troost EG, Lambin P, et al. PET-based dose painting in non-small cell lung cancer: Comparing uniform dose escalation with boosting hypoxic and metabolically active sub-volumes. Radiother Oncol 2015; 116: 281–6. doi: 10.1016/j.radonc.2015.07.013 [DOI] [PubMed] [Google Scholar]
- 25.Knudtsen IS, van Elmpt W, Öllers M, Malinen E. Impact of PET reconstruction algorithm and threshold on dose painting of non-small cell lung cancer. Radiother Oncol 2014; 113: 210–4. doi: 10.1016/j.radonc.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 26.Frood R, Prestwich R, Tsoumpas C, Murray P, Franks K, Scarsbrook A. Effectiveness of respiratory-gated PET/CT for radiotherapy planning in patients with lung carcinoma–a systematic review. Clin Oncol 2017; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.Riou O, Serrano B, Azria D, Paulmier B, Villeneuve R, Fenoglietto P, et al. Integrating respiratory-gated PET-based target volume delineation in liver SBRT planning, a pilot study. Radiat Oncol 2014; 9: 127. doi: 10.1186/1748-717X-9-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aristophanous M, Berbeco RI, Killoran JH, Yap JT, Sher DJ, Allen AM, et al. Clinical utility of 4D FDG-PET/CT scans in radiation treatment planning. Int J Radiat Oncol Biol Phys 2012; 82: e99–e105. doi: 10.1016/j.ijrobp.2010.12.060 [DOI] [PubMed] [Google Scholar]
- 29.Chang G, Chang T, Pan T, Clark JW, Mawlawi OR, Chnnag T. Implementation of an automated respiratory amplitude gating technique for PET/CT: clinical evaluation. J Nucl Med 2010; 51: 16–24. doi: 10.2967/jnumed.109.068759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farid K, Poullias X, Alifano M, Regnard JF, Servois V, Caillat-Vigneron N, et al. Respiratory-gated imaging in metabolic evaluation of small solitary pulmonary nodules: 18F-FDG PET/CT and correlation with histology. Nucl Med Commun 2015; 36: 722–7. doi: 10.1097/MNM.0000000000000311 [DOI] [PubMed] [Google Scholar]
- 31.Grootjans W, de Geus-Oei LF, Meeuwis AP, van der Vos CS, Gotthardt M, Oyen WJ, et al. Amplitude-based optimal respiratory gating in positron emission tomography in patients with primary lung cancer. Eur Radiol 2014; 24: 3242–50. doi: 10.1007/s00330-014-3362-z [DOI] [PubMed] [Google Scholar]
- 32.Guerra L, De Ponti E, Elisei F, Bettinardi V, Landoni C, Picchio M, et al. Respiratory gated PET/CT in a European multicentre retrospective study: added diagnostic value in detection and characterization of lung lesions. Eur J Nucl Med Mol Imaging 2012; 39: 1381–90. doi: 10.1007/s00259-012-2148-2 [DOI] [PubMed] [Google Scholar]
- 33.Nehmeh SA, Erdi YE, Ling CC, Rosenzweig KE, Schoder H, Larson SM, et al. Effect of respiratory gating on quantifying PET images of lung cancer. J Nucl Med 2002; 43: 876–81. [PubMed] [Google Scholar]
- 34.Suzawa N, Ichikawa Y, Ishida M, Tomita Y, Nakayama R, Sakuma H. Respiratory-gated time-of-flight PET/CT during whole-body scan for lung lesions: feasibility in a routine clinical setting and quantitative analysis. Ann Nucl Med 2016; 30: 722–30. doi: 10.1007/s12149-016-1118-3 [DOI] [PubMed] [Google Scholar]
- 35.van Elmpt W, Hamill J, Jones J, De Ruysscher D, Lambin P, Öllers M. Optimal gating compared to 3D and 4D PET reconstruction for characterization of lung tumours. Eur J Nucl Med Mol Imaging 2011; 38: 843–55. doi: 10.1007/s00259-010-1716-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García Vicente AM, Soriano Castrejón AM, Talavera Rubio MP, León Martín AA, Palomar Muñoz AM, Pilkington Woll JP, et al. 18F-FDG PET-CT respiratory gating in characterization of pulmonary lesions: approximation towards clinical indications. Ann Nucl Med 2010; 24: 207–14. doi: 10.1007/s12149-010-0345-2 [DOI] [PubMed] [Google Scholar]
- 37.García Vicente AM, Castrejón AS, León Martín AA, García BG, Pilkington Woll JP, Muñoz AP. Value of 4-dimensional 18F-FDG PET/CT in the classification of pulmonary lesions. J Nucl Med Technol 2011; 39: 91–9. doi: 10.2967/jnmt.110.082719 [DOI] [PubMed] [Google Scholar]
- 38.Werner MK, Parker JA, Kolodny GM, English JR, Palmer MR. Respiratory gating enhances imaging of pulmonary nodules and measurement of tracer uptake in FDG PET/CT. AJR Am J Roentgenol 2009; 193: 1640–5. doi: 10.2214/AJR.09.2516 [DOI] [PubMed] [Google Scholar]
- 39.Salavati A, Borofsky S, Boon-Keng TK, Houshmand S, Khiewvan B, Saboury B, et al. Application of partial volume effect correction and 4D PET in the quantification of FDG avid lung lesions. Mol Imaging Biol 2015; 17: 140–8. doi: 10.1007/s11307-014-0776-6 [DOI] [PubMed] [Google Scholar]
- 40.Kruis MF, van de Kamer JB, Vogel WV, Belderbos JS, Sonke JJ, van Herk M. Clinical evaluation of respiration-induced attenuation uncertainties in pulmonary 3D PET/CT. EJNMMI Phys 2015; 2: 4. doi: 10.1186/s40658-014-0107-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nehmeh SA, Erdi YE, Pan T, Yorke E, Mageras GS, Rosenzweig KE, et al. Quantitation of respiratory motion during 4D-PET/CT acquisition. Med Phys 2004; 31: 1333–8. doi: 10.1118/1.1739671 [DOI] [PubMed] [Google Scholar]
- 42.Lowe VJ, DeLong DM, Hoffman JM, Coleman RE. Optimum scanning protocol for FDG-PET evaluation of pulmonary malignancy. J Nucl Med 1995; 36: 883–7. [PubMed] [Google Scholar]
- 43.Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer--a PET study. J Nucl Med 1993; 34: 1–6. [PubMed] [Google Scholar]
- 44.Zasadny KR, Wahl RL. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose: variations with body weight and a method for correction. Radiology 1993; 189: 847–50. doi: 10.1148/radiology.189.3.8234714 [DOI] [PubMed] [Google Scholar]
- 45.Nagel CC, Bosmans G, Dekker AL, Ollers MC, De Ruysscher DK, Lambin P, et al. Phased attenuation correction in respiration correlated computed tomography/positron emitted tomography. Med Phys 2006; 33: 1840–7. doi: 10.1118/1.2198170 [DOI] [PubMed] [Google Scholar]
- 46.Callahan J, Kron T, Schneider ME, Hicks RJ. A prospective investigation into the clinical impact of 4D-PET/CT in the characterisation of solitary pulmonary nodules. Cancer Imaging 2014; 14: 24. doi: 10.1186/1470-7330-14-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grootjans W, Hermsen R, van der Heijden EH, Schuurbiers-Siebers OC, Visser EP, Oyen WJ, et al. The impact of respiratory gated positron emission tomography on clinical staging and management of patients with lung cancer. Lung Cancer 2015; 90: 217–23. doi: 10.1016/j.lungcan.2015.09.016 [DOI] [PubMed] [Google Scholar]
- 48.Dawood M, Büther F, Lang N, Schober O, Schäfers KP. Respiratory gating in positron emission tomography: a quantitative comparison of different gating schemes. Med Phys 2007; 34: 3067–76. doi: 10.1118/1.2748104 [DOI] [PubMed] [Google Scholar]
- 49.Crivellaro C, De Ponti E, Elisei F, Morzenti S, Picchio M, Bettinardi V, et al. Added diagnostic value of respiratory-gated 4D 18F-FDG PET/CT in the detection of liver lesions: a multicenter study. Eur J Nucl Med Mol Imaging 2018; 45: 102–9. doi: 10.1007/s00259-017-3795-0 [DOI] [PubMed] [Google Scholar]
- 50.Büther F, Vehren T, Schäfers KP, Schäfers M. Impact of data-driven respiratory gating in clinical PET. Radiology 2016; 281: 229–38. doi: 10.1148/radiol.2016152067 [DOI] [PubMed] [Google Scholar]
- 51.Kesner AL, Chung JH, Lind KE, Kwak JJ, Lynch D, Burckhardt D, et al. Validation of software gating: a practical technology for respiratory motion correction in PET. Radiology 2016; 281: 239–48. doi: 10.1148/radiol.2016152105 [DOI] [PubMed] [Google Scholar]
- 52.Kesner AL, Kuntner C. A new fast and fully automated software based algorithm for extracting respiratory signal from raw PET data and its comparison to other methods. Med Phys 2010; 37: 5550–9. doi: 10.1118/1.3483784 [DOI] [PubMed] [Google Scholar]
- 53.Cuplov V, Holman BF, McClelland J, Modat M, Hutton BF, Thielemans K. Issues in quantification of registered respiratory gated PET/CT in the lung. Phys Med Biol 2017; 63: 015007. doi: 10.1088/1361-6560/aa950b [DOI] [PubMed] [Google Scholar]
- 54.Howard BA, Morgan R, Thorpe MP, Turkington TG, Oldan J, James OG, et al. Comparison of Bayesian penalized likelihood reconstruction versus OS-EM for characterization of small pulmonary nodules in oncologic PET/CT. Ann Nucl Med 2017; 31: 623–8. doi: 10.1007/s12149-017-1192-1 [DOI] [PubMed] [Google Scholar]
- 55.Teoh EJ, McGowan DR, Bradley KM, Belcher E, Black E, Gleeson FV. Novel penalised likelihood reconstruction of PET in the assessment of histologically verified small pulmonary nodules. Eur Radiol 2016; 26: 576–84. doi: 10.1007/s00330-015-3832-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vallot D, Caselles O, Chaltiel L, Fernandez A, Gabiache E, Dierickx L, et al. A clinical evaluation of the impact of the Bayesian penalized likelihood reconstruction algorithm on PET FDG metrics. Nucl Med Commun 2017; 38: 1–84. doi: 10.1097/MNM.0000000000000729 [DOI] [PubMed] [Google Scholar]
- 57.Miles KA, Ganeshan B, Hayball MP. CT texture analysis using the filtration-histogram method: what do the measurements mean? Cancer Imaging 2013; 13: 400–6. doi: 10.1102/1470-7330.2013.9045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scalco E, Rizzo G. Texture analysis of medical images for radiotherapy applications. Br J Radiol 2017; 90: 20160642. doi: 10.1259/bjr.20160642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grootjans W, Tixier F, van der Vos CS, Vriens D, Le Rest CC, Bussink J, et al. The impact of optimal respiratory gating and image noise on evaluation of intratumor heterogeneity on 18F-FDG PET imaging of lung cancer. J Nucl Med 2016; 57: 1692–8. doi: 10.2967/jnumed.116.173112 [DOI] [PubMed] [Google Scholar]
- 60.Yip S, McCall K, Aristophanous M, Chen AB, Aerts HJ, Berbeco R. Comparison of texture features derived from static and respiratory-gated PET images in non-small cell lung cancer. PLoS One 2014; 9: e115510–4. doi: 10.1371/journal.pone.0115510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oliver JA, Budzevich M, Zhang GG, Dilling TJ, Latifi K, Moros EG. Variability of image features computed from conventional and respiratory-gated PET/CT images of lung cancer. Transl Oncol 2015; 8: 524–34. doi: 10.1016/j.tranon.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]