Abstract
Objective:
To correlate clinicoradiologic and pathological features of breast cancer with quantitative and qualitative shear wave elastographic parameters.
Methods:
82 breast cancers in 75 patients examined by B-mode ultrasound and shear wave elastography (SWE) were included. SWE parameters including quantitative factors [maximum elasticity (Emax), mean elasticity (Emean), elasticity ratio (Eratio) and standard deviation (SD)] and qualitative factor (color pattern) were correlated with clinicoradiologic and pathological features using univariate and multivariate linear regression analyses.
Results:
Presence of symptoms and larger tumor size on ultrasound were significantly associated with higher Emax, Emean, Eratio, and SD (all p < 0.05) on univariate analysis. Older age was significantly correlated with higher Emax and Emean (p = 0.026, 0.018). Lymphovascular invasion and larger pathologic size were significantly associated with higher Emax (p = 0.036, 0.043) and SD (p < 0.001, 0.019). No immunohistochemical biomarkers were significantly correlated with SWE parameters. There was no significant correlation between color pattern and any variable. Multivariate logistic regression analysis showed that the symptom, tumor size on ultrasound and lymphovascular invasion were independent factors that influenced the SWE values.
Conclusion:
Tumor stiffness as measured by SWE and B-mode ultrasound could help predict cancer prognosis.
Advances in knowledge:
Clinicoradiologic factors had correlation with quantitative and qualitative SWE parameters. Using SWE parameters and B-mode ultrasound, we can predict breast cancer prognosis.
INTRODUCTION
Breast cancer is considered as a heterogeneous disease entity with various histological types, clinical courses, and prognoses. Pre-operative prediction of prognosis is important not only to know the natural course of the disease, but also to decide the proper treatment. Prognostic factors of breast cancer are related to tumor size, histological type, histological grade, lymphovascular invasion (LVI), and lymph node status.1, 2 More recently, gene expression analyses using DNA microarrays have classified different molecular subtypes that are significantly associated with different disease prognoses.3 Immunohistochemical study is based on the following cancer biomarkers: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Instead of molecular classification, immunohistochemical profiles of breast cancer for evaluating the expression of hormonal receptors (ER, PR), HER2, and Ki-67 have been used.4 Several studies report that breast cancer subtypes classified by immunohistochemical expression of ER, PR, HER2, and Ki-67 show different clinical, radiologic and pathological features.5, 6
Breast ultrasound elastography is a method used to measure the stiffness of breast lesions. Many studies have shown that US elastography improves differentiation between benign and malignant lesions in breast tissue.7–9 There are two main methods for assessment of stiffness: strain elastography and shear wave elastography (SWE).10 Strain elastography assesses stiffness from the degree of strain caused by manual compression. The efficacy of strain elastography to assess stiffness is limited by the operator’s ability to provide adequate repetitive compression, and SWE was introduced to overcome this limitation.9 The SWE system uses acoustic radiation to induce mechanical vibrations and quantifies the stiffness of a lesion by capturing propagating shear waves.11 This technique results in quantitative measurement of tissue elasticity in kilopascals (kPa) or meters per second (m s–1).9, 11 Moreover, the color overlay image obtained using B-mode imaging can provide information about stiffness of the breast lesion from the color pattern or from quantitative parameters.8,10,12–15
Relationships between prognostic factors including immunohistochemical features of breast cancer and their SWE values have been the subject of ongoing research. Evans et al16 demonstrated a significant correlation between cancer size and mean elasticity (Emean) value. Youk et al17 reported that palpable abnormality, histologic grade, and LVI were significantly associated with Emean, but immunohistochemical factors were not. Ganau et al18 concluded that there were no significant differences among the subtypes of invasive tumors in maximal elasticity (Emax) and Emean. Several groups have reported that breast cancers with poorer prognosis based on histologic prognostic features, immunohistochemical profiles, and subtypes have a higher Emean19, 20 and Eratio.21 However, there have been different conclusions about the relationship between prognostic clinicopathologic factors of breast cancer and SWE parameters [Emax, Emean, elasticity ratio (Eratio), and standard deviation (SD)]. Moreover, to our knowledge, few published studies have compared all SWE parameters, including both quantitative and qualitative factors, with the prognostic features of breast cancer.16, 17,20
Therefore, the purpose of this study was to correlate quantitative and qualitative SWE parameters with clinical, radiologic and pathologic factors, including immunohistochemical biomarker profiles, in breast cancer.
METHODS AND MATERIALS
Patients
The Institutional Review Board of our hospital approved this retrospective study, and neither patient approval nor informed consent was required for the review of medical records or radiological images. Signed informed consent was obtained from all patients before ultrasound-guided biopsy procedures or surgery. From August 2013 to May 2015, 129 patients were diagnosed with breast cancer. Among them, patients who had no SWE images (n = 31), patients who underwent neoadjuvant chemotherapy before surgery (n = 16) or had lack of data on immunohistochemical factors such as ER, PR, HER2, and Ki-67 (n = 7) were excluded from the study. Ultimately, 82 breast cancers in 75 females (age range: 32–80 years, mean age: 57.4 years) were included in the final study.
Ultrasound examinations
Conventional ultrasound and SWE images were obtained using the Aixplorer system (Supersonic Imagine, Aix-en Provence, France) equipped with a 4–15 MHz linear array transducer by one radiologist (Y-MS) with 10 years of experience and other radiologist (MS) with 6 years of experience in breast imaging. Final assessments based on B-mode ultrasound findings were made and recorded by the American College of Radiology Breast Imaging Reporting and Data System.22 After B-mode ultrasound, SWE imaging before biopsy was performed by the same radiologist with no pressure induced by the transducer. The region of interest (3 × 3 mm) was set to include the lesion and surrounding normal breast parenchyma. The B-mode semitransparent color map revealed stiffness with a range from dark blue to red (0–180 kPa). After a few seconds of immobilization to let the image stabilize, the SWE image was obtained.
Image analysis
The following data from B-mode images were retrospectively measured and recorded by one radiologist (EJS) with 2 years of experience in breast imaging: lesion size (maximum diameter on ultrasound images), breast thickness (maximum vertical distance from the skin to the pectoralis muscle on the ultrasound image including the breast mass targeted for biopsy), and lesion depth (vertical diameter from the skin to the center of the breast mass).
For quantitative SWE analysis, we recorded the following parameters: maximum elasticity (Emax), mean elasticity (Emean), elasticity ratio (Eratio), and SD. Emax and Emean represent the general stiffness of the lesion. Eratio is the ratio between elasticity values of the tumor and the reference tissue. SD refers to the internal heterogeneity of the lesion. For qualitative analysis from SWE, we (EJS and Y-MS) independently recorded color patterns according to the classifications proposed by Tozaki and Fukuma using a four-color overlay.12 Disagreements were resolved by consensus. Images were classified as follows: “Pattern 1,” the color around the lesion was not different from the margin of the lesion or its interior, showing a homogeneously blue pattern; “Pattern 2,” the color extending beyond the lesion was different from the color around the lesion, showing continuous vertical stripes on the cutaneous or thoracic wall side; “Pattern 3,” the localized colored area was at the margin of the lesion; and “Pattern 4,” the colored areas were heterogeneously present in the interior of the lesion.12 Of the qualitative SWE pattern classifications, patterns 1 and 2 were considered benign, while patterns 3 and 4 were considered malignant.12
Clinical and pathologic analysis
Each patient’s medical records were reviewed and data on age and presence of clinical symptoms such as palpability and breast pain were collected. Pathologic reports of surgical specimens were also reviewed to determine tumor type, tumor size, histological grade, lymphovascular invasion (LVI), and lymph node status. Tumor size was defined as the largest diameter in the formalin-fixed pathologic specimen. Histological grade was determined by the Elston modification of the Scarff–Bloom–Richardson criteria.23, 24 Immunohistochemical staining was performed to determine ER (Novocastra, Newcastle upon Tyne, UK), PR (Novocastra), HER2 (Ventana Medical Systems, Tucson, AZ), and Ki-67 (MIB-1; Dako, Glostrup, Denmark) status. ER and PR status were assessed by nuclear staining, which was graded from 0 to 8 using the Allred score.25 The results were categorized as positive when the total score, expressed as the sum of the proportion score and immunointensity score, was 3 or more. For HER2 evaluation, membranous staining was graded as follows: score 0, 1, 2, and 3 +.26 HER2 status was regarded as positive with a score of 3 + and negative with a score of 0 or 1 +. Tumors with a score of 2 + were evaluated using the Zytolight Spec Her2/Cen17 dual color probe kit (ZytoVision, Bremerhaven, Germany) for fluorescence in situ hybridization testing, which determines HER2 amplification in the event that the ratio of the HER2 gene signal to chromosome 17 signal is more than 2, which is considered positive. Ki-67 ≥ 14% was considered positive.
Statistical analyses
Quantitative SWE parameter values (Emax, Emean, Eratio, and SD) were correlated with clinical, radiologic and pathologic factors using simple linear regression models. For qualitative analysis of color patterns, we used logistic regression. Multiple linear regression analysis with statistically significant variables from the univariate analysis was used to determine variables independently correlated with SWE parameters. All statistical analyses were performed with statistical software SAS (v. 9.2, SAS Institute Inc., Cary, NC). Differences were considered statistically significant at p-value < 0.05.
RESULTS
Demographic and clinical characteristics
The mean age of the 75 females was 57.4 years (range, 32–80 years). The mean pathologic size of tumors was 20.88 ± 17.10 mm and radiologic cancer size was 16.2 ± 7.89 mm. The histologic cancer types were as follows: invasive ductal carcinoma (IDC, n = 59), ductal carcinoma in situ (DCIS, n = 10), invasive lobular carcinoma (ILC, n = 5), invasive mucinous carcinoma (IMC, n = 1), mixed invasive ductal and lobular carcinoma (n = 1), invasive apocrine carcinoma (n = 1), histiocytoid variant metaplastic carcinoma (n = 1), and papillary carcinoma (n = 1).
The quantitative SWE parameters of the 82 cancers were as follows: Emax 169 ± 70.3 kPa, Emean 131.17 ± 52.76 kPa, Eratio 8.00 ± 8.16, and SD 23.60 ± 19.45 kPa. For qualitative analysis, color patterns 1 and 2 were present in 3 breast cancers and color patterns 3 and 4 were present in 79 masses (Figures 1 and 2).
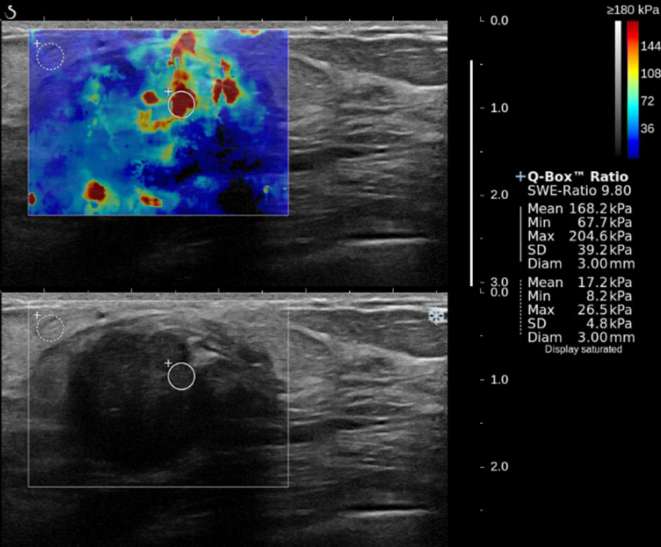
Figure 1.
A 75-year-old female with a 26.5 mm, Grade 3, intraductal carcinoma in the left breast. SWE color map (top) shows that the colored area was heterogeneously present in the interior of the mass (Pattern 4), and the lesion had high stiffness (Emax, 204.6 kPa; Emean, 168.2 kPa; Eratio, 9.8; SD, 39.2 kPa). On pathology, the cancer showed lymphovascular invasion, positive ER and PR, negative HER2, and positive Ki-67. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor; SD, standard deviation; SWE, shear wave elastography.
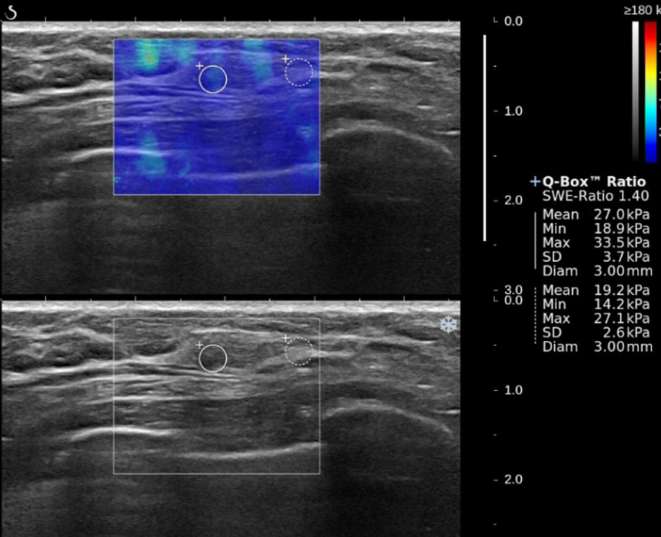
Figure 2.
A 51-year-old female with a 4 mm, Grade 1, ductal carcinoma in situ in the left breast. B-mode ultrasound (bottom) shows that mass has irregular shape and non-circumscribed margin. SWE color map (top) shows that blue color around the lesion continues vertically on the cutaneous side (Pattern 2) and low SWE values (Emax, 33.5 kPa; Emean, 27.0 kPa; Eratio, 1.4; SD, 3.7 kPa). On pathology, the cancer showed no lymphovascular invasion, negative ER, negative PR, negative HER2, and negative Ki-67.
Correlation of clinicoradiologic factors with quantitative SWE parameters on univariate analysis
Tumor size measured on ultrasound was significantly correlated with higher Emax (p < 0.001), Emean (p < 0.001), Eratio (p = 0.001), and SD (p < 0.001). The presence of symptoms such as breast pain and palpability was also significantly associated with Emax (p = 0.003), Emean (p = 0.018), Eratio (p = 0.035), and SD (p = 0.050). Older age showed a significant association with higher Emax (p = 0.026) and Emean (p = 0.018). There was no significant correlation with lesion depth or breast thickness and SWE parameter values (Table 1).
Table 1.
Correlation of clinicoradiologic factors with quantitative SWE parameters on univariate analysis
| Emax | Emean | Eratio | SD | ||||||||||
| Variable | n | Mean ± SD | β coefficient | p | Mean ± SD | β coefficient | p | Mean ± SD | β coefficient | p | Mean ± SD | β coefficient | p |
| Age | 82 | 169.16 ± 70.30 | 1.49 ± 0.66 | 0.026 | 131.17 ± 52.76 | 1.19 ± 0.49 | 0.018 | 8.00 ± 8.16 | 0.09 ± 0.08 | 0.237 | 23.60 ± 19.45 | 0.24 ± 0.19 | 0.200 |
| Symptom | |||||||||||||
| Absent | 40 | 135.20 ± 59.32 | 0 | 108.89 ± 47.62 | 0 | 6.11 ± 4.10 | 0 | 16.66 ± 12.55 | 0 | ||||
| Present | 41 | 199.59 ± 64.56 | 64.39 ± 13.79 | <0.001 | 151.48 ± 49.20 | 42.59 ± 10.76 | <0.001 | 9.93 ± 10.53 | 3.82 ± 1.78 | 0.035 | 30.24 ± 22.76 | 13.58 ± 4.10 | 0.001 |
| Ultrasound size | 82 | 169.16 ± 70.30 | 4.18 ± 0.88 | <0.001 | 131.17 ± 52.76 | 2.79 ± 0.68 | <0.001 | 8.00 ± 8.16 | 0.36 ± 0.11 | 0.001 | 23.60 ± 19.45 | 1.06 ± 0.25 | <0.001 |
| Ultrasound size | |||||||||||||
| <10 mm | 15 | 129.85 ± 60.22 | 0 | 103.82 ± 47.59 | 0 | 5.76 ± 4.04 | 0 | 13.85 ± 6.51 | 0 | ||||
| 10–20 mm | 45 | 152.02 ± 61.67 | 22.17 ± 17.74 | 0.215 | 119.18 ± 47.24 | 15.36 ± 13.70 | 0.266 | 5.79 ± 3.49 | 0.03 ± 2.20 | 0.989 | 20.90 ± 16.36 | 7.05 ± 5.37 | 0.193 |
| >20 mm | 22 | 231.04 ± 54.16 | 101.19 ± 19.92 | <0.001 | 174.34 ± 41.91 | 70.52 ± 15.39 | <0.001 | 14.05 ± 12.96 | 8.30 ± 2.47 | 0.001 | 35.77 ± 25.12 | 21.91 ± 6.03 | <0.001 |
| Thickness | |||||||||||||
| 10–20 mm | 45 | 166.46 ± 66.07 | 0 | 129.35 ± 46.81 | 0 | 6.62 ± 5.91 | 0 | 22.88 ± 19.13 | 0 | ||||
| >20 mm | 37 | 172.44 ± 75.93 | 5.98 ± 15.68 | 0.704 | 133.39 ± 59.80 | 4.04 ± 11.77 | 0.733 | 9.67 ± 10.09 | 3.05 ± 1.79 | 0.092 | 24.48 ± 20.05 | 1.60 ± 4.34 | 0.714 |
| Lesion depth | |||||||||||||
| <10 mm | 21 | 155.89 ± 69.91 | 0 | 123.54 ± 51.98 | 0 | 8.91 ± 8.00 | 0 | 20.41 ± 16.74 | 0 | ||||
| ≥10 mm | 61 | 173.73 ± 70.43 | 17.85 ± 17.79 | 0.319 | 133.80 ± 53.20 | 10.26 ± 13.38 | 0.446 | 7.68 ± 8.26 | −1.23 ± 2.07 | 0.555 | 24.70 ± 20.31 | 4.28 ± 4.93 | 0.387 |
Emax, max elasticity; Emean, mean elasticity; Eratio, elasticity ratio; SD, standard deviation; SWE, shear wave elastography.
p-values < 0.05 were considered statistically significant.
Correlation of pathologic factors with quantitative SWE parameters on univariate analysis
Table 2 provides a detailed summary of the results. Larger surgical specimen size had a significantly higher Emax (p = 0.043) and SD (p = 0.019). Lymphovasular invasion (LVI) was also associated with Emax (p = 0.036) and SD (p < 0.001). There were no significant differences related to lymph nodal status or histologic grade. Although no statistically significant differences were found, histological Grade 3 had higher values of quantitative SWE parameters than the rest. There was no significant difference in SWE parameters between different pathologic types. However, DCIS tended to have lower quantitative values (Emax 119.37 kPa, Emean 85.45 kPa, Eratio 3.78, and SD 19.73 kPa) than IDC or ILC. In terms of immunohistochemical biomarkers, there was no significant correlation with SWE parameters and hormonal receptors such as ER and PR. Although there was no significant difference in SWE parameters between Ki-67 positive and negative cancers, Ki-67 positive cancers tended to have higher values for all SWE parameters compared to Ki-67 negative cancers.
Table 2.
Correlation of pathologic factors with quantitative shear wave elastographic parameters on univariate analysis
| Emax | Emean | Eratio | SD | ||||||||||
| Variable | n | Mean ± SD | β coefficient | p | Mean ± SD | β coefficient | p | Mean ± SD | β coefficient | p | Mean ± SD | β coefficient | p |
| Pathologic size | 82 | 169.16 ± 70.30 | 0.92 ± 0.45 | 0.043 | 131.17 ± 52.76 | 0.44 ± 0.34 | 0.206 | 8.00 ± 8.16 | 0.03 ± 0.05 | 0.578 | 23.60 ± 19.45 | 0.29 ± 0.12 | 0.019 |
| Pathologic size | |||||||||||||
| <10 mm | 17 | 137.56 ± 74.87 | 0 | 108.69 ± 57.83 | 0 | 6.53 ± 8.53 | 0 | 18.13 ± 17.26 | 0 | ||||
| 10–20 mm | 31 | 155.66 ± 54.81 | 18.10 ± 20.11 | 0.371 | 124.62 ± 45.32 | 15.93 ± 15.39 | 0.304 | 6.84 ± 5.04 | 0.31 ± 2.45 | 0.899 | 18.71 ± 9.15 | 0.58 ± 5.64 | 0.918 |
| >20 mm | 34 | 197.27 ± 72.00 | 59.70 ± 19.80 | 0.003 | 148.38 ± 52.33 | 39.69 ± 15.15 | 0.011 | 9.79 ± 9.97 | 3.27 ± 2.41 | 0.179 | 30.80 ± 24.83 | 12.67 ± 5.55 | 0.025 |
| Histologic grade | |||||||||||||
| 1 | 17 | 170.06 ± 64.85 | 0 | 133.30 ± 45.99 | 0 | 6.91 ± 4.79 | 0 | 20.55 ± 11.04 | 0 | ||||
| 2 | 41 | 160.55 ± 72.59 | −5.84 ± 19.49 | 0.765 | 124.90 ± 54.59 | −5.77 ± 14.67 | 0.695 | 6.24 ± 4.26 | −0.46 ± 2.17 | 0.832 | 23.88 ± 22.80 | 3.89 ± 5.43 | 0.476 |
| 3 | 22 | 187.60 ± 71.84 | 21.21 ± 22.00 | 0.338 | 143.30 ± 56.15 | 12.63 ± 16.55 | 0.448 | 12.40 ± 13.29 | 5.70 ± 2.44 | 0.022 | 26.20 ± 18.78 | 6.22 ± 6.13 | 0.314 |
| Lymph node status | |||||||||||||
| Negative | 66 | 170.51 ± 67.30 | 0 | 134.14 ± 51.00 | 0 | 7.36 ± 5.98 | 0 | 21.54 ± 14.69 | 0 | ||||
| Positive | 16 | 163.59 ± 83.80 | −6.93 ± 19.70 | 0.726 | 118.93 ± 59.68 | −15.21 ± 14.70 | 0.304 | 10.62 ± 13.97 | 3.26 ± 2.26 | 0.153 | 32.11 ± 31.80 | 10.57 ± 5.32 | 0.05 |
| LVI | |||||||||||||
| Negative | 75 | 164.21 ± 70.53 | 0 | 129.12 ± 54.55 | 0 | 7.54 ± 6.11 | 0 | 21.21 ± 15.84 | 0 | ||||
| Positive | 7 | 222.20 ± 42.31 | 57.99 ± 27.20 | 0.036 | 153.10 ± 16.31 | 23.98 ± 20.81 | 0.253 | 12.96 ± 20.19 | 5.43 ± 3.19 | 0.092 | 49.20 ± 34.28 | 27.99 ± 7.07 | <0.001 |
| Pathologic type | |||||||||||||
| IDC | 59 | 180.83 ± 65.61 | 12.75 ± 27.22 | 0.641 | 140.50 ± 50.02 | 0.69 ± 19.91 | 0.973 | 9.34 ± 9.30 | 3.13 ± 3.21 | 0.333 | 24.95 ± 18.98 | 5.88 ± 7.88 | 0.458 |
| ILC | 5 | 167.18 ± 53.52 | −0.89 ± 39.80 | 0.982 | 131.56 ± 44.08 | −8.25 ± 29.11 | 0.778 | 6.19 ± 2.64 | −0.02 ± 4.70 | 0.996 | 24.64 ± 12.41 | 5.57 ± 11.52 | 0.63 |
| DCIS | 13 | 119.37 ± 90.85 | −48.70 ± 31.86 | 0.131 | 85.45 ± 56.54 | −54.36 ± 23.31 | 0.022 | 3.78 ± 2.49 | −2.43 ± 3.76 | 0.521 | 19.73 ± 27.50 | 0.66 ± 9.22 | 0.943 |
| Etc | 5 | 168.07 ± 38.18 | 0 | 139.81 ± 33.02 | 0 | 6.21 ± 3.65 | 0 | 19.07 ± 7.18 | 0 | ||||
| ER | |||||||||||||
| Negative | 22 | 160.57 ± 74.42 | 0 | 120.20 ± 54.32 | 0 | 5.87 ± 3.58 | 0 | 21.73 ± 18.78 | 0 | ||||
| Positive | 60 | 172.31 ± 69.11 | 11.75 ± 17.58 | 0.506 | 135.19 ± 52.06 | 14.99 ± 13.13 | 0.257 | 8.78 ± 9.20 | 2.91 ± 2.02 | 0.154 | 24.29 ± 19.80 | 2.55 ± 4.87 | 0.601 |
| PR | |||||||||||||
| Negative | 25 | 159.42 ± 65.29 | 0 | 126.89 ± 55.54 | 0 | 6.36 ± 3.68 | 0 | 18.58 ± 10.96 | 0 | ||||
| Positive | 57 | 173.43 ± 72.53 | 14.01 ± 16.90 | 0.41 | 133.05 ± 51.89 | 6.16 ± 12.72 | 0.629 | 8.72 ± 9.42 | 2.36 ± 1.95 | 0.23 | 25.81 ± 21.89 | 7.23 ± 4.62 | 0.122 |
| HER2 | |||||||||||||
| Negative | 61 | 169.96 ± 65.05 | 0 | 132.09 ± 48.09 | 0 | 7.40 ± 7.93 | 0 | 23.09 ± 17.21 | 0 | ||||
| Positive | 21 | 166.86 ± 85.53 | −3.10 ± 17.89 | 0.863 | 128.49 ± 65.78 | −3.60 ± 13.43 | 0.789 | 9.74 ± 8.77 | 2.33 ± 2.06 | 0.261 | 25.08 ± 25.30 | 1.98 ± 4.95 | 0.690 |
| Ki67 | |||||||||||||
| <14% | 38 | 166.59 ± 78.51 | 0 | 127.91 ± 57.01 | 0 | 6.74 ± 4.88 | 0 | 22.91 ± 20.54 | 0 | ||||
| ≥14% | 32 | 176.00 ± 63.84 | 9.41 ± 17.32 | 0.589 | 140.19 ± 53.13 | 12.29 ± 13.26 | 0.357 | 10.68 ± 11.43 | 3.94 ± 2.04 | 0.058 | 24.37 ± 18.20 | 1.46 ± 4.68 | 0.756 |
DCIS, ductal carcinoma in situ; Emax, max elasticity; Emean, mean elasticity; Eratio, elasticity ratio; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IDC, invasiveductal carcinoma; ILC, invasive lobular carcinoma; LVI, lymphovascular invasion; PR, progesterone receptor; SD, standard deviation.
p-values < 0.05 were considered statistically significant.
Correlation of clinicoradiologic and pathologic factors and color pattern on univariate analysis
There was no significant correlation between color pattern and any variable (Tables 3 and 4). Although there was no significant correlation between pathologic cancer size and color pattern, larger tumors (greater than 20 mm) tended to have a positive color pattern [odd ratio = 11.13, 95% confidence interval (0.478–259.383)] compared to lesions of other sizes. Moreover, IDC with higher histologic grade tended to have a positive color pattern.
Table 3.
Correlation of clinicoradiologic factors with color pattern on univariate analysis
| Variable | Color pattern | OR (95% CI) | p-value | |
| Negative | Positive | |||
| Age | 3 (100.0) | 79 | 1.041 (0.932, 1.163) | 0.475 |
| Symptom | ||||
| Absent | 2 (66.7) | 38 (48.7) | 0 | |
| Present | 1 (33.3) | 40 (51.3) | 1.753 (0.215, 14.276) | 0.600 |
| Ultrasound size | ||||
| <10 mm | 1 (33.3) | 14 (17.7) | 0 | |
| 10–20 mm | 2 (66.7) | 43 (54.4) | 1.800 (0.207, 15.643) | 0.594 |
| >20 mm | 0 (0.0) | 22 (27.9) | 4.655 (0.163, 132.726) | 0.368 |
| Thickness | ||||
| 10–20 mm | 2 (66.7) | 43 (54.4) | 0 | |
| >20 mm | 1 (33.3) | 36 (45.6) | 1.398 (0.172, 11.391) | 0.754 |
| Lesion depth | ||||
| <10 mm | 2 (66.7) | 19 (24.1) | 0 | |
| ≥10 mm | 1 (33.3) | 60 (75.9) | 5.171 (0.622, 42.972) | 0.128 |
CI, confidence interval; OR, odd ratio.
p-values < 0.05 were considered statistically significant.
Table 4.
Correlation of pathologic factors with color pattern on univariate analysis
| Variable | Color pattern | OR (95% CI) | p-value | |
| Negative | Positive | |||
| Pathologic size | 3 (100.0) | 79 (100.0) | 1.262 (0.981, 1.624) | 0.070 |
| Pathologic size | ||||
| <10 mm | 2 (66.7) | 15 (19.0) | 0 | |
| 10–20 mm | 1 (33.3) | 30 (38.0) | 3.280 (0.379, 28.423) | 0.281 |
| >20 mm | 0 (0.0) | 34 (43.0) | 11.131 (0.478, 259.383) | 0.134 |
| Histologic grade | ||||
| 1 | 0 (0.0) | 17 (22.1) | 0 | |
| 2 | 3 (100.0) | 38 (49.3) | 0.314 (0.014, 6.958) | 0.464 |
| 3 | 0 (0.0) | 22 (28.6) | 1.286 (0.022, 75.402) | 0.904 |
| Lymph node status | ||||
| Negative | 3 (100.0) | 63 (79.8) | 0 | |
| Positive | 0 (0.0) | 16 (20.2) | 1.819 (0.082, 40.209) | 0.705 |
| LVI | ||||
| Negative | 3 (100.0) | 72 (91.1) | 0 | |
| Positive | 0 (0.0) | 7 (8.9) | 0.724 (0.028, 18.595) | 0.846 |
| Pathologic type | ||||
| IDC | 0 (0.0) | 59 (71.9) | 7.667 (0.120, 488.030) | 0.337 |
| ILC | 0 (0.0) | 5 (6.3) | 0.733 (0.009, 60.153) | 0.890 |
| DCIS | 3 (100.0) | 10 (12.7) | 0.200 (0.007, 5.465) | 0.340 |
| Etc | 0 (0.0) | 5 (6.1) | 0 | |
| ER | ||||
| Negative | 1 (33.3) | 21 (26.6) | 0 | |
| Positive | 2 (66.7) | 58 (73.4) | 1.633 (0.196, 13.597) | 0.650 |
| PR | ||||
| Negative | 1 (33.3) | 24 (30.4) | 0 | |
| Positive | 2 (66.7) | 55 (69.6) | 1.359 (0.164, 11.233) | 0.776 |
| HER2 | ||||
| Negative | 2 (66.7) | 59 (74.7) | 0 | |
| Positive | 1 (33.3) | 20 (25.3) | 0.574 (0.069, 4.798) | 0.609 |
| Ki67 | ||||
| <14% | 2 (100.0) | 36 (52.9) | 0 | |
| ≥14% | 0 (0.0) | 32 (47.1) | 4.452 (0.197, 100.748) | 0.348 |
CI, confidence interval; DCIS, ductal carcinoma in situ; ER,estrogen receptor; HER2, humanepidermal growth factor receptor 2; IDC, invasive ductal carcinoma; ILC, invasivelobular carcinoma; LVI, lymphovascularinvasion; OR, odd ratio; PR, progesterone receptor.
p-values < 0.05 were considered statistically significant.
Multivariate analysis
For the variables significantly associated with SWE parameters on univariate analysis, multiple linear regression analysis demonstrated that the presence of symptoms was an independent factor for Emax (p = 0.003) and Emean (p = 0.018). The β regression coefficient for the presence of symptoms was higher at Emax and Emean (46.05, 28.91, respectively) than that without symptoms (Table 5). Mass size on US independently influenced Emean (β coefficient = 1.85, p = 0.035) and Eratio (β coefficient = 0.30, p = 0.035). LVI independently influenced SD (p = 0.009) on multivariate regression analysis. Table 5 provides detailed results.
Table 5.
Results of multivariate logistic regression analysis for clinicoradiologic and pathologic factors
| Variable | Mean ± SD | Emax | Emean | Eratio | SD | |||||||
| β coefficient | p | Mean ± SD | β coefficient | p | Mean ± SD | β coefficient | p | Mean ± SD | β coefficient | p | ||
| Age | 169.16 ± 70.30 | 0.97 ± 0.58 | 0.097 | 131.17 ± 52.76 | 0.89 ± 0.46 | 0.055 | 8.00 ± 8.16 | 0.08 ± 0.08 | 0.318 | 23.60 ± 19.45 | 0.12 ± 0.17 | 0.489 |
| Symptom | ||||||||||||
| Absent | 135.20 ± 59.32 | 0 | 108.89 ± 47.62 | 0 | 6.11 ± 4.10 | 0 | 16.66 ± 12.55 | 0 | ||||
| Ppresent | 199.59 ± 64.56 | 46.05 ± 15.06 | 0.003 | 151.48 ± 49.20 | 28.91 ± 11.94 | 0.018 | 9.93 ± 10.53 | 0.37 ± 1.97 | 0.851 | 30.24 ± 22.76 | 8.68 ± 4.36 | 0.050 |
| Ultrasound size | 169.16 ± 70.30 | 2.12 ± 1.08 | 0.055 | 131.17 ± 52.76 | 1.85 ± 0.86 | 0.035 | 8.00 ± 8.16 | 0.30 ± 0.14 | 0.035 | 23.60 ± 19.45 | 0.49 ± 0.31 | 0.123 |
| Pathologic size | 169.16 ± 70.30 | 0.07 ± 0.44 | 0.878 | 131.17 ± 52.76 | −0.18 ± 0.35 | 0.612 | 8.00 ± 8.16 | −0.09 ± 0.06 | 0.121 | 23.60 ± 19.45 | 0.11 ± 0.13 | 0.388 |
| LVI | ||||||||||||
| Negative | 164.21 ± 70.53 | 0 | 129.12 ± 54.55 | 0 | 7.54 ± 6.11 | 0 | 21.21 ± 15.84 | 0 | ||||
| Positive | 222.20 ± 42.31 | 19.16 ± 24.73 | 0.441 | 153.10 ± 16.31 | −4.54 ± 19.62 | 0.818 | 12.96 ± 20.19 | 2.15 ± 3.23 | 0.507 | 49.20 ± 34.28 | 19.13 ± 7.17 | 0.009 |
Emax, max elasticity; Emean, mean elasticity; Eratio, elasticity ratio; LVI, lymphovascular invasion; SD, standard deviation.
p-values < 0.05 were considered statistically significant
Comparison of small (≤20 mm) and large tumors (>20 mm)
Mass size on ultrasound was an independent factor for Emean (p = 0.035) and Eratio (p = 0.035) in the multivariate analysis. When tumors were classified by sizes of <10, 10–20 mm, and >20, >20 mm larger tumors had the most significant association with higher stiffness (Table 2). Accordingly, tumors were regrouped into small tumors (≤20 mm) and large tumors (>20 mm) for analysis of pathologic factors, since no pathologic factor other than LVI showed a significant association with SWE parameters. In large tumors (>20 mm), positive lymph node status was significantly correlated with higher SD (p = 0.039). Positive LVI was also significantly associated with higher SD (p = 0.001). No significant difference was found among other factors with quantitative SWE parameters (Table 6).
Table 6.
Correlation of pathologic factors withquantitative shear wave elastographic parameters in large tumor (>20 mm)
|
Variable |
n |
Emax | Emean | Eratio | SD | ||||||||
| Mean ± SD | β coefficient | p | Mean ± SD | β coefficient | p | Mean ± SD | β coefficient | p | Mean ± SD | β coefficient | p | ||
| Histologic grade | |||||||||||||
| 1 | 5 | 235.44 ± 44.18 | 171.36 ± 36.73 | 10.78 ± 6.73 | 34.24 ± 8.36 | ||||||||
| 2 | 9 | 201.8 ± 88.92 | –33.64 ± 36.05 | 0.361 | 150.72 ± 59.23 | –20.64 ± 26.7 | 0.448 | 8.39 ± 6.54 | –2.39 ± 6.85 | 0.730 | 38.64 ± 38.97 | 4.4 ± 14.38 | 0.762 |
| 3 | 10 | 233.42 ± 43.06 | –2.02 ± 35.41 | 0.955 | 185.13 ± 40.34 | 13.77 ± 26.22 | 0.605 | 18.78 ± 17.15 | 8 ± 6.73 | 0.248 | 29.08 ± 13.07 | –5.16 ± 14.12 | 0.719 |
| Lymph node status | |||||||||||||
| Negative | 17 | 221.94 ± 63.67 | 173.15 ± 48.83 | 11.23 ± 9.01 | 27.09 ± 13.02 | ||||||||
| Positive | 7 | 222.09 ± 69.25 | 0.14 ± 29.3 | 0.996 | 160.14 ± 49.59 | -13.01 ± 22.02 | 0.561 | 18.05 ± 19.1 | 6.82 ± 5.65 | 0.241 | 49.9 ± 38.96 | 22.81 ± 10.41 | 0.039 |
| LVI | |||||||||||||
| Negative | 20 | 217.53 ± 67.03 | 173.33 ± 51.78 | 12.26 ± 8.67 | 26.74 ± 16.96 | ||||||||
| Positive | 4 | 244.25 ± 44.29 | 26.72 ± 35.27 | 0.457 | 149.5 ± 17.42 | -23.83 ± 26.59 | 0.380 | 18.04 ± 26.95 | 5.78 ± 7.01 | 0.419 | 68.78 ± 31.85 | 42.04 ± 10.77 | 0.001 |
| Pathologic type | |||||||||||||
| IDC | 21 | 230.07 ± 55 | 40.87 ± 56.29 | 0.476 | 174.43 ± 42.02 | 23.93 ± 43.01 | 0.584 | 14.2 ± 13.28 | 7.1 ± 13.59 | 0.607 | 34.66 ± 25.97 | 3.26 ± 26.58 13.1 ± 36.73 -25.4 ± 36.73 | 0.904 |
| ILC | 1 | 256.70 | 67.5 ± 77.78 | 0.396 | 208.20 | 57.7 ± 59.43 | 0.343 | 9.79 | 2.69 ± 18.78 | 0.888 | 44.50 | 13.1 ± 36.73 | 0.725 |
| DCIS | 1 | 50.30 | –138.9 ± 77.78 | 0.089 | 42.80 | –107.7 ± 59.43 | 0.085 | 2.13 | –4.97 ± 18.78 | 0.794 | 6.00 | –25.4 ± 36.73 | 0.497 |
| Etc | 1 | 189.20 | 150.50 | 31.0 | 31.40 | ||||||||
| ER | |||||||||||||
| Negative | 6 | 210.25 ± 22.01 | 166.35 ± 21.43 | 8.26 ± 4.74 | 24.55 ± 14.11 | ||||||||
| Positive | 18 | 225.89 ± 72.8 | 15.64 ± 30.57 | 0.614 | 170.36 ± 54.97 | 4.01 ± 23.28 | 0.865 | 14.87 ± 14.16 | 6.61 ± 5.96 | 0.280 | 36.81 ± 27.36 | 12.26 ± 11.77 | 0.309 |
| PR | |||||||||||||
| Negative | 6 | 219.08 ± 41.61 | 180.03 ± 41.42 | 8.94 ± 4.46 | 22.92 ± 10.97 | ||||||||
| Positive | 18 | 222.95 ± 70.67 | 3.87 ± 30.74 | 0.901 | 165.8 ± 51.02 | –14.23 ± 23.1 | 0.544 | 14.64 ± 14.29 | 5.7 ± 6.01 | 0.353 | 37.35 ± 27.5 | 14.43 ± 11.66 | 0.229 |
| HER2 | |||||||||||||
| Negative | 16 | 224.49 ± 64.9 | 165.4 ± 47.11 | 11.89 ± 13.55 | 34.01 ± 23.99 | ||||||||
| Positive | 8 | 216.96 ± 65.62 | –7.53 ± 28.2 | 0.792 | 177.28 ± 53.04 | 11.88 ± 21.25 | 0.582 | 15.88 ± 11.2 | 4 ± 5.56 | 0.480 | 33.2 ± 28.69 | –0.81 ± 11.08 | 0.942 |
| Ki67 | |||||||||||||
| <14 % | 8 | 244.1 ± 70.81 | 168.74 ± 46.63 | 11.64 ± 7.1 | 42.29 ± 30.23 | ||||||||
| ≥14 % | 14 | 222.93 ± 43.66 | –21.17 ± 24.25 | 0.393 | 179.27 ± 40.26 | 10.53 ± 18.88 | 0.583 | 15.38 ± 15.48 | 3.74 ± 5.84 | 0.529 | 31.63 ± 22.3 | –10.66 ± 11.24 | 0.354 |
DISCUSSION
This study investigated correlations between various clinicoradiologic and pathologic features and SWE parameters. For the evaluation of clinical factors, older age was significantly correlated with higher Emax and Emean (p = 0.026, 0.018, respectively). The presence of symptoms such as breast pain and palpability was significantly associated with Emax (p = 0.003), Emean (p = 0.018), Eratio (p = 0.035), and SD (p = 0.050) on univariate analysis. Multivariate logistic regression analysis showed that the presence of symptoms was significantly associated with higher Emax (p = 0.003) and Emean (p = 0.018). These findings are consistent with previous studies. Youk et al17 demonstrated that palpable abnormalities were significantly associated with Emean. Berg et al reported that Eratio was significantly greater in palpable masses than in nonpalpable masses.27
We found a significant correlation between ultrasound cancer size and SWE parameters, with larger tumors demonstrating significantly higher values of Emax, Emean, Eratio and SD. Larger pathologic size of surgical specimens was also correlated with higher Emax (p = 0.043) and SD (p = 0.019). LVI was significantly associated with higher Emax (p = 0.036) and SD (p < 0.001). These findings are similar to other studies. Youk et al17 reported that larger cancer size (p = 0.013) and LVI (p < 0.0001) were significantly associated with higher Emean. Choi et al21 found that larger cancer size (p = 0.009) was significantly correlated with higher Eratio. We analyzed cancer size (maximal diameter) separately as measured by either ultrasound images or pathologic reports, whereas previous studies only used one method for size measurement. Au et al11 determined mean cancer size from ultrasound images by averaging length x height x width and mean cancer size was significantly correlated with higher Emax, Emean and Eratio (all p < 0.001). Chang et al20 also determined the maximal tumor diameter from ultrasound, while several groups determined cancer size from pathologic reports.16, 17,21 The results of our study showed the difference between tumor size measured on ultrasound and pathologically confirmed tumor size. Most differences were found in DCIS, a complex pathologic entity, and could be explained by the lack of morphologic changes detected on ultrasound.28 Previous studies have reported that the presence of DCIS has a significant impact on the accuracy of tumor size measurement.28, 29
In the multiple linear regression analysis, mass size on US was an independent factor for Emean (β coefficient = 1.85, p = 0.035) and Eratio (β coefficient = 0.30, p = 0.035). However, pathologic cancer size was not independently associated with SWE parameters. LVI was significantly positively correlated with higher SD (p = 0.009). LVI, which means invasion of the tumor into lymphatic spaces or vessels in the peritumoral area, has been associated with poor prognosis in patients with breast cancer.30 In this study, SD was an independent factor in SWE parameters. Evans et al15 suggested that SD was useful measurement of heterogeneity, differentiating benign from malignant lesions, because the value was significantly higher in patients with malignant histopathology.
Although there was no significant difference among histologic cancer grades in this study, we found that Grade 3 breast cancers tended to have higher SWE values compared to Grade 1 and 2 cancers, but there was no significant difference between the three histologic grades. Youk et al17 and Chang et al20 demonstrated that a higher cancer grade was significantly correlated with higher Emean. Choi et al21 reported that a higher grade was associated with higher E ratio (p = 0.015). However, Ganau et al18 found no significant correlation between stiffness and histologic grade. In this study, the relatively smaller number of Grade 3 cancers could explain the lack of a significant association of histologic grade with SWE parameters. We demonstrated that DCIS appeared to be softer than IDC or ILC with lower quantitative SWE values. There was only one case of IMC, which was relatively soft (Emax: 109.9 kPa, Emean: 89.3 kPa). This observation is expected because IMC has been described as a soft cancer.11 However, Evans et al31 reported there were no significant differences in Emean or Emax among tumor types such as lobular, mucinous, papillary and metaplastic cancers.
In this study, no immunohistochemical profile of breast cancers was associated with SWE parameters. There was no significant correlation with SWE parameters and hormonal receptors such as ER and PR. In this study, positive Ki-67 expression tended to be associated with higher quantitative SWE values (Emax, Emean, Eratio and SD), although this trend did not reach statistical significance. We postulated that Ki-67 expression could be explained by high stiffness on SWE, as it is considered a marker of cellularity.32
Since none of the pathologic factors except LVI were significantly associated with SWE parameters, tumors were regrouped into small tumors (≤20 mm) and large tumors (>20 mm) for analysis of pathologic factors. In large tumors (>20 mm), positive lymph node status was significantly correlated with higher SD (p = 0.039). Positive LVI was also significantly associated with higher SD (p = 0.001). No significant difference was found among other factors with quantitative SWE parameters. A recent study by Denis et al33 found that tumor size was an independent factor for Emean, but the other pathologic factors, including histologic grade and immunohistochemical profiles, were not significantly associated with Emean.
There was no significant correlation between color pattern and any variable. To date, recent studies have focused on correlation of quantitative SWE factors and clinicopathologic factors in breast cancer. However, there have been a few previous studies concerning color map patterns in breast cancer.34 Lee et al34 demonstrated an association between color map patterns and clinicopathologic factors in breast cancer. They divided color map patterns into three main categories: Type 1 (diffuse pattern), Type 2 (lateral pattern), and Type 3 (rim-off pattern). In our study, the color map pattern was classified into two main categories: color patterns “1” and “2” vs color patterns “3” and “4”. Although we observed no significant differences among color patterns, this research has significance in its efforts to investigate correlations between qualitative SWE features and clinicopathologic factors. Further studies on the prognostic value of color map patterns are necessary with a larger study population.
There were several limitations of this study. First, the retrospective design might unavoidably have selection bias, because only excised breast lesions with preoperative SWE and available immunohistochemical profiles were included. Second, only one radiologist measured SWE parameters of each breast cancer. However, SWE parameters have been proven to be highly reproducible with high intra-and interobserver agreement.35, 36 Third, the number of cancers was relatively small. In particular, the qualitative analysis of color patterns was limited because there were only three cases with a color pattern of “1” or “2”, precluding definite conclusions about the correlation of SWE parameters and color patterns. However, the strength of this study is that it was a comprehensive assessment of both quantitative and qualitative SWE parameters. Further study with a larger number of patients may enlarge the clinical significance of our findings.
CONCLUSION
The presence of symptoms, older age, larger tumor size on US, larger pathologic cancer size, and LVI were significantly correlated with higher quantitative SWE factors on univariate analysis. Among these variables, the presence of symptoms was an independent factor for Emax and Emean on multivariate analysis. Mass size on US was independently correlated with Emean and Eratio. LVI was independently correlated with SD. Accordingly, tumor stiffness measured by SWE and B-mode US could help predict cancer prognosis.
Contributor Information
Eun Jee Song, Email: kslisa85@naver.com.
Yu-Mee Sohn, Email: sonyumee@naver.com.
Mirinae Seo, Email: s1434@hanmail.net.
REFERENCES
- 1.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991; 19: 403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x [DOI] [PubMed] [Google Scholar]
- 2.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989; 63: 181–7. doi: [DOI] [PubMed] [Google Scholar]
- 3.Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol 2005; 23: 7350–60. doi: 10.1200/JCO.2005.03.3845 [DOI] [PubMed] [Google Scholar]
- 4.Tang P, Skinner KA, Hicks DG. Molecular classification of breast carcinomas by immunohistochemical analysis: are we ready? Diagn Mol Pathol 2009; 18: 125–32. doi: 10.1097/PDM.0b013e31818d107b [DOI] [PubMed] [Google Scholar]
- 5.Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, et al. . Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast 2012; 21: 50–7. doi: 10.1016/j.breast.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 6.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res 2009; 7: 4–13. doi: 10.3121/cmr.2008.825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko KH, Jung HK, Kim SJ, Kim H, Yoon JH. Potential role of shear-wave ultrasound elastography for the differential diagnosis of breast non-mass lesions: preliminary report. Eur Radiol 2014; 24: 305–11. doi: 10.1007/s00330-013-3034-4 [DOI] [PubMed] [Google Scholar]
- 8.Gweon HM, Youk JH, Son EJ, Kim JA. Clinical application of qualitative assessment for breast masses in shear-wave elastography. Eur J Radiol 2013; 82: e680–e685. doi: 10.1016/j.ejrad.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 9.Lee EJ, Jung HK, Ko KH, Lee JT, Yoon JH. Diagnostic performances of shear wave elastography: which parameter to use in differential diagnosis of solid breast masses? Eur Radiol 2013; 23: 1803–11. doi: 10.1007/s00330-013-2782-5 [DOI] [PubMed] [Google Scholar]
- 10.Au FW, Ghai S, Moshonov H, Kahn H, Brennan C, Dua H, et al. . Diagnostic performance of quantitative shear wave elastography in the evaluation of solid breast masses: determination of the most discriminatory parameter. AJR Am J Roentgenol 2014; 203: W328–36. doi: 10.2214/AJR.13.11693 [DOI] [PubMed] [Google Scholar]
- 11.Au FW, Ghai S, Lu FI, Moshonov H, Crystal P. Quantitative shear wave elastography: correlation with prognostic histologic features and immunohistochemical biomarkers of breast cancer. Acad Radiol 2015; 22: 269–77. doi: 10.1016/j.acra.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 12.Tozaki M, Fukuma E. Pattern classification of ShearWave™ Elastography images for differential diagnosis between benign and malignant solid breast masses. Acta Radiol 2011; 52: 1069–75. doi: 10.1258/ar.2011.110276 [DOI] [PubMed] [Google Scholar]
- 13.Athanasiou A, Tardivon A, Tanter M, Sigal-Zafrani B, Bercoff J, Deffieux T, et al. . Breast lesions: quantitative elastography with supersonic shear imaging--preliminary results. Radiology 2010; 256: 297–303. doi: 10.1148/radiol.10090385 [DOI] [PubMed] [Google Scholar]
- 14.Chang JM, Moon WK, Cho N, Yi A, Koo HR, Han W, et al. . Clinical application of shear wave elastography (SWE) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res Treat 2011; 129: 89–97. doi: 10.1007/s10549-011-1627-7 [DOI] [PubMed] [Google Scholar]
- 15.Evans A, Whelehan P, Thomson K, McLean D, Brauer K, Purdie C, et al. . Quantitative shear wave ultrasound elastography: initial experience in solid breast masses. Breast Cancer Res 2010; 12: 1. doi: 10.1186/bcr2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans A, Whelehan P, Thomson K, McLean D, Brauer K, Purdie C, et al. . Invasive breast cancer: relationship between shear-wave elastographic findings and histologic prognostic factors. Radiology 2012; 263: 673–7. doi: 10.1148/radiol.12111317 [DOI] [PubMed] [Google Scholar]
- 17.Youk JH, Gweon HM, Son EJ, Kim JA, Jeong J. Shear-wave elastography of invasive breast cancer: correlation between quantitative mean elasticity value and immunohistochemical profile. Breast Cancer Res Treat 2013; 138: 119–26. doi: 10.1007/s10549-013-2407-3 [DOI] [PubMed] [Google Scholar]
- 18.Ganau S, Andreu FJ, Escribano F, Martín A, Tortajada L, Villajos M, et al. . Shear-wave elastography and immunohistochemical profiles in invasive breast cancer: evaluation of maximum and mean elasticity values. Eur J Radiol 2015; 84: 617–22. doi: 10.1016/j.ejrad.2014.12.020 [DOI] [PubMed] [Google Scholar]
- 19.Evans A, Rauchhaus P, Whelehan P, Thomson K, Purdie CA, Jordan LB, et al. . Does shear wave ultrasound independently predict axillary lymph node metastasis in women with invasive breast cancer? Breast Cancer Res Treat 2014; 143: 153–7. doi: 10.1007/s10549-013-2747-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang JM, Park IA, Lee SH, Kim WH, Bae MS, Koo HR, et al. . Stiffness of tumours measured by shear-wave elastography correlated with subtypes of breast cancer. Eur Radiol 2013; 23: 2450–8. doi: 10.1007/s00330-013-2866-2 [DOI] [PubMed] [Google Scholar]
- 21.Choi WJ, Kim HH, Cha JH, Shin HJ, Kim H, Chae EY, et al. . Predicting prognostic factors of breast cancer using shear wave elastography. Ultrasound Med Biol 2014; 40: 269–74. doi: 10.1016/j.ultrasmedbio.2013.09.028 [DOI] [PubMed] [Google Scholar]
- 22.D’Orsi C, Sickles E, Mendelson E.et al. . ACR BI-RADS atlas, breast imaging reporting and data system. Reston, VA: The British Institute of Radiology.; 2013. [Google Scholar]
- 23.Carriaga MT, Henson DE. The histologic grading of cancer. Cancer 1995; 75(1 Suppl): 406–21. doi: [DOI] [PubMed] [Google Scholar]
- 24.Frierson HF, Wolber RA, Berean KW, Franquemont DW, Gaffey MJ, Boyd JC, et al. . Interobserver reproducibility of the Nottingham modification of the Bloom and Richardson histologic grading scheme for infiltrating ductal carcinoma. Am J Clin Pathol 1995; 103: 195–8. doi: 10.1093/ajcp/103.2.195 [DOI] [PubMed] [Google Scholar]
- 25.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998; 11: 155–68. [PubMed] [Google Scholar]
- 26.Moeder CB, Giltnane JM, Harigopal M, Molinaro A, Robinson A, Gelmon K, et al. . Quantitative justification of the change from 10% to 30% for human epidermal growth factor receptor 2 scoring in the American Society of Clinical Oncology/College of American Pathologists guidelines: tumor heterogeneity in breast cancer and its implications for tissue microarray based assessment of outcome. J Clin Oncol 2007; 25: 5418–25. doi: 10.1200/JCO.2007.12.8033 [DOI] [PubMed] [Google Scholar]
- 27.Berg WA, Cosgrove DO, Doré CJ, Schäfer FK, Svensson WE, Hooley RJ, et al. . Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology 2012; 262: 435–49. doi: 10.1148/radiol.11110640 [DOI] [PubMed] [Google Scholar]
- 28.Soliman AA, Wojcinski S, Degenhardt F. The effect of accompanying in situ ductal carcinoma on accuracy of measuring malignant breast tumor size using B-mode ultrasonography and real-time sonoelastography. Int J Breast Cancer 2012; 2012: 1–5. doi: 10.1155/2012/376032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chagpar AB, McMasters KM, Sahoo S, Edwards MJ. Does ductal carcinoma in situ accompanying invasive carcinoma affect prognosis? Surgery 2009; 146: 561–8. doi: 10.1016/j.surg.2009.06.039 [DOI] [PubMed] [Google Scholar]
- 30.Rakha EA, Martin S, Lee AH, Morgan D, Pharoah PD, Hodi Z, et al. . The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer 2012; 118: 3670–80. doi: 10.1002/cncr.26711 [DOI] [PubMed] [Google Scholar]
- 31.Evans A, Sim YT, Thomson K, Jordan L, Purdie C, Vinnicombe SJ, et al. . Shear wave elastography of breast cancer: sensitivity according to histological type in a large cohort. Breast 2016; 26: 115–8. doi: 10.1016/j.breast.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 32.Choi SY, Chang YW, Park HJ, Kim HJ, Hong SS, Seo DY, et al. . Correlation of the apparent diffusion coefficiency values on diffusion-weighted imaging with prognostic factors for breast cancer. Br J Radiol 2012; 85: e474–e479. doi: 10.1259/bjr/79381464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denis M, Gregory A, Bayat M, Fazzio RT, Whaley DH, Ghosh K, et al. . Correlating tumor stiffness with immunohistochemical subtypes of breast cancers: prognostic value of comb-push ultrasound shear elastography for differentiating luminal subtypes. PLoS One 2016; 11: e0165003. doi: 10.1371/journal.pone.0165003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S, Jung Y, Bae Y. Clinical application of a color map pattern on shear-wave elastography for invasive breast cancer. Surg Oncol 2016; 25: 44–8. doi: 10.1016/j.suronc.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 35.Cosgrove DO, Berg WA, Doré CJ, Skyba DM, Henry JP, Gay J, et al. . BE1 Study Group Shear wave elastography for breast masses is highly reproducible. Eur Radiol 2012; 22: 1023–32. doi: 10.1007/s00330-011-2340-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans A, Whelehan P, Thomson K, Brauer K, Jordan L, Purdie C, et al. . Differentiating benign from malignant solid breast masses: value of shear wave elastography according to lesion stiffness combined with greyscale ultrasound according to BI-RADS classification. Br J Cancer 2012; 107: 224–9. doi: 10.1038/bjc.2012.253 [DOI] [PMC free article] [PubMed] [Google Scholar]