Abstract
Objective
The Childhood Health and Asthma Management Program (CHAMP) is a behavioral family lifestyle intervention for youth with overweight or obesity (OV/OB) and asthma. This pilot randomized controlled trial examined the feasibility, acceptability, and preliminary efficacy of CHAMP.
Methods
A sample of 24 children (Mage = 8.67) with asthma and a BMI ≥ 85th percentile and their caregivers participated in a pilot randomized controlled trial. Families were recruited from local pediatrician offices and pediatric pulmonary and allergy clinics and randomized to CHAMP or a health education attention control condition. Children’s height, weight, lung function, asthma control, and asthma-related quality of life (QOL) were collected at baseline, post-intervention, and 6-months post-treatment. Analysis of covariance and standardized mean differences were used to assess changes in outcome variables among participants attending > 50% of sessions (n = 12).
Results
Families participating in CHAMP reported high satisfaction; however, there were a number of barriers to recruitment and regular session attendance. There were no statistically significant between group differences at post-intervention or long-term follow-up. From baseline to post-intervention, there were small to large effect sizes favoring CHAMP for BMI z-scores, asthma control, and measures of lung function. There were small to medium effect sizes favoring CHAMP at long-term follow-up for BMI z-scores, asthma control, and asthma-related QOL.
Conclusions
CHAMP had adequate acceptability in this trial. We did not find significant results favoring CHAMP in comparison to the control group, however, lessons learned provide important directions for modifications in anticipation of a larger trial.
Keywords: asthma, obesity, intervention, family
Youth with overweight or obesity (OV/OB) and asthma experience increased morbidity than their non-obese peers (Belamarich et al., 2000; van Gent et al., 2007). National asthma management guidelines call for weight loss to improve asthma outcomes in individuals who are OV/OB (National Asthma Education and Prevention Program, 2007). Weight loss is linked to improved lung function, asthma control, and decreased morbidity in individuals with OV/OB and asthma (Lan Xiao, Xiao, & Ma, 2015).
To date, four randomized controlled trials (RCTs) have been conducted, all outside of the United States, to target weight management in youth with OV/OB and asthma (El- Kader, Al-Jiffri, & Ashmawy, 2013; Jensen, Gibson, Collins, Hilton, & Wood, 2013; Luna-Pech et al., 2014; Willeboordse et al., 2016). These studies used either prescriptive, dietary-based interventions (Jensen et al., 2013; Luna-Pech et al., 2014) or intensive diet and exercise programs (El- Kader et al., 2013; Willeboordse et al., 2016). Each of these interventions were successful in reducing body mass index (BMI). In addition, positive changes in asthma control, asthma-related quality of life, lung function, and inflammatory markers (e.g., tumor necrosis factor-alpha, interleukin-6) have been found in one or more studies.
Children with OV/OB are at significant risk for becoming adults who are obese (Simmonds, Llewellyn, Owen, & Woolacott, 2016). To forestall poor long-term health outcomes, the American Academy of Pediatrics and others recommend that weight management interventions for youth with OV/OB should occur early on during childhood and promote active parent involvement to facilitate health behavior change and weight loss (Spear et al., 2007). While effective, the previously developed weight management interventions for youth with OV/OB and asthma may not be ideally suited for school-age children for two reasons. First, they were predominantly focused on older youth (Mage > 12 years-old) who are likely to have unique developmental needs and obesogenic risk factors (Adair, 2008). Second, given the substantial control and modeling power that parents have over the eating environment of school-age children (Golan, Fainaru, & Weizman, 1998), active parent involvement is likely critical. However, only half of these studies included parents in the intervention. Jensen and colleagues (2013) invited parents to attend sessions whereas parents took an active role in receiving intervention content in the Willeboordse et al. (2016) study.
Behavioral family-based interventions (BFIs) are a gold standard intervention method to equip parents and school-age children with self-management skills that can lead to sustainable health behavior changes and elicit long-term weight loss (Janicke et al., 2014). BFIs function by assisting parents in acquiring: 1) evidence-based self-management strategies such as self-monitoring, goal setting, action planning, and problem solving, and 2) behavioral parenting strategies. Parents then work together with their child to gradually utilize these self-management strategies and ultimately establish sustainable healthy habits (Epstein, Paluch, Roemmich, & Beecher, 2007; Janicke et al., 2008). Despite their effectiveness in youth with OV/OB, to date, BFIs for school-age children with OV/OB and asthma have not been developed.
We recently developed the Childhood Health and Asthma Management Program (CHAMP), the first BFI for school-age children with OV/OB and asthma, by modifying our previously successful BFI for weight management in youth with OV/OB (Janicke et al., 2008). CHAMP was developed via an iterative process that involved incorporating asthma education and management skills into existing sessions, adding additional content related to common asthma management difficulties, and gathering qualitative feedback from the target intervention population. Our primary aims for this pilot study were: 1) to assess the feasibility and acceptability of CHAMP and 2) conduct a pilot RCT to examine the preliminary effectiveness of CHAMP to improve weight and asthma outcomes compared to an attention control in a sample of 6–12 year-old children with OV/OB and asthma.
Methods
Participants
Study inclusion criteria were that children were 6–12 years-old, had a physician-verified persistent asthma diagnosis, and had a BMI ≥ 85th percentile for Centers for Disease Control and Prevention age and gender norms (Kuczmarski et al., 2000). Youth were excluded if they had history positive for dietary restrictions, medical conditions in which physical activity is contraindicated, prescribed antipsychotic agents, or significant developmental delay. Families could not be enrolled in another weight loss program. Youth were recruited from local pediatrician’s offices and pediatric pulmonary and allergy clinics. Participants in the current study were 24 parent-child dyads. The average age of children in the sample was 8.67 (SD = 1.9) and the majority of the children were female (54.2%) and African American (66.7%). Median family income was $25,000-$34,999. Demographic information is presented in Table 1. The Institutional review board approved the current study.
Table 1.
Participant Baseline Characteristics of Randomized Participants
| CHAMP (n = 14) | HEC (n = 10) | χ2 | Mann- Whitney U |
P | |
|---|---|---|---|---|---|
| Child Age | 8.64 (1.78) | 8.70 (2.16) | 68.50 | .93 | |
| Sessions Attended | 7.07 (5.34) | 8.70 (5.81) | 86.00 | .37 | |
| Sex | .12 | .73 | |||
| Male | 6 (43%) | 5 | |||
| Female | 8 (57%) | 5 | |||
| Race/ethnicity | 5.49 | .24 | |||
| White/Caucasian | 3 (21%) | 1 | |||
| Black/African American | 10 (71%) | 6 | |||
| Other | 1 (7%) | 3 | |||
| Incomea | 8.89 | .71 | |||
| Less than | 3 (25%) | 1 (12%) | |||
| $10,000 | |||||
| $10,000–$19,999 | 2 (17%) | 1 (12%) | |||
| $20,000–$34,999 | 3 (25%) | 4 (50%) | |||
| $35,000–$44,999 | 1 (8%) | 1 (12%) | |||
| $45,000 and over | 3 (25%) | 1 (12%) | |||
| BMIz | 2.16 (.50) | 2.26 (.58) | 81.00 | .55 | |
| ACT | 17.58 (5.23) | 19.40 (4.95) | 79.50 | .20 | |
| PACQLQ | 5.57 (1.09) | 4.96 (1.59) | 53.50 | .48 | |
| PAQLQ | 5.28 (1.30) | 4.83 (1.48) | 56.50 | .61 | |
| FVC | 104.71 (10.59) | 97.80 (11.53) | 43.00 | .12 | |
| FEV1 | 98.21 (11.60) | 86.50 (16.07) | 35.00 | .04 | |
| Ratio | 92.36 (10.31) | 86.40 (9.11) | 52.50 | .31 | |
| FEF25–75 | 91.21 (38.67) | 63.10 (24.79) | 38.50 | .06 | |
| FEFMax | 89.64 (19.68) | 74.10 (13.58) | 33.50 | .03 |
Note. Body Mass Index z-score (BMIz), Asthma Control Test (ACT), Pediatric Asthma Caregiver Quality of Life Questionnaire (PACQLQ), Pediatric Asthma Quality of Life Questionnaire (PAQLQ), forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), FEV1/FVC ratio (Ratio), forced expiratory flow 25–75%, (FEF25–75), maximum forced expiratory flow (FEFMax).
Measures
Body mass index
Children’s height was measured via stadiometer to the nearest 0.1 centimeter. Weight was measured using a certified digital scale to the nearest 0.1 kilogram. Children were dressed in light clothing and without shoes for measurements. Values were converted to z-scores for child body mass index (BMIz) using age- and gender-specific norms published by the CDC (Kuczmarski et al., 2000).
Pulmonary function
Lung function measures assessed via spirometry included forced vital capacity (FVC), forced expiratory volume in one second (FEV1), FEV1/FVC, maximal forced expiratory flow (FEFmax), and forced expiratory flow 25–75% (FEF25–75). Spirometry was conducted per American Thoracic Society guidelines (Miller et al., 2005). Predicted values were used in analyses, with higher values corresponding to better lung function.
Asthma control
Youth asthma control was assessed with the Asthma Control Test (ACT; Liu et al., 2007). The ACT assesses the frequency of children’s asthma symptoms, activity limitations, and perceptions of disease control. Higher total ACT scores represent better asthma control, with the clinical cut off of ≤ 19 signifying “poor control.”
Asthma-related quality of life
Youth asthma-related quality of life was measured via the Standardised Paediatric Asthma Quality of Life Questionnaire (PAQLQ; Juniper et al., 1996). The PAQLQ(s) consists of 23 items related to three domains, symptoms, activity limitations, and emotional functioning, spanning the previous week. Overall mean scores were calculated and ranged from 1–7, such that higher scores indicated higher quality of life.
Participant satisfaction
Parents completed satisfaction surveys at the end of each session and at the conclusion of the intervention using a 5-point scale ranging from Strongly Disagree to Strongly Agree, such that higher scores indicated greater satisfaction. A 10-item session satisfaction survey at the end of each session was designed to assess interest in materials, participants’ ability to understand information, and the appropriateness and relevance of content given their family’s concerns. A satisfaction survey at the end of the intervention was used to measure the parents’ perceptions regarding the difficulty of the program, amount of information learned, pace of the program, intervention setting, usefulness and value of content, and likelihood of sharing information with others. Additionally, families completed a targeted semi-structured interview regarding the value of the program, recommendations for improvement, appropriateness of the intervention, family behavioral changes since completing the program (e.g., changes in physical activity and eating habits) and barriers to making changes, and long-term utility of topics discussed in sessions.
Procedure
Assessments and randomization
Outcome variables were collected at three time points: baseline, post-intervention, and long-term follow-up (6 months post-treatment). An advanced registered nurse practitioner who was blind to intervention condition conducted spirometry. Trained doctoral level graduate students collected all other outcomes. All assessment and intervention sessions took place at either a local community health center or a pediatrician’s office. Dyads completed baseline assessment materials approximately 2–4 weeks before the start of treatment. Prior to randomization, a pediatric pulmonologist verified study eligibility. After a baseline assessment, individual families were randomly assigned to CHAMP or a health education control (HEC) group. Both groups were assigned 16-week interventions delivered in-person by trained interventionists over 4 months. Each parent-child dyad participated in simultaneous but separate groups. Outcomes were measured at treatment completion and at 6-months post-treatment. The intervention was conducted across two waves at a local community health center or local pediatrician’s office. Groups typically consisted of three to five participants.
Intervention development
CHAMP is a BFI that was tailored from our previous work (Janicke et al., 2008) for children with OV/OB and asthma in an iterative process. First, the investigative team modified our existing BFI by incorporating asthma education and management skills into existing sessions (e.g. incorporating physical activity with asthma, removing triggers as part of creating a healthy environment, setting goals related to sharing medication responsibility) and adding a session addressing common asthma management difficulties. Second, we conducted a focus group with 3 youth-caregiver dyads from the target intervention population. The first half of the focus group centered on delineating asthma-specific and contextual barriers to weight management in children with OV/OB and asthma and their families. Core topics included: 1) beliefs about asthma and physical activity, 2) concerns with physical activity due to asthma, and 3) ways to engage in physical activity with asthma. In the second half, we presented preliminary CHAMP and HEC intervention content to solicit feedback and gauge participant interest, relevance, and potential feasibility and acceptability. Families provided feedback on intervention components and provided suggestions on prioritizing intervention targets. Feedback was integrated into final intervention content. Supplemental Table 1 contains a session-by-session description of intervention content for both groups.
Childhood health and asthma management program
Dyads randomized to CHAMP were asked to attend 12 group-based sessions (3 sessions per month) and 4 individual family sessions (1 session per month) that occurred on weekday evenings. Groups emphasized modeling and providing support to work together to establish healthier eating and exercise patterns. Both parent and child sessions were designed to last approximately 90 minutes and include three segments: 1) a review of parent and child progress in implementing the strategies recommended for changing their eating or exercise in the previous session; 2) skills training and implementation; and 3) goal setting, feedback, and encouragement from group leaders and members. Parent group topics included benefits of weight loss, basics of energy balance and nutrition, appropriate methods for increasing physical activity, establishing healthy eating patterns, proper portion size for foods, healthy cooking strategies, meal planning on a limited budget, eating healthy when eating out, improving self-esteem and body image, behavior management, and positive parenting skills (e.g., goal setting, self-monitoring, stimulus control, etc.). Guidelines driven asthma management education components including asthma physiology, medication administration guidance, trigger control, and effective collaboration with the health care system were reviewed. Notably, CHAMP highlighted the advantages of weight management for OV/OB children with asthma throughout the intervention and targeted children’s perceived competence and parental misperceptions surrounding their child’s safety in engaging in physical activity (Matricardi, Gruber, Wahn, & Lau, 2007) by teaching families how to manage their asthma while engaging in physical activity.
Children were gradually exposed to increased exercise through in-vivo activities during the intervention (e.g. stoplight tag based on red, yellow, and green foods) and families were provided pedometers to track steps. Children also participated in activities related to parent group content, such as estimating portion sizes of common foods, brainstorming replacements for red foods, and building models of airways. Child and group leaders prepared and sampled healthy snacks each session to teach children about nutrition (e.g., recognizing calorie and fat content of foods) and encourage trying new foods. In between sessions, families set weekly SMART goals based on session topics (e.g. going for one additional family walk per week, adding in one vegetable to dinner each night). Monthly individual family sessions were used to discuss and problem solve individual barriers to implementing intervention strategies, provide reinforcement for participation and trying behavioral skills, and provide general support.
Health education control
Dyads randomized to the HEC condition received national guidelines on asthma management, proper nutrition, physical activity, stress management, dental hygiene, and school-related difficulties, among other health-related topics. Families in the HEC condition did not receive training in behavioral self-regulation strategies, such as goal-setting, self-monitoring, or problem-solving. Children participated in fun, physically active games during each session (e.g. freeze tag, relay races) but there was no discussion of implementing these activities in other settings. Similar to the CHAMP condition, sessions were approximately 90 minutes. In between sessions, families were asked to complete tasks related to session content (e.g. finding a newspaper article related to healthy eating).
Interventionists
Interventionists were doctoral level psychologists or trained graduate level psychology students. Interventionists underwent 12 hours of training and certification in the treatment protocols and collection of anthropometric data with two study authors (D.F. and D.J.) prior to implementation. Booster training sessions occurred halfway through the intervention protocol to sustain consistency of effort and adherence to the treatment protocol. Weekly intervention team meetings led by the same authors were dedicated to practice in the application of intervention procedures and to case management reviews of participant progress. Intervention group sessions were audio-taped, and biweekly reviews were carried out by the lead author to assess treatment fidelity and determine when additional training is required.
Data Analyses
We examined feasibility by calculating enrollment and session attendance rates; acceptability was assessed via descriptive statistics of satisfaction surveys. We also examined feedback from one-on-one exit interviews at the post-intervention assessment visit in an attempt to extract themes related to: 1) the perceived value of CHAMP, 2) recommendations for improving CHAMP, 3) appropriateness of CHAMP for their family, 4) behavioral changes since completing CHAMP, and 5) barriers to making changes. Analysis of covariance (ANCOVA), with baseline values entered as covariates, was used to assess changes in outcome variables. Due to the small samples size in our pilot study, we relied on standardized mean difference effect sizes (i.e., Cohen’s d; Cohen, 1988) and 95% confidence intervals (CIs) to interpret findings (Coe, 2002). Participants attending ≥ 9 sessions (n = 12) were categorized as completers and were included in analyses. Given the pilot nature of the current study, this post hoc decision was made in order to investigate the efficacy of CHAMP among families who received a reasonable dose of the intervention. Cell sizes vary slightly due to missing data. Descriptive statistics of outcome variables by intervention group and study time point are available in Supplemental Table 2.
Results
Feasibility
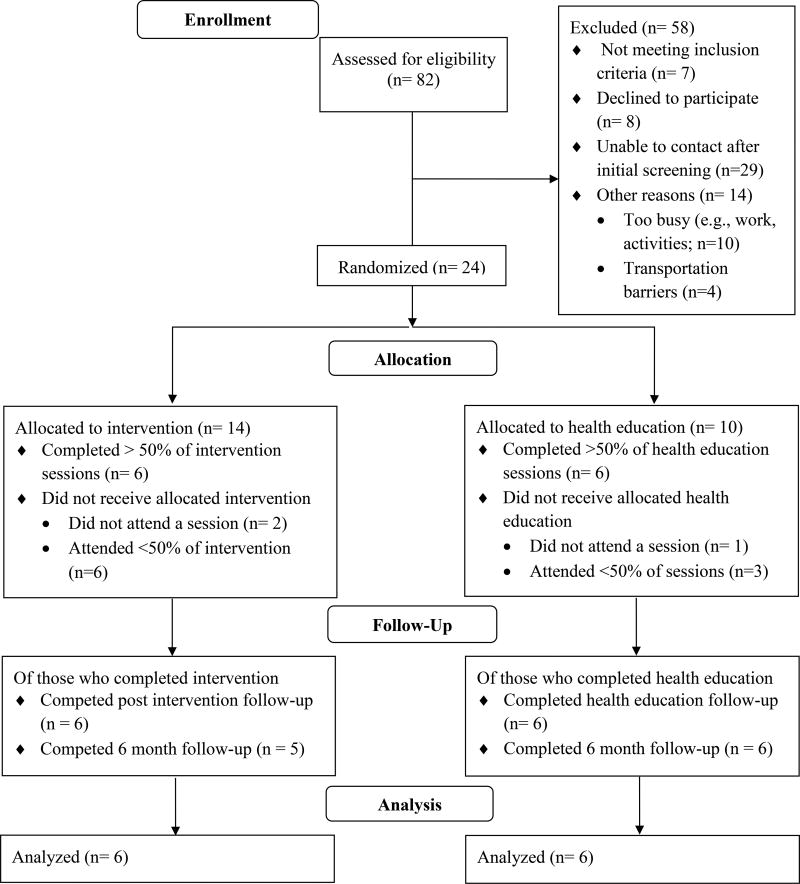
Figure 1 includes participant flow through the study. We assessed 82 families for study eligibility. Approximately 10% of families (n = 8) declined to participate and 17% (n = 14) were interested in the study but unable to participate due to work conflicts or transportation difficulties (e.g., resided a significant distance from study location). We were unable to re-contact 35% of families (n = 29) after obtaining their contact information during in-clinic recruitment. We did not meet our targeted enrollment of 32 families. Session attendance rates did not differ between the CHAMP (M = 7.07, SD = 5.34) and HEC (M = 8.70, SD = 5.81) groups, t(22) = .71, p = .49.
Figure 1.
Overview of Study Flow
Acceptability
Average session satisfaction was calculated across all participants that completed at least one session (CHAMP, n =10; HEC: n = 8). Average satisfaction levels across individual sessions was high in both the CHAMP (M = 4.84, SD = .24) and HEC (M = 4.80, SD = .37) groups. Likewise, overall intervention satisfaction was very high in the CHAMP (M = 4.78, SD = .67) and HEC (M = 5.00, SD = .00) groups. Parents in both the CHAMP (M = 4.78, SD = .67) and HEC (M = 5.00, SD = .00) groups reported that they were likely to share information from the program with their family and friends. Further, families in both groups felt that the program presented valuable information about managing their child’s asthma (CHAMP: M = 4.78, SD = .44; HEC: M = 5.00, SD = .00) and weight (CHAMP: M = 4.78, SD = .44; HEC: M = 4.83, SD = .41).
Families valued the variety of topics that were covered during CHAMP, the education about the relationship between asthma and weight management, and discussion of serving sizes and portion control. Recommendations for future changes included more hands-on activities (e.g., cooking class, group exercise) and booster sessions to sustain motivation. All families reported that CHAMP was developmentally appropriate and that they enjoyed having separate parent and child sessions. Families noted a number of changes they made during the course of CHAMP including making physical activity a priority (e.g., encouraging walking as a family, reducing television time), increasing vegetable intake, being more conscious about serving sizes, and eating out less frequently. With regards to barriers to behavior change, several families noted difficulties sustaining motivation and trying to balance work demands.
Between-Group Comparisons of Change
Baseline demographic characteristics were not significantly different between participants randomized to CHAMP and HEC. There were significant differences favoring CHAMP for FEV1 (Mann-Whitney U = 35.00, p =.04) and FEFMax (Mann-Whitney U = 33.50, p =.03), however, other measures of lung functioning were not significantly different between groups at baseline.
Post-intervention
There were no statistically significant differences in outcome variables between CHAMP and HEC groups from baseline to post-intervention. There were medium to large effect size estimates for some outcomes (see Table 2). BMI z-score change was greater for children in CHAMP compared to those in the HEC group (d = −.43). ACT scores (d = .45) and several lung function outcomes including FVC (d = .94), FEV1 (d = .66), FEFmax (d = .40), and FEF25–75 (d = .54) were also higher among children in CHAMP.
Table 2.
Outcome Data for Completers by Treatment Condition
| Outcome | CHAMP | HEC | F | p | Cohen’s (95% CI) |
|---|---|---|---|---|---|
| BMI z-score | |||||
| Δbaseline-post | −.08 (.16) | −.02 (.07) | .58 | .47 | −.43 (−1.58, .71) |
| Δbaseline-follow-up | −.12 (.24) | .01 (.20) | 1.11 | .32 | −.55 (−1.75, .67) |
| ACT | |||||
| Δbaseline-post | 2.17 (1.60) | 1.17 (2.32) | .29 | .61 | .45 (−.71, 1.58) |
| Δbaseline-follow-up | 3.20 (3.42) | 1.67 (1.75) | .43 | .53 | .50 (−.70, 1.71) |
| PACQLQ total | |||||
| Δbaseline-post | −.01 (1.13) | .67 (.77) | .83 | .39 | −.61 (−1.77, .55) |
| Δbaseline-follow-up | .22 (1.36) | .73 (.83) | .17 | .69 | −.41 (−1.60, .80) |
| PAQLQ total | |||||
| Δbaseline-post | .35 (1.52) | .28 (.42) | .02 | .88 | .05 (−1.07, 1.18) |
| Δbaseline-follow-up | 1.10 (1.21) | .69 (1.02) | .75 | .41 | .34 (−.86, 1.53) |
| FVC | |||||
| Δbaseline-post | 4.80 (7.94) | −2.40 (5.64) | 3.21 | .11 | .94 (−.31, 2.19) |
| Δbaseline-follow-up | 4.20 (11.88) | 3.50 (10.82) | .38 | .56 | .06 (−1.13, 1.24) |
| FEV1 | |||||
| Δbaseline-post | 6.00 (10.90) | −1.80 (10.47) | 1.75 | .22 | .66 (−.56, 1.88) |
| Δbaseline-follow-up | 0.80 (11.23) | 4.83 (12.83) | .04 | .85 | −.29 (−1.48, .90) |
| FEV1/FVC ratio | |||||
| Δbaseline-post | .83 (7.03) | .60 (10.21) | .85 | .38 | .03 (−1.16, 1.21) |
| Δbaseline-follow-up | −3.40 (5.81) | 2.33 (7.66) | .40 | .55 | −.68 (−1.90, .54) |
| FEF25–75 | |||||
| Δbaseline-post | 10.50 (26.17) | −4.60 (22.80) | 1.07 | .33 | .54 (−.66, 1.75) |
| Δbaseline-follow-up | −3.80 (17.66) | 5.33 (21.91) | .18 | .68 | −.39 (−1.59, .81) |
| FEFmax | |||||
| Δbaseline-post | 10.33 (10.75) | 6.40 (6.43) | 1.21 | .30 | .40 (−.80, 1.60) |
| Δbaseline-follow-up | 9.20 (11.43) | 12.83 (12.30) | .05 | .84 | −.27 (−1.46, .93) |
Note. All analyses included controlling for baseline level of dependent variable. Standard deviations appear in parentheses by means of change score. CI = confidence interval. Body Mass Index z-score (BMIz), Asthma Control Test (ACT), Pediatric Asthma Caregiver Quality of Life Questionnaire (PACQLQ), Pediatric Asthma Quality of Life Questionnaire (PAQLQ), forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), FEV1/FVC ratio (Ratio), forced expiratory flow 25–75%, (FEF25–75), maximum forced expiratory flow (FEFMax).
Long-term follow-up
Similarly, there were no statistically significant levels of change between the CHAMP and HEC groups from baseline to long-term follow-up. BMI z-score change remained higher among children in CHAMP compared to children in the HEC group (d = −.55). Medium effect sizes favoring CHAMP were also found for ACT scores (d = .50) and PAQLQ scores (d = .34). Children in the HEC group had higher lung function outcomes including FEV1 (d = −.29), FEV1/FVC (d = −.68), FEF25–75 (d = −.39), and FEFmax (d = −.27).
Discussion
The current study builds upon existing RCTs for youth with OV/OB and asthma by assessing the feasibility, acceptability, and preliminary efficacy of CHAMP, a BFI in children with OV/OB and asthma, compared to a rigorous attention control. Feasibility and acceptability data for CHAMP were mixed. Participant satisfaction with CHAMP was high, suggesting that CHAMP may be an acceptable intervention. However, a number of participants only attended the baseline study visit (n = 4) or ≤ 50% of the sessions (n = 9). Attendance difficulties occurred despite conducting the study at community health clinics and provider offices, providing travel compensation at each session, and offering free child care. Encountered participation barriers were not formally assessed. It is possible that time delays between baseline study visits and the start of groups, approximately one month for some families, may have contributed to attrition. Anecdotally, a number of families were also unable to attend sessions due to transportation barriers and work schedule conflicts. Sessions were only provided on designated weekday evenings which resulted in some families being unable to participate. These barriers are consistent with existing research in BFIs (Golley, Magarey, Baur, Steinbeck, & Daniels, 2007; Lim & Janicke, 2013). Additionally, while longer treatment duration has been associated with larger treatment effects in BFIs (Janicke et al., 2014), few families were able to attend all 16 sessions of CHAMP. Furthermore, known sociodemographic factors linked to attrition in BFIs include minority status and low income (Williams et al., 2010). The majority of families in the current study identified as African American and reported an annual income of <$35,000. These results indicate a need for future asthma and obesity interventions to be less burdensome on participants, especially when focusing on socioeconomically disadvantaged minority families.
Statistically significant differences between CHAMP and the HEC group were not found but should be interpreted in the context of our small pilot study design. The HEC comparator in the current study was an active attention control condition where we delivered education on physical activity, nutrition, and asthma management. Therefore, we expected high satisfaction ratings and modest improvements in outcomes within the control group. Examination of standardized mean differences among completers revealed small to medium effect sizes favoring CHAMP for BMI-z scores, asthma control, and several lung function outcomes from baseline to post-intervention. Similar effect size estimates were maintained for weight, asthma control, and asthma-related quality of life at the six month follow-up time point. Effect size estimates among completers from the current study are consistent with other weight management intervention modalities in youth with OV/OB and asthma (e.g., Jensen et al., 2014) and indicate that differences in weight and asthma control may exist several months post-intervention. The maintenance of effect sizes after six months of no contact is encouraging given the well documented challenge of maintaining health behavior and weight change improvements after the completion of format treatment contacts (Mead et al., 2017). Notably, however, lung function outcomes favored children in the HEC group at the six month follow-up time point. It is unclear why this shift in lung function findings between groups occurred. We plan to revisit these findings in the context of a larger, fully powered trial in the future.
Strengths of this study include a RCT design and use of a rigorous attention control condition. The attrition rate limits inferences from the current study. Additional limitations include only collecting satisfaction data from parents and not collecting information from families on reasons for attrition. A formal a priori power analysis was not conducted and we acknowledge that a modest sample size limits our statistical approach and inferences. Interventionists were not the same across intervention conditions which may have influenced study findings. Notably, all interventionists had similar training backgrounds, received the same level of intervention training, and fidelity monitoring was conducted throughout the trial. Interventionists also participated in collecting study outcomes. Although their participation in study visits was minimal (i.e., distributing questionnaire packets), this may have influenced findings. Finally, we did not assess change in healthy eating behaviors as an outcome.
Lessons Learned
The feasibility difficulties we encountered during this pilot trial are largely consistent with previous literature on barriers to BFI (Mead et al., 2017). Given the high satisfaction ratings of CHAMP and the effectiveness of BFIs for health behavior change (Janicke et al., 2014), future studies should consider ways to balance active BFI content while concurrently focusing on increasing feasibility. We are considering two ways to modify our BFI for children with OV/OB and asthma. First, we are in the beginning stages of transitioning CHAMP to an individual family model that involves nurse interventionists completing a small number of home visits with families and a series of telephone counseling sessions. Home visits will focus on delivering personalized feedback regarding activity and nutrition and reviewing core BFI behavior change strategies. Phone counseling sessions will be used to reinforce family progress towards behavior change goals and troubleshoot barriers. We hope that this design will increase feasibility by allowing for more convenient scheduling and reducing a need for participant travel. Furthermore, the widespread availability of nurses offers downstream dissemination potential. Second, given that mobile health (mHealth) interventions have demonstrated efficacy in improving health outcomes in youth (Fedele, Cushing, Fritz, Amaro, & Ortega, 2017), we are exploring ways to leverage mHealth as an alternative intervention modality that eliminates barriers related to transportation and scheduling conflicts. BFIs typically include a high number of contact hours. Therefore, cost effectiveness and sustainability of these future intervention modalities will be important factors to consider.
Conclusion
Overall, findings from the current study were mixed. Families reported high satisfaction with CHAMP and some modestly positive outcomes were found with regards to weight change and asthma outcomes when examining change in effect size estimates. However, families reported several barriers to intervention attendance, suggesting the need for feasible interventions to improve outcomes within this population. While significant results in comparison to the control group were not found, lessons learned from this pilot RCT provide direction for future research for weight management interventions in youth with asthma and OV/OB.
Supplementary Material
Implications for Impact.
Children with overweight or obesity (OV/OB) and asthma are at increased risk for experiencing negative health outcomes than their non-obese peers. We developed Childhood Health and Asthma Management Program (CHAMP), a 16- week family-based intervention for school-aged youth with overweight or obesity and asthma. Families reported high satisfaction with CHAMP and some modestly positive outcomes were found with regards to weight change and asthma outcomes; however, families reported several barriers to intervention attendance, suggesting the need for feasible interventions to improve outcomes within this population.
Acknowledgments
Financial Support: This work was supported by grant ALASB88692 from the American Lung Association and UL1TR001427 from the National Institutes of Health.
References
- Adair LS. Child and adolescent obesity: Epidemiology and developmental perspectives. Physiology & Behavior. 2008;94(1):8–16. doi: 10.1016/J.PHYSBEH.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Adeniyi FB, Young T. Weight loss interventions for chronic asthma. Cochrane Database Syst Rev. 2012;7 doi: 10.1002/14651858.CD009339.pub2. CD009339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belamarich PF, Luder E, Kattan M, Mitchell H, Islam S, Lynn H, Crain EF. Do obese inner-city children with asthma have more symptoms than nonobese children with asthma? Pediatrics. 2000;106(6):1436–1441. doi: 10.1542/peds.106.6.1436. [DOI] [PubMed] [Google Scholar]
- Coe R. It’s the Effect Size, Stupid: What effect size is and why it is important; Annual Conference of the British Educational Research Association.2002. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Statistical Power Analysis for the Behavioral Sciences. 1988 doi: 10.1234/12345678. [DOI]
- El- Kader M, Al-Jiffri O, Ashmawy E. Impact of weight loss on markers of systemic inflammation in obese Saudi children with asthma. African Health Sciences. 2013;13(3):682–8. doi: 10.4314/ahs.v13i3.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: Twenty-five years of pediatric obesity treatment. Health Psychology : Official Journal of the Division of Health Psychology, American Psychological Association. 2007;26(4):381–91. doi: 10.1037/0278-6133.26.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele DA, Cushing CC, Fritz A, Amaro CM, Ortega A. Mobile health interventions for improving health outcomes in youth: A meta-analysis. JAMA Pediatrics. 2017 doi: 10.1001/jamapediatrics.2017.0042. https://doi.org/doi:10.1001/jamapediatrics.2017.0042. [DOI] [PMC free article] [PubMed]
- Golan M, Fainaru M, Weizman A. Role of behaviour modification in the treatment of childhood obesity with the parents as the exclusive agents of change. International Journal of Obesity. 1998;22(12):1217–1224. doi: 10.1038/sj.ijo.0800749. [DOI] [PubMed] [Google Scholar]
- Golley RK, Magarey AM, Baur LA, Steinbeck KS, Daniels LA. Twelve-month effectiveness of a parent-led, family-focused weight-management program for prepubertal children: a randomized, controlled trial. Pediatrics. 2007;119(3):517–25. doi: 10.1542/peds.2006-1746. [DOI] [PubMed] [Google Scholar]
- Janicke DM, Sallinen BJ, Perri MG, Lutes LD, Huerta M, Silverstein JH, Brumback B. Comparison of parent-only vs family-based interventions for overweight children in underserved rural settings: outcomes from project STORY. Arch Pediatr Adolesc Med. 2008;162(12):1119–1125. doi: 10.1001/archpedi.162.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke DM, Steele RG, Gayes LA, Lim CS, Clifford LM, Schneider EM, Westen S. Systematic Review and Meta-Analysis of Comprehensive Behavioral Family Lifestyle Interventions Addressing Pediatric Obesity. Journal of Pediatric Psychology. 2014;39(8):809–825. doi: 10.1093/jpepsy/jsu023. [DOI] [PubMed] [Google Scholar]
- Jensen ME, Gibson PG, Collins CE, Hilton JM, Wood LG. Diet-induced weight loss in obese children with asthma: A randomized controlled trial. Clinical & Experimental Allergy. 2013;43(7):775–784. doi: 10.1111/cea.12115. [DOI] [PubMed] [Google Scholar]
- Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res. 1996;5(1):35–46. doi: 10.1007/BF00435967. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Johnson CL. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- Lan Xiao NL, Xiao L, Ma J. Weight management interventions in adult and pediatric asthma populations: A systematic review. Journal of Pulmonary & Respiratory Medicine. 2015;5(1) doi: 10.4172/2161-105X.1000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CS, Janicke DM. Barriers related to delivering pediatric weight management interventions to children and families from rural communities. Children’s Health Care. 2013;42:214–230. doi: 10.1080/02739615.2013.816596. [DOI] [Google Scholar]
- Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, Manjunath R. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007;119(4):817–825. doi: 10.1016/j.jaci.2006.12.662. [DOI] [PubMed] [Google Scholar]
- Luna-Pech JA, Torres-Mendoza BM, Luna-Pech JA, Garcia-Cobas CY, Navarrete-Navarro S, Elizalde-Lozano AM. Normocaloric diet improves asthma-related quality of life in obese pubertal adolescents. International Archives of Allergy and Immunology. 2014;163(4):252–258. doi: 10.1159/000360398. [DOI] [PubMed] [Google Scholar]
- Matricardi PM, Gruber C, Wahn U, Lau S. The asthma-obesity link in childhood: open questions, complex evidence, a few answers only. Clin Exp Allergy. 2007;37(4):476–484. doi: 10.1111/j.1365-2222.2007.02664.x. [DOI] [PubMed] [Google Scholar]
- Mead E, Brown T, Rees K, Azevedo LB, Whittaker V, Jones D, Ells LJ. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. In: Ells LJ, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Force AET. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- National Asthma Education and Prevention Program (NAEPP) Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obesity Reviews. 2016;17(2):95–107. doi: 10.1111/obr.12334. [DOI] [PubMed] [Google Scholar]
- Spear BA, Barlow SE, Ervin C, Ludwig DS, Saelens BE, Schetzina KE, Taveras EM. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl):S254–88. doi: 10.1542/peds.2007-2329F. [DOI] [PubMed] [Google Scholar]
- van Gent R, van der Ent CK, Rovers MM, Kimpen JLL, van Essen-Zandvliet LEM, de Meer G. Excessive body weight is associated with additional loss of quality of life in children with asthma. Journal of Allergy and Clinical Immunology. 2007;119(3):591–596. doi: 10.1016/j.jaci.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Willeboordse M, Kant KDG, van de Tan FES, Mulkens S, Schellings J, Crijns Y, Dompeling E. A multifactorial weight reduction programme for children with overweight and asthma: A randomized controlled trial. PLOS ONE. 2016;11(6):e0157158. doi: 10.1371/journal.pone.0157158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NA, Coday M, Somes G, Tylavsky FA, Richey PA, Hare M. Risk factors for poor attendance in a family-based pediatric obesity intervention program for young children. Journal of Developmental and Behavioral Pediatrics : JDBP. 2010;31(9):705–12. doi: 10.1097/DBP.0b013e3181f17b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.