Abstract
Quantum dot light-emitting devices (QLEDs), originally developed for displays, were recently demonstrated to be promising light sources for various photomedical applications, including photodynamic therapy cancer cell treatment and photobimodulation cell metabolism enhancement. With exceptional emission wavelength tunability and potential flexibility, QLEDs could enable wearable, targeted photomedicine with maximized absorption of different medical photosensitizers. In this paper, we report, for the first time, the in vitro study to demonstrate that QLEDs-based photodynamic therapy can effectively kill Methicillin-resistant Staphylococcus aureus, an antibiotic-resistant bacterium. We then present successful synthesis of highly efficient quantum dots with narrow spectra and specific peak wavelengths to match the absorption peaks of different photosensitizers for targeted photomedicine. Flexible QLEDs with a peak external quantum efficiency of 8.2% and a luminance of over 20,000 cd/m2 at a low driving voltage of 6 V were achieved. The tunable, flexible QLEDs could be employed for oral cancer treatment or diabetic wound repairs in the near future. These results represent one fresh stride toward realizing QLEDs’ long-term goal to enable the wide clinical adoption of photomedicine.
Keywords: flexible quantum dot light-emitting devices, photomedicine
1 Background
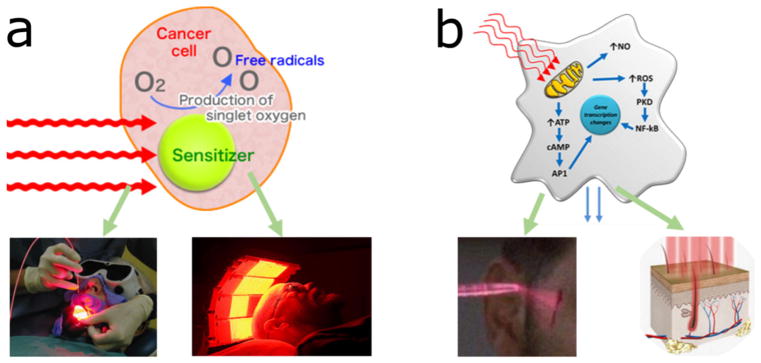
Photodynamic therapy (PDT) and photobiomodulation (PBM) are two branches of photomedicine that involve the application of light with respect to disease and health. In PDT (Fig. 1a), light of specific wavelength is used to excite photosensitizers (i.e., drugs that are nontoxic themselves, but can be activated by light exposure) and turn molecular oxygen into singlet oxygen that can kill unwanted tissues, cells (including cancer cells, bacteria, fungi, and viruses) and thus lead to the treatments of cancers, infections, etc. In PBM (Fig. 1b), light can enhance cellular function leading to beneficial clinical effects, such as wound repair or hair regrowth.1
FIGURE 1.
Scheme of working mechanism of (a) photodynamic therapy and (b) photobiomodulation.
Although PDT and PBM have already been clinically demonstrated as effective minimally invasive or noninvasive strategies to treat cancers and infections, improve wound repair, reduce pain, grow hair etc., they still have not received widespread acceptance mainly because of the challenging light source requirements: the ideal light source needs to have right color with narrow emission spectrum to match the absorption peaks of photosensitizers, high enough power density for sufficient excitation, but low heat to avoid pain for the patients, and flexible form factors with homogeneous emission so that can be easily applied to the patients without worrying about over or under treatments.
Currently, laser and LED arrays are dominating light sources in photomedicine field because they can provide sufficient power density at the proper wavelength window.2 However, these expensive, hot, rigid, heavy, and inhomogeneous light sources are not commonly available in small clinics, and treatments can only be carried out in limited places and require expensive hospital visits, limiting their further penetration into practical clinical use.
Organic light-emitting diodes (OLEDs) were once proposed to work as light-emitting bandages for PDT3 because of their unique form factors as thin, flexible, lightweight, and uniformly large area luminaire. But this method was later abandoned, mainly because relatively high light brightness (>20,000 cd/m2 or ~10 mW/cm2) at wavelengths of deep red region is demanded in photomedical field in order to have deep tissue penetration while still maintaining sufficient energy for molecular excitations.1 Existing OLEDs with either fluorescent or phosphorescent emitters cannot achieve such high brightness at the right wavelength windows because of significant efficiency roll-off problems of OLEDs at high current density4 and the lack of efficient deep red emitters with narrow spectra.5
Our group reported ultrabright and efficient deep red quantum dot light-emitting devices (QLEDs).6 The devices show peak emission wavelength of 620 nm, narrow bandwidth of 22 nm and can achieve high current efficiency (20.5 cd/A at ~20, 000 cd/m2) and small efficiency roll-off at high driving current density. Ultrahigh brightness of 165,000 cd/m2 can be achieved at current density of 1000 mA/cm2, which sets a brightness record for existing organic related red light-emitting devices.
With the potential to be low cost, wearable, disposable light-emitting bandage products, these ultrabright deep red QLEDs enjoy all form factor merits like OLEDs, while having emission peak width 3–5 times narrower, and power density 2–4 times higher (under similar driving conditions) than OLEDs for the mid-deep red spectral range. These advantages can translate into over one order improvement in photomedical treatment efficacy over traditional OLEDs. Being solution processable at low costs, QLEDs represent the ideal photomedical light sources with all desired features over other lighting strategies as summarized in Table 1.
TABLE 1.
Competitive advantages of QLEDs over other light source technologies for photomedicine
Preliminary PBM and PDT tests with these ultrabright QLEDs as light sources have been carried out in vitro and were reported last year in this journal. The experimental results demonstrated that QLED PBM can increase cell metabolism in multiple cell lines by ~11–25% over control systems,7,8 and QLED PDT can kill cancerous cells, in a similar fashion as inorganic LED arrays.8 The demonstrations of ultrabright deep red QLEDs and their effectiveness for PBM and PDT warrant further studies investigating QLED devices for photomedical applications.
In this paper, we present the results of in vitro study for QLEDs-based PDT Methicillin-resistant Staphylococcus aureus (MRSA) treatment, the developments of new quantum dot (QD) materials and the demonstration of flexible QLEDs. PDT using QLEDs as excitation source could effectively kill MRSA. QDs with precisely controlled emission peaks at the absorption wavelengths of photosensitizers for wound repair, inflammation, and cancer treatment applications were synthesized. QLED devices on flexible substrates have been fabricated, encapsulated with laminated barrier films, and characterized as potentially low cost, wearable, disposable light-emitting bandage products. Initial photomedicine markets for these flexible QLEDs have been identified and the potential impacts of these results to OLEDs, QLEDs, and photomedicine have been outlined.
2 Experiment and results
2.1 Preliminary photodynamic therapy Methicillin-resistant Staphylococcus aureus (MRSA) treatment results
Infections caused by multidrug-resistant bacteria such as MRSA are extremely resistant to conventional therapies including conservative antibiotic treatment. Laser-based PDT has been demonstrated as an efficient way to cure MRSA infections without invasive treatments.9 To evaluate the potential of red QLEDs as a light source for PDT infection treatment, we conducted an in vitro experiment to investigate the effectiveness of QLEDs-based PDT for killing MRSA.
MRSA bacterium was treated with 10 uM photofrin and 100 mM potassium iodide and then illuminated with QLEDs powered by a simple battery pack with two 3 V coin cells. As shown in Fig. 2, the survival fraction of MRSA dropped to less than 10−6 after 1 h illumination. The result not only demonstrated that QLEDs can kill MRSA efficiently for infection treatment but also showed the extreme simplicity of QLED-based treatment, that is, no optical fibers, sophisticated drivers, lens, mirrors, or any other supporting components are needed.
FIGURE 2.
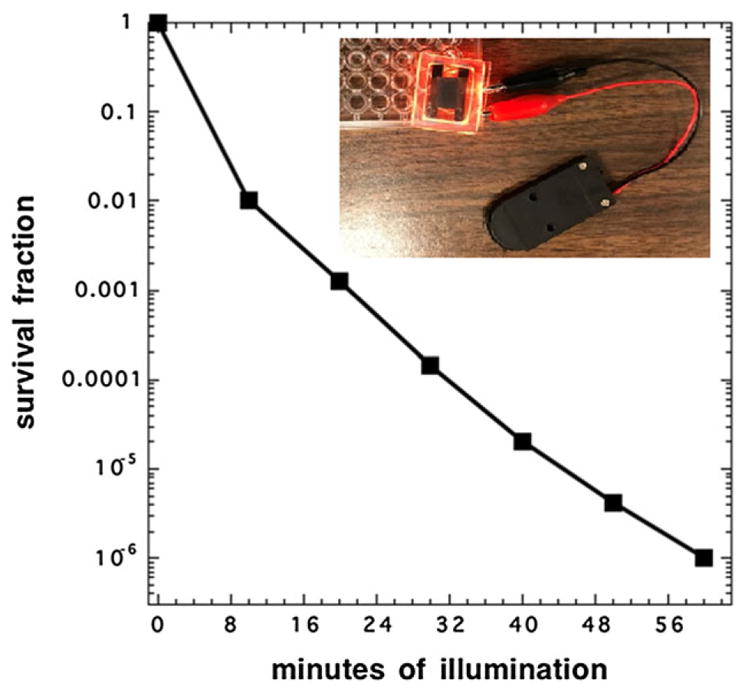
Survival fraction evolution of MRSA with continuous red quantum dot light-emitting device illumination assisted photodynamic therapy, inset: experimental setup.
2.2 Synthesis of quantum dots with different target wavelengths
While we had reported promising results of QLEDs-based PDT and PBM, it should be noted that the emission wavelength of the QLEDs we used was 620 nm. Although it falls into the favorite range for most photomedical applications (620–670 nm), highly effective phototherapy calls for better spectral control to maximize the absorption of specific photosensitizers for targeted photomedicine.
Thanks to quantum confinement effect, by tuning the QD synthesis conditions (QD’s size and composition), we have achieved highly efficient QD materials with precisely controlled emission peaks at the following wavelengths for targeted medical applications (shown in Fig. 3): (1) 631 nm for porfimer sodium (Photofrin®), an FDA approved photosensitizer widely used for various PDT cancer treatments; (2) 646 nm that is close to the absorption peak (652 nm) of Temoporfin, a photosensitizer (based on chlorin) used in PDT for the treatment of squamous cell carcinoma of the head and neck.
FIGURE 3.
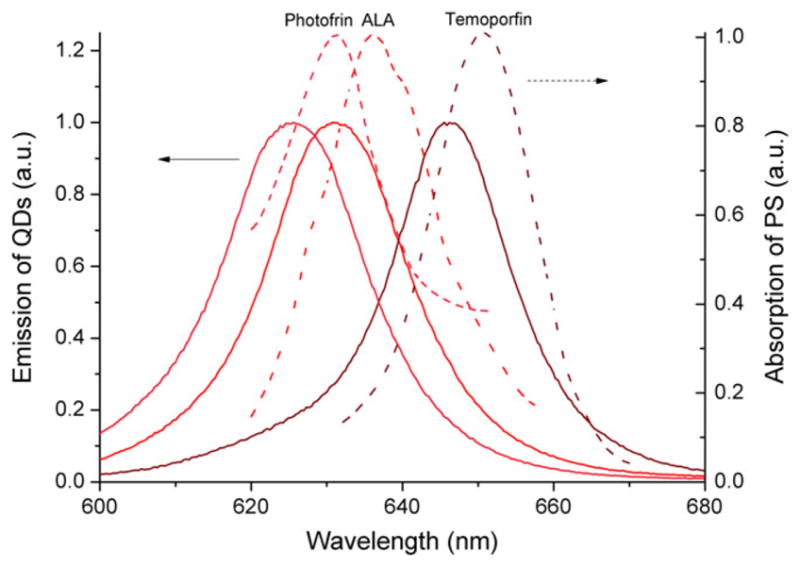
The absorption spectra (dashed line) of some common photosensitizers and the experimental emission spectra of quantum dots under UV excitation. Porfimer sodium (Photofrin®), aminolevulinic acid (ALA), and temoporfin are three photosensitizers widely used for various photodynamic therapy cancer treatments. QDs, quantum dots. Journal of the SID, 2018
Quantum dots with different wavelengths of 625, 631, and 646 nm all exhibit narrow spectra (full width at half maximum (FWHM) <22 nm) as shown in Fig. 3. With these QDs that fit well with the absorption of photosensitizers, better results of QLEDs-based PDT and PBM are expected.
The demonstrated tunability indicates that QDs’ emission should also be able to be tuned to the absorption of newly developed photosensitizers. Currently, such wavelength control is realized by expensive, bulky lasers, although the laser light needs to be waveguided with optical fibers and spread out with diffusers for large area applications. QLEDs have clear advantages over lasers in term of lower expense and less complexity.
2.3 Flexible devices
Flexible light sources are in obligatory demand for various medical situations when wearability and integrability are of uttermost importance, but have been challenging to achieve with existing expensive, hot, bulky, heavy, rigid, and inhomogeneous lasers or LED arrays. As a prominent example, Dr. Serge Mordon of French National Institute of Health and Medical Research developed a light-emitting fabric in which light from expensive lasers was guided through numerous leaky optical fibers that were woven into a fabric sheet for flexible, homogeneous light delivery for PDT.10 Compared with the light-emitting fabric system, flexible QLEDs have clear advantages as low cost, thin, lightweight, and inherently wearable or integrable light sources for demanding medical applications.
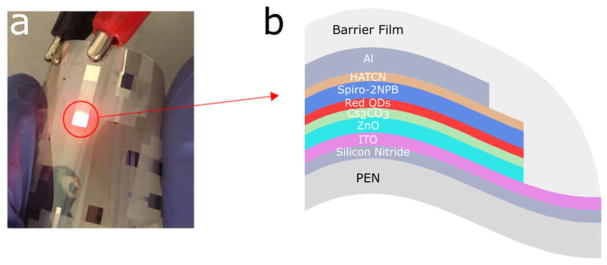
For flexible QLED fabrication, polyethylene naphthalate (PEN) film with transparent indium tin oxide (ITO) cathode conductor and silicon nitride barrier layer was employed as the substrate. The device stack consists of multiple layers as ITO/ZnO nanoparticles/Cs2CO3/CdSe-ZnS-CdZnS core-shell-shell QDs/2,2′,7,7′-tetrakis[N-naphthalenyl(phenyl)-amino]-9,9-spirobifluorene/1,4,5,8,9,11-hexaazatriphenylene-hexacarbonitrile/Al anode (Fig. 4b). These organic–inorganic hybrid QLED devices were fabricated by a combination of solution-processing and vacuum evaporation techniques as reported in Dong et al.6 The completed devices were later encapsulated with laminated barrier film developed by Holst Center (a hybrid thin-film encapsulation stack consisting of two inorganic barrier layers of silicon nitride deposited at low temperature with an organic layer in between11) (Fig. 4).
FIGURE 4.
(a) Photograph of one flexible quantum dot light-emitting device lighting up in air and (b) structure of the flexible devices on PEN substrate. ITO, indium tin oxide; PEN, polyethylene naphthalate; QDs, quantum dots.
After encapsulation, the devices were tested in ambient condition. The electroluminescence spectrum (as exhibited in Fig. 5a) displays a saturated QD emission profile (FWHM of 28 nm with a peak wavelength of 630 nm), falling right into the absorption range of Photofrin® as shown in Fig. 3.
FIGURE 5.
Flexible inverted quantum dot light-emitting devices. (a) Spectra of quantum dot light-emitting device electroluminescence at 3 V; (b) luminance and current density versus driving voltage; and (c) external quantum efficiency and luminous power efficiency versus luminance for typical devices. Device was tested at ambient condition (temperature: ~25°C; humidity: ~70%).
Figure 5b demonstrates the current-density/luminance/voltage (J-L-V) characteristics of the flexible devices. With a turn-on voltage of 1.9 V, the QLEDs could achieve a peak external quantum efficiency (EQE) of 8.2% at the current density of 19 mA/cm2, corresponding to a luminance of 1800 cd/m2. Luminance can reach up to over 20,000 cd/m2, which is sufficient for photomedicine treatment, at a current density of 283 mA/cm2 and a driving voltage of only 6 V. It should be noted that the devices exhibit relatively low efficiency roll-off at high current densities, compared with typical OLEDs/QLEDs (Fig. 5c). QLEDs’ EQE only dropped 1.5% when luminance increased from 1100 nits (8.2%) to 20,000 nits (6.7%).
Although the lifetime of these flexible QLEDs are limited in air at current status, it is expected that established flexible OLED encapsulation technologies (e.g., atomic layer deposition (ALD) encapsulations) could prolong the lifetime of QLEDs to a level to satisfy clinical requirements of photomedical treatments. Ultrabright flexible QLEDs represent the ideal light sources to work as highly efficient, low-cost, wearable, and disposable bandage products for photomedical treatment in terms of the high luminance, wavelength tunability, and narrow spectra.
3 Flexible QLEDs for treatment of two specific diseases: Diabetic wound and oral cancer
The vast opportunities of photomedicine posed a special challenge for research decisions. After careful evaluation, oral cancer and diabetic wounds treatments were selected as initial QLED treatment targets because of the technical feasibility, high social impacts, and commercialization potential. The treatment of oral cancer or diabetic wounds has urgent requirements of device flexibility, light homogeneity, while the device size can be small (<2 cm2) and can thus be fabricated in research or pilot scale laboratory at low cost (<$10/piece).
Oral cancer has been considered as a global health crisis because of its high incidence in India. Although largely preventable, cancers of the oral cavity account for over 30% of cancers reported in India. This is one of the highest oral cancer rates in the world and is largely due to the widespread popularity of chewing gutka, a tobacco mixture with crushed betel nut and acacia extract. Treatment typically consists of surgery and/or radiotherapy, which require expertise and medical infrastructure that are often not available in the settings where they are most needed. Even if the disease is detected relatively early, these interventions can be disfiguring and present major quality of life issues including the ability to chew, swallow, speak, and work, thus increasing the societal economic burden on an already burdened economy. On the other hand, early clinical studies showed that PDT is a safe and effective approach, with remarkable healing and is especially effective for early stage cancerous and precancerous lesions of the oral cavity. While PDT photosensitizer is readily available, the expensive laser light source that is currently main stream treatment option is not.12,13
Wound healing in diabetes mellitus is often impaired and results in nonhealing or long-lasting chronic skin ulcers. Current treatment of the diabetic wound includes systemic glycemic control, local wound care and infection control, revascularization, and pressure relieving strategies. However, results from existing multidisciplinary treatments are often unsatisfactory. PBM with red light has been demonstrated to improve diabetic wound healing by accelerating collagen production, enhancing angiogenesis, increasing wound closure rate, and increasing growth factor expression. While OLEDs have been applied to improve diabetic cutaneous wound healing in rats, their broad emission peaks and relatively low power density remain limiting the treatment effects.14,15
For both oral cancer treatment and diabetic wound repairs, flexible QLED light source is expected to greatly simplify the light source setup, lower the overall treatment cost, and enhance quality of life of patients.16,17
Although the public concern of cadmium contained in QD materials could be a political challenge without solid biomedical evidences, it should be noted that QLEDs with hermetic encapsulations leave little chance of cadmium leakage. The concern for cadmium can be eased out through serious medical evaluation of the QLED’s beneficial treatment effect and close monitoring of any possible side effects.
4 Impact and perspectives
In this paper, we demonstrated that QLEDs-based PDT can effectively kill MRSA bacteria. QDs with high efficiency, narrow spectra, and specific wavelengths of interests to photomedicine were successfully synthesized. The emission spectra of these QDs can match well the absorption of different photosensitizers, thus could improve the photomedical treatment efficacy. Flexible QLEDs, which could be perfect light sources for photomedicine, were fabricated and demonstrated peak EQE up to 8.2% and high luminance over 20,000 cd/m2 at low driving voltage.
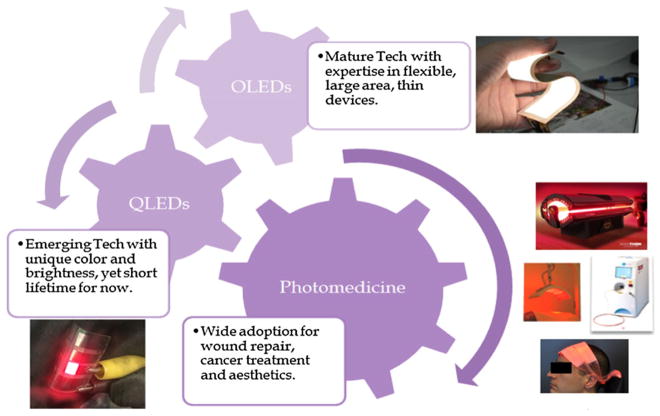
These progresses further demonstrate the feasibility of using QLEDs for photomedical applications and prepared us well for future flexible QLED-based targeted photomedicine developments. To move the project forward to clinical adoptions of QLEDs in photomedicine, we envisioned that technical challenges lie on the inherently multidisciplinary nature of this project that requires deep understanding and close collaborative progress on QLED device performance, medical treatments, and regulatory approval process. These challenges, on the other hand, could create a huge opportunity for OLEDs, QLEDs and photomedicine with tremendous potential technical, economic, and social impacts (Fig. 6). For mature OLED technology, their established knowledge of flexible devices will be important for further development of flexible
FIGURE 6.
Potential impact of QLEDs-photomedicine. Gearing up three industries: OLEDs, QLEDs, and photomedicine. OLEDs, organic light-emitting diodes; QLEDs, quantum dot light-emitting devices.
QLEDs which, as an emerging technology, have clear advantages in color tunability, color purity, and high power density over state-of-the art OLEDs. The joint efforts of OLED and QLED communities will enable advanced thin, flexible, lightweight, homogeneously large area QLED devices that will gear up the adoption of photomedicine in multiple hundred-billion-dollar healthcare markets,18,19 helping manage cancer, acute and chronic wounds, inflammation, and antimicrobial resistance among others.
Acknowledgments
We thank Dr. Gal Shafirstein of Roswell Park Cancer Institute for helpful discussion on photomedicine and Prof. Ho-Kyoon Chung of Sungkyunkwan University for insightful suggestions on flexible QLEDs developments. Y. D. thanks the American Society for Laser Medicine and Surgery (ASLMS) and Community Foundation of North Central Wisconsin for A. Ward Ford Memorial Research Grant support.
Biographies

Hao Chen received his BS degree in Optical Information Science and Technology from Huazhong University of Science and Technology in 2011, his MS degree in Physical Electronics from Wuhan Research Institute of Posts and Telecommunications in 2014, and is currently working toward his PhD degree from the College of Optics and Photonics, University of Central Florida, Orlando. His current research in Dr. Yajie Dong’s group focuses on synthesis of colloidal quantum dots, development of QLEDs, and the applications of QLEDs in display, lighting, and photomedicine. He was a recipient of SID Distinguished Student Paper Award in 2016 and 2017.

Tzu-Hung Yeh received his MS degree from the Department of Electronic Engineering at Ming Chi University of Technology in 2016. Then, he is currently working toward his PhD at National Taiwan University of Science and Technology. His research focuses on intrinsic characteristics of organic/inorganic materials and their relevant devices, including light-emitting diodes, photovoltaic cells, and novel optoelectronic applications. Besides, he is the member of Organic Electronics Research Center under the relationship with Prof. Shun-Wei Liu.

Juan He received her BS and MS degrees from the Department of Physics at Peking University, Beijing, China, in 2011 and 2014, respectively. She is currently working toward her PhD degree at the College of Optics and Photonics, University of Central Florida, Orlando, FL, USA. Her research focuses on quantum dots and perovskite materials for display, lighting, sensing, and lasing application. She was a recipient of SID Distinguished Student Paper Award in 2016 and 2017.

Caicai Zhang received her BS degree in Physical Science and Technology department from Lanzhou University in 2013, her MS degree in Materials Science and Engineering from University of Florida, Gainesville, in 2016, and is currently working toward her PhD degree in Materials Science and Engineering at University of Central Florida, Orlando. Her current research in Dr. Yajie Dong’s group focuses on metal halide perovskite luminescent materials and devices for display, lighting, and other novel applications.

Robert Abbel studied chemistry at the universities of Mainz (Germany) and Toronto (Canada). In 2004, he joined Eindhoven University of Technology (the Netherlands) for his PhD research project on supramolecular chemistry. Since 2009, he is working at Holst Center, first in the research group “Printed Functional Structures,” and since 2015, in the technology program “Lighting and Signage.” His core activities are technology development for the large area coating of functional inks and LED processing from solution.

Michael R Hamblin PhD is a Principal Investigator at the Wellman Center for Photomedicine, Massachusetts General Hospital, an Associate Professor of Dermatology, Harvard Medical School, and affiliated faculty at Harvard-MIT Division of Health Science and Technology. He directs a laboratory of around a dozen scientists who work in photodynamic therapy and photobiomodulation. He has published 346 peer-reviewed articles, is Editor or Associate Editor for 10 journals, and serves on NIH Study-Sections. He has an h-factor of 77 and over 21,700 citations. He has authored/edited 11 proceedings volumes together with 10 other major textbooks on PDT and photomedicine. Dr. Hamblin was honored by election as a Fellow of SPIE in 2011 and received the 1st Endre Mester Lifetime Achievement Award from NAALT in 2017.

Yingying Huang, MD, PhD is an instructor at Harvard Medical School in Dr. Hamblin’s laboratory. Dr. Huang received her MD degree from Xiangya Medical School of Central South University. She received her MS degree in dermatology in Sun Yat-sen University. She received her PhD in pathology from Guangxi Medical University. Dr. Huang’s research interests lie in photodynamic therapy (PDT) for infections, cancer, and mechanisms of low-level light therapy (LLLT) on injured cortical neurons. She has published over 60 peer-reviewed articles and 15 conference proceedings and book chapters.

Raymond J. Lanzafame, MD, MBA, FACS received a Bachelor of Science with Honors and Distinction from Cornell, MD from George Washington University, and MBA from the Simon School of University of Rochester. He has specialized expertise in credentialing, medicolegal consulting, lasers, and safety and holds key leadership and board positions in multiple professional and scientific organizations and task forces, including consultant to the General and Plastic Surgery Devices Advisory Committee of FDA. Publications include over 250 papers and three textbooks. His research interests include light-tissue interactions, imaging, host-tumor immunity, wound healing, photobiomodulation, photodynamic therapy, biomaterials, medical device and product development, technology assessment, clinical outcomes, and education.

Istvan Stadler is a Research (Surgical) Scientist of Laser Center at Rochester General Hospital. He has a PhD degree of Biochemistry/Biophysics from the School of Natural Sciences, Eotvos Lorand University, Budapest Hungary, and is Research Assistant Professor in Medicine at SUNY Buffalo School of Medicine. His research focuses on biological mechanisms of photobiomodulation, developing new treatment modalities for impaired wound healing, and evaluating surgical and biomedical applications of laser technologies. His research is published in 70 peer-reviewed publications and in 72 presentations at national meetings. His translational research has received international attention of doctors, dentists, veterinarians, research scientists among others, providing new basic information on photobiomodulation mechanisms. Journal of the SID, 2018

Jonathan Celli is an Associate Professor in the Department of Physics at the University of Massachusetts Boston. His lab is focused on research at the intersection of cancer biology, biophysics, and photomedicine and is pursuing several projects on photodynamic therapy for cancer treatment. Prior to joining the University of Massachusetts, Prof. Celli was junior faculty at the Wellman Center for Photomedicine at Massachusetts General Hospital and Harvard Medical School.

Shun-Wei Liu is an Associate Professor in the Department of Electronic Engineering at the Ming Chi University of Technology (MCUT) as well as a Director of Organic Electronics Research Center in MCUT. His current research interests are in the area of understanding the fundamental physics of organic materials and their relevance in novel optoelectronic devices, that is, organic photovoltaic driven image devices, ITO-free organic light-emitting devices, and transparent electronics. Until now, Dr. Liu has published over 120 international journal papers. The details of laboratory’s core facility can be found in the website (http://organicelectronics.mcut.edu.tw).

Shin-Tson Wu is a Pegasus professor at College of Optics and Photonics, University of Central Florida. He is among the first six inductees of the Florida Inventors Hall of Fame (2014), and a Charter Fellow of the National Academy of Inventors (2012). He is a Fellow of the IEEE, OSA, SID, and SPIE, and the recipient of 2014 OSA Esther Hoffman Beller medal, 2011 SID Slottow-Owaki prize, 2010 OSA Joseph Fraunhofer award, 2008 SPIE G. G. Stokes award, and 2008 SID Jan Rajchman prize. Presently, he is serving as SID honors and awards committee chair.

Yajie Dong is an assistant professor in NanoScience Technology Center of University of Central Florida, with joint appointment in College of Optics and Photonics and Department of Materials Science & Engineering. He received his BS and MS degrees in Chemistry from Tsinghua University of Beijing, China, and his PhD degree from Harvard University. Before joining NSTC of UCF in August 2014, he worked as a Senior Scientist at QD Vision Inc and a postdoc associate at Massachusetts Institute of Technology. He is an associate editor of Optics Express and a member of Emissive Display (EMD) subcommittee of SID Technical Program Committee.
Contributor Information
Hao Chen, College of Optics and Photonics, University of Central Florida, Orlando, FL, USA. Nanoscience Technology Center, University of Central Florida, Orlando, FL, USA.
Tzu-Hung Yeh, Department of Electronic Engineering, National Taiwan University of Science and Technology, Taipei City, Taiwan. Organic Electronics Research Center, Ming Chi University of Technology, New Taipei City, Taiwan.
Juan He, College of Optics and Photonics, University of Central Florida, Orlando, FL, USA.
Caicai Zhang, Nanoscience Technology Center, University of Central Florida, Orlando, FL, USA. Department of Materials Science & Engineering, University of Central Florida, Orlando, FL, USA.
Robert Abbel, Holst Centre-TNO, Eindhoven, Netherlands.
Michael R. Hamblin, Harvard Medical School, Wellman Center for Photomedicine, Boston, MA, USA
Yingying Huang, Harvard Medical School, Wellman Center for Photomedicine, Boston, MA, USA.
Raymond J. Lanzafame, Raymond J Lanzafame MD PLLC, Rochester, NY, USA. Laser Surgical Research Laboratory, Rochester General Hospital, Rochester, NY, USA
Istvan Stadler, Laser Surgical Research Laboratory, Rochester General Hospital, Rochester, NY, USA.
Jonathan Celli, Department of Physics, University of Massachusetts Boston, Boston, MA, USA.
Shun-Wei Liu, Organic Electronics Research Center, Ming Chi University of Technology, New Taipei City, Taiwan.
Shin-Tson Wu, College of Optics and Photonics, University of Central Florida, Orlando, FL, USA.
Yajie Dong, College of Optics and Photonics, University of Central Florida, Orlando, FL, USA. Nanoscience Technology Center, University of Central Florida, Orlando, FL, USA. Department of Materials Science & Engineering, University of Central Florida, Orlando, FL, USA.
References
- 1.Hamblin MR, Huang YY, editors. Handbook of Photomedicine. Taylor and Francis; Oct 15, 2013. [Google Scholar]
- 2.Evans J. High-tech bandages lighten the load of light therapy. Nat Med. 2009;15:713. doi: 10.1038/nm0709-713a. [DOI] [PubMed] [Google Scholar]
- 3.http://www.ambicarehealth.com/ambulight-pdt/
- 4.Murawski C, et al. Efficiency Roll-Off in Organic Light-Emitting Diodes. Adv Mater. 2013;25:6801–6827. doi: 10.1002/adma.201301603. [DOI] [PubMed] [Google Scholar]
- 5.Chen CH, et al. Highly efficient orange and deep-red organic light emitting diodes with long operational lifetimes using carbazole–quinoline based bipolar host materials. J Mater Chem C. 2014;2:6183–6191. doi: 10.1039/c4tc00523f. [DOI] [Google Scholar]
- 6.Dong YJ, et al. 20.2: Ultra-Bright, Highly Efficient, Low Roll-Off Inverted Quantum-Dot Light Emitting Devices (QLEDs) SID Symp Dig Tech Pap. 2015;46:270–273. doi: 10.1002/sdtp.10462. [DOI] [Google Scholar]
- 7.Lanzafame RJ, et al. Preliminary studies of a novel red-emitting quantum dot LED source for PBM applications. 2017 conference of American Society for Laser Medicine and Surgery (ASLMS). [Google Scholar]
- 8.Chen H, et al. Quantum dot light emitting devices for photomedical applications. J Soc Inf Disp. 2017;25:177–184. doi: 10.1002/jsid.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka M, et al. Photodynamic Therapy Using Intra-Articular Photofrin for Murine MRSA Arthritis: Biphasic Light Dose Response for Neutrophil-Mediated Antibacterial Effect. Lasers Surg Med. 2012;43:221–229. doi: 10.1002/lsm.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mordon S, et al. Light emitting fabric technologies for photodynamic therapy. Photodiagnosis Photodyn Ther. 2015;12:1–8. doi: 10.1016/j.pdpdt.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Weijer PV, et al. High-performance thin-film encapsulation for organic light-emitting diodes. Org Electron. 2017;44:94–98. doi: 10.1016/j.apsusc.2017.12.007. [DOI] [Google Scholar]
- 12.Hempstead J, et al. Low-cost photodynamic therapy devices for global health settings: Characterization of battery-powered LED performance and smartphone imaging in 3D tumor models. Sci Rep. 2015;5:10093. doi: 10.1038/srep10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mallidi S, et al. In vivo evaluation of battery-operated light-emitting diode-based photodynamic therapy efficacy using tumor volume and biomarker expression as endpoints. J Biomed Opt. 2015;20:048003. doi: 10.1117/1.JBO.20.4.048003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.https://www.youtube.com/watch?v=pfYTnvE1LRc
- 15.Wu X, et al. Organic light emitting diode improves diabetic cutaneous wound healing in rats. Wound Repair Regen. 2015;23:104–114. doi: 10.1111/wrr.12258. [DOI] [PubMed] [Google Scholar]
- 16.Oakley E, et al. A new finite element approach for near real-time simulation of light propagation in locally advanced head and neck tumors. Lasers Surg Med. 2015;47:60–67. doi: 10.1002/lsm.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanzafame RJ, Stadler I. In: Chapter 32 Handbook of Low-Level Laser Therapy. Hamblin MR, Agrawal T, de Sousa M, editors. CRC Press; Pan Stanford: 2016. [Google Scholar]
- 18.Sen CK, et al. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. [accessed 11/03/17]; http://costprojections.cancer.gov.