Abstract
Increasing concerns on environmental and economic issues linked to fossil fuel use has driven great interest in cyanobacteria as third generation biofuel agents. In this study, the biodiesel potential of a model photosynthetic cyanobacterium, Fremyella diplosiphon, was identified by fatty acid methyl esters (FAME) via direct transesterification. Total lipids in wild type (Fd33) and halotolerant (HSF33-1 and HSF33-2) strains determined by gravimetric analysis yielded 19% cellular dry weight (CDW) for HSF33-1 and 20% CDW for HSF33-2, which were comparable to Fd33 (18% CDW). Gas chromatography-mass spectrometry detected a high ratio of saturated to unsaturated FAMEs (2.48-2.61) in transesterified lipids, with methyl palmitate being the most abundant (C16:0). While theoretical biodiesel properties revealed high cetane number and oxidative stability, high cloud and pour point values indicated that fuel blending could be a viable approach. Significantly high FAME abundance in total transesterified lipids of HSF33-1 (40.2%) and HSF33-2 (69.9%) relative to Fd33 (25.4%) was identified using comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry, indicating that robust salt stress response corresponds to higher levels of extractable FAME. Alkanes, a key component in conventional fuels, were present in F. diplosiphon transesterified lipids across all strains confirming that natural synthesis of these hydrocarbons is not inhibited during biodiesel production. While analysis of photosynthetic pigments and phycobiliproteins did not reveal significant differences, FAME abundance varied significantly in wild type and halotolerant strains indicating that photosynthetic pathways are not the sole factors that determine fatty acid production. We characterize the potential of F. diplosiphon for biofuel production with FAME yields in halotolerant strains higher than the wild type with no loss in photosynthetic pigmentation.
Keywords: Alkane, Biofuel, Lipid, Phycobiliprotein
Introduction
Adverse environmental effects caused by the use of fossil fuels have resulted in an increased demand for renewable energy in recent years [1]. While first generation biofuels produced by fermentation of edible crops such as sugarcane [2] and corn [3] are the most common alternative to fossil fuels, these are controversial as land designated for food supply is diverted to fuel production. Third generation biofuel sources such as cyanobacteria and microalgae offer a viable and renewable hydrocarbon feedstock without compromising agricultural land. In particular, photosynthetic cyanobacteria can be exploited as cost-effective biofuel agents due to readily available genome sequences, high growth rate, ability to thrive in marginal areas, and minimal nutrient requirements [4]. Recently, cyanobacteria engineered through manipulation of genes that regulate photosynthetic processes and carbohydrate/fatty acid synthesis pathways have gained great importance in high-value biofuels such as biogas, cellulosic ethanol, and biodiesel [5].
Transesterification of lipids and oils is the primary process for conversion of metabolic products to generate biodiesel and jet fuel [6]. The reaction yields fatty acid methyl esters (FAMEs), which are the major component in biodiesel resulting in fuels containing lower sulfur and carbon monoxide emissions relative to conventional diesel [7, 8]. Since lipids store twice as much energy per gram as carbohydrates, they are highly valuable for biofuel production, signifying a greater energy capacity than other potential products [9]. The process can also be applied to a wide range of feedstocks including algal oils [10], terrestrial plant lipids [11], and waste cooking oil [12] for biodiesel production. In algae and terrestrial plants, neutral triacylglycerols (TAGs) contain fatty acids which are essential precursors for FAME synthesis via traditional two-step extraction/transesterification reaction [7, 13]. Unlike eukaryotic microalgae, cyanobacteria produce minimal amounts of TAG in their photosynthetic membranes since free fatty acids (FFA) are directly shuttled to the membrane lipid synthesis pathway [14]. The development of a single-step direct transesterification process ensures high FAME yields through efficient FFA extraction from lipid molecules [15, 16]. In addition, alkanes are an essential component of petroleum-based fuel that are naturally synthesized from fatty acids in cyanobacteria [17, 18]. Specifically, fatty acids are first converted into a fatty aldehyde intermediate, then synthesized by aldehyde decarbonylase into an alkane [17]. This process provides an additional benefit to cyanobacteria-based biofuels since conventional fuels also contain alkanes, thus enabling the biodiesel product to be more compatible with vehicle engines [19].
The hydrocarbon and fatty acid profile of several microalgal species has been characterized as they have a great potential for biodiesel production [19, 20]. Fremyella diplosiphon, a freshwater cyanobacterium, is a widely studied model organism due to its ability to absorb different wavelengths of light and acclimate to various environmental conditions and water depths [21]. This unique process known as complementary chromatic adaptation enhances light capture conversion capacity reducing the artificial light input required in a cultivation system [22]. Recent efforts to enhance halotolerance in F. diplosiphon has resulted in a strain that tolerates exposure to 20 g L−1 sodium chloride (NaCl) [23]. An attempt to further increase salt tolerance by gene overexpression has resulted in strains that thrive in 35 g L−1 NaCl, the average salinity of marine waters [24]. While several studies report direct transesterification leading to FAME production in other cyanobacteria [7, 16], to the best of our knowledge, no such information is available in F. diplosiphon. Here, we report the total lipid content in F. diplosiphon, and FAME composition and theoretical biodiesel properties of lipids subjected to direct transesterification. Additional structural information from comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC-TOFMS) highlights compositional differences between wild type and halotolerant strains, specifically with regards to FAME and alkane profiles. Chlorophyll a (chla), carotenoid, and phycobiliprotein levels were compared to assess the impact of photosynthetic pigment accumulation on fatty acid yield.
Materials and Methods
Strain and Culture Conditions
Strains HSF33-1 and HSF33-2 engineered by overexpression of hlyB and mdh halotolerant genes [21] in short filamentous F. diplosiphon wild type strain Fd33 [25] were used in this study. Cultures were grown in liquid BG-11 medium [26] with 20 mM HEPES (hereafter referred as BG-11/HEPES) supplemented with 35 g L−1 NaCl at 170 rpm and 28 °C under continuous white light adjusted to 30 μmol m−2 s−1 (model LI-190SA quantum Sensor, Li-Cor, USA). Non-transformed Fd33 grown in BG11/HEPES without NaCl under similar conditions served as control.
Total Lipid Content in Wild Type and Halotolerant Strains using Gravimetric Analysis
Total lipid content in F. diplosiphon was determined using a chloroform: methanol extraction method based on Folch et al. [27] reported in Wahlen et al. [7]. Dried samples (100-200 mg) were sonicated in 5 mL of chloroform: methanol (2:1 by volume) for 30 s and centrifuged at 6000 rpm before transfer to a new tube, washed with 1 mL distilled water, and centrifuged at 2000 rpm to facilitate phase separation. While methanol and sulfuric acid partitioned with water in the upper phase, FAME and lipids separated with chloroform in the lower phase. The organic phase was pooled into a preweighed vial and re-extracted twice as mentioned above. Organic extracts were dried in a rotary evaporator and weighed to determine lipid yield of each sample. Three biological replicates were maintained, and the exact experiment repeated once. Significance among cumulative treatment means was determined using one-way analysis of variance (ANOVA) and Tukey’s Honest Significant Differences post-hoc test at 95% confidence intervals (P<0.05). The single factor, fixed effect ANOVA model, Yij = μ + αSi + εij, was used where Y is the total lipid yield content in strain i and biological replicate j. The μ represents overall total lipid content mean with adjustments from the effects of strain (αS), and εij is the experimental error from genotype i and biological replicate j.
Simultaneous Extraction and Transesterification
Lipids in F. diplosiphon strains were extracted and converted to FAMEs through direct transesterification that combines extraction and in situ biofuel production [7]. Lyophilized cells (100 mg) were dissolved in 2 mL methanol containing 1.8% (v/v) sulfuric acid and microwaved at 80 °C (1378.95 kPa for 20 min) in a commercial multimode scientific microwave (CEM Corporation, USA) with a maximum power output set at 25W per sample. The reaction was quenched with 4 ml chloroform, the mixture washed with 5 mL distilled water, and centrifuged at 2000 rpm for phase separation. The organic (chloroform) phase containing FAMEs and lipids was isolated and transferred to a new flask. Remaining biomass was washed twice with 2 mL chloroform and the combined organic phase mixed by inversion.
Gas Chromatography-Mass Spectrometry of Transesterified Product for FAME Analysis
Fatty acid composition of transesterified material obtained from in situ transesterification was determined using a Shimadzu GC17A/QP5050A GC/MS combination (Shimadzu Instruments, USA) at the Mass Spectrometry Facility at Johns Hopkins University (Baltimore, MD). The GC17A was equipped with a low polarity (5% phenyl-, 95% methyl-siloxane) capillary column (30 m length, 0.25 mm ID, 0.25 μm film thickness, and 10 m length guard column). Products were dissolved in chloroform and 1 μL injected into the instrument using an autosampler. The injector temperature and transfer interface were maintained at 280 °C. The oven temperature was initially held at 130 °C for 10 min, then ramped to 160 °C (hold for 7 min); from 160 °C to 190 °C (hold for 7 min), 190 °C to 220 °C (hold for 22 min) and 220 °C to 250 °C (hold for 17 min) at a rate of 10 °C min−1 for each step [28]. The QP5050A EI quadrupole had a mass scan range from m/z 40 to 900 and electron-impact ionization at 70 eV. Peak identification was accomplished by comparing mass spectra to the American Oil Chemists Society Lipid Library Spectra of FAME. Three biological replicates of each sample were analyzed and the experiment repeated once. In addition, biodiesel properties of the transesterified lipids were analyzed theoretically from fatty acid composition (w%) using BiodieselAnalyzer© software Version 2.2 [29].
GC×GC-TOFMS Analysis of Total Transesterified Lipids
High resolution GC×GC-TOFMS from LECO (USA) was used to identify FAMEs from the wild type and halotolerant strains. Total lipids were extracted, subjected to direct transesterification as described above [7], dried under dry N2 (g), and reconstituted in 2 ml dichloromethane with cholestane (50 μg mL−1) spiked as an internal standard. For each sample, 1 μL transesterified lipid extract was injected splitless. The first dimension column was a BP-1 (60 m × 0.25 mm ID, 0.25 μm film thickness, 100% polysiloxane, SGE. Inc.), and the second dimension was a BPX50 (1.5 m × 0.1 mm ID, 0.1 μm film thickness, 50% Phenyl, SGE, Inc.). A flow rate of 1 mL min−1 was used for the helium carrier gas, and the GC inlet temperature was 300 °C. The GC oven temperature was initially held at 40 °C for 0.5 min, then ramped at 2 °C min−1 to 340 °C for 10 min. The secondary oven had a temperature offset of +5 °C from the first oven. The modulator had an offset of +10 °C with a modulation period of 6 s and hot pulse of 0.8 s. Electron-impact ionization at 70 eV was used with the ion source set at 225 °C and the transfer line at 280 °C. MS data were collected with an acquisition rate of 100 spectra/s and mass range from m/z 40 to 550.
Photosynthetic Pigment Accumulation in Wild Type and Halotolerant F. diplosiphon
Photosynthetic pigment (chla, and carotenoids) and phycobiliproteins (phycocyanin, phycoerythrin, and allophycocyanin) were extracted and quantified as previously described [30, 31]. Cultures were grown in liquid BG-11/HEPES to an optical density of 0.6 at a wavelength of 750 nm (OD750) under fluorescent white light. Samples were incubated on ice for 1 h, centrifuged at 13,000 × g for 5 min, and the supernatant removed. For chla and carotenoid extraction, flash frozen pellets were resuspended in 500 μl of 90% methanol and incubated in the dark at 4 °C for 1 h with rocking. Samples were centrifuged at 10,000 × g for 10 min and the process repeated. The supernatant was pooled into preweighed microfuge tubes and the weight of each sample recorded. Absorbance values of the supernatant were read at 470 and 665 nm for carotenoids and chla respectively.
For phycobiliproteins extraction, pellets were resuspended in 1 ml of ice cold STES (50 mM Tris-HCl, 50 mM NaCl, 10 mM EDTA, 250 mM sucrose) containing 5 mg/ml (w/v) lysozyme and incubated in dark at room temperature for 30 min with rocking. Cells were centrifuged at 13,000 × g for 5 min at room temperature and optical density of the supernatant measured at 565, 620, and 650 nm. Phycobiliprotein levels were calculated according to Tandeau de Marsac and Houmard [31] and reported relative to chla as described [32].
Results and Discussion
Gravimetric Analysis for Total Lipid Extraction
Total cellular lipid content is a prerequisite to determining the capacity of a candidate organism for high quality biodiesel production. In particular, gravimetric analysis offers highly precise and reproducible measurements of total lipid content in microalgae and cyanobacteria [7]. While total lipid yield in wild type and halotolerant F. diplosiphon detected by gravimetric analysis ranged between 18 and 20% cellular dry weight (CDW), no significant difference in yield was observed (Table 1; Fig. 1). This suggests that overexpression of halotolerance genes did not significantly alter lipid biosynthesis. Total lipid yield in F. diplosiphon was similar to that of Synechocystis sp. PCC 6803 (18.4% CDW) and Synechococcus elongatus (17.7% CDW) [7] indicating that F. diplosiphon yield is comparable to other cyanobacteria. Comparable lipid yields in the halotolerant strains and its freshwater counterpart indicate that HSF33-1 and HSF33-2 can be cultivated in sea water, thus maximizing its potential for biodiesel production.
Table 1.
Partitioning of variance for gravimetric analysis of total lipid content in Fremyella diplosiphon wild type and halotolerant strains using one-way class I analysis of variance.
| Source | Sum of squares (SS) | Degrees of freedom (ν) | Mean square (MS) | F-statistic | p-value |
|---|---|---|---|---|---|
| Strain | 5.5764 | 2 | 2.7882 | 0.6385 | 0.5419 |
| Error | 65.5072 | 15 | 4.3671 | ||
| Total | 71.0836 | 17 |
Fig. 1.
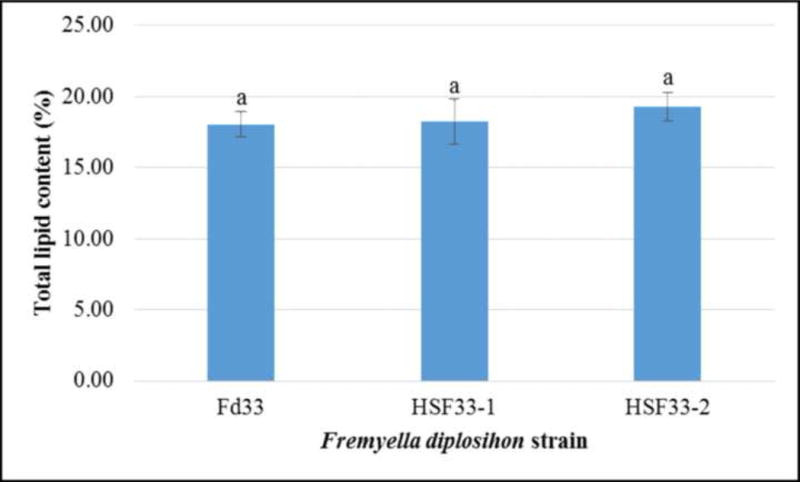
Comparison of total lipid content in wild type (Fd33) and halotolerant (HSF33-1 and HSF33-2) Fremyella diplosiphon. Average percent lipid content (± standard error) of six biological replicates for each strain is shown. Data were analyzed using ANOVA and Tukey’s HSD test. No significant difference was observed (P>0.05).
FAME Characterization by GC-MS
FAME abundance and compositional distribution are essential parameters for evaluating production capacity of any potential biofuel source [33], since total lipid yields include not only FAMEs but all cellular lipids [7]. In this study, FAME composition of F. diplosiphon transesterified lipids underscores comparable fatty acid profiles in both halotolerant and wild type strains indicating that gene overexpression did not impact the fatty acid synthesis pathway (Fig. 2). The most abundant FAME in all three strains was methyl palmitate, the methyl ester of hexadecanoic acid (C16:0), which accounted for ~61-66% of total FAMEs produced (Table 1). Similar results were reported in the microalgae Scenedesmus obliquus (33%) and Spirulina maxima (40%) where methyl palmitate was the most abundant FAME component detected in the transesterified lipids [34, 35]. In addition to methyl palmitate, other FAMEs including methyl dodecanoate (C12:0), methyl myristate (C14:0), methyl hexadecenoate (C16:1), methyl octadecanoate (C18:0), methyl octadecenoate (C18:1), and methyl octadecadienoate (C18:2) were identified (Fig. 3, Table 2). These results indicate a high proportion of saturated FAME in the transesterified lipid profile (Table 3) that could result in biodiesel with high cetane number and oxidative stability [36]. In addition, the two most abundant FAME components detected in our study, methyl palmitate and methyl octadecenoate, are known to yield high quality biodiesel (ASTM standard D6751; EN 14214) [37].
Fig. 2.
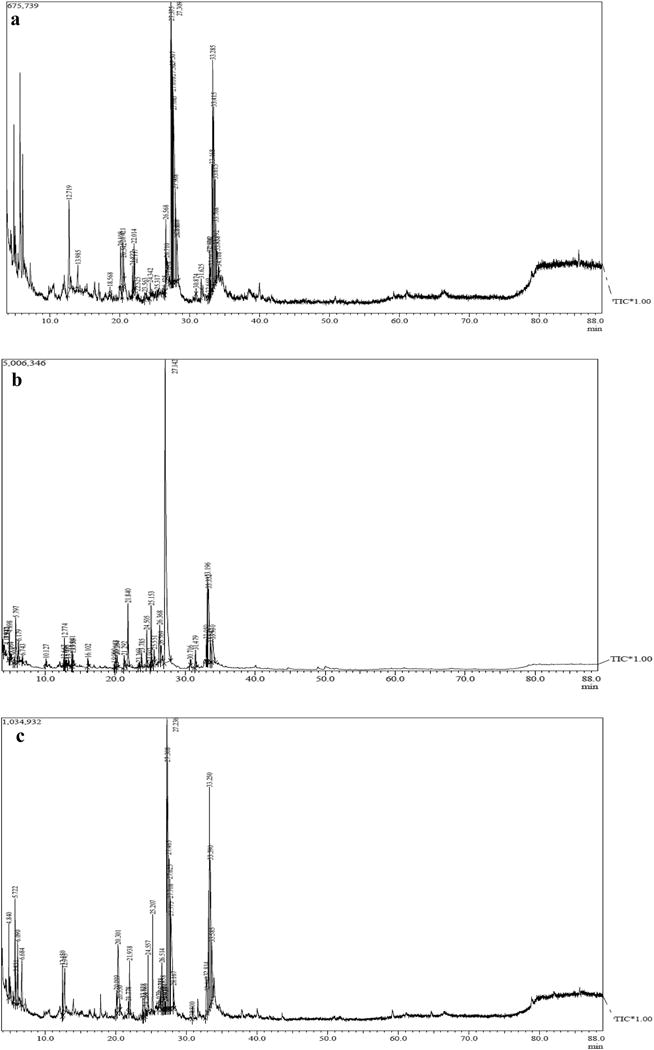
Representative one dimensional gas chromatogram of Fremyella diplosiphon strains (a) wild type (Fd33) and halotolerant (b) HSF33-1 and (c) HSF33-2 total lipids subjected to direct transesterification.
Fig. 3.
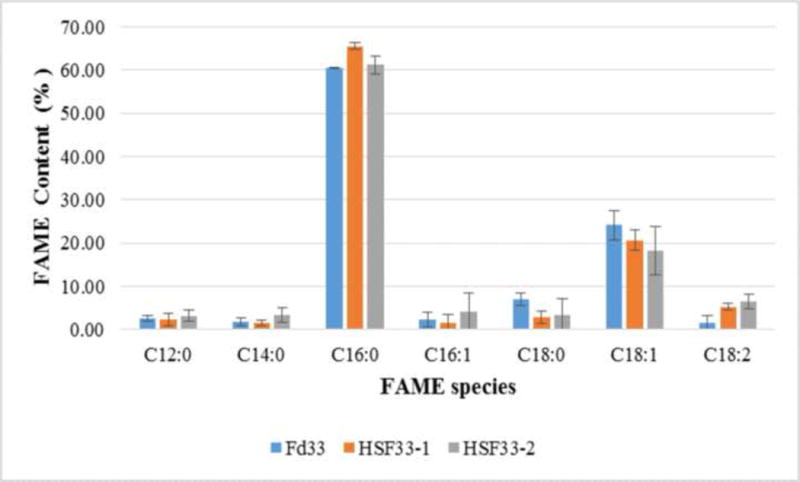
Comparison of fatty acid methyl ester (FAME) composition of wild type (Fd33) and halotolerant (HSF33-1 and HSF33-2) Fremyella diplosiphon total lipids subjected to direct transesterification. Average percent FAME content (± standard error) for three biological replicates of each strain is shown.
Table 2.
Quantitative composition of fatty acid methyl ester in transesterified lipids of Fremyella diplosiphon wild type (Fd33) and halotolerant (HSF33-1 and HSF33-2) strains.
| Fd33 | HSF33-1 | HSF33-2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| :0b | :1 | :2 | SUM | :0 | :1 | :2 | SUM | :0 | :1 | :2 | SUM | |
| C12a | 2.48 | − | − | 2.48 | 2.36 | − | − | 2.36 | 3.14 | − | − | 3.14 |
| C14 | 1.76 | – | – | 1.76 | 1.46 | – | – | 1.46 | 3.44 | – | – | 3.44 |
| C16 | 60.6 | 2.43 | − | 63.0 | 65.5 | 1.46 | – | 67.0 | 61.2 | 4.20 | – | 65.4 |
| C18 | 7.02 | 24.2 | 1.5 | 32.7 | 2.91 | 20.7 | 5.29 | 28.9 | 3.42 | 18.2 | 6.45 | 28.1 |
| SUM | 71.9 | 26.6 | 1.5 | 100 | 72.3 | 22.1 | 5.3 | 100 | 71.2 | 22.4 | 6.45 | 100 |
Column represents length of carbon chain.
Row represents degree of saturation (number of double bonds in chain).
Table 3.
Breakdown of saturated and unsaturated fatty acid methyl ester (FAME) proportions in wild type (Fd33) and halotolerant (HSF33-1 and HSF33-2) Fremyella diplosiphon.
| Strain | FAME Type (%) | Ratio of FAME | |
|---|---|---|---|
| Saturated | Unsaturated | Saturated/Unsaturated | |
| Fd33 | 71.86 | 28.14 | 2.55 |
| HSF33-1 | 71.59 | 27.42 | 2.61 |
| HSF33-2 | 71.16 | 28.84 | 2.48 |
For a more comprehensive assessment of the candidate’s potential as a biodiesel agent, we determined various chemical and physical properties of F. diplosiphon transesterified lipids. Our results revealed a wide array of theoretical biodiesel properties (Table 4) confirming a product with very high cetane number (64.34–66.67) and oxidative stability (81.21–7.89 h). By comparison, minimum American and European fuel standards are 47 and 51 for cetane number, and 3 h and 6h for oxidative stability, respectively [38], indicating that F. diplosiphon-derived biodiesel significantly exceeds minimum acceptable levels. In addition, values for density (0.868-0.870 g/cm3), viscosity (3.627-3.850 mm2/s), and iodine (21.053-32.243 g I2/100 g) were above the minimum or within the acceptable range for both American and European fuel standards. As expected in fuel with high saturated fatty acids, cold filter plugging point (6.634-13.589 °C), cloud point (26.884-29.461 °C), and pour point (22.363-25.160 °C) were very high. This suggests that blending with other biodiesel, conventional diesel, or additives could lower these attributes making this a more viable option rather than using it “straight” into an engine, particularly in colder weather. While there are several studies where biodiesel was derived from cyanobacteria and microalgae [5, 7], this was the first report of F. diplosiphon total lipids subjected to direct transesterification and evaluated for biodiesel production.
Table 4.
Theoretical biodiesel properties of Fremyella diplosiphon wild type (Fd33) and halotolerant strains (HSF33-1 and HSF33-2) transesterified lipids.
| Biodiesel Properties | Fd33 | HSF33-1 | HSF33-2 |
|---|---|---|---|
| Saponification Value (mg KOH/g fat) | 217.014 | 218.347 | 220.251 |
| Iodine Value (g I2/100 g) | 21.258 | 24.418 | 21.053 |
| Cetane number | 66.667 | 65.803 | 66.344 |
| Long Chain Saturated Factor | 8.481 | 7.729 | 7.356 |
| Cold Filter Plugging Point (°C) | 10.168 | 7.806 | 6.634 |
| Cloud Point (°C) | 29.153 | 28.097 | 28.613 |
| Pour Point (°C) | 24.826 | 23.68 | 24.24 |
| Allylic Position Equivalent | 20.983 | 23.43 | 18.853 |
| Bis-Allylic Position Equivalent | 2.111 | 2.774 | 1.613 |
| Oxidation Stability (h) | 58.455 | 45.103 | 75.702 |
| Higher Heating Value (mJ/kg) | 39.188 | 39.139 | 39.086 |
| Kinematic Viscosity (mm2/s) | 3.749 | 3.68 | 3.627 |
| Density (g/cm3) | 0.868 | 0.869 | 0.868 |
Lipid Characterization by GC×GC-TOFMS
High resolution GCxGC-TOFMS revealed the presence of 480 (Fd33), 545 (HSF33-1) and 458 (HSF33-2) components and a range of 8–18 FAMEs in each strain was derived from the transesterified lipid (TL) profile. Significantly higher FAME abundance (69.9% TL) was observed in HSF33-2 compared to HSF33-1 (40.2% TL); however, FAMEs in both strains were significantly greater than that of the wild type (25.4% TL) (Fig. 4). Prior studies in our laboratory have reported that HSF33-2 and HSF33-1 exhibit a 20 and 9-fold increase in mdh and hlyB gene transcript levels respectively [23], indicating that gene overexpression in the halotolerant strains enhanced fatty acid production. In particular, the higher fatty acid yield in HSF33-2 paves the way for its application as a more efficient production-level biofuel agent. It is also interesting to note that FAME compounds C15:0, C18:3, and C18:4 which were not detected in 1D GC-MS were identified by GC×GC due to increased resolution and sensitivity.
Fig. 4.
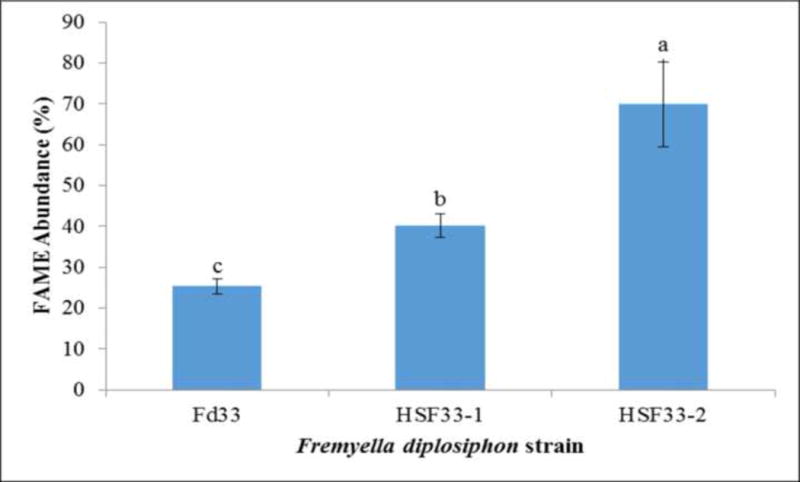
Fatty acid methyl ester (FAME) abundance in transesterified extractable lipids of wild type (Fd33) and halotolerant (HSF33-1 and HSF33-2) Fremyella diplosiphon determined using GC×GC-TOFMS. Average percent lipid content (± coefficient of variation) of each strain for three biological replicates is shown. Data were analyzed using ANOVA and Tukey HSD test. Different letters above bars indicate significance among treatment means (P< 0.05).
Alkane Distribution in F. diplosiphon Strains
Detectability and resolving power in GC-MS limits the identification of trace and co-eluted elements. To overcome this challenge, GC×GC-TOFMS has been developed for comprehensive characterization of unresolved complex mixtures [39]. Using this approach, high levels of hydrocarbons were detected in the cyanobacteria Prochlorococcus and Synechococcus sp. [40, 41]. Contrary to conventional GC-MS, high-resolution GC×GC-TOFMS separates volatile compounds by molecular weight (carbon number) on the first dimension and degree of unsaturation (number of double bonds) on the second dimension (Fig. 5). Our results revealed the presence of alkanes (normal and iso-) in transesterified products confirming that photosynthetic biodiesel production does not interfere with natural synthesis of these hydrocarbons required for fuel combustion of conventional petroleum-derived diesel [42]. Alkanes were most abundant in HSF33-2 suggesting a value-added enhancement that maximizes the quality of biofuel produced by this strain. Analysis of hydrocarbon profiles among diverse filamentous cyanobacteria has reported detectable levels of these compounds contrary to unicellular species [43]. Additionally, we identified highly abundant C7->C35 alkanes which are not typically observed in plant-derived biomass [44] suggesting that the lipid profile of F. diplosiphon could be particularly amenable for biofuel production. According to US Department of Energy, pure biodiesel (B100) is a common blendstock to produce lower blends with petroleum diesel due to the potential negative impacts on the conventional diesel engines. Currently, B5 and B20 are the most commonly used biodiesel blends [45]. With the high amount of alkanes found in cyanobacteria derived biodiesel, the fuel properties are expected to be improved significantly and, higher percentage (>20%) blend is possible.
Fig. 5.
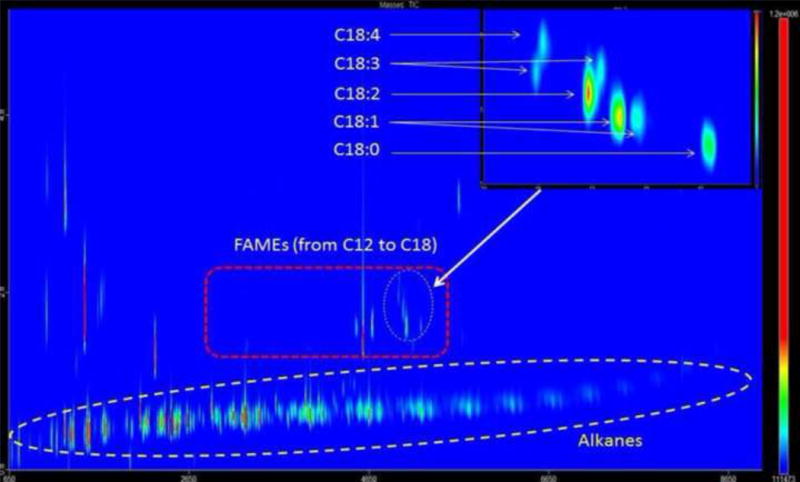
Representative GC×GC-TOFMS chromatogram of Fremyella diplosiphon halotolerant HSF33-1 indicating that all fatty acid methyl esters (FAMEs) were separated from alkanes and other components. The inset shows the isomers of C18 FAMEs with various degrees of unsaturation.
Normal Photosynthetic Pigment Accumulation in HSF33-1 and HSF33-2
Compared to terrestrial plants that convert 1% of available solar energy into biomass, cyanobacteria exhibit significantly higher photosynthetic capacity of up to 10% [46]. This is advantageous for biofuel production since allocation of photosynthetic carbon mediates the synthesis of metabolic products such as carbohydrates and fatty acids [47]. In this study, analysis of photosynthetic pigments and phycobiliproteins revealed no significant differences in wild type and halotolerant strains (Table 5) indicating that HSF33-1 and HSF33-2 photosynthetic activity was unaltered when exposed to 35 g L−1 NaCl, the average salinity of sea water. These results correlate to total lipid content in the F. diplosiphon strains; however, varying FAME abundances suggest that increased fatty acid production is not exclusively related to photosynthetic pathways.
Table 5.
Phycobiliprotein and photosynthetic pigment levels of wild type (Fd33) and halotolerant (HSF33-1 and HSF33-2) Fremyella diplosiphon.
| Strain | Phycobiliprotein levels (μg/μg chla)a | Photosynthetic Pigments (μg/μl) | |||
|---|---|---|---|---|---|
|
| |||||
| PEb | PCc | APd | Chlae | Carotenoids | |
| Fd33 | 8.66±0.34a | 20.89±0.29a | 16.03 ±0.40a | 0.0012±1.67E-4a | 0.7007±0.1062a |
| HSF33-1 | 10.78±0.27a | 21.92±0.29a | 17.96±0.25a | 0.0011±2.12E-4a | 0.6918±0.0448a |
| HSF33-2 | 7.49±0.47a | 20.20±0.54a | 1.02±0.50a | 0.0011±3.71E-4a | 0.6148±0.1185a |
Mean pigment levels (±standard errors) for three independent biological replicates were calculated. Identical letters followed by values denote no significant difference between treatments within the same column (P> 0.05).
PE= phycoerythrin,
PC= phycocyanin
AP= allophycocyanin
Chla= Chlorophyll a.
By contrast, Liu et al. [48] reported that fatty acid production in cyanobacteria is directly influenced by carbon dioxide fixation. Thus, measuring alterations in pigment accumulation is a necessary consideration when assessing the production potential of a candidate biofuel agent, since the loss in photosynthetic capacity corresponds to reduced fatty acid yield.
Conclusions
This is the first report of FAME production and biodiesel properties in direct transesterified lipids of F. diplosiphon, as determined by GC-MS and high resolution GC×GC-TOFMS analysis. The results indicate the potential of this organism as an efficient biofuel component due to a transesterified lipid profile with desirable biodiesel properties, high in saturated fatty acids, and dominated by methyl palmitate, methyl octadecenoate, and enhanced alkane production. Higher FAME abundance with no loss in lipid content and photosynthetic pigmentation in halotolerant strains, particularly HSF33-2, maximizes their potential for biofuel production. Scale-up studies to identify blends which provide optimal biodiesel properties are being pursued. Additionally, studies comparing wet and dry biomass as starting material, and viability of microwave-assisted and traditional transesterification methods for larger quantities of cyanobacteria will be explored. Ultimately, pilot-scale testing of F. diplosiphon-derived biodiesel will be conducted using optimized parameters, which will pave the way for its application as a cost-effective alternative energy source.
Acknowledgments
The study was supported by TEDCO MII [0913-001-3] and the National Institutes of Health [UL1GM118973] awarded to Morgan State University, and the National Science Foundation [DMR 11-57490] awarded to the National High Magnetic Field Laboratory and the State of Florida. Assistance provided by the Future Fuels Institute in the Ion Cyclotron Resonance Facility is gratefully acknowledged. The authors thank Dr. Beronda L. Montgomery at Michigan State University for providing the Fd33 strain and Dr. Solomon Tadesse at Morgan State University for technical help.
References
- 1.Martens P. Health and climate change: modelling the impacts of global warming and ozone depletion. Routledge; New York: 2014. [Google Scholar]
- 2.Goldemberg J. Ethanol for a sustainable energy future. Sci. 2007;315:808–810. doi: 10.1126/science.1137013. [DOI] [PubMed] [Google Scholar]
- 3.Hill J, Nelson E, Tilman D, Polasky S, Tiffany D. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Natl Acad Sci. 2006;103:11206–11210. doi: 10.1073/pnas.0604600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low‐cost photobioreactor. Biotechnol Bioeng. 2009;102:100–112. doi: 10.1002/bit.22033. [DOI] [PubMed] [Google Scholar]
- 5.Machado IM, Atsumi S. Cyanobacterial biofuel production. J Biotechnol. 2012;162:50–56. doi: 10.1016/j.jbiotec.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Peralta-Yahya PP, Zhang F, Del Cardayre SB, Keasling JD. Microbial engineering for the production of advanced biofuels. Nat. 2012;488:320. doi: 10.1038/nature11478. [DOI] [PubMed] [Google Scholar]
- 7.Wahlen BD, Willis RM, Seefeldt LC. Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour Technol. 2011;102:2724–2730. doi: 10.1016/j.biortech.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Koberg M, Gedanken A. Direct transesterification of castor and Jatropha seeds for FAME production by microwave and ultrasound radiation using a SrO catalyst. BioEnergy Res. 2012;5:958–968. [Google Scholar]
- 9.Dismukes GC, Carrieri D, Bennette N, Ananyev GM, Posewitz MC. Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr Opin Biotechnol. 2008;19:235–240. doi: 10.1016/j.copbio.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Dong T, Wang J, Miao C, Zheng Y, Chen S. Two-step in situ biodiesel production from microalgae with high free fatty acid content. Bioresour Technol. 2011;136:8–15. doi: 10.1016/j.biortech.2013.02.105. [DOI] [PubMed] [Google Scholar]
- 11.Lim S, Hoong SS, Teong LK, Bhatia S. Supercritical fluid reactive extraction of Jatropha curcas L. seeds with methanol: a novel biodiesel production method. Bioresour Technol. 2010;101:7169–7172. doi: 10.1016/j.biortech.2010.03.134. [DOI] [PubMed] [Google Scholar]
- 12.Canakci M. The potential of restaurant waste lipids as biodiesel feedstocks. Bioresour Technol. 2007;98:183–190. doi: 10.1016/j.biortech.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Atabani AE, Silitonga AS, Badruddin IA, Mahlia TMI, Masjuki HH, Mekhilef S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sustain Energy Rev. 2012;16:2070–2093. [Google Scholar]
- 14.Hu P, Borglin S, Kamennaya NA, Chen L, Park H, Mahoney L, Quinn NW. Metabolic phenotyping of the cyanobacterium Synechocystis 6803 engineered for production of alkanes and free fatty acids. Appl Energy. 2013;102:850–859. [Google Scholar]
- 15.Vicente G, Bautista LF, Rodríguez R, Gutiérrez FJ, Sádaba I, Ruiz-Vázquez RM, Garre V. Biodiesel production from biomass of an oleaginous fungus. Biochem Engn J. 2009;48:22–27. [Google Scholar]
- 16.Lewis T, Nichols PD, McMeekin TA. Evaluation of extraction methods for recovery of fatty acids from lipid-producing microheterotrophs. J Microbiol Methodol. 2000;43:107–116. doi: 10.1016/s0167-7012(00)00217-7. [DOI] [PubMed] [Google Scholar]
- 17.Schirmer A, Rude MA, Li X, Popova E, Del Cardayre SB. Microbial biosynthesis of alkanes. Sci. 2010;329:559–562. doi: 10.1126/science.1187936. [DOI] [PubMed] [Google Scholar]
- 18.Han J, McCarthy ED, Calvin M, Benn MH. Hydrocarbon constituents of the blue-green algae Nostoc muscorum, Anacystis nidulans, Phormidium luridium and Chlorogloea fritschii. J Chem Soc C. 1968:2785–2791. [Google Scholar]
- 19.Lu X. A perspective: photosynthetic production of fatty acid-based biofuels in genetically engineered cyanobacteria. Biotechnol Adv. 2010;28:742–746. doi: 10.1016/j.biotechadv.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Lang I, Hodac L, Friedl T, Feussner I. Fatty acid profiles and their distribution patterns in microalgae: a comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol. 2011;11:124. doi: 10.1186/1471-2229-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutu A, Kehoe DM. Emerging perspectives on the mechanisms, regulation, and distribution of light color acclimation in cyanobacteria. Mol Plant. 2012;5:1–13. doi: 10.1093/mp/ssr054. [DOI] [PubMed] [Google Scholar]
- 22.Singh SP, Montgomery BL. Salinity impacts photosynthetic pigmentation and cellular morphology changes by distinct mechanisms in Fremyella diplosiphon. Biochem Biophys Res Commun. 2013;433:84–89. doi: 10.1016/j.bbrc.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 23.Tabatabai B, Arumanayagam AS, Enitan O, Mani A, Natarajan SS, Sitther V. Overexpression of hlyB and mdh genes confers halotolerance in Fremyella diplosiphon, a freshwater cyanobacterium. Enzy Microb Technol. 2017;103:12–17. doi: 10.1016/j.enzmictec.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Tabatabai B, Arumanayagam AS, Enitan O, Mani A, Natarajan SS, Sitther V. Identification of a halotolerant mutant via in vitro mutagenesis in the cyanobacterium Fremyella diplosiphon. Curr Microbiol. 2017;74:77–83. doi: 10.1007/s00284-016-1156-z. [DOI] [PubMed] [Google Scholar]
- 25.Cobley G, Zerweck E, Reyes R, Mody A, Seludo-Unson JR, Jaeger HR, Weerasuriya S, Navankasattusas S. Construction of shuttle plasmids which can be efficiently mobilized from Escherichia coli into the chromatically adapting cyanobacterium Fremyella diplosiphon. Plasmid. 1990;30:90–105. doi: 10.1006/plas.1993.1037. [DOI] [PubMed] [Google Scholar]
- 26.Allen MJ. Simple conditions for growth of unicellular blue-green algae on plates. Phycol. 1968;4:1–4. doi: 10.1111/j.1529-8817.1968.tb04667.x. [DOI] [PubMed] [Google Scholar]
- 27.Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 28.Rosenberg JN, Kobayashi N, Barnes A, Noel EA, Betenbaugh MJ, Oyler GA. Comparative analyses of three Chlorella species in response to light and sugar reveal distinctive lipid accumulation patterns in the microalga C. sorokiniana. PloS One. 2014;9:e92460. doi: 10.1371/journal.pone.0092460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talebi AF, Tabatabaei M, Chisti Y. BiodieselAnalyzer: a user-friendly software for predicting the properties of prospective biodiesel. Biofuel Res J. 2014;1:55–57. [Google Scholar]
- 30.Kahn K, Mazel D, Houmard J, Tandeau de Marsac N, Schaefer M. A role for cpeYZ in cyanobacterial phycoerythrin biosynthesis. J Bacteriol. 1997;179:998–1006. doi: 10.1128/jb.179.4.998-1006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tandeau de Marsac N, Houmard J. Complementary chromatic adaptation: physiological conditions and action spectra. Methods Enzymol. 1988;167:318–328. [Google Scholar]
- 32.Whitaker M, Bordowitz J, Montgomery B. CpcF-dependent regulation of pigmentation and development in Fremyella diplosiphon. Biochem Biophys Res Commun. 2009;389:602–606. doi: 10.1016/j.bbrc.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Liao JC, Mi L, Pontrelli S, Luo S. Fueling the future: microbial engineering for the production of sustainable biofuels. Nature Rev Microbiol. 2016;14:288–304. doi: 10.1038/nrmicro.2016.32. [DOI] [PubMed] [Google Scholar]
- 34.Abou-Shanab RA, Ji MK, Kim HC, Paeng KJ, Jeon BH. Microalgal species growing on piggery wastewater as a valuable candidate for nutrient removal and biodiesel production. J Environ Manag. 2013;115:257–264. doi: 10.1016/j.jenvman.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 35.Gouveia L, Oliveira AC. Microalgae as a raw material for biofuels production. J Indust Microbiol Biotechnol. 2009;36:269–274. doi: 10.1007/s10295-008-0495-6. [DOI] [PubMed] [Google Scholar]
- 36.Rittmann BE. Opportunities for renewable bioenergy using microorganisms. Biotechnol Bioengn. 2008;100:203–212. doi: 10.1002/bit.21875. [DOI] [PubMed] [Google Scholar]
- 37.Rashid U, Anwar F, Moser BR, Knothe G. Moringa oleifera oil: a possible source of biodiesel. Bioresour Technol. 2008;99:8175–8179. doi: 10.1016/j.biortech.2008.03.066. [DOI] [PubMed] [Google Scholar]
- 38.Masera K, Hossain AK. Production, characterisation and assessment of biomixture fuels for compression ignition engine application. World Acad Sci Engin Technol Internat J Mech Aero Indust Mecha Manufact Engn. 2017;11:1852–1858. [Google Scholar]
- 39.Frysinger GS, Gaines RB, Xu L, Redd CM. Resolving the unresolved complex mixture in petroleum-contaminated sediments. Environ Sci Technol. 2003;37:1653–1662. doi: 10.1021/es020742n. [DOI] [PubMed] [Google Scholar]
- 40.Lea-Smith JD, Biller SJ, Davey MP, Cotton CA, Sepulveda BMP, Turchyn AV, Howe CJ. Contribution of cyanobacterial alkane production to the ocean hydrocarbon cycle. Pro Nat Acad Sci. 2015;112:13591–13596. doi: 10.1073/pnas.1507274112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valentine DL, Reddy CM. Latent hydrocarbons from cyanobacteria. Pro Nat Acad Sci. 2015;112:13434–13435. doi: 10.1073/pnas.1518485112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peramuna A, Morton R, Summers ML. Enhancing alkane production in cyanobacterial lipid droplets: a model platform for industrially relevant compound production. Life. 2015;5:1111–1126. doi: 10.3390/life5021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu A, Zhu T, Lu X, Song LH. Hydrocarbon profiles and phylogenetic analyses of diversified cyanobacterial species. Appl Energy. 2013;111:383–393. [Google Scholar]
- 44.Ladygina N, Dedyukhina EG, Vainshtein MG. A review on microbial synthesis of hydrocarbons. Process Biochem. 2006;41:1001–1014. [Google Scholar]
- 45.Department of Energy. Biodiesel Blends. Alternative Fuels Data Center; 2018. https://www.afdc.energy.gov/fuels/biodiesel_blends.html. Accessed 10 April 2018. [Google Scholar]
- 46.Parmar A, Singh NK, Pandey A, Gnansounou E, Madamwar D. Cyanobacteria and microalgae: a positive prospect for biofuels. Bioresour Technol. 2011;102:10163–10172. doi: 10.1016/j.biortech.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 47.Lindberg P, Park S, Melis A. Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab Engin. 2010;12:70–79. doi: 10.1016/j.ymben.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Sheng J, Curtiss R., III Fatty acid production in genetically modified cyanobacteria. Pro Nat Acad Sci. 2011;108:6899–6904. doi: 10.1073/pnas.1103014108. [DOI] [PMC free article] [PubMed] [Google Scholar]


