Abstract
Objective:
To compare the N- and M-staging accuracy of PET vs CT, as per the American Joint Committee on Cancer (AJCC) eighth edition in patients with malignant pleural mesothelioma (MPM) being considered for multimodality therapy in a tertiary referral center. A secondary aim was to assess survival outcome of patients chosen for surgical management after PET.
Methods:
A retrospective, single institution comparison of PET and CT was performed in patients with histologically proven MPM being considered for multimodality therapy. Performance of each modality in identifying nodal category and presence or absence of distant metastases was abstracted from electronic patient records. The standard of reference was surgical histopathology for nodal stage and histopathology or clinical and imaging follow-up of >3 months for distant metastases.
Results:
There were 101 eligible patients with complete data sets; 82 males, 19 females with a mean age of 66.6 years (range: 39–85). Most patients (n = 68) had epithelioid histology. Surgery was performed in 61/101 patients (60.4%), most of whom had multimodality therapy. Nodal category was concordant to surgical histopathology in 38/60 patients (63.3%) on PET, compared to 27/60 (45%) on CT (p = 0.001). For detection of ≥N1 disease only, PET and CT correctly staged 15/37 patients (40.5%) and 8/37 (21.6%), respectively (p = 0.023). Distant metastases were identified uniquely on PET in eight patients and on CT only in one patient. Overall, PET and CT correctly identified 11/12 (91.6%) and 4/12 (33.3%) patients with distant metastases, respectively (p = 0.0391).
Conclusion:
PET identifies significantly more patients with nodal or distant metastatic disease than CT and may contribute to more appropriate selection of patients with MPM for surgery or multimodality therapy.
Advances in knowledge:
In patients with MPM, fludeoxyglucose-PET/CT detects significantly more patients with distant metastases than CT. PET/CT can help in the selection of patients with MPM who would benefit from surgery or multimodality therapy.
INTRODUCTION
Malignant pleural mesothelioma (MPM) is an aggressive malignancy with a dismal outcome. MPM is locally aggressive and may invade the diaphragm, the chest wall, or vital mediastinal structures. It may metastasize to mediastinal and hilar lymph nodes and approximately 10–20% of patients have distant metastases.1 Accurate assessment of local extent, nodal status and presence or absence of distant metastases are crucial for meticulous selection of patients who may benefit from macroscopic complete surgical resection, extrapleural pneumonectomy (EPP), or pleurectomy and decortication, in a combined modality approach.2 With multimodality treatment (surgery, chemotherapy and radiotherapy), reported median survival is approximately 9–19 months; with 2- and 5-year survivals of 38 and 15%, respectively.3, 4
The routine staging of MPM is performed with CT, although it underestimates local invasion and lymph node metastases. Due to its exquisite contrast resolution, MRI is superior to CT in assessing local extent including chest wall invasion (sensitivity of 69 vs 46%, respectively) and invasion of the diaphragm (sensitivity of 82 vs 55%, respectively).5 Despite this, MRI is limited in detecting microscopic diaphragmatic invasion or small volume peritoneal disease.6 Detection of nodal involvement is of importance due to the poor outcome of patients with extrapleural nodal involvement who undergo radical resection. However, all imaging modalities, including PET are limited in assessing metastatic nodal disease due to limited sensitivity and specificity. Therefore, invasive nodal sampling is important in patient selection.3 Prior small scale studies have suggested that PET may improve selection of patients with MPM for multimodality therapy by detecting occult metastatic mediastinal nodes such as internal mammary nodes, or by detecting metastatic supraclavicular nodes or other extrathoracic metastases.1,7–12 The main objective of this study was to compare the N- and M-staging accuracy of PET versus CT, as per the American Joint Committee on Cancer eighth edition13 in patients with MPM being considered for multimodality therapy in a tertiary referral center. A secondary aim was to assess survival outcome of patients chosen for surgical management after PET.
PATIENTS AND METHODS
This is a single institution, retrospective study on patients with MPM who underwent staging with CT and 18F-fludeoxyglucose (FDG) PET/CT (=PET) in a tertiary referral center with a multidisciplinary program for the management of MPM. The study was approved by the institutional ethics review board and informed consent was waived. All patients included in the study had a histological diagnosis of MPM, and staging with contrast-enhanced CT of the chest and abdomen and PET (median time difference between contrast-enhanced CT and PET was 14 days). All imaging reports and clinical data were abstracted from electronic patient records by a single reviewer (EH). Recorded parameters included demographic data (age, gender), histology, patient management (surgical vs non-surgical), and patient outcome, including survival data, if available. For each patient, the N and M category (as per American Joint Committee on Cancer eighth edition; Table 1) was determined for each imaging modality. The standard of reference for nodal staging was histopathology, and for distant metastases were histopathology and/or clinical and imaging follow-up with CT and/or PET of at least 3 months to confirm suspected metastatic sites on initial imaging. Treatment decisions were made by a multidisciplinary team including thoracic surgeons and medical and radiation oncologists with all clinical and imaging data available.
Table 1.
N & M category of MPM 13
| Regional lymph nodes (N) : | |
| Nx | Regional lymph nodes cannot be assessed. |
| N0 | No regional lymph node metastases. |
| N1 | Metastases in the ipsilateral bronchopulmonary/hilar lymph nodes or in the subcarinal or the ipsilateral mediastinal lymph nodes including the ipsilateral internal mammary and peridiaphragmatic nodes. |
| N2 | Metastases in the contralateral mediastinal, contralateral internal mammary, ipsilateral or contralateral supraclavicular lymph nodes. |
| Distant metastases (M): | |
| M0 | No distant metastases |
| M1 | Distant metastases present |
MPM, malignant pleural mesothelioma.
From AJCC Cancer Staging Manual 8th edition.13
Imaging protocols
CT protocol
CT scans of the chest and abdomen were acquired on a multidetector CT scanner with 64 parallel detector rows (Aquilion 64, Toshiba America Medical Systems, New York, NY). Scan parameters included: detector collimation = 0.5 mm × 64; thickness × reconstruction interval = 5 × 2.5 mm; mA determined by automated tube modulation (at a 12.5–15 h noise index, with minimum and maximum tube currents of 10 and 510 mA, respectively); kVp = 120; coronal reformation reconstruction thickness of 3 mm and reconstruction interval of 3 mm. Non-ionic intravenous contrast material was used unless contraindicated [Omnipaque ™300 (iohexol), 30 mg of iodine ml−1; Amersham Health, Buckinghamshire, UK) was administered with the use of a power injector (Medrad, Indianola, TX) at a dose of 2 ml kg–1 up to a maximum of 180 ml after a 60–70 s delay.
PET/CT protocol
PET scans were performed in three-dimensional mode with a dedicated inline PET/CT scanner [Siemens, Biograph Duo (2009–2013; n = 49) and Siemens Biograph mCT 40 (2013–2016; n = 52)] (Siemens Healthcare, Erlagen, Germany). Patients were asked to fast for at least 6 h before undergoing the examination. Data were acquired 60–70 min after an intravenous injection of approximately 5 MBq kg–1 body weight of FDG (up to 550 MBq). First, a spiral CT scan from the skull base to the upper thighs was obtained using the following parameters: 120-kVp; 40–105 mAs; scan width, 5.0 mm reconstructed section thickness, 2.0 mm overlap. On completion of CT, PET scans of the same area were acquired for 3 min/ bed position, with 5–7 bed positions per patient. PET was interpreted on a dedicated fused imaging workstation (Thinking Systems, Petersburg, FL).
Imaging data abstraction
Data regarding N- and M-staging from CT and PET reports were collected for each patient from the Radiology Information System and compared to the standard of reference.
Statistical analysis
Comparison of performance of CT and PET in determining N and M category of patients was performed using McNemar’s test without continuity correction. Comparison of sensitivity between tests and lesion-level comparison of the detection of distant metastases with PET and CT was performed using two-tailed Fisher’s exact test. For all statistical analysis, p < 0.05 was considered significant. Statistical analysis was performed using SAS statistical software v. 9.4 (SAS Institute, Cary, NC).
RESULTS
The radiology information system database identified 124 PET scans performed for patients with MPM between November 2007 and March 2016. There were 116 unique patients. No clinical and/or imaging data were available for 11 patients who were treated at other institutions and staging CT was not available for 4 other patients. Therefore, the study cohort consisted of 101 patients, including 82 males and 19 females with median age of 69 years (range: 39–85). The histological subtypes were epithelioid (n = 68), biphasic (n = 22), sarcomatoid (n = 5), and not otherwise specified (n = 6).
Patient management and outcome
Surgery was performed in 61/101 patients (60.4%) with 51/61 patients (83.6%) receiving neoadjuvant radiotherapy, most of whom received a short accelerated course of high-dose hemithoracic intensity-modulated radiation therapy followed by surgery (Surgery for Mesothelioma after Radiation Therapy; “SMART” protocol). Chemotherapy was given to 54/101 (53.5%) of patients, including prior to surgery in 8/61 patients (13.1%), adjuvant chemotherapy in 17/61 (27.9%) and in 29/40 patients (72.5%) who were not treated surgically. Of the eight patients who received chemotherapy prior to surgery, four had invasive lymph node sampling prior to surgery [mediastinoscopy (n = 2) or endoscopic bronchial ultrasound guided biopsy (n = 2)] and four additional patients had no suspicious nodes on preoperative staging.
For the cohort of patients treated surgically, at time of censor, 13/61 were alive (at a median surveillance period of 23 months; range: 13–61), 47/61 patients died (with a median survival time of 16 months; range: 3–76) and one patient was lost to follow up after 34 months of surveillance. Nearly half of the patients treated with surgery (29/61; 47.5%) had a survival time of at least 2 years. Outcome data for the nonsurgical group were limited, as patients were often managed at other medical centers. At time of censor, for those who were not treated surgically, 20/40 (50%) were known to have died (with a median survival time of 10 months; range: 1–30) and 20/40 (50%) were treated at a local cancer center and lost to our follow up after a median time of 9.5 months (range 1–58).
N & M category
Data from pathological lymph node staging are available for 60 surgical patients (Table 2). In one patient, the pleural tumor was found to be technically non-resectable and nodal staging was not performed. In one of eight patients treated with neoadjuvant chemotherapy, there were positive hilar and ipsilateral mediastinal nodes on PET that were negative at final pathology, but the node was sampled previously at mediastinoscopy and found to be positive. Overall PET and CT correctly staged 38/60 (63.3%) patients, and 27/60 (45%) patients, respectively (p = 0.001). PET and CT correctly staged N0 disease in 23/23 (100%) and 19/23 (82.6%) patients; N1 disease in 15/36 (41.7%) and 8/36 (22.2%) patients and N2 disease in 0/1 and 0/1 patients, respectively. For detection of ≥N1 disease only, PET and CT correctly staged 15/37patients (40.5%) and 8/37 (21.6%), respectively (p = 0.023).
Table 2.
N & M category according to the standard of reference
| N= | |
| N-category | |
| Nx | 1a |
| N0 | 23 |
| N1 | 36 |
| N2 | 1 |
| M-category | |
| M0 | 89 |
| M1 | 12 |
Please note that N category refers to patients treated surgically for which histopathology was available. M category refers to the entire cohort.
One patient underwent surgery but did not have histopathology for lymph nodes (Nx).
There were 12/101 patients (11.9%) with distant metastases as per the standard of reference (pathology: n = 1 or clinical and imaging follow-up: n = 11). Distant metastases were identified uniquely on PET in eight patients and on CT only in one patient [Figure 1]. Overall, PET and CT correctly identified 11/12 (91.6%) and 4/12 (33.3%) patients with distant metastases, respectively (p = 0.0391). The sensitivity, specificity, negative predictive value, positive predictive value for detecting distant metastases for PET and CT is provided in Table 3. Confirmed metastatic sites included: extrathoracic lymph nodes (n = 8), bone (n = 2), liver (n = 2), peritoneum (n = 2), contralateral lung (n = 1), and adrenal (n = 1). There was one rib lesion thought to represent a metastatic deposit on CT which was negative on PET and shown to represent an old fracture on subsequent imaging. On a lesion-level analysis, PET correctly identified 15/16 (93.8%) of distant metastases, compared with 4/16 (25%) for CT (p = 0.0002).
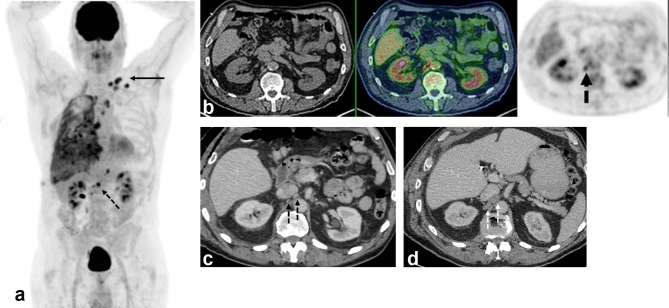
Figure 1.
A 70 year-old male with epithelioid malignant pleural mesothelioma. (a) Maximum intensity projection FDG-PET image shows extensive right pleural malignancy and metastatic adenopathy in the mediastinum and left supraclavicular fossa (arrow). Further metabolically active nodes are seen below the diaphragm (dotted arrow). (b) PET/CT image (CT, left; fused PET/CT, middle; PET, right) shows two foci of abnormal FDG uptake in tiny retrocaval lymph nodes. (c) Concurrent contrast-enhanced CT shows normal sized retroperitoneal nodes, with no size significant lymphadenopathy. (d) Follow-up contrast–enhanced CT performed 7 weeks later shows interval development of enlarged lymph nodes in same location, indicating progressive metastatic disease and confirming baseline PET/CT findings. FDG, fludeoxyglucose.
Table 3.
Performance measures of PET and CT for detection of distant metastases (patient-level data)
| PET | CT | |
| Sensitivity (95% CI) | 91.7 (61.52–99.79) | 33.3 (9.92–65.11) |
| Specificity (95% CI) | 100 (95.9–100) | 98.88 (93.8–99.9) |
| PPV (95% CI) | 100 | 80 (32.7–97.1) |
| NPV (95% CI) | 98.89 (93.2–99.8) | 91.7 (88.1–94.3) |
CI, confidence interval; NPV, negative-predictive value; PPV, positive-predictive value.
DISCUSSION
The management of patients with MPM is extremely challenging and overall reported survival ranges between 9 and 17 months.14–18 The role of surgery in this disease and specifically the most appropriate surgical technique remains controversial. Many surgeons choose lung-sparing technique of pleurectomy/decortication,19 while others, including thoracic surgeons and oncologists at our center, advocate for EPP as part of a multimodality therapy regimen.20 The use of an accelerated radiotherapy regime of 25 Gy in five daily fractions over a week with EPP, the following week has been associated with an exceptional median survival of 36 months in select patients.21 From that study, de Perrot et al have suggested that EPP after induction hemithoracic radiation therapy should be reserved for patients with epithelial mesothelioma with clinical stage T1-3 N0 M0.21 In these selected patients, multimodality therapy appears to be associated with a significant survival benefit. Patients with biphasic histology or metastatic mediastinal nodal disease are currently excluded from this approach, given significant associated morbidities and lack of evidence of survival benefit. Therefore, accurate patient selection for multimodality therapy is crucial.
In the current study, PET N-category correlated with surgical pathology in nearly two-thirds of the patients, and performed significantly better than CT. However, PET detected nodal metastases in less than half of the patients with ipsilateral hilar of mediastinal nodal metastases at histopathology. This may be due to difficulty in discriminating nodal metastases from pleural tumor, especially in bulky tumors invading the mediastinum, or due to the limited resolution of PET for small volume metastatic disease. Overall, PET detected ipsilateral hilar or mediastinal nodal metastases missed on CT in 7 of the 61 surgical patients (11.5%). For the detection of distant metastases, PET identified distant metastases missed on CT in nearly 8% of the patient population. These results suggest that PET may more reliably triage patients for surgical or multimodality therapeutic approach and exclude a significant number of patients, who would be presumed eligible for surgery if staged by CT alone. Our results are concordant with previously published studies.22, 23
This study has several limitations. First, it is retrospective and based on data from a single institution. However, to the best of our knowledge, this is the largest report in the literature on the role of PET in management of patients with MPM being considered for surgical or multimodal management. Second, imaging reports were used to create the database and imaging studies were not interpreted by two independent readers. However, all imaging was interpreted by fellowship-trained subspecialized radiologists. We believe the results of this study reflect the impact of PET and CT on the staging of MPM in clinical practice. Third, surgical nodal staging is available for only 60% of the cohort (as others did not undergo surgery). Although the sensitivity of PET in detecting nodal metastases appears limited, the size of this cohort was sufficient to show a significant advantage of PET over CT. Fourth, survival data for the entire cohort are incomplete, especially for patients who did not undergo surgery, and comparison of survival between the group of patients treated surgically and those who had non-surgical management cannot be performed. However, we were able to show that for the surgical group the documented survival is better than the reported median survival in MPM, and previous data from our group have shown that the median survival in select patients treated with Surgery for Mesothelioma after Radiation Therapy approach may reach 36 months. Lastly, we could not document the downstream effect of PET on patient outcome given the retrospective single arm design of this study; however, it is likely that better patient selection with exclusion of patients with occult distant metastases or N1/N2 disease would improve the overall survival of patients who do undergo surgery or multimodality therapy.
In conclusion, FDG PET/CT is able to identify significantly more patients with nodal or distant metastatic disease than CT and may contribute to more appropriate selection of patients with MPM for surgery or multimodality therapy.
Contributor Information
Ur Metser, Email: ur.metser@uhn.ca.
Marc de Perrot, Email: Marc.DePerrot@uhn.ca.
John Cho, Email: John.Cho@rmp.uhn.ca.
REFERENCES
- 1.Otsuka H, Terazawa K, Morita N, Otomi Y, Yamashita K, Nishitani H. Is FDG-PET/CT useful for managing malignant pleural mesothelioma? J Med Invest 2009; 56: 16–20. doi: 10.2152/jmi.56.16 [DOI] [PubMed] [Google Scholar]
- 2.Rusch V, Baldini EH, Bueno R, De Perrot M, Flores R, Hasegawa S, et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: meeting summary of the International Mesothelioma Interest Group Congress, September 11-14, 2012, Boston, Mass. J Thorac Cardiovasc Surg 2013; 145: 909–10. doi: 10.1016/j.jtcvs.2013.01.039 [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker DJ, Flores RM, Jaklitsch MT, Richards WG, Strauss GM, Corson JM, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999; 117: 54–65. doi: 10.1016/S0022-5223(99)70469-1 [DOI] [PubMed] [Google Scholar]
- 4.Tsao AS, Wistuba I, Roth JA, Kindler HL. Malignant pleural mesothelioma. J Clin Oncol 2009; 27: 2081–90. doi: 10.1200/JCO.2008.19.8523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almuhaideb A, Papathanasiou N, Bomanji J. 18F-FDG PET/CT imaging in oncology. Ann Saudi Med 2011; 31: 3–13. doi: 10.4103/0256-4947.75771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heelan RT, Rusch VW, Begg CB, Panicek DM, Caravelli JF, Eisen C, et al. Staging of malignant pleural mesothelioma: comparison of CT and MR imaging. AJR Am J Roentgenol 1999; 172: 1039–47. doi: 10.2214/ajr.172.4.10587144 [DOI] [PubMed] [Google Scholar]
- 7.Rice DC, Erasmus JJ, Stevens CW, Vaporciyan AA, Wu JS, Tsao AS, et al. Extended surgical staging for potentially resectable malignant pleural mesothelioma. Ann Thorac Surg 2005; 80: 1988–93. doi: 10.1016/j.athoracsur.2005.06.014 [DOI] [PubMed] [Google Scholar]
- 8.Seo MJ, Lee JJ, Kim HO, Chae SY, Park SH, Ryu JS, et al. Detection of internal mammary lymph node metastasis with 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with stage III breast cancer. Eur J Nucl Med Mol Imaging 2014; 41: 438–45. doi: 10.1007/s00259-013-2600-y [DOI] [PubMed] [Google Scholar]
- 9.Pilling J, Dartnell J-A, Lang-Lazdunski L. Integrated positron emission tomography-computed tomography does not accurately stage intrathoracic disease of patients undergoing trimodality therapy for malignant pleural mesothelioma. Thorac Cardiovasc Surg 2010; 58: 215–9. doi: 10.1055/s-0029-1241029 [DOI] [PubMed] [Google Scholar]
- 10.Erasmus JJ, Truong MT, Smythe WR, Munden RF, Marom EM, Rice DC, et al. Integrated computed tomography-positron emission tomography in patients with potentially resectable malignant pleural mesothelioma: staging implications. J Thorac Cardiovasc Surg 2005; 129: 1364–70. doi: 10.1016/j.jtcvs.2004.10.034 [DOI] [PubMed] [Google Scholar]
- 11.Frauenfelder T, Kestenholz P, Hunziker R, Nguyen TD, Fries M, Veit-Haibach P, et al. Use of computed tomography and positron emission tomography/computed tomography for staging of local extent in patients with malignant pleural mesothelioma. J Comput Assist Tomogr 2015; 39: 160–5. doi: 10.1097/RCT.0000000000000174 [DOI] [PubMed] [Google Scholar]
- 12.Ambrosini V, Rubello D, Nanni C, Farsad M, Castellucci P, Franchi R, et al. Additional value of hybrid PET/CT fusion imaging vs. conventional CT scan alone in the staging and management of patients with malignant pleural mesothelioma. Nucl Med Rev Cent East Eur 2005; 8: 111–5. [PubMed] [Google Scholar]
- 13.Valerie R. ED05.02 update on the mesothelioma staging system. J Thorac Oncol 2017; 12: S30–S31. [Google Scholar]
- 14.Rice DC, Stevens CW, Correa AM, Vaporciyan AA, Tsao A, Forster KM, et al. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg 2007; 84: 1685–93. doi: 10.1016/j.athoracsur.2007.04.076 [DOI] [PubMed] [Google Scholar]
- 15.Sugarbaker DJ, Jaklitsch MT, Bueno R, Richards W, Lukanich J, Mentzer SJ, et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg 2004; 128: 138–46. doi: 10.1016/j.jtcvs.2004.02.021 [DOI] [PubMed] [Google Scholar]
- 16.Martino D, Pass HI. Integration of multimodality approaches in the management of malignant pleural mesothelioma. Clin Lung Cancer 2004; 5: 290–8. doi: 10.3816/CLC.2004.n.008 [DOI] [PubMed] [Google Scholar]
- 17.Jaklitsch MT, Grondin SC, Sugarbaker DJ. Treatment of malignant mesothelioma. World J Surg 2001; 25: 210–7. doi: 10.1007/s002680020021 [DOI] [PubMed] [Google Scholar]
- 18.Rusch VW, Venkatraman E. The importance of surgical staging in the treatment of malignant pleural mesothelioma. J Thorac Cardiovasc Surg 1996; 111: 815–26. doi: 10.1016/S0022-5223(96)70342-2 [DOI] [PubMed] [Google Scholar]
- 19.Wolf AS, Flores RM. Current treatment of mesothelioma: extrapleural pneumonectomy versus pleurectomy/decortication. Thorac Surg Clin 2016; 26: 359–75. doi: 10.1016/j.thorsurg.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 20.Cho BC, Feld R, Leighl N, Opitz I, Anraku M, Tsao MS, et al. A feasibility study evaluating surgery for mesothelioma after radiation therapy: the “SMART” approach for resectable malignant pleural mesothelioma. J Thorac Oncol 2014; 9: 397–402. doi: 10.1097/JTO.0000000000000078 [DOI] [PubMed] [Google Scholar]
- 21.de Perrot M, Feld R, Leighl NB, Hope A, Waddell TK, Keshavjee S, et al. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2016; 151: 468–75. doi: 10.1016/j.jtcvs.2015.09.129 [DOI] [PubMed] [Google Scholar]
- 22.Plathow C, Staab A, Schmaehl A, Aschoff P, Zuna I, Pfannenberg C, et al. Computed tomography, positron emission tomography, positron emission tomography/computed tomography, and magnetic resonance imaging for staging of limited pleural mesothelioma: initial results. Invest Radiol 2008; 43: 737–44. doi: 10.1097/RLI.0b013e3181817b3d [DOI] [PubMed] [Google Scholar]
- 23.Flores RM. The role of PET in the surgical management of malignant pleural mesothelioma. Lung Cancer 2005; 49(Suppl 1): S27–S32. doi: 10.1016/j.lungcan.2005.03.007 [DOI] [PubMed] [Google Scholar]