Abstract
Background
Immune thrombocytopenia (ITP) is an auto-immune disorder characterized by enhanced platelet destruction and, subsequently, the potential for increased bleeding. Thrombopoietin receptor (TPO-R) agonists have recently emerged as promising therapies for ITP patients who are refractory to other treatments. While eltrombopag (EPAG) is the only TPO-R agonist US Food and Drug Administration approved for use in pediatric patients, romiplostin (ROMI) has been used in Phase III clinical studies.
Methods
A cost-consequence model (CCM) was developed to evaluate the costs of EPAG, ROMI, and watch-and-rescue (W&R) in relation to their respective treatment outcomes in previously-treated pediatric chronic ITP (cITP) over a 26-week time horizon. The costs of drugs, administration, routine care, rescue medications, adverse events, and mortality were included. Data on platelet count response rate, bleeding events, and adverse events were derived from all relevant identified Phase III-registered clinical trials, health outcomes were compared via indirect treatment comparison.
Results
The overall estimated cost of EPAG per patient was US$66,550, compared to US$101,056 for ROMI and US$32,720 for W&R. EPAG’s lower cost compared to ROMI was largely due to lower drug costs (US$62,202 vs US$84,396), administration costs (US$0 vs US$1,955), and significantly lower costs due to severe bleeding (US$354 vs US$10,191). When assessing cost per severe bleeding event avoided, EPAG was dominant over ROMI (less expensive and more effective). EPAG was again dominant over ROMI when assessing the cost per responder and per bleeding event (any grade). Sensitivity analysis was consistent with the base case findings.
Conclusion
EPAG was the preferred TPO-R agonist to treat cITP when indirectly compared to ROMI, largely driven by its favorable severe bleeding outcomes and lower drug and administration costs.
Keywords: chronic immune thrombocytopenia, eltrombopag, romiplostim, cost-consequence, USA
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disorder in which platelets are disproportionately destroyed, resulting in a potential risk of increased bleeding. In children, ITP is a common cause of platelet deficiencies and when platelet counts drop below 10–20 × 109/L, clinically significant bleeding may occur.1,2 Approximately 40% of all patients diagnosed with ITP are children younger than 10 years.3 In most of these children, approximately 70%, ITP is a self-limiting disease that resolves naturally within 6 months.2,4,5 The disease becomes chronic in 20%–30% of pediatric patients, for whom spontaneous remission is unlikely.2,6 In the United States, the average estimate of the incidence of chronic ITP (cITP) is 5 children per 100,000 per year.7 Few children are affected by cITP but it may limit their activities and those who do not respond sufficiently to conventional therapies may be at risk for potentially life-threatening bleeding complications.2,5
Individuals with cITP face an increased risk of bleeding due to their diminished platelet counts. Bleeding episodes commonly manifest as minor symptoms such as bruising, nosebleeds, and petechiae.2 Additionally, cITP may be detrimental to quality of life, some patients experience depression and a fear of bleeding that limits routine activities.8,9 In rare cases, cITP is also associated with serious complications that include internal bleeding and major external bleeding. Intracranial bleeding is the most serious complication of ITP: although infrequent, it is considered to be life-threatening.3
To help prevent bleeding episodes, ITP therapies increase platelet counts. Many first-line therapies curb immune system-mediated platelet destruction. Thrombopoietin receptor (TPO-R) agonists, such as eltrombopag (EPAG) and romiplostim (ROMI), stimulate platelet production.10 These emerging therapies may provide a solution for patients whose first-line treatment with immunoglobulins, corticosteroids, or splenectomy proves ineffective.5
The efficacy of EPAG in pediatric patients was demonstrated in the randomized, double-blind, multi-center, Phase II and III trials PETIT and PETIT-2. In these trials, patients treated with EPAG had significantly higher platelet response rates (PETIT) and sustained platelet response rates (PETIT-2) than placebo-treated patients.11,12 Orally-administered EPAG was well-tolerated and successful in maintaining platelet counts during longer-term therapy. This evidence supported US Food and Drug Administration (FDA) approval of EPAG for pediatric patients who are refractory or who had an inadequate response to first-line therapies. ROMI was similarly evaluated in a Phase III study of pediatric patients and high rates of platelet response were reported; however, its US approval is pending.
To date, no head-to-head trials have compared EPAG and ROMI and few indirect treatment comparisons have assessed their relative efficacy and safety.13 Several studies have assessed the cost of ROMI per patient who responded to treatment.14–16 However, these studies did not consider pediatric patients in a US setting and costs were not compared to the costs for EPAG treatment. One study compared EPAG and ROMI to “watch-and-rescue” (W&R) in a cost per response analysis: the TPO-R agonists proved cost-effective.17
Additional studies are required to better understand the role of TPO-R agonists in cITP treatment strategies. We present the results of a cost-consequences model (CCM) comparing EPAG to ROMI and W&R in previously-treated pediatric cITP patients in the US.
Materials and methods
Model perspective, patient populations and horizon/discounting
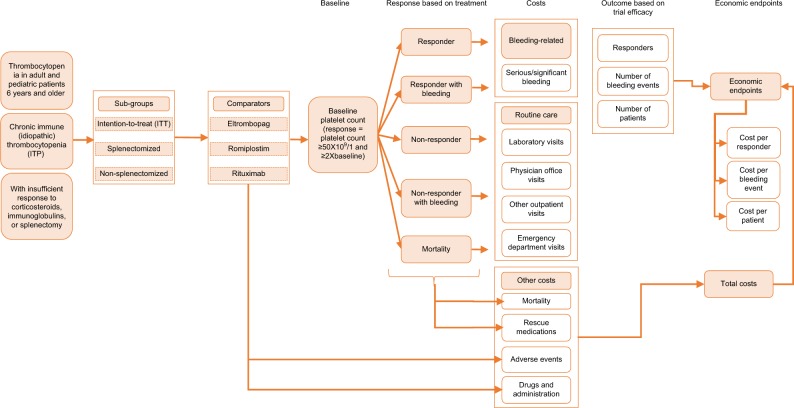
The model was constructed from a general US payer’s perspective and incorporated a time horizon of 26 weeks. The population consisted of pediatric patients (1–17 years) with cITP who responded insufficiently to corticosteroids, immunoglobulins, and/or splenectomy. The trial period of ROMI (26 weeks) was incorporated as the time horizon because certain model endpoints are bound to within-trial data. As such, evidence from the published literature could be incorporated without relying on extrapolation techniques and additional assumptions. The model horizon was less than 1 year so no discounting was applied. A diagram of the model is presented in Figure 1.
Figure 1.
Model structure.
Comparators
EPAG, ROMI, and W&R were selected as comparators following a targeted literature review. W&R treatment was based on the respective trials’ placebo groups, as these patients would receive rescue therapy as needed. Rituximab and splenectomy, while common treatment options for cITP, were not included as comparators due to lack of comparability of the available subjects recruited to the trials. The two identified randomized trials assessing rituximab,17,18 which were pooled, only included adult cITP patients who were treated in in the first line, differing from the EPAG and ROMI trials, which only included pediatric patients treated in the second line or greater. Furthermore, the rituximab trials only included non-splenectomized patients, which further limited the comparability among these trials and those used for EPAG and ROMI. Splenectomy was not included as a comparator due to the absence of any randomized clinical trials and because the population of the only identified retrospective study19 was not comparable to those used in the other treatments.
Indirect treatment comparison
The EPAG (PETIT2) clinical trial followed 63 EPAG-treated patients and 29 placebo-treated patients. The ROMI trial followed 42 ROMI-treated patients and 20 placebo-treated patients. By design, the two studies compared had similar baseline characteristics.11,20 The most notable differences at baseline were in the proportion of patients’ ethnic origins (32% “East Asian” in the EPAG trial vs 7% “Asian” in the ROMI trial), the amount of time since diagnosis (3.4 years in the EPAG trial vs 1.9 years in the ROMI trial) and the proportion of male patients (52% in the EPAG trial vs 43% in the ROMI trial). Overall, the two trials were considered comparable enough to be entered into an indirect treatment comparison (ITC).
The ITC technique and results were identical to those reported in the Evidence Review Group (ERG) report, where ROMI efficacy data were adjusted to match the EPAG trial. Therefore, EPAG and W&R (no drug treatment) efficacy data were taken directly from the trial.21 Frequentist ITC was employed and 95% confidence intervals were calculated. ROMI results were pooled using random effects models (Mantel-Haenszel method), which generated odds ratios for ROMI vs placebo. Odds ratios for EPAG were obtained from the National Institute for Health and Care Excellence (NICE) Single Technology Appraisal.22 Odds ratios were estimated for splenectomized and non-splenectomized patients. Classical frequentist ITC was implemented using the Bucher (1997) method and 95% confidence intervals.23
An additional endpoint was included to provide a more complete comparative effectiveness profile. In the ITC, bleeding events were the primary endpoint and platelet response was also considered.
Efficacy measures
A summary of the efficacy values and the resulting odds ratios for all clinical trials used in this analysis are presented in the Supplementary material.11,20 The raw values presented for ROMI were not used in the model and were instead matched to EPAG trial data for ITC, as described above. Therefore, W&R and EPAG results are untouched in the model, while the efficacy results for ROMI are adjusted.
Severe bleeding (WHO grades 3–5) was the primary measurement of efficacy in this analysis. The WHO bleeding scale is the most common measurement applied to quantify thrombocytopenia, and its validity is accepted. Mortality rates were derived as the product of treatment-specific severe bleeding rates, mortality of standard discharge in the US (2.39%) and relative mortality ratio for ITP (1.50).24,25 Moderate bleeding and platelet response were also assessed as secondary efficacy endpoints in our analysis. Cost-effectiveness was calculated using the severe bleeding, all bleeding, and platelet response endpoints.
Costs
The total cost of each treatment included the costs of drugs, administration, bleeding events, routine care, rescue medications, adverse events (all grades, affecting >20% of patients in at least one of the included trials), and mortality. The proportion of patients associated with these costs in each treatment arm was derived from clinical trial data if available, or from other published literature (Table 2).26,27
Table 2.
Probabilistic sensitivity analysis parameters (intent-to-treat population)
| Parameter | Point estimate | SE |
|---|---|---|
|
| ||
| Efficacy | ||
| Overall response – EPAG | 0.746 | 5.48% |
| Overall response – ROMI | 0.714 | 6.97% |
| Severe bleeding (WHO 3–5) – EPAG | 0.008 | 1.12% |
| Severe bleeding (WHO 3–5) – ROMI | 0.119 | 5.00% |
| Use of rescue medication – EPAG | 0.180 | 4.84% |
| Use of rescue medication – ROMI | 0.405 | 7.57% |
| Costs | ||
| Drug costs, administration costs, routine care costs, cost of bleeding (severe and moderate), adverse events costs, mortality costs | Variable point estimate | Standard error assumed at 20% |
Abbreviations: EPAG, eltrombopag; ROMI, romiplostim.
All costs are listed in USD. All cost data were derived from US-based sources (no currency conversions were required) and US healthcare inflation (3.6%) was applied where no up-to-date data were available.28
The available pack sizes of drugs may not provide the exact doses of drug required. Wastage was applied to dose calculations based on the received doses (ie, doses were rounded up to the nearest pack/vial size when necessary). Wastage and dose reduction accommodations were only applied for the primary therapies (EPAG and ROMI). It should be noted that ROMI is not yet FDA approved in pediatric patients and as such, the final costs are uncertain.
Costs incorporated in the analysis and their sources are summarized in Table 1.
Table 1.
Costs incorporated and resources used
| Resource | Unit Cost (inflation-adjusted) | Source |
|---|---|---|
| Comparators • Eltrombopag • Romiplostim |
US$10,253.00 (per pack) US$6,492.00 (per pack) |
AnalySource29 AnalySource29 |
| Routine medical costs • Laboratory visits • Office Visits • Other outpatient visits • Emergency department visits |
US$27.00 (per visit) US$78.00 (per visit) US$367.00 (per visit) US$260.00 (per visit) |
Saleh, Fisher30 |
| Rescue treatments ▪ Blood transfusion ▪ IVIg ▪ IV methylprednisolone |
US$424.90 (per transfusion) US$37.89 (per “pack”) US$3.73 (per “pack”) |
Utilization Lee, Thornton 31 Costs Blood transfusions32; IV Ig and IV methylprednisolone33 |
| Bleeding costs (with and without hospitalization) |
US$2,196.00 (without hospitalization) US$44,590.00 (with hospitalization) |
Lin34 |
| Mortality costs |
US$55,238.00 (for ITP) |
Centers for Medicare & Medicaid Services35 |
| Administration costs ▪ Subcutaneous injection ▪ IV ▪ Oral |
US$75.19 (per use) US$136.41 (per use) US$0.00 (per use) |
CPT code 96413 (Chemotherapy administration, intravenous infusion technique; up to 1 hour, single or initial substance/drug); 96401 (chemotherapy administration, subcutaneous or intramuscular, non-hormonal anti-neoplastic) |
| Adverse Events | (See Supplementary Material for specific prevalence and costs) |
Prevalence26,27; Costs25 |
Note: All costs are listed in USD.
Abbreviations: IV, intravenous; IV Ig, intravenous immunoglobulin.
Probabilistic sensitivity analysis
Probabilistic sensitivity analyses (PSA) were used to address uncertainty in the analysis. To perform these analyses, probabilistic distributions were directly applied to the base case model. The variations used in the PSA are presented in Table 2.
Results
Table 3 presents the overall costs and efficacy results for each primary therapy. Indirect treatment comparison efficacy data were used in the model (Table 3), while raw data are presented in the Supplementary material.
Table 3.
Efficacy and costs
| EPAG | ROMI (naïve trial data) | ROMI (ITC data used in model) | Watch-and- rescue (W&R) | Adjusted ITC OR (EPAG/ROMI) (95% CI) | ∆ EPAG - ROMI | ∆ EPAG – W&R | |
|---|---|---|---|---|---|---|---|
| Efficacy | |||||||
| Overall response | 74.6% (47/63) | 71.4% (30/42) | 72.3% | 20.7% (6/29) | 1.13 (0.21, 5.96) | 2.3% | 53.9% |
| Severe bleeding (WHO 3–5) | 0.8% (0/63 – Cochrane adjustment to 0.5/63) | 11.9% (5/42) | NA | 10.3% (3/29) | 0.03 (0.00,1.15) | −22.1% | −9.6% |
| Moderate bleeding only (WHO 2) | 36.5% (23/63) | 71.4% (30/42) | 52.2% | 44.8% (13/29) | 0.53 (0.12, 2.23) | −15.7% | −8.3% |
| Use of rescue medication | 19.0% (12/63) | 40.5% (17/42) | 20.9% | 24.1% (7/29) | 0.89 (0.20, 4.02) | −1.9% | −5.1% |
| Mortality (derived from severe bleeding) | 0.03% | NA | 0.82% | 0.37% | NA | 0.79% | 0.34% |
| Costs ($) | NA | NA | NA | NA | NA | NA | NA |
| Drug costs | 62,202 | NA | 84,396 | 22,024 | NA | −22,194 | 40,178 |
| Administration costs | 0 | NA | 1,955 | 889 | NA | −1,955 | −889 |
| Routine care costs | 486 | NA | 486 | 486 | NA | 0 | 0 |
| Rescue medication costs | 983 | NA | 569 | 2,184 | NA | 414 | −1,201 |
| Cost of severe bleeding (G3–5) | 354 | NA | 10,191 | 4,613 | NA | −9,837 | −4,259 |
| Cost of moderate bleeding (G2) | 802 | NA | 1,147 | 984 | NA | −345 | −183 |
| Adverse events costs | 1,709 | NA | 1,860 | 1,335 | NA | −151 | 373 |
| Mortality costs | 16 | NA | 453 | 205 | NA | −437 | −189 |
| Total costs | 66,550 | NA | 101,056 | 32,720 | NA | −34,506 | 33,830 |
Abbreviations: EPAG, eltrombopag; ROMI, romiplostim; ITC, indirect treatment comparison; NA, not applicable; W&R, watch-and-rescue.
EPAG, ROMI, and W&R had total estimated costs of US$66,550, US$101,056, and US$32,720, respectively. In this population, EPAG was estimated to cost US$34,506 less than ROMI. Compared with W&R, EPAG cost US$33,830 more, while ROMI cost US$68,336 more. Drug costs comprised the largest share of the cost for all comparators.
Severe bleeding (WHO grade 3–5) was assessed as the primary endpoint. EPAG demonstrated a 22.1% incremental benefit over ROMI and a 9.6% benefit over W&R, while ROMI showed an incremental benefit of 12.5% over W&R. Moderate bleeding (WHO grade 2) was also assessed, EPAG demonstrated a 15.7% and 8.3% incremental benefit over ROMI and W&R, respectively.
Additionally, platelet response was assessed as a secondary endpoint. After ITC, EPAG again showed a benefit over the comparators, with incremental benefits of 2.3% and 53.9% over ROMI and W&R, respectively.
Incremental cost-effectiveness ratios (ICER) were derived to further explore cost per event (presented in Table 4). When assessing cost per bleeding event avoided, EPAG dominated ROMI for both severe and all grade bleeding events with ICERs of US$354,197 and US$189,303, respectively, when compared to W&R. Regarding cost per responder, EPAG dominated ROMI and had an ICER of US$62,749 when compared with W&R. Additionally, exploratory analyses were performed using the raw (non-adjusted) data in the model (presented in the Supplementary material), which generated results consistent with the base case findings.
Table 4.
Incremental cost-effectiveness ratios for severe bleeding events avoided
| Endpoint | ICER
|
|
|---|---|---|
| EPAG/ROMI | EPAG/W&R | |
|
| ||
| Incremental cost per responder | Dominant | US$62,749 |
| Incremental cost per severe bleeding event avoided (WHO 3–5) | Dominant | US$354,197 |
| Incremental cost per bleeding event avoided (WHO 2–5) | Dominant | US$189,303 |
| Incremental cost per patient | Dominant | US$62,749 |
Note: All costs are listed in USD.
Abbreviations: ICER, incremental cost-effectiveness ratio; EPAG, eltrombopag; ROMI, romiplostim; W&R, watch-and-rescue.
Probabilistic sensitivity analysis
Uncertainty in these results was assessed via PSA, in which probabilistic distributions were directly applied to the base case model. The parameters explored in the PSA are presented in Table 5.
Table 5.
Probabilistic sensitivity analysis results – ICERs for cost per severe bleeding avoided
| EPAG/ROMI | EPAG/W&R | |
|---|---|---|
|
| ||
| Mean | − 169,568 | 526,907 |
|
| ||
| Proportion below threshold ($) | ||
| 25,000 | 97.5% | 0.3% |
| 50,000 | 98.3% | 0.5% |
| 100,000 | 99.2% | 0.8% |
| 150,000 | 99.6% | 1.5% |
| 250,000 | 99.9% | 14.6% |
| 500,000 | 100.0% | 79.8% |
| 1,000,000 | 100.0% | 98.8% |
| 10,000,000 | 100.0% | 99.7% |
Note: All costs are listed in USD.
Abbreviations: ICER, incremental cost-effectiveness ratio; EPAG, eltrombopag; ROMI, romiplostim; W&R, watch-and-rescue.
The results of the PSA were relatively consistent with base case findings (Table 5 and Figure 2). ICERs of 99.2% and 0.8% vs ROMI and W&R, respectively, were under a cost-effectiveness threshold of US$100,000, while 99.6% and 1.5% were under a cost-effectiveness threshold of US$150,000.
Figure 2.
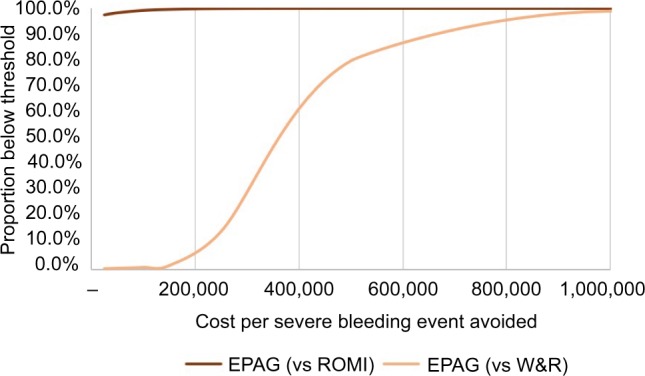
Cost-effectiveness acceptability curve (per severe bleeding event avoided).
Note: All costs are listed in USD.
Abbreviations: EPAG, eltrombopag; ROMI, romiplostim; W&R, watch-and-rescue.
Deterministic sensitivity analyses were also intended to assess incremental cost-effectiveness for severe bleeding. However, these analyses were not feasible because EPAG was dominant over ROMI for severe bleeding and therefore the relevant base case ICER was unavailable.
Discussion
In our model, TPO-R agonists were assessed for the treatment of pediatric patients with chronic ITP after comparing two robust randomized clinical trials. EPAG showed favorable rates in all endpoints assessed compared to both ROMI and W&R. EPAG-treated patients had fewer severe bleeding events than ROMI and W&R (0.8% vs 22.9% vs 10.3%, respectively), in addition to fewer moderate bleeding events (36.5% vs 52.2% vs 44.8%, respectively) which resulted in significantly lower estimated bleeding-related costs. Additionally, EPAG showed higher platelet response rates and lower mortality relative to the comparators.
EPAG, ROMI, and W&R had total estimated costs of US$66,550, US$101,056, and US$32,720, respectively, with drug costs comprising most of the price for all comparators. The lower total cost of EPAG and improved outcomes led EPAG to dominate ROMI in all endpoints assessed. When compared to W&R in our assessment, EPAG had a higher total cost but had improved outcomes. Comparing EPAG to W&R yielded ICERs of US$354,197 per severe bleeding event avoided, US$189,303 per any grade bleeding event avoided, and US$62,749 per platelet response. Probabilistic sensitivity analysis results were relatively consistent with the base case findings.
This analysis, while robust, is accompanied by some minor limitations. Trial endpoint definitions sometimes varied in the literature, direct matching and data selection were therefore challenging. Rituximab and splenectomy, two common treat ments for cITP, could not be included as comparators due to a lack of available data. The time horizon used in this model was relatively short but allowed modeling of within-trial endpoints without the need for extrapolation techniques.
We present the most comprehensive CCM to date comparing EPAG, ROMI, and W&R in children with cITP who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy. This data is of interest to physicians and healthcare decision-makers who wish to determine the best available treatment options for pediatric ITP patients. The results of our analysis indicate that EPAG is cost-effective for the treatment of pediatric cITP patients in terms of cost per severe bleeding event avoided, though the results describing the costs and benefits of other outcomes are mixed.
Conclusion
Overall, EPAG was preferred over ROMI and W&R. Additional analyses incorporating long-term extrapolation and preference-based outcomes would complete the evidence base for the treatment of pediatric cITP.
Acknowledgments
Thanks to Jaclyn Hearnden for contributions to writing the manuscript and support in preparing it for publication. This analysis was previously presented, in part, at the 59th annual meeting of the American Society of Hematology (Tremblay et al, 2017). Available at: http://www.bloodjournal.org/content/130/Suppl_1/2146)
This study was funded by Novartis Pharmaceuticals.
Footnotes
Author contributions
GT and MD made substantial contributions to the conception and design of this study and also analyzed and interpreted data. MB, QS, BE, AR and AB contributed to conception and development of the analysis and evaluation and interpretation of the data. All authors contributed by drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
GT and MD are employees of Purple Squirrel Economics. MB, QS, AR, and BE are employees of Novartis Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
- 1.Ballem PJ, Segal GM, Stratton JR, Gernsheimer T, Adamson JW, Slichter SJ. Mechanisms of thrombocytopenia in chronic autoimmune thrombocytopenic purpura. Evidence of both impaired platelet production and increased platelet clearance. J Clin Invest. 1987;80(1):33–40. doi: 10.1172/JCI113060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neunert CE, Buchanan GR, Imbach P, et al. Bleeding manifestations and management of children with persistent and chronic immune thrombocytopenia: data from the Intercontinental Cooperative ITP Study Group (ICIS) Blood. 2013;121(22):4457–4462. doi: 10.1182/blood-2012-12-466375. [DOI] [PubMed] [Google Scholar]
- 3.Immune Thrombocytopenia: National Organization for Rare Disorders. 2015. [Accessed September 20, 2018]. Available from: https://rarediseases.org/rare-diseases/immune-thrombocytopenia/
- 4.Watts RG. Idiopathic thrombocytopenic purpura: a 10-year natural history study at the children’s Hospital of Alabama. Clin Pediatr. 2004;43(8):691–702. doi: 10.1177/000992280404300802. [DOI] [PubMed] [Google Scholar]
- 5.Kühne T. Idiopathic thrombocytopenic purpura of childhood: a problem-oriented review of the management. Transfus Apher Sci. 2003;28(3):243–248. doi: 10.1016/S1473-0502(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 6.Bolton-Maggs PH. Idiopathic thrombocytopenic purpura. Arch Dis Child. 2000;83(3):220–222. doi: 10.1136/adc.83.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am J Hematol. 2010;85(3):NA–80. doi: 10.1002/ajh.21616. [DOI] [PubMed] [Google Scholar]
- 8.Mathias SD, Gao SK, Miller KL, et al. Impact of chronic Immune Thrombocytopenic Purpura (ITP) on health-related quality of life: a conceptual model starting with the patient perspective. Health Qual Life Outcomes. 2008;6:13. doi: 10.1186/1477-7525-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons S, Bennett C, Mahoney D, et al. Health-related quality of life (HRQL) in children with severe, chronic immune thrombocytopenia (cITP) treated with rituximab. Blood. 2005;106(11):5575. [Google Scholar]
- 10.Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. The Lancet. 2011;377(9763):393–402. doi: 10.1016/S0140-6736(10)60959-2. [DOI] [PubMed] [Google Scholar]
- 11.Grainger JD, Locatelli F, Chotsampancharoen T, et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;386(10004):1649–1658. doi: 10.1016/S0140-6736(15)61107-2. [DOI] [PubMed] [Google Scholar]
- 12.Bussel JB, de Miguel PG, Despotovic JM, et al. Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (PETIT): a randomised, multicentre, placebo-controlled study. Lancet Haematol. 2015;2(8):e315–e325. doi: 10.1016/S2352-3026(15)00114-3. [DOI] [PubMed] [Google Scholar]
- 13.Allen R, Brainsky A, Grotzinger K, Roccia T. A comment on Boyers et al.: “eltrombopag for the treatment of chronic immune or idiopathic thrombocytopenic purpura: a NICE single technology appraisal”. Pharmacoeconomics. 2013;31(1):87–89. doi: 10.1007/s40273-012-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López MF, Mingot ME, Valcárcel D, Vicente García V, Perrin A, Campos Tapias I. Cost-per-responder analysis comparing romiplostim to rituximab in the treatment of adult primary immune thrombocytopenia in Spain. Med Clin. 2015;144(9):389–396. doi: 10.1016/j.medcli.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Chiche L, Perrin A, Stern L, Kutikova L, Cohen-Nizard S, Lefrère F. Cost per responder associated with romiplostim and rituximab treatment for adult primary immune thrombocytopenia in France. Transfus Clin Biol. 2014;21(2):85–93. doi: 10.1016/j.tracli.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 16.dos Santos RF, Vargas-Valencia JJ, Giannopoulou A, Campioni M. Cost-effectiveness analysis of romiplostim for the treatment of adult chronic immune thrombocytopenic purpura (Itp) in Brazil. Value in Health. 2015;18(7):A668. [Google Scholar]
- 17.Ghanima W, Khelif A, Waage A, et al. Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9978):1653–1661. doi: 10.1016/S0140-6736(14)61495-1. [DOI] [PubMed] [Google Scholar]
- 18.Arnold DM, Heddle NM, Carruthers J, et al. A pilot randomized trial of adjuvant rituximab or placebo for nonsplenectomized patients with immune thrombocytopenia. Blood. 2012;119(6):1356–1362. doi: 10.1182/blood-2011-08-374777. [DOI] [PubMed] [Google Scholar]
- 19.Moulis G, Sailler L, Sommet A, Lapeyre-Mestre M, Derumeaux H, Adoue D. Rituximab versus splenectomy in persistent or chronic adult primary immune thrombocytopenia: an adjusted comparison of mortality and morbidity. Am J Hematol. 2014;89(1):41–46. doi: 10.1002/ajh.23580. [DOI] [PubMed] [Google Scholar]
- 20.Tarantino MD, Bussel JB, Blanchette VS, et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388(10039):45–54. doi: 10.1016/S0140-6736(16)00279-8. [DOI] [PubMed] [Google Scholar]
- 21.Boyers D, Jia X, Crowther M, Jenkinson D, Fraser C, Mowatt G. Eltrombopag for the treatment of chronic idiopathic (immune) thrombocytopenic purpura (ITP) Health Technol Assess. 2011;15(Suppl 1):23–32. doi: 10.3310/hta15suppl1/03. [DOI] [PubMed] [Google Scholar]
- 22.Eltrombopag for treating chronic immune (idiopathic) thrombocytopenic purpura. Technology appraisal guidance [TA293] National Institute for Health and Care Excellence. 2013. [Accessed September 26, 2018]. Available from: https://www.nice.org.uk/guidance/ta293.
- 23.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 24.Danese MD, Lindquist K, Gleeson M, Deuson R, Mikhael J. Cost and mortality associated with hospitalizations in patients with immune thrombocytopenic purpura. Am J Hematol. 2009;84(10):631–635. doi: 10.1002/ajh.21500. [DOI] [PubMed] [Google Scholar]
- 25.Healthcare Cost and Utilization Project (HCUP) US Department of Health & Human Services; 2013. [Accessed September 26, 2018]. Available from: https://www.hcup-us.ahrq.gov/ [Google Scholar]
- 26.Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377(9763):393–402. doi: 10.1016/S0140-6736(10)60959-2. [DOI] [PubMed] [Google Scholar]
- 27.Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371(9610):395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]
- 28.United States Census Bureau . Inflation Rate. US Bureau of Labor Statistics (CPI); 2015. [Accessed September 26, 2018]. Available from: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=PEP_2015_PEPANNRES&src=pt. [Google Scholar]
- 29.Drug Pricing Services [Internet] 2016. [Accessed September 26, 2018]. Available from: https://www.analysource.com/
- 30.Saleh MN, Fisher M, Grotzinger KM, Saleh, Fisher G. Analysis of the impact and burden of illness of adult chronic ITP in the US. Curr Med Res Opin. 2009;25(12):2961–2969. doi: 10.1185/03007990903362388. [DOI] [PubMed] [Google Scholar]
- 31.Lee D, Thornton P, Hirst A, Kutikova L, Deuson R, Brereton N. Cost effectiveness of romiplostim for the treatment of chronic immune thrombocytopenia in Ireland. Appl Health Econ Health Policy. 2013;11(5):457–469. doi: 10.1007/s40258-013-0044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toner RW, Pizzi L, Leas B, Ballas SK, Quigley A, Goldfarb NI. Costs to hospitals of acquiring and processing blood in the US. Appl Health Econ Health Policy. 2011;9(1):29–37. doi: 10.2165/11530740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Medicare & Medicaid Services Medicare – ASP Drug Pricing Files [Internet] Medicare. 2016. [Accessed September 26, 2018]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2016ASPFiles.html.
- 34.Lin J, Zhang X, Li X, et al. Cost of bleeding-related episodes in adult patients with primary immune thrombocytopenia: a population-based retrospective cohort study of administrative claims data for commercial payers in the United States. Clin Ther. 2017;39(3):603–609. doi: 10.1016/j.clinthera.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 35.CMS Medicare Fee Schedule [Internet] Medicare. 2017. [Accessed September 26, 2018]. Available from: https://www.findacode.com/