Abstract
Research over recent years has demonstrated that curative external-beam radiotherapy can be safely and efficaciously delivered with roughly half the number of treatments which was previously considered standard. We review the data supporting this change in practice, methods for implementation, as well as emerging future directions.
Introduction
External beam radiotherapy (EBRT) has been proven to have equivalent efficacy compared with radical prostatectomy for the definitive management of prostate cancer.1 Prostate EBRT has also undergone several major refinements over the last 20 years designed to improve accuracy, reduce dose to the rectum, and increase efficacy. One by-product of these efforts is that the duration of a conventional course of prostate radiotherapy now involves around 8 weeks of daily treatment, with clear implications for patient convenience and hospital resource utilization. Recent work has built on historical experience showing that shorter, or hypofractionated, courses of prostate radiotherapy are non-inferior to conventional schedules. Here, we review the biology underlying such work, the key clinical trials which have recently been reported, the implementation of hypofractionated regimens and future research directions.
Prostate cancer radiobiology
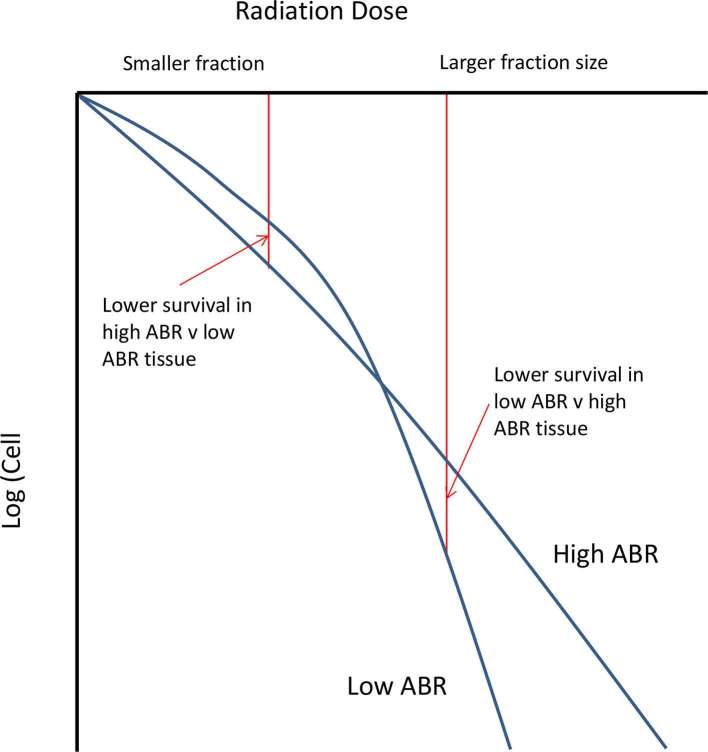
A key concept underlying the practice of radiation oncology is that both total radiation dose as well as the dose per fraction influence outcomes. In the laboratory, this is demonstrated by cell survival curves, whereas the dose of a single fraction of radiation increases in a linear manner, there is a greater than exponential rate of cell kill. This is often described by the linear-quadratic model, where an individual cell survival curve can be defined by the alpha–beta ratio (ABR). Most mucosal malignancies, typified by head and neck squamous cell carcinoma, have a higher ABR in the range of 8–10 Gy. On the cell survival curve, the slope for such tumours is relatively constant, as demonstrated in Figure 1. This is in contradistinction to several late reacting normal tissues such as spinal cord, where the ABR is approximately 2–3 Gy, resulting in a “bendier” cell survival curve. Since, at low doses per fraction there is greater cell kill of a high ABR tumour than a lower ABR normal tissue, we have a sound biological rationale for conventional fraction sizes of 1.8–2 Gy per fraction.
Figure 1.
Idealized cell survival curves showing the difference between a high and low ABR tissue corresponding to many mucosal malignancies (high ABR), some late responding normal tissues and prostate cancer (low ABR) respectively. ABR, alpha–beta ratio.
Data from several avenues suggests that the ABR for prostate cancer is very low. In ground-breaking work from the 1990s, Brenner and Hall used data from low dose rate brachytherapy and EBRT tumour responses to calculate an ABR of 1.5 Gy [95% confidence interval (CI) (0.8–2.2)].2 Very large combined institutional experiences have also been explored for EBRT, showing mean ABRs of 1.6–3.7 Gy.3, 4 Finally, older clinical trial data has been interrogated, with a 936 patient Canadian Randomized trial of conventional (66 Gy in 33 fractions) vs hypofractionated (52.5 Gy in 20 fractions) radiotherapy yielding an ABR estimate of 1.12.5, 6 Due to 7% worse biochemical–clinical failure, this particular trial could not confirm non-inferiority of the hypofractionated approach, possibly because of the relatively low biological equivalent dose in the experimental arm. There are caveats with all of this data given the number of other uncontrollable variables at work using either retrospective data or older and less accurate radiotherapy techniques. However, if the ABR of prostate cancer is as low, or lower, than surrounding normal tissues, there would be scope to increase the fraction size to exploit the enhanced tumour cell kill compared with effects on neighbouring critical structures. This observation underpins the work in the field of hypofractionated prostate radiotherapy.
Conventional prostate radiotherapy
Radiation doses of 60–64 Gy in 1.8–2 Gy fractions were standard until at least the 1990s. However, another key tenet of radiobiology, is that the greater the dose of radiotherapy delivered to a volume, the higher the probability of tumour control.7 Technological advances, especially in the use of conformal radiotherapy techniques, have enabled higher radiation doses to be investigated. A series of randomized controlled trials have asked whether dose escalated radiotherapy has any clinical advantage compared with standard doses. In brief summary, they have generally shown that doses of 74–80 Gy are superior in terms of biochemical disease control compared with doses of 64–70.2 Gy, without achieving a survival benefit, despite follow-up now extended beyond 10 years.8, 9 There is also a higher risk of late toxicity, usually in the form of radiation proctitis. This latter issue has largely been managed through a combination of newer technologies (expanded as “Hypofractionated prostate radiotherapy” in the next section) as well as approaches to temporarily distance the rectum from the prostate such as the surgical insertion of hydrogel, rectal balloons or a rectal rod. On this basis, radiation doses of ~74–80 Gy have become widely adopted not only in clinical practice, but also within the control arms of contemporary clinical trials.10–12
There are issues with both patient access and convenience as well as resource utilization with treatment regimens which extend for around 8 weeks. Some evidence suggests that radiotherapy is underutilized as a treatment for prostate cancer, although the causes for this extend beyond access and convenience.13 Conversely, given the high incidence of prostate cancer, there is a multiplicative effect where high incidence multiplied by a long treatment schedule results in clear implications regarding the resourcing of radiotherapy. Concurrently, there are several other ablative approaches available including cryotherapy, high intensity focussed ultrasound and electroporation, most of which involve a single treatment administered without the need for an overnight stay in hospital.14 Although the evidence supporting most of these ablative strategies is non-randomized, and some of these tensions tend to be opposing, both from a patient access aspect as well as resourcing and retaining appeal amongst numerous competing treatment strategies, there is merit in exploring hypofractionated approaches beyond the biological and technological rationales already outlined.
Hypofractionated prostate radiotherapy
Newer technologies have emerged which made the deliverability of hypofractionated prostate radiotherapy regimens feasible.15 Image-guided radiotherapy (IGRT) ensures more accurate targeting of the prostate, while intensity modulated radiotherapy (IMRT) enables greater control over the focussing of the radiation dose within a volume, allowing more dose to the tumour, and less to normal tissues.16, 17 Some early Phase II data showed the integration of IGRT and IMRT allows the deployment of hypofractionated radiotherapy.18, 19 This led to several randomized trials being undertaken comparing conventional and hypofractionated approaches.20–22 Most showed no clear enduring benefit for hypofractionation, and as such need to be considered negative trials. However, they were all relatively small, single centre experiences and hence, underpowered to detect small differences in efficacy. One 206 male study from the MD Anderson Cancer Centre presented, thus far in abstract form only, showed an absolute benefit of 4.7% in prostate cancer recurrence after a median follow up of 8.4 years in favour of the hypofractionated arm of 72 Gy in 30 fractions compared with 75.6 Gy in 42 fractions (10.7 vs 15.4%, p = 0.034)23 Another notable exception was the large collaborative Dutch HYPRO trial, which accrued 820 males with intermediate- or high-risk disease, randomizing between 78 Gy in 39 fractions and 64.6 Gy in 19 fractions.24 Although the hazard ratio was 0.86 in favour of the shorter arm, similar to the smaller trials, the 95% CI crossed 1 and was hence non-significant under the superiority requirement defined at the outset [95% CI (0.63–1.16), p = 0.36].
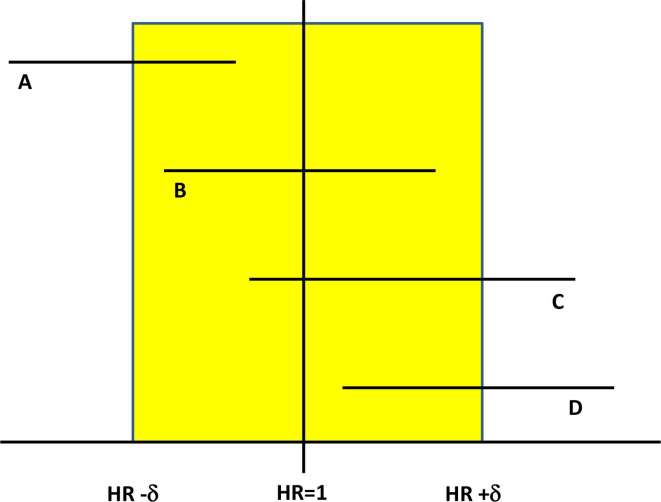
The desire for superior efficacy itself is questionable, as even equivalence between the two approaches would see the hypofractionated approaches preferred. A more appropriate trial design to explore this is a non-inferiority randomized study. This model takes the initial view that the experimental arm has other factors beyond efficacy, which makes it more desirable, such as improved convenience or lower cost. It, therefore, hypothesizes that the experimental treatment is no worse than the standard approach. Rather than comparing the point estimate of an outcome such as 5 year biochemical control rates, it compares the upper limit of the 95% CI of the hazard ratio (the failure rate from the experimental arm is divided by the same figure from the control arm). This is because the true figure for the hazard ratio can lie anywhere within that CI, and to deem an experimental treatment non-inferior, it is necessary to be confident that it is not unacceptably worse than the standard arm. Figure 2 illustrates this difference between superiority and non-inferiority studies.
Figure 2.
Illustration of how significance is determined for both superiority and non-inferiority trial designs. If two arms of a study are equivalent, the HR will be 1. A range is defined around the HR corresponding to the magnitude of the differences between the two arms (HR ± δ). From the trial, a CI is constructed for the HR, usually with HR <1 implying the experimental arm had less events than the control arm. Trial (a) shows the experimental arm to be superior, and Trial (d) the experimental arm is in, as their CIs are all less than and greater than 1 respectively. Trial (b) shows non-inferiority of the experimental arm, since the upper limit of the CI is less than the pre-defined HIR + δ. The trial (c) CI straddles both 1 and HR+δ, meaning it is either not superior or not non-inferior depending on the initial trial design. CI, confidence interval; HR, Hazard ratio.
Evidence from non-inferiority trials
Three large, well-powered, non-inferiority randomized controlled trials have all reported non-inferior efficacy for hypofractionated regimens compared to conventionally fractionated control arms.25–27 Follow-up is a median of 5–6 years in all studies, a mixture of low-, intermediate- and high-risk males were treated, and one study including androgen deprivation therapy for all patients. Their findings are summarized in Table 1. Although efficacy was non-inferior, there were differences in toxicity profiles, with PROFIT showing reduced late gastrointestinal toxicity in favour of the hypofractionated arm, whilst RTOG 0415 demonstrated an increase in late gastrointestinal and genitourinary Grade 2 toxicity for the abbreviated regimen. Whether this is due to different fraction sizes in the conventional arm, differing methods of radiation dose prescribing or merely chance findings will await further analyses. Summary patient quality of life showed no significant differences between arms in the three studies, with a more detailed report currently being prepared for the PROFIT cohort. On the basis of non-inferior efficacy, many centres are now beginning to offer hypofractionated regimens as a standard approach, as well as including hypofractionation as a component of standard treatment for currently accruing clinical trials.12
Table 1.
Summary of the three large non-inferiority randomized controlled trials of conventional vs hypofractionated radiotherapy for prostate cancer
| Trial | Risk groups included | Number of patients | Median follow-up | ADT? | Dose/fractions | 5-year control | Late G2–4 GI toxicity | Late G2–4 GU toxicity |
| RTOG 0415 | Low | 1115 | 5.8 years | No | 73.8 Gy/39 | 85.3% | 14% | 22.8% |
| 70 Gy/28 | 86.3% | 22.4% | 29.7% | |||||
| CHHiP | All, but majority intermediate | 3216 | 5.1 years | Yes 6–9 months | 74 Gy/37 | 88.3% | 13.7% | 9.1% |
| 60 Gy/20 | 90.6% | 11.9% | 11.7% | |||||
| 57 Gy/19 | 85.9% | 11.3% | 6.6% | |||||
| PROFIT | Intermediate | 1205 | 6 years | No | 78 Gy/39 | 85% | 13.9% | 22% |
| 60 Gy/20 | 85% | 8.9% | 22.2% |
ADT, androgen deprivation therapy.
Of the three trials, PROFIT had some characteristics which increase the relevance of hypofractionation in contemporary practice.25 It was the only one of the three studies to mandate IGRT for all patients, and although IMRT was not essential on the study, the vast majority of the males managed on PROFIT did receive IMRT rather than three-dimensional conformal radiotherapy. The control arm of PROFIT received 78 Gy in 39 fractions prescribed so that the 95% isodose covered 99% of the planning target volume (PTV), an approach that remains very contemporary. Alongside CHHiP, which included accrual from the UK, as well as New Zealand, PROFIT enjoyed international cooperation, with patient accrual from Canada, Australia and France. This widespread engagement alongside the wide eligibility criteria means that the findings can confidently be applied broadly, rather than uniquely to a particular jurisdiction.
Data from these trials can also be used to infer both a dose response relationship as well as estimate an ABR. CHHiP had three arms: 74 Gy/37, 60 Gy/20 and 57 Gy/19. Using the conventional arm as the reference, the hazard ratios for the two hypofractionated arms were 0.84 and 1.2 respectively (p = NS), and 0.7 in favour of the 60 Gy/20 arm when directly compared with 57 Gy/19 (p = 0.0026). Furthermore, PROFIT showed essentially equivalence between 60 Gy/20 and 78 Gy/39, with a hazard ratio of 0.96. Assuming these fractionation schedules are indeed equivalent, allows an estimate of the ABR of 1.33. Furthermore, accepting the caveats associated with intertrial comparisons these data suggest an efficacy hierarchy of 57/19, 74/37, 78/39 and 60/20, again supporting a dose response relationship.
Patient selection
Although the findings from the non-inferiority studies are broadly applicable, there are some caveats worthy of mention. Obvious exclusion criteria such as previous pelvic radiotherapy or active colitis are relevant beyond hypofractionation to any consideration of prostate radiotherapy for a particular patient. PROFIT excluded males with a total hip joint replacement (THJR), and CHHiP males with bilateral THJRs. More modern planning techniques such as the fusion of MRI to aid prostate delineation and volumetric modulated arc therapy mean that, in our experience, hypofractionated regimens can usually be delivered to males with even bilateral THJRs, whilst respecting critical structure dose constraints.28, 29 One relatively small single institutional study suggested on post-hoc analysis that males with more obstructive urinary symptoms (international prostate symptom score >12) had worse late urinary toxicity following hypofractionated radiotherapy.22 These findings have not been replicated in other randomized studies of moderately hypofractionated regimens. Our approach is to optimally manage lower urinary tract symptoms prior to prostate radiotherapy, either medically or surgically, but not to consider them a validated contraindication to offering a hypofractionated schedule. Similarly, prostate volume alone was not an exclusion criteria in any of the three non-inferiority trials, meaning that achieving dose homogeneity across the PTV and respecting critical structure dose constraints should be key considerations in the deliverability of hypofractionated regimens.
Planning considerations
The three non-inferiority randomized trials were all launched prior to 2006, meaning that many of the planning approaches have evolved significantly in the interim. CHHiP had three separate phases, including a final boost phase with no posterior expansion around the prostate.26 PROFIT only had 2 rectal dose volume histogram constraints—a D30 and D50.25 RTOG 0415 dosed so that 100% of the dose was applied to at least 98% of the PTV.27 In addition, the identification of critical structures varied such as rectal wall verses volume, and whole organ contour verses only contouring in the vicinity of the PTV. Although all of these approaches have sound rationales, it is also helpful to attempt to make hypofractionated treatments more contemporary.
Institutions are going through this exercise in various ways. In Newcastle, we asked other centres around the world what their approaches were, modelled a series of patient plans including several scenarios such as THJRs, hydrogel insertion and elective proximal seminal vesicle irradiation, and shared our protocol for feedback through various avenues including social media. In brief summary, we:
Prescribe 60 Gy in 20 fractions to 98% of the prostate/CTV, and concurrently 57 Gy to 98% of the PTV.
Treat as a single phase, with a uniform 7mm expansion around the clinical target volume.
Use IGRT for all patients, IMRT for most, and volumetric modulated arc therapy for patients with THJRs or lateral separation greater than 45 cm.
Contour the whole of the bladder and rectum as solid organs, as well as a urethral PRV to prevent hot spots occurring in this region.
Utilize a range of critical structure dose criteria, with both mandatory and ideal constraints (Table 2).
Take care with minimizing intermediate dose wash (especially 50% dose through the rectum) as well as excessive monitor units per fraction (aim for less than 600 for prostate only treatments).
Table 2.
Dose volume histogram constraints for a 60 Gy in 20 fraction plan in Newcastle
| Structure | Mandatory | Ideal |
| Clinical target volume | D98% > 60 Gy and < 60.2 Gy | |
| Planning target volume | D98% > 57 Gy and < 57.2 Gy V63 < 1% |
|
| Rectum/ bladder |
V60 < 3% | V60 < 1% |
| V57 < 15% | V57 < 10% | |
| V54 < 20% | V54 < 15% | |
| V46 < 35% | V46 < 20% | |
| V38 < 50% | V38 < 30% | |
| V15 < 80% | V15 < 60% | |
| Necks of femurs | V30 < 5% | |
| Urethra | Dmax < 61 Gy | |
Implementation
The large number of centres who participated in the various clinical trials means that the infrastructure, knowledge and confidence to deliver hypofractionated regimens was widespread, making adoption relatively straight-forward. Some clinicians are more cautious in embracing hypofractionation, mainly due to concerns regarding longer term toxicity. This can be seen with hypofractionated breast radiotherapy, which despite initial publications appearing in 2002, as of 2013 only 34.5% of female eligible for such treatment were receiving it in the USA.30, 31 Reassuringly, the Phase II trial which predated PROFIT has recently been updated with greater than 10 years of median follow-up, and it confirmed very low rates of physician graded 2–4 late gastrointestinal (4%) and genitourinary (12%) toxicity at the 8-year time point.32 Where whole pelvic radiotherapy is being prescribed, there can be a tendency to conventionally fractionate treatment. There is, however, literature showing it is safe to delivered regimens to pelvic lymph nodes in conjunction with a 28-day schedule similar to that utilized in RTOG 0415.33, 34 A more insidious barrier to uptake remains the financial incentives in some jurisdictions incentivizing more prolonged treatment schedules. As clinicians, we need to engage with our funding bodies to ensure our practices are not penalized for offering prostate radiotherapy hypofractionation.
Future directions
Stereotactic monotherapy
The regimens explored up until this point hypofractionate relatively mildly, and much larger doses per fraction has become a very active area of investigation. Largely using the Cyberknife platform, many series have been published demonstrating the feasibility of a five-fraction stereotactic treatment schedule. A large compilation of over 1100 patients worth of data showed excellent patient reported quality of life as well as high rates of disease control for such an approach, albeit after only 3 years of median follow-up.35, 36 More mature data of a large 515 patient series with median follow-up extending beyond 7 years is now also available which appears to confirm these promising findings.37 Although there is some interest in reducing the number of fractions all the way down to one, a cautionary note should be sounded from the Sunnybrook randomised trial of 1 vs 2 fractions of high-dose rate (HDR) brachytherapy monotherapy. Despite promising early data,38 a more recent update showed much worse efficacy outcomes for the single fraction approach.39 A small ongoing randomized controlled trial is currently comparing 45 Gy in 5 fractions of EBRT with 24 Gy in 1 fraction.40 Several randomized studies are comparing 5–7 fraction experimental stereotactic schedules with conventionally or moderately hypofractionated alternatives (Table 3). Other smaller Phase II studies are investigating minimizing radiation dose to structures involved in erectile function,41 the integration of newer agents,42 the use of tumour nodule boosting43 or technological advances such as real time tumour tracking.44 The results of such research are eagerly anticipated, and will undoubtedly inform future practice.
Table 3.
Current and pending randomized trials investigating stereotactic EBRT for prostate cancer
| Trial | Control arm(s) | Experimental arm | Number of patients |
| HYPO-RT-PC (ISRCTN45905321) | 78 Gy/39 | 42.7 Gy/7 | 592 |
| PACE (NCT01584258) | 78 Gy/39 or 62 Gy/20 | 36.25 Gy/5 | 858 |
| HEAT (NCT01794403) | 70.2 Gy/26 | 36.25 Gy/5 | 456 |
| NRG GU005 (pending) | 70 Gy/28 | 36.25 Gy/5 | 622 |
| RPAH2 (NCT02361515) | 62 Gy/20 | 37.5 Gy/5 | 96 |
Intrafraction motion management
With increasing doses per fraction of radiotherapy, treatment accuracy becomes an even higher priority. IGRT traditionally has focussed on correcting any prostate movement prior to commencing treatment, so called interfraction motion. More recently, we have gained a greater appreciation of prostate movement during a treatment session, or intrafraction motion, with novel methods being implemented to address both translations and rotations.45 There are multiple vendor and investigative strategies which have been developed to manage this, with many now widely available and integrated into routine practice.46
Virtual HDR boost
Radiation dose escalation remains an area of active interest, with three randomized trials of brachytherapy boosting showing improved biochemical control which has not translated into an overall survival benefit at the cost of increased genitourinary morbidity.47–49 One novel way this is currently being explored is through so called virtual HDR boost, where 1–3 stereotactic treatments are given prior to a more conventionally fractionated course.50 This blended approach borrows from the very favourable results reported in the brachytherapy literature, but holds the promise of being more widely implementable given the challenges of maintaining sufficient expertize with brachytherapy.
Rectal protection
As previously alluded to, there are several mechanisms to physically displace the rectum from the prostate in an effort of reducing rectal toxicity.51 Rectal balloons and rectal rods have data suggesting improved dosimetry, but the practice of inserting a device into the rectum for every fraction of radiotherapy has limited their widespread uptake. An alternative approach is to insert Hydrogel between the rectum and prostate. A 212 patient randomized trial has shown that this results in reduced rectal toxicity for patients with low and favourable–intermediate-risk disease, with 3 year physician Grade 2 or greater toxicity reduced from 5.7 to 0%.52 This has led to national recommendations in the United Kingdom to embrace this technique and should lead to further confidence in the wider use of hypofractionated prostate radiotherapy.53 A different approach is the use heavy ions such as protons to both reduce dose to the rectum as well as lower the integral dose, with promising low toxicity results reported in large institutional series.54
Imaging
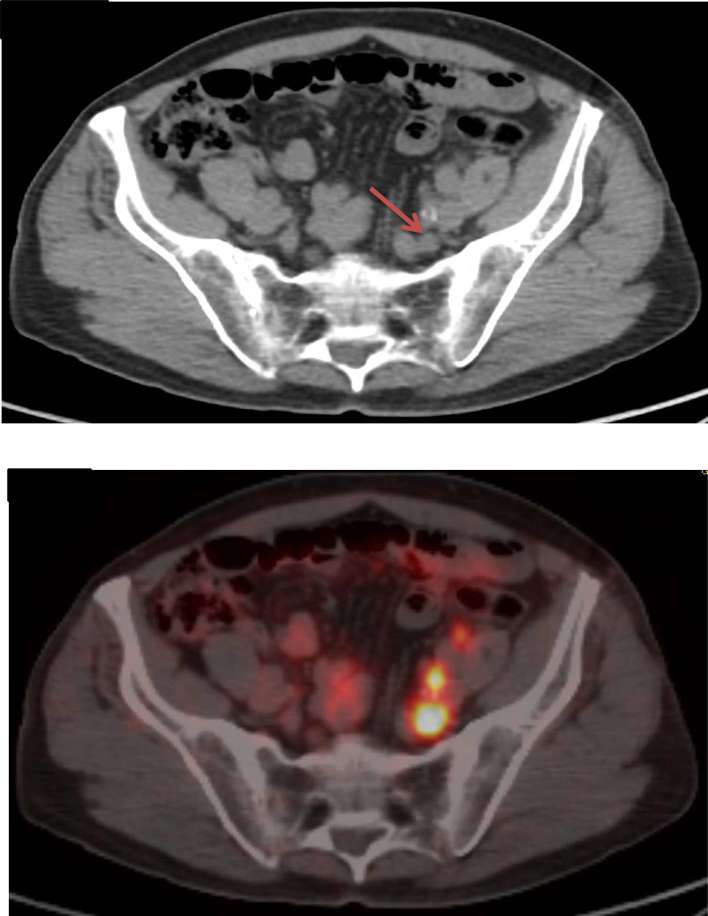
Advancing imaging capabilities are transforming the practice of prostate radiotherapy. Prostate MRI can help with local tumour detection and staging as well as prostate delineation.55 Applications under investigation include boosting MRI nodules as well as MRI-only planning, where a synthetic CT is created to allow treatment planning system dose calculation with the need for a dedicated CT.56 Prostate specific membrane antigen PET is emerging as having great potential as a highly sensitive and specific single staging investigation (Figure 3).57 The integration of such new imaging will further optimize both patient selection as well as treatment delivery of hypofractionated radiotherapy.
Figure 3.
Staging CT showing a pelvic lymph node (arrowed) which was negative by size criteria. Subsequent PSMA PET showed high avidity in both this lymph node, as well as several others which were no more than 5 mm in maximal diameter. PSMA, prostate specific membrane antigen.
Conclusions
The practice of prostate radiotherapy continues to evolve, and the growing evidence of the safety and efficacy of mildly hypofractionated schedules is a key part of that evolution. The technology is available to implement, and the barriers to adoption are surmountable. Numerous promising avenues of enquiry are already flowing from and towards hypofractionated treatment as a springboard to ongoing improvements in the care of our patients.
Contributor Information
Jarad M Martin, Email: Jarad.Martin@calvarymater.org.au.
Stephane Supiot, Email: Stephane.Supiot@ico.unicancer.fr.
Paul J Keall, Email: paul.keall@sydney.edu.au.
Charles N Catton, Email: Charles.Catton@rmp.uhn.ca.
REFERENCES
- 1.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375: 1415–24. doi: 10.1056/NEJMoa1606220 [DOI] [PubMed] [Google Scholar]
- 2.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 1999; 43: 1095–101. doi: 10.1016/S0360-3016(98)00438-6 [DOI] [PubMed] [Google Scholar]
- 3.Williams SG, Taylor JM, Liu N, Tra Y, Duchesne GM, Kestin LL, et al. Use of individual fraction size data from 3756 patients to directly determine the alpha/beta ratio of prostate cancer. Int J Radiat Oncol Biol Phys 2007; 68: 24–33. doi: 10.1016/j.ijrobp.2006.12.036 [DOI] [PubMed] [Google Scholar]
- 4.Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivities of low/middle/high-risk prostate cancer deduced from seven international primary institutional datasets. Int J Radiat Oncol Biol Phys 2009; 75: S81. doi: 10.1016/j.ijrobp.2009.07.202 [DOI] [PubMed] [Google Scholar]
- 5.Lukka H, Hayter C, Julian JA, Warde P, Morris WJ, Gospodarowicz M, et al. Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J Clin Oncol 2005; 23: 6132–8. doi: 10.1200/JCO.2005.06.153 [DOI] [PubMed] [Google Scholar]
- 6.Bentzen SM, Ritter MA. The α/β ratio for prostate cancer: what is it, really? Radiother Oncol 2005; 76: 1–3. doi: 10.1016/j.radonc.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 7.Martin JM, Supiot S, Berthold DR. Pharmacotherapeutic management of locally advanced prostate cancer: current status. Drugs 2011; 71: 1019–41. doi: 10.2165/11591500-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 8.Dearnaley DP, Jovic G, Syndikus I, Khoo V, Cowan RA, Graham JD, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol 2014; 15: 464–73. doi: 10.1016/S1470-2045(14)70040-3 [DOI] [PubMed] [Google Scholar]
- 9.Trada Y, Plank A, Martin J. Defining a dose-response relationship for prostate external beam radiotherapy. J Med Imaging Radiat Oncol 2013; 57: 237–46. doi: 10.1111/1754-9485.12008 [DOI] [PubMed] [Google Scholar]
- 10.Lehman M, Hayden AJ, Martin JM, Christie D, Kneebone AB, Sidhom M, et al. FROGG high-risk prostate cancer workshop: patterns of practice and literature review: part I: intact prostate. J Med Imaging Radiat Oncol 2014; 58: 257–65. doi: 10.1111/1754-9485.12142 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen H, Frantzis J, Sisson T, Jones M, Martin J, Middleton M. The impact of IGRT for prostate radiotherapy on dosimetry and the traditional workflow practice of focus to skin distance measurements. Radiographer 2009; 56: 15–20. doi: 10.1002/j.2051-3909.2009.tb00098.x [DOI] [Google Scholar]
- 12.Gillett J, Ientile C, Hiscock J, Plank A, Martin JM. Complementary and alternative medicine use in radiotherapy: what are patients using? J Altern Complement Med 2012; 18: 1014–20. doi: 10.1089/acm.2011.0334 [DOI] [PubMed] [Google Scholar]
- 13.Thompson SR, Delaney GP, Jacob S, Shafiq J, Wong K, Hanna TP, et al. Estimation of the optimal utilisation rates of radical prostatectomy, external beam radiotherapy and brachytherapy in the treatment of prostate cancer by a review of clinical practice guidelines. Radiother Oncol 2016; 118: 118–21. doi: 10.1016/j.radonc.2015.12.023 [DOI] [PubMed] [Google Scholar]
- 14.Habibian DJ, Katz AE. Emerging minimally invasive procedures for focal treatment of organ-confined prostate cancer. Int J Hyperthermia 2016; 32: 795–800. doi: 10.1080/02656736.2016.1195925 [DOI] [PubMed] [Google Scholar]
- 15.Citrin DE. Recent developments in radiotherapy. N Engl J Med 2017; 377: 1065–75. doi: 10.1056/NEJMra1608986 [DOI] [PubMed] [Google Scholar]
- 16.Ratnayake G, Martin J, Plank A, Wong W. Incremental changes verses a technological quantum leap: the additional value of intensity-modulated radiotherapy beyond image-guided radiotherapy for prostate irradiation. J Med Imaging Radiat Oncol 2014; 58(Suppl 3): 503–10. doi: 10.1111/1754-9485.12153 [DOI] [PubMed] [Google Scholar]
- 17.Martin JM, Frantzis J, Eade T, Chung P. Clinician”s guide to prostate IMRT plan assessment and optimisation. J Med Imaging Radiat Oncol 2010; 54: 569–75. doi: 10.1111/j.1754-9485.2010.02217.x [DOI] [PubMed] [Google Scholar]
- 18.Martin JM, Rosewall T, Bayley A, Bristow R, Chung P, Crook J, et al. Phase II trial of hypofractionated image-guided intensity-modulated radiotherapy for localized prostate adenocarcinoma. Int J Radiat Oncol Biol Phys 2007; 69: 1084–9. doi: 10.1016/j.ijrobp.2007.04.049 [DOI] [PubMed] [Google Scholar]
- 19.Kupelian PA, Willoughby TR, Reddy CA, Klein EA, Mahadevan A. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: cleveland clinic experience. Int J Radiat Oncol Biol Phys 2007; 68: 1424–30. doi: 10.1016/j.ijrobp.2007.01.067 [DOI] [PubMed] [Google Scholar]
- 20.Yeoh EE, Botten RJ, Butters J, Di Matteo AC, Holloway RH, Fowler J. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase III randomized trial. Int J Radiat Oncol Biol Phys 2011; 81: 1271–8. doi: 10.1016/j.ijrobp.2010.07.1984 [DOI] [PubMed] [Google Scholar]
- 21.Arcangeli G, Saracino B, Arcangeli S, Gomellini S, Petrongari MG, Sanguineti G, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol 2017; 35: 1891–7. doi: 10.1200/JCO.2016.70.4189 [DOI] [PubMed] [Google Scholar]
- 22.Pollack A, Walker G, Horwitz EM, Price R, Feigenberg S, Konski AA, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol 2013; 31: 3860–8. doi: 10.1200/JCO.2013.51.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman KE, Voong KR, Levy LB, Pugh TJ, Choi S, Du W, et al. Randomized trial of hypofractionated dose-escalated intensity modulated radiation therapy versus conventionally fractionated intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2016; 96: S32. doi: 10.1016/j.ijrobp.2016.06.091 [DOI] [Google Scholar]
- 24.Incrocci L, Wortel RC, Alemayehu WG, Aluwini S, Schimmel E, Krol S, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2016; 17: 1061–9. doi: 10.1016/S1470-2045(16)30070-5 [DOI] [PubMed] [Google Scholar]
- 25.Catton CN, Lukka H, Gu CS, Martin JM, Supiot S, Chung PWM, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol 2017; 35: 1884–90. doi: 10.1200/JCO.2016.71.7397 [DOI] [PubMed] [Google Scholar]
- 26.Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 2016; 17: 1047–60. doi: 10.1016/S1470-2045(16)30102-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee WR, Dignam JJ, Amin M, Bruner D, Low D, Swanson GP, et al. NRG oncology RTOG 0415: a randomized phase III non-inferiority study comparing two fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol 2016; 34(2_suppl): 1. doi: 10.1200/jco.2016.34.2_suppl.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosewall T, Kong V, Vesprini D, Catton C, Chung P, Ménard C, et al. Prostate delineation using CT and MRI for radiotherapy patients with bilateral hip prostheses. Radiother Oncol 2009; 90: 325–30. doi: 10.1016/j.radonc.2008.11.015 [DOI] [PubMed] [Google Scholar]
- 29.Prabhakar R, Kumar M, Cheruliyil S, Jayakumar S, Balasubramanian S, Cramb J. Volumetric modulated arc therapy for prostate cancer patients with hip prosthesis. Rep Pract Oncol Radiother 2013; 18: 209–13. doi: 10.1016/j.rpor.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whelan T, MacKenzie R, Julian J, Levine M, Shelley W, Grimard L, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst 2002; 94: 1143–50. doi: 10.1093/jnci/94.15.1143 [DOI] [PubMed] [Google Scholar]
- 31.Bekelman JE, Malin J, Emanuel EJ. Hypofractionated whole breast irradiation for early-stage breast cancer-reply. JAMA 2015; 313: 1371. doi: 10.1001/jama.2015.1644 [DOI] [PubMed] [Google Scholar]
- 32.Lieng H, Pintilie M, Bayley A, Berlin A, Bristow R, Chung P, et al. Long-term outcomes of a phase II trial of moderate hypofractionated image-guided intensity modulated radiotherapy (IG-IMRT) for localized prostate cancer. Radiother Oncol 2017; 122: 93–8. doi: 10.1016/j.radonc.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 33.Wu R, Woodford H, Capp A, Hunter P, Cowin G, Tai KH, et al. A prospective study of nomogram-based adaptation of prostate radiotherapy target volumes. Radiat Oncol 2015; 10: 243. doi: 10.1186/s13014-015-0545-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald AM, Baker CB, Popple RA, Shekar K, Yang ES, Jacob R, et al. Different rectal toxicity tolerance with and without simultaneous conventionally-fractionated pelvic lymph node treatment in patients receiving hypofractionated prostate radiotherapy. Radiat Oncol 2014; 9: 129. doi: 10.1186/1748-717X-9-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King CR, Freeman D, Kaplan I, Fuller D, Bolzicco G, Collins S, et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol 2013; 109: 217–21. doi: 10.1016/j.radonc.2013.08.030 [DOI] [PubMed] [Google Scholar]
- 36.King CR, Collins S, Fuller D, Wang PC, Kupelian P, Steinberg M, et al. Health-related quality of life after stereotactic body radiation therapy for localized prostate cancer: results from a multi-institutional consortium of prospective trials. Int J Radiat Oncol Biol Phys 2013; 87: 939–45. doi: 10.1016/j.ijrobp.2013.08.019 [DOI] [PubMed] [Google Scholar]
- 37.Katz A, Formenti SC, Kang J. Predicting biochemical disease-free survival after prostate stereotactic body radiotherapy: risk-stratification and patterns of failure. Front Oncol 2016; 6: 168. doi: 10.3389/fonc.2016.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morton G, Chung HT, McGuffin M, Helou J, D'Alimonte L, Ravi A, et al. Prostate high dose-rate brachytherapy as monotherapy for low and intermediate risk prostate cancer: early toxicity and quality-of life results from a randomized phase II clinical trial of one fraction of 19 Gy or two fractions of 13.5 Gy. Radiother Oncol 2017; 122: 87–92. doi: 10.1016/j.radonc.2016.10.019 [DOI] [PubMed] [Google Scholar]
- 39.Morton G, Chung H, McGuffin M, Ravi A, Liu S, Tseng E, et al. Prostate HDR monotherapy: initial efficacy results from a randomized trial of one versus two fractions. Brachytherapy 2017; 16: S19–S20. doi: 10.1016/j.brachy.2017.04.018 [DOI] [Google Scholar]
- 40.Greco C, Pares O, Pimentel N, Louro V, Arcangeli S, Pinzi V, et al. Acute toxicity following single-dose radiation therapy in the management of intermediate risk prostate cancer: results from a phase 2 randomized trial. Int J Radiat Oncol Biol Phys 2017; 99: E236. doi: 10.1016/j.ijrobp.2017.06.1167 [DOI] [Google Scholar]
- 41.Spratt DE, Lee JY, Dess RT, Narayana V, Evans C, Liss A, et al. Vessel-sparing radiotherapy for localized prostate cancer to preserve erectile function: a single-arm phase 2 trial. Eur Urol 2017; 72: 617–24. doi: 10.1016/j.eururo.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 42.Robson K, Alizart M, Martin J, Nagel R. Coeliac patients are undiagnosed at routine upper endoscopy. PLoS One 2014; 9: e90552. doi: 10.1371/journal.pone.0090552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ratnayake G, Martin J, Plank A, Wong W. Incremental changes verses a technological quantum leap: the additional value of intensity-modulated radiotherapy beyond image-guided radiotherapy for prostate irradiation. J Med Imaging Radiat Oncol 2014; 58: 503–10. doi: 10.1111/1754-9485.12153 [DOI] [PubMed] [Google Scholar]
- 44.Keall P, Nguyen DT, O’rien R, Booth J, Greer P, Poulsen P, et al. Stereotactic prostate adaptive radiotherapy utilising kilovoltage intrafraction monitoring: the TROG 15.01 SPARK trial. BMC Cancer 2017; 17: 180. doi: 10.1186/s12885-017-3164-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen DT, O’Brien R, Kim JH, Huang CY, Wilton L, Greer P, et al. The first clinical implementation of a real-time six degree of freedom target tracking system during radiation therapy based on kilovoltage intrafraction monitoring (KIM). Radiother Oncol 2017; 123: 37–42. doi: 10.1016/j.radonc.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 46.Zaorsky NG, Showalter TN, Ezzell GA, Nguyen PL, Assimos DG, D’Amico AV, et al. ACR appropriateness criteria for external beam radiation therapy treatment planning for clinically localized prostate cancer, part II of II. Adv Radiat Oncol 2017; 2: 437–54. doi: 10.1016/j.adro.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris WJ, Tyldesley S, Rodda S, Halperin R, Pai H, McKenzie M, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT trial): an analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017; 98: 275–85. doi: 10.1016/j.ijrobp.2016.11.026 [DOI] [PubMed] [Google Scholar]
- 48.Rodda S, Tyldesley S, Morris WJ, Keyes M, Halperin R, Pai H, et al. ASCENDE-RT: an analysis of treatment-related morbidity for a randomized trial comparing a low-dose-rate brachytherapy boost with a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017; 98: 286–95. doi: 10.1016/j.ijrobp.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 49.Dayes IS, Parpia S, Gilbert J, Julian JA, Davis IR, Levine MN, et al. Long-term results of a randomized trial comparing iridium implant plus external beam radiation therapy with external beam radiation therapy alone in node-negative locally advanced cancer of the prostate. Int J Radiat Oncol Biol Phys 2017; 99: 90–3. doi: 10.1016/j.ijrobp.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 50.Sidhom M, Arumugam S, Bucci J. Early results of Australian multicentre trial of stereotactic “virtual HDR’ radiography boost for intermediate and high risk prostate cancer. J Med Imaging Radiat Oncol 2016; 60(Suppl 1): 48. [Google Scholar]
- 51.Wilton L, Richardson M, Keats S, Legge K, Hanlon MC, Arumugam S, et al. Rectal protection in prostate stereotactic radiotherapy: a retrospective exploratory analysis of two rectal displacement devices. J Med Radiat Sci 2017; 64: 266–73. doi: 10.1002/jmrs.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamstra DA, Mariados N, Sylvester J, Shah D, Karsh L, Hudes R, et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncol Biol Phys 2017; 97: 976–85. doi: 10.1016/j.ijrobp.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 53.Supiot S, Créhange G, Latorzeff I, Pommier P, Paumier A, Rio E, et al. Hypofractionated radiotherapy in prostate cancer. Cancer Radiother 2013; 17: 349–54. doi: 10.1016/j.canrad.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 54.Bryant C, Smith TL, Henderson RH, Hoppe BS, Mendenhall WM, Nichols RC, et al. Five-year biochemical results, toxicity, and patient-reported quality of life after delivery of dose-escalated image guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2016; 95: 422–34. doi: 10.1016/j.ijrobp.2016.02.038 [DOI] [PubMed] [Google Scholar]
- 55.Khoo EL, Schick K, Plank AW, Poulsen M, Wong WW, Middleton M, et al. Prostate contouring variation: can it be fixed? Int J Radiat Oncol Biol Phys 2012; 82: 1923–9. doi: 10.1016/j.ijrobp.2011.02.050 [DOI] [PubMed] [Google Scholar]
- 56.Dowling JA, Sun J, Pichler P, Rivest-Hénault D, Ghose S, Richardson H, et al. Automatic substitute computed tomography generation and contouring for magnetic resonance imaging (MRI)-alone external beam radiation therapy from standard MRI sequences. Int J Radiat Oncol Biol Phys 2015; 93: 1144–53. doi: 10.1016/j.ijrobp.2015.08.045 [DOI] [PubMed] [Google Scholar]
- 57.Gupta SK, Watson T, Denham J, Shakespeare TP, Rutherford N, McLeod N, et al. Prostate-specific membrane antigen positron emission tomography-computed tomography for prostate cancer: distribution of disease and implications for radiation therapy planning. Int J Radiat Oncol Biol Phys 2017; 99: 701–9. doi: 10.1016/j.ijrobp.2017.06.2448 [DOI] [PubMed] [Google Scholar]