Abstract
The establishment of a simple, rapid and efficient transient expression system is a necessary tool for the functional validation of candidate genes in coffee biotechnology. The effects of Agrobacterium strain, age of the donor plant, infiltration method, and infiltration medium on transgene expression in detached coffee leaves were evaluated. Regarding the effect of Agrobacterium strain, the expression of uidA was higher in GV3101-treated coffee disks than in LBA4404 and ATHV-treated samples. On the other hand, transient expression of uidA was significantly higher in leaf disks from young plants (6-weeks-old) (13.1 ± 1.4%) than in mature tissue (12-weeks-old) (1.6 ± 1.2%). Transient uidA expression was higher in detached coffee leaf disks from young plants infiltrated with one injection of 15 µL of Agrobacterium strain GV3101::1303 suspended in MS salts supplemented with 30 g/L sucrose, 1.9 g/L MES and 200 uM AS with subsequent sanding of the abaxial epidermis. Using the optimized protocol, expression of the uidA gene was observed 6, 24 and 48 h and 5 weeks after bacterial injection. DNA was extracted from coffee disks with positive GUS expression and specific mgfp5 and uidA fragments were amplified 5 weeks post-agroinfiltration. On the other hand, using the optimized protocol, a specific cry10Aa (500 bp) fragment was amplified in the agro-infiltrated coffee leaf disks 5 weeks post-agroinfiltration with the plasmid pB427-35S-cry10Aa. Moreover, the expression of the gene cry10Aa in two infiltrated coffee leaf disks was verified by RT-PCR and an expected 500 bp fragment was amplified.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1495-5) contains supplementary material, which is available to authorized users.
Keywords: Leaf agroinfection, Genetic transformation, In planta genetic transformation, Agrobacterium tumefaciens, Bacillus thuringiensis, Cry10Aa
Introduction
Coffee (Coffea spp.) is an economically important crop and one of the main export products of several countries in Latin America, Africa and Asia (Ivamoto et al. 2017). Recently, the genomes of C. canephora (Denoeud et al. 2014) and C. arabica (Van Deynze et al. 2017) as well as the transcriptomes of Arabica coffee (Ivamoto et al. 2017) and an Arabica coffee ancestor Coffea eugenioides (Yuyama et al. 2016) were reported. These advances open possibilities for the study of candidate genes related to agronomical traits and metabolic pathways and for assisted breeding applications through the introduction of new desirable traits by genetic engineering (Ribas et al. 2011).
Two genetic transformation strategies, stable transformation and transient transformation, have been used in plants for gene function analysis, protein production, protein–protein interaction and promoter activity evaluation (Cao et al. 2017; Zhao et al. 2017). Protocols for stable genetic transformation of C. arabica and C. canephora have been reported using Agrobacterium tumefaciens (Hatanaka et al. 1999; Leroy et al. 2000; Mishra et al. 2002; Ogita et al. 2002; Canche-Moo et al. 2006; Ribas et al. 2011), Agrobacterium rhizogenes (Alpizar et al. 2006; Kumar et al. 2006) electroporation (Fernandez-Da Silva and Menendez Yuffa 2003) and biolistics (van Boxtel et al. 1995; Rosillo et al. 2003; Ribas et al. 2005; Albuquerque et al. 2009).
Genetic engineering research on coffee has been done in the past 15 years to express herbicide resistance genes, insect resistance genes, genes controlling biochemical and physiological traits and reporter genes (Mishra and Slater 2012). Nevertheless, up to date, coffee plants expressing cry genes from Bacillus thuringiensis, which are effective against coffee berry borer (CBB), one of the major threats for its production, have not been produced. In this sense, Espinoza et al. (2010) indicated that cry10Aa and cyt1Aa genes might be effective against CBB. The results reported by these authors showed that bioassays against first-instar of H. hampei of cry10Aa and cyt1Aa gave estimated larval mortality of 20 and 50%, respectively.
Nevertheless, stable transformation of coffee under in vitro conditions is still a time consuming, inefficient and laborious process that requires the establishment of an efficient regeneration system (Ribas et al. 2011). Therefore, rapid, simple and efficient techniques for the functional study of candidate genes are particularly attractive (Rosillo et al. 2003). Transient transformation avoids the limitations of the stable transformation process such as transformation efficiency, selection, and regeneration of putative transgenic material (Zhao et al. 2017). A transient transformation system has been described for coffee by van Boxtel et al. (1995) and Rosillo et al. (2003). These authors evaluated factors affecting the transient expression of transgenes introduced into suspension cells using particle bombardment. To the best of our knowledge, an ex vitro protocol for transient expression using A. tumefaciens-mediated infiltration of coffee leaves has not been reported.
The delivery of genes into plant tissues for transient expression using A. tumefaciens has been reported in various species using vacuum or syringe-based infiltration methods (Shah et al. 2013; Cao et al. 2017; Zhao et al. 2017). Vacuum infiltration requires specialized equipment. Due to its speed, yield, and cost, this method has been used for the production of large quantities of recombinant proteins (Zhao et al. 2017). On the other hand, the syringe-based method requires only a needleless syringe. Due to its simplicity and speed, it has been widely used for promoter and gene function analyses, plant-pathogen interaction studies, abiotic stress-tolerance assays, and for the production of low quantities of recombinant proteins (Cao et al. 2017). In order to increase transformation efficiency, several factors need to be optimized (Matsuo et al. 2016; Cao et al. 2017). We studied the effects of the infiltration method, Agrobacterium strain, and age of the donor plant on Agrobacterium infiltration of coffee leaves to produce an efficient protocol for transient and stable expression of genes in coffee.
Materials and methods
Plant material
Seedlings of C. arabica L. variety Catuaí provided by CICAFE (Coffee Research Center, Barva de Heredia, Costa Rica) were grown in pots containing peat (Nutripeat, V-J Centroamericana S.A, Costa Rica) in a culture room at 100% relative humidity in darkness at 30 °C. The second fully expanded leaf below the apex was collected from 6 and 12-week-old plants and leaf disks were cut using a cork borer. Prior to agroinfiltration, leaf disks were rinsed in 95% (v/v) ethanol for 1 min and subsequently surface sterilized with a 1% (v/v) NaOCl solution for 1 min. After three washes with distilled water, the leaf disks were injected with bacterial suspensions as described below.
Bacterial strains and binary plasmids
For the optimization of the agroinfiltration method, three Agrobacterium strains with different chromosomal backgrounds and opines were used: A. tumefaciens GV3101 (50 mg/L rifampicin + 50 mg/L spectinomycin + 50 mg/L streptomycin + 50 mg/L kanamycin), LBA4404 (50 mg/L rifampicin + 50 mg/L ampicillin + 50 mg/L kanamycin) and ATHV (50 mg/L rifampicin + 50 mg/L kanamycin) containing the binary plasmid pCAMBIA 1303 (acquired from CAMBIA, Canberra, Australia). The T-DNA contained reporter genes mgfp5 (which codes for green fluorescent protein) and uidA (which codes for beta-glucuronidase) and the selectable marker gene hptII coding for hygromycin phosphotransferase under the control of the CAMV 35S promoter (Fig. 1a). For the stable genetic transformation experiments, the A. tumefaciens GV3101 strain containing the binary plasmid pB427-35S-cry10Aa was used (acquired from DNA Cloning Service, Hamburg, Germany). The T-DNA cassette harbors the cry10Aa and hptII gene under the control of the CAMV 35S promoter (Fig. 7a). Plasmids were introduced into the agrobacteria by the freeze–thaw method. Their presence was verified by restriction analysis and by PCR (polymerase chain reaction) using specific primers for the hptII and cry10Aa gene.
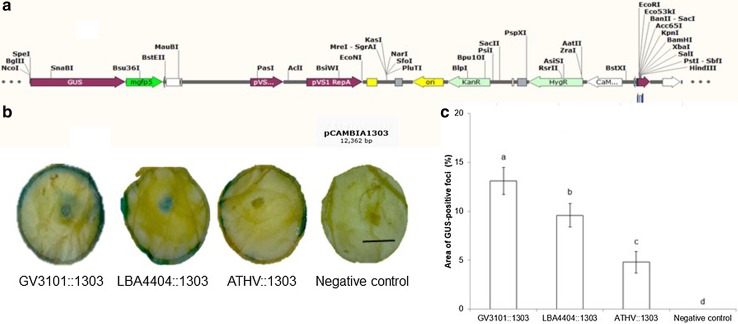
Fig. 1.
Transient GUS expression in coffee leaf disks injected with three strains of Agrobacterium tumefaciens. a Linear map of pCAMBIA1303 plant expression vector b GUS staining observed 48 h after infiltration c Percentage of GUS-stained area detected using Image J measurements. Each value represents the mean ± SD of fifteen repetitions. Means with the same letter are not significantly different (Duncan test p = 0.05). Bar: 1 cm
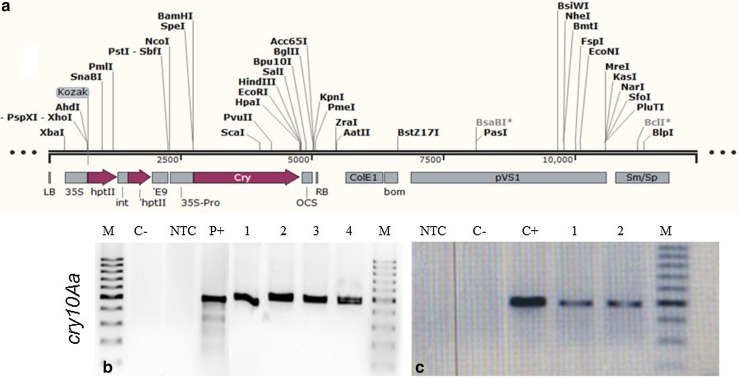
Fig. 7.
a Linear map of cry10Aa gene cassette cloned in pB427-35S-cry10Aa plant expression vector. b PCR analysis showing the presence of the cry10Aa gene in agroinfiltrated coffee leaf disks (amplicon size 500 bp). M molecular weight marker (100 bp, Fermentas), NTC negative control (PCR reaction mix without template), P+ positive control (pB427-35S-cry10Aa DNA), C- wildtype leaf disks (non-infiltrated control), 1, 2, 3, and 4: putative transgenic coffee leaf disks. c RT-PCR analysis demonstrating cry10Aa expression in agroinfiltrated coffee leaf disks (amplicon size 500 bp). M molecular weight marker (100 bp, Fermentas), NTC: negative control (RT-PCR reaction mix without template), P+ positive control (pB427-35S-cry10Aa DNA), C- wildtype leaf disks (non-infiltrated control), 1, and 2: putative transgenic coffee leaf disks
Prior to transformation experiments, a single colony of each Agrobacterium strain was grown overnight at 26 °C in liquid LB medium supplemented with 20 µM acetosyringone (AS) (5-dimethoxy-4-hydroxyaceto-phenone) and the respective antibiotics on an orbital shaker at 250 rpm. The bacterial suspensions were centrifuged at 3500 rpm for 20 min. Pellets were resuspended in Murashige and Skoog (MS) medium supplemented with 30 g/L sucrose, 1.9 g/L MES and 200 uM AS to a final optical cell density (OD600) of 0.6.
Optimization of agroinfiltration mediated transformation
To establish an agroinfiltration protocol for detached coffee leaf disks, four variables were evaluated: the strain of Agrobacterium tumefaciens, the age of the plant (6 or 12 weeks old) from which leaf disks were obtained, the type of wound made in the leaf disks and the infiltration medium in which the bacteria were resuspended.
In the first experiment, a 1 mL needleless syringe was used to inject surface sterilized coffee leaf disks (1 cm) with a suspension of the Agrobacterium strains GV3101, LBA4404 or ATHV (OD600: 0.6) carrying the pCAMBIA 1303 plasmid. Injection was done as follows: a small wound was made in the abaxial epidermis of the leaf using the needle of the syringe. Subsequently, 15 µL of the bacteria solution was injected using a needleless syringe in the wound until the complete disk was covered. As the solution was injected, the disk becomes darker, which shows proper infiltration. In the second experiment, once the optimal Agrobacterium strain was chosen, coffee leaf disks from young (6-week-old) and adult (12-week-old) plants were injected with a solution of A. tumefaciens GV3101::pCAMBIA 1303 using a 1 mL needleless syringe. In the third experiment, once the best age of the donor plant was selected, a 1 mL needleless syringe was used to inject leaf disks from 6-week-old plants with A. tumefaciens GV3101::pCAMBIA 1303 using one of the following methods: (A) one injection of 15 µL of the bacterial suspension and subsequent sanding of the abaxial epidermis, (B) one injection of the bacterial suspension (15 µL) without sanding, (C) four equidistant injections of 15 µL of the bacterial suspension without sanding, (D) sonication for 1 min of the leaf disks together with the bacteria suspended in infiltration medium, and (E) sonication of the disks in infiltration medium for 1 min and then one injection of 15 µL of the bacterial suspension. Sanding of the abaxial epidermis was done with a 2000 grit sandpaper.
Finally, in the fourth experiment, coffee leaf disks from 6-week-old plants were infiltrated with one injection of Agrobacterium strain GV301::1303 suspended in MS salts supplemented with 30 g/L sucrose, 1.9 g/L MES and 200 uM AS or 10 mM MgCl2 supplemented with 1.9 g/L MES and 200 uM AS. In all experiments, the leaf disks were placed on wet tissue in Petri dishes with the adaxial side up and maintained at 25 °C in darkness for 48 h. Then, the explants were washed with a 1% (v/v) NaOCl solution for 1 min followed by three washes with distilled water of 10 min each one. All experiments were performed in triplicate using five leaf disks. Explants not exposed to A. tumefaciens were included as negative controls.
Transformation efficiency was evaluated 48 h after infiltration by observing GUS histochemical staining. The relative area of blue staining per explant was quantified using the program ImageJ (https://imagej.nih.gov/ij/index.html) (Rasband 1997–2016) as follows: [(area with blue foci/total area of the leaf disk) × 100]. Data were subjected to one-way analysis of variance (ANOVA) and means were compared using Duncan’s (1955) multiple range tests (p = 0.05).
Histochemical GUS assays
GUS assays were performed by immersing tissues in staining buffer [50 mM sodium phosphate (pH 7.0), 0.5 mM potassium ferrocyanide, 0.5 mM potassium ferricyanide, 10 mM EDTA, 0.1% Triton, 1 mM X-Gluc (5-bromo-4-chloro-3-indolyl-b-d-glucuronide)] for 24 h at 37 °C in the dark (Jefferson 1987). Leaf disks were then washed with 95% ethanol (EtOH) and incubated at 65 °C for 12 h until the green color was removed. GUS-stained tissue was observed under 10-fold magnification using a stereomicroscope (Nikon SM2 8000) equipped with a digital camera (Moticam 5).
Transformation of the cry10Aa gene using the optimized method
Coffee leaf disks from 6-week-old plants were infiltrated with 15 µL of Agrobacterium strain GV301::1303 or GV301::pB427-35S-cry10Aa suspended in MS salts supplemented with 30 g/L sucrose, 1.9 g/L MES and 200 uM AS. After agroinfiltration, the explants were placed on wet tissue in Petri dishes with the adaxial side up and maintained at 25 °C in darkness for 48 h. Then, the explants were washed with a 1% (v/v) NaOCl solution for 1 min followed by three washes with distilled water of 10 min each one. Following washing, the infiltrated disks were placed on wet tissue in Petri dishes with the adaxial side up and kept in plant tissue culture room with 16 h day light regime at 25 °C for 5 weeks. Explants not exposed to A. tumefaciens were included as negative controls.
DNA extraction and PCR analysis
Genomic DNA was extracted using the CTAB method outlined by Doyle and Doyle (1990) with minor modifications according to Bolívar-González et al. (2018). The concentration and purity (ratio 260/280) of genomic DNA was measured by spectrophotometric analysis and adjusted to 100 ng/µL. The integrity of the extracted DNA was verified by electrophoresis on 0.8% (w/v) agarose gels prepared in 1X TBE buffer.
PCR was used to test for the presence of the mgfp5, uidA, cry10Aa, and virG genes. The PCR reaction was performed in a mixture (25 µl) containing 1X Taq Buffer + (NH4)SO4 (Thermo Scientific), 1.5 mM MgCl2, 0.2 mM dNTPs mix, 0.3 µM of each primer, 0.5 U Taq Polymerase (Thermo Scientific) and 3 µL of genomic DNA. The primer sequences, as well as the amplification conditions, are shown in Supplementary Table 1. PCR products (10 µl) were separated on 1% (w/v) agarose gels stained with GelRed® (Phenix Research) in 1X TBE buffer at 50 V for 1 h. The gels were visualized in a Darkhood DH-20 transilluminator and photographed with the Gerix 1000 system (Biostep, Germany). The plasmid pCAMBIA 1303 and pB427-35S-cry10Aa were used as a positive control for the presence of the uidA and cry10Aa genes, respectively. Whereas DNA isolated from A. tumefaciens was used as positive control for virG.
RNA isolation and RT-PCR
Total RNA was isolated from two infiltrated and one non-infiltrated coffee leaf disks using the TRI Reagent (Sigma–Aldrich Chemie, Steinheim, Germany) following the manufactures’ instructions. After RNA extraction, DNA was removed by DNase I treatment (MBI Fermentas, St. Leon-Rot). The total RNA was quantified using a spectrophotometer (NanoPhotometer TM, Germany) at wavelengths of 260 and 280 nm, and RNA integrity was verified by analyzing samples on a 1.2% (w/v) denaturing agarose gel. RNA (1 µg) was transcribed to DNAc using the RevertAid H Minus First Strand cDNA Synthesis Kit® (Thermo Scientific) according to the manufactures’ instructions. For detecting possible DNA contaminations in RNA preparations, a negative control was included in which no reverse transcriptase was employed.
The transcript of the gene cry10Aa was analyzed by PCR in a mixture (20 µl) containing 1X Taq Buffer + (NH4)SO4 (Thermo Scientific), 1.5 mM MgCl2, 0.2 mM dNTPs mix, 0.3 µM of each primer [cry10Aa-f: (5′-aactacaagagaaccgattcataca-3′) and cry10Aa-r: (5′-acggtacgtgggaaagtaaca-3′)], 0.5 U Taq Polymerase (Thermo Scientific) and 2 µL of DNAc. Amplification conditions for were as follows: 94 °C for 3 min followed by 35 cycles at 94 °C for 1 min, 60 °C for 1 min, 72 °C for 1 min and a final step of 72 °C for 10 min. The RT-PCR products (10 µl) were evaluated on 1.2% (w/v) agarose gel stained with GelRed® (Phenix Research) in 1X TBE buffer at 50 V for 1 h, visualized in a Darkhood DH-20 transilluminator and photographed with the Gerix 1000 system (Biostep, Germany).
Results and discussion
Several factors, including plant genotype, type of explant, Agrobacterium strain, cell density in inoculation medium, and the conditions of inoculation and co-culture, influence the transfer of T-DNA from Agrobacterium to plant cells. Each of these elements needs to be optimized for each transformation system (Amoah et al. 2001).
Effect of the A. tumefaciens strain on transient expression of uidA
To determine the most suitable strain for the transient expression of the uidA gene, fifteen coffee disks were injected using A. tumefaciens strain GV3101 (C58, nopaline), LBA4404 (Ach5, octopine) or ATHV harboring the plasmid pCAMBIA 1303. We observed histochemical staining 48 h post infection in 100% of the explants injected with either GV3101 or LBA4404. A positive reaction was seen in 86.7% of the explants injected with ATHV (Fig. 1b). Expression of uidA was 0.7 and 2.7 times higher in GV3101-treated coffee disks than in LBA4404 and ATHV-treated samples, respectively (Fig. 1c). The A. tumefaciens strain has been shown to affect the efficiency of transient expression in grapevine (Zottini et al. 2008), rose (Yasmin and Debener 2010), Maesa lanceolata (Faizal and Geelen 2012), and spinach (Cao et al. 2017). In coffee, Mishra et al. (2008) demonstrated that the Agrobacterium strain influenced the transient expression of uidA and gfp in embryogenic tissues. The virulence of Agrobacterium strains can be dependent on the target plant species (Faizal and Geelen 2012). This may be due to the ability of the bacteria to attach to plant cells or to differences in the T-DNA transfer mechanism (Wroblewski et al. 2005).
Effect of plant age on transient GUS expression
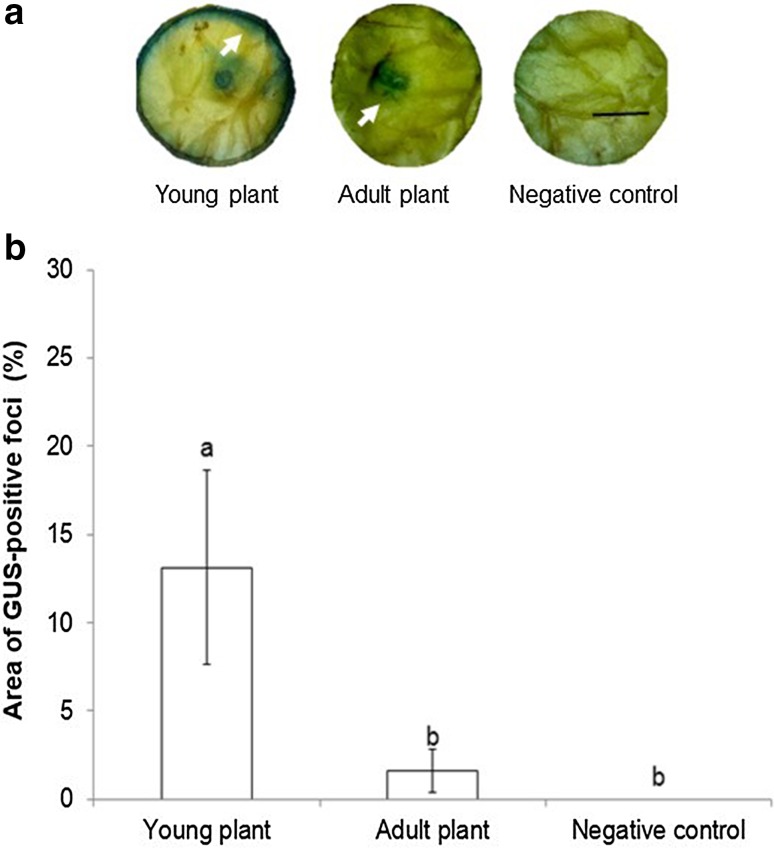
To determine the effect of leaf age on the transient expression of the uidA gene, fifteen coffee leaf disks from young (6-week-old) and adult (12-week-old) plants were injected with A. tumefaciens GV3101::pCAMBIA 1303. Transient expression of uidA was observed 48 h post infection in leaves of both stages injected with the bacterial suspension but not in the non-inoculated control (Fig. 2a). Transient expression of uidA was significantly higher in leaf disks from young plants than in mature tissue. Histochemical staining was observed in 100% of the leaf disks from 6-week-old plants and the relative area of blue-stained tissue per explant was 13.1 ± 1.4% (Fig. 2b). Similarly, leaf age affected transformation efficiency in cacao (Theobroma cacao L) (Fister et al. 2016) and strawberry (Fragaria vesca) (Cui et al. 2017). Transient expression of yfp and gfp genes decreased with increasing leaf age (Fister et al. 2016; Cui et al. 2017). Fister et al. (2016) attribute this result to both physiological and physical (toughness, rigidity) differences between leaves of different ages.
Fig. 2.
Transient GUS expression in coffee leaf disks from young and adult plants injected with A. tumefaciens GV3101::pCAMBIA 1303. a GUS staining observed 48 h after infiltration. b Percentage of GUS-stained area detected using Image J measurements. Each value represents the mean ± SD of fifteen repetitions. Means with the same letter are not significantly different (Duncan test p = 0.05). Bar: 1 cm
Effect of infiltration method on transient expression of uidA
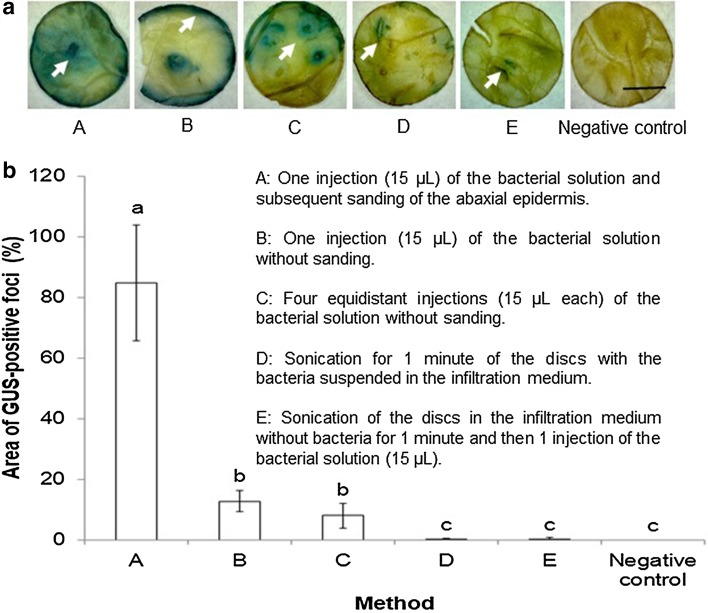
Fifteen coffee leaf disks from young (6-week-old) plants were injected with A. tumefaciens GV3101::pCAMBIA 1303 using different infiltration methods. Transient expression of uidA was observed 48 h post-infection in leaf disks injected with the bacterial suspension using the different methods, but not in the control (Fig. 3a). Agroinfiltration using one injection followed by sanding of the abaxial epidermis resulted in significantly higher transient uidA expression than the other injection methods (Fig. 3b). Histochemical staining was observed in 100% of the explants and the relative area of GUS-stained tissue was 84.8 ± 19.0% (Fig. 3b). Target genes have been effectively introduced into plants by agroinfiltration using syringe- and vacuum-based methods. Expression of target genes within tissue cells may differ between the two methods due to differences in the penetration and spread of the bacteria (Cao et al. 2017).
Fig. 3.
Effect of A. tumefaciens GV3101::pCAMBIA 1303 infiltration method on transient GUS expression in coffee leaf disks. a GUS staining observed 48 h after infiltration. b Percentage of GUS-stained area detected using Image J measurements. Each value represents the mean ± SD of fifteen repetitions. Means with the same letter are not significantly different (Duncan test p = 0.05). Bar: 1 cm
The use of surfactants, antioxidants, and abrasive substances such as Tween 20, Silwet L-77, ascorbate acid, and carborundum influences transformation efficiency (Zhao et al. 2017). Surfactants reduce surface tension, thereby allowing penetration of bacteria into plant tissues (Zhao et al. 2017). Here, we demonstrated that damaging the coffee leaf epidermis using sandpaper increased transient expression of the uidA gene (Fig. 3a). Wounding the tissue probably triggered the Agrobacterium infection apparatus, resulting in an increased number of transformed cells. Andrieu et al. (2012) showed that wounding the rice leaf surface using a small apparatus carrying many 600 µm diameter needles favored the penetration of Agrobacterium and enhanced the expression of the uidA gene relative to unwounded infiltrated leaves.
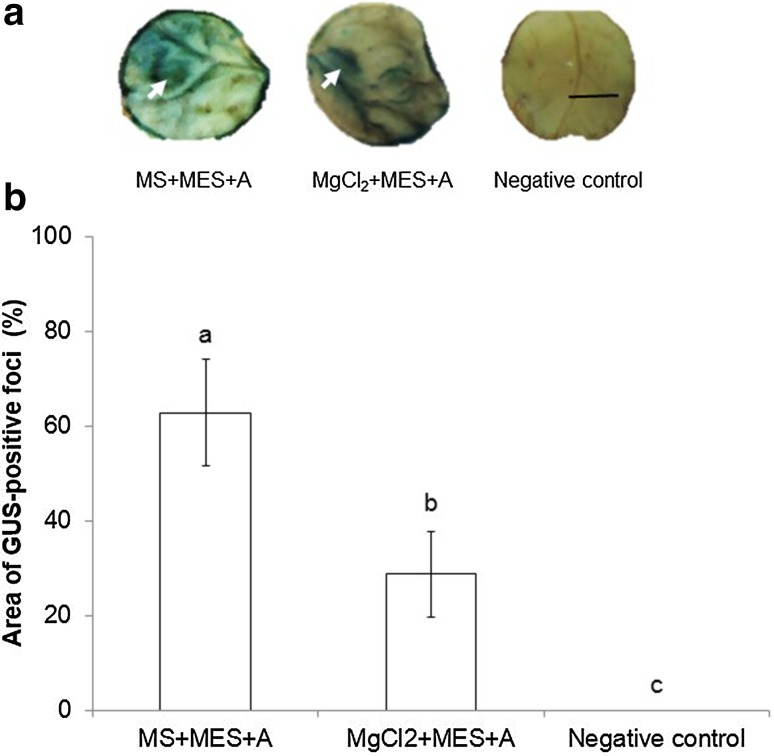
Effect of infiltration medium on transient GUS expression
The infiltration medium is an important parameter to be considered for gene delivery in plant systems (Du et al. 2010; Kumar et al. 2017). In order to define the appropriate infiltration medium for the transient expression of the uidA gene, fifteen coffee leaf disks from young (6-week-old) plants were injected with A. tumefaciens GV3101::pCAMBIA 1303 using the best infiltration method described above and two different infiltration media (MS salts supplemented with 30 g/L sucrose, 1.9 g/L MES and 200 uM AS or 10 mM MgCl2 supplemented with 1.9 g/L MES and 200 uM AS). Transient expression of uidA was observed 48 h post-infection in coffee leaf disks injected with both media but not in the control (Fig. 4a). Transient expression of uidA was significantly higher in explants injected with bacteria suspended in MS salts supplemented with 30 g/L sucrose, 1.9 g/L MES and 200 uM AS (Fig. 4b). GUS-positive foci were observed in 93% of the explants with an average stained leaf area of 62.8 ± 11.2%. Both MS and MgCl2 media have been used successfully for genetic transformation by infiltration. The former has been used with Nicotiana tabacum (Yang et al. 2000), Arabidopsis thaliana (Dehestani et al. 2010), Vigna unguiculata (Adesoye et al. 2010) and Pupulus sp (Takata and Eriksson 2012). Infiltration media based on MgCl2 have been used with Nicotiana benthamiana, Nicotiana tabacum (Koscianska et al. 2005), Solanum melongena and Solanum lycopersicum (Pratap et al. 2011).
Fig. 4.
Effect of infiltration medium on transient GUS expression in coffee leaf disks injected with A. tumefaciens GV3101::pCAMBIA 1303. a GUS staining observed 48 h after infiltration. b Percentage of GUS-stained area detected using Image J measurements. Each value represents the mean ± SD of fifteen repetitions. Means with the same letter are not significantly different (Duncan test p = 0.05). Bar: 1 cm
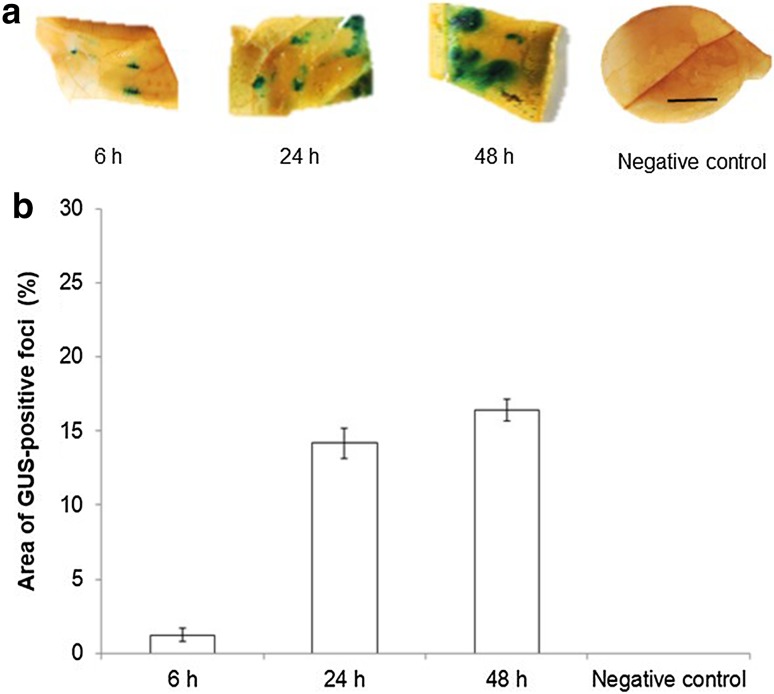
Expression of the uidA gene
Expression of the uidA gene was evaluated over time using images of coffee leaves acquired 6, 24 and 48 h after infiltration with Agrobacterium using the optimized protocol and no phenolic damage due to sanding was observed (Fig. 5a). Transient GUS expression was first detected 6 h after bacterial injection. The relative area of GUS-stained tissue per explant was highest 48 h post-infiltration (Fig. 5b). The duration of co-cultivation of rose and cacao explants had a significant effect on the level of expression of the transgene over time (Yasmin and Debener 2010; Fister et al. 2016). In grapevine, rose and cacao, expression of the reporter gene increased over time until reaching a limit, after which it decreased (Zottini et al. 2008; Yasmin and Debener 2010; Fister et al. 2016).
Fig. 5.
Time course of GUS staining after infiltration of coffee leaf disks with A. tumefaciens GV3101::pCAMBIA 1303. a GUS staining observed 6, 24, and 48 h after infiltration. b Percentage of GUS-stained area detected using Image J measurements. Each value represents the mean ± SD of fifteen repetitions. Bar: 1 cm
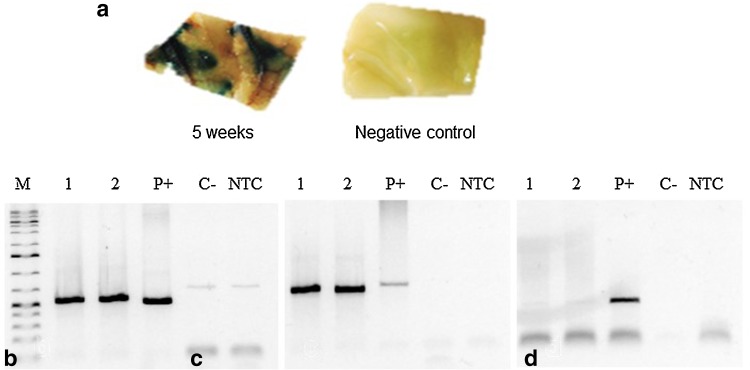
After establishing a transient transformation assay, the expression of the uidA gene was monitored for 5 weeks after injection of coffee leaf disks with A. tumefaciens GV3101::pCAMBIA 1303. As shown in Fig. 6a, a positive reaction, indicated by blue staining of coffee leaves and no damage of the tissue due to phenolic exudation, was observed. As demonstrated in grapevine (Zottini et al. 2008) and Maesa lanceolate (Faizal and Geelen 2012), coffee leaves could potentially be infiltrated under in vitro conditions and cut from the plant after 5 weeks and cultured on shoot or embryogenic callus induction medium.
Fig. 6.
a Expression of the uidA gene 5 weeks after infiltration. PCR analysis showing the presence of the genes b mgfp5 (amplicon size 790 bp) and c uidA (amplicon size 710 bp) and the absence of the gene d virG in agroinfiltrated coffee leaf disks (amplicon size 390 bp). M molecular weight marker (1 Kb Plus, Fermentas), NTC negative control (PCR reaction mix without template), P+ positive control (pCAMBIA 1303 DNA), C- wildtype leaf disks (non-injected control), 1 and 2: putative transgenic coffee leaf disks. Bar: 1 cm
PCR was used to test for the presence of the mgfp5, uidA, and virG genes 5 weeks post-agroinfiltration. Specific mgfp5 (790 bp) (Fig. 6b) and uidA (710 bp) (Fig. 6c) fragments were amplified in the agro-injected coffee leaf disks. No DNA fragments were amplified by PCR in wild-type leaf disks (non-injected control). Moreover, the virG gene was not detected in the agroinfiltrated coffee leaf disks, indicating the lack of contamination with A. tumefaciens (Fig. 6d).
Expression of the cry10Aa gene
Specific cry10Aa (500 bp) fragment was amplified in the agro-infiltrated coffee leaf disks 5 weeks post-agroinfiltration with the plasmid pB427-35S-cry10Aa (Fig. 7b). No DNA fragments were amplified by PCR in wild-type leaf disks (non-injected control). Moreover, the virG gene was not detected in the agroinfiltrated coffee leaf disks, indicating the lack of contamination with A. tumefaciens (data not shown).
The expression of the gene cry10Aa in two infiltrated coffee leaf disks was verified by RT-PCR and an expected 500 bp fragment was amplified (Fig. 7c). No signal was detected in the non-infiltrated leaf disk. In the controls where the reverse transcriptase was added to the reaction mixture, no amplicons were detected (data not shown). Production of insect-resistant coffee plants is one of the major objectives of the breeding programs. Previously, transgenic coffee plants expressing synthetic cry genes (cry1Ac) from Bacillus thuringiensis, which is effective against the coffee leaf miner, were regenerated (Leroy et al. 2000). In another experiment, an α-amylase inhibitor from Phaseolus vulgaris was tested against coffee berry borer and found to have an inhibitory effect on its growth and development (Barbosa et al. 2010). The effectiveness of Bacillus thuringiensis genes in controlling CBB has been well reported (Mendez-Lopez et al. 2003). Therefore, the development of coffee varieties resistant to coffee berry borer using transgenic technology will be a great benefit for the coffee industry.
In conclusion, the rapid, simple and economic transformation strategy reported in this study could be used to modify the expression of multiple genes in coffee. Potential uses include the development of control strategies for coffee berry borer (Hypothenemus hampei Ferrari), coffee rust (Hemileia vastatrix) or leaf miner (Leucoptera coffeella), as well as CRISPR/Cas9 mediated genome editing in coffee leaves.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was financed by the Vicerrectoría de Investigación of the University of Costa Rica and MKTPLACE (project No. 111-B3-206).
Abbreviations
- 2, 4-D
2, 4-Dichlorophenoxyacetic acid
- ABA
Abscisic acid
- AS
Acetosyringone
- PCR
Polymerase chain reaction
Author contributions
CVG and CVS and JVV designed and performed the experiments; AGA conceived and proposed the project for financing, designed and coordinated the experiments, analyzed data and wrote the paper; LFPP proposed the project for financing and edited the paper.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest. All authors read and approved the final manuscript.
References
- Adesoye A, Togun A, Machuka J. Transformation of cowpea (Vigna unguiculata L. Walp.) by Agrobacterium infiltration. J Appl Biosc. 2010;30:1845–1860. [Google Scholar]
- Albuquerque EVS, Cunha WG, Barbosa AEAD, Costa PM, Teixeira JB, Vianna GR, Cabral GB, Fernandez D, Grossi-de-Sa MF. Transgenic coffee fruits from Coffea arabica genetically modified by bombardment. In Vitro Cell Dev Biol Plant. 2009;45:532–539. doi: 10.1007/s11627-009-9254-2. [DOI] [Google Scholar]
- Alpizar E, Dechamp E, Espeout S, Royer M, Lecouls AC, Nicole M, Bertrand B, Lashermes P, Etienne H. Efficient production of Agrobacterium rhizogenes-transformed roots and composite plants for studying gene expression in coffee roots. Plant Cell Rep. 2006;25(9):959–967. doi: 10.1007/s00299-006-0159-9. [DOI] [PubMed] [Google Scholar]
- Amoah BK, Wu H, Sparks C, Jones HD. Factors influencing Agrobacterium-mediated transient expression of uidA in wheat inflorescence tissue. J Exp Bot. 2001;52(358):1135–1142. doi: 10.1093/jexbot/52.358.1135. [DOI] [PubMed] [Google Scholar]
- Andrieu A, Breitler JC, Siré C, Meynard D, Gantet P, Guiderdoni E. An in planta, Agrobacterium-mediated transient gene expression method for inducing gene silencing in rice (Oryza sativa L.) leaves. Rice. 2012;5:23. doi: 10.1186/1939-8433-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa AEAD, Albuquerque EVS, Silva MCM, Souza DSL, Oliveira-Neto OB, Valencia A, Rocha TL, Grossi-de-Sa MF. αe- Amylase inhibitor-1 gene from Phaseolus vulgaris expressed in Coffea arabica plants inhibits α-amylases from the coffee berry borer pest. BMC Biotechnol. 2010;10:44. doi: 10.1186/1472-6750-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolívar-González A, Valdez-Melara M, Gatica-Arias A. Responses of Arabica coffee (Coffea arabica L. var. Catuaí) cell suspensions to chemically induced mutagenesis and salinity stress under in vitro culture conditions. In Vitro Cell Dev Biol Plant. 2018 doi: 10.1007/s11627-018-9918-x. [DOI] [Google Scholar]
- Canche-Moo RLR, Ku-Gonzalez A, Burgeff C, Loyola-Vargas VM, Rodriguez-Zapata LC, Castaño E. Genetic transformation of Coffea canephora by vacuum infiltration. Plant Cell Tiss Org Cult. 2006;84(3):373–377. doi: 10.1007/s11240-005-9036-4. [DOI] [Google Scholar]
- Cao DV, Pamplona RS, Kim J, Oh YK, Cho SK, Ahn J, Yang SW, Riu KZ, Boo KH. Optimization of Agrobacterium-mediated transient expression of heterologous genes in spinach. Plant Biotechnol Rep. 2017 doi: 10.1007/s11816-017-0457-4. [DOI] [Google Scholar]
- Cui MY, Wei W, Gao K, Xie YG, Guo Y, Feng JY. A rapid and efficient Agrobacterium-mediated transient gene expression system for strawberry leaves and the study of disease resistance proteins. Plant Cell Tiss Organ Cult. 2017;131:233–246. doi: 10.1007/s11240-017-1279-3. [DOI] [Google Scholar]
- Dehestani A, Ahmadian G, Salmanian H, Jelodar N, Kazemitabar K. Transformation efficiency enhancement of Arabidopsis vacuum infiltration by surfactant application and apical inflorescence removal. Trakia J Sci. 2010;8(1):19–26. [Google Scholar]
- Denoeud F, Carretero-Paulet L, Dereeper A, Droc G, Guyot R, Pietrella M, Zheng C, Alberti A, Anthony F, Aprea G, Aury JM, Bento P, Bernard M, Bocs S, Campa C, Cenci A, Combes MC, Crouzillat D, Da Silva C, Daddiego L, De Bellis F, Dussert S, Garsmeur O, Gayraud T, Guignon V, Jahn K, Jamilloux V, Joët T, Labadie K, Lan T, Leclercq J, Lepelley M, Leroy T, Li LT, Librado P, Lopez L, Muñoz A, Noel B, Pallavicini A, Perrotta G, Poncet V, Pot D, Priyono, Rigoreau M, Rouard M, Rozas J, Tranchant-Dubreuil C, VanBuren R, Zhang Q, Andrade AC, Argout X, Bertrand B, de Kochko A, Graziosi G, Henry RJ, Jayarama, Ming R, Nagai C, Rounsley S, Sankoff D, Giuliano G, Albert VA, Wincker P, Lashermes P. The coffee genome provides insight into the convergent evolution of caffeine biosynthesis. Science. 2014;345(6201):1181–1184. doi: 10.1126/science.1255274. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Du H, Wu H, Yang J, Li J. Effects of basal media, salt concentrations, antioxidant supplements and co-effects on the Agrobacterium -mediated transformation efficiency in maize. Afr J Biotechnol. 2010;9:1135–1143. doi: 10.5897/AJB09.1276. [DOI] [Google Scholar]
- Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- Espinoza AM, Hernández A, Ibarra JE, Bergman MR, Monnerart SPRG, Soares ME, Obando A, Wagner AR. Derived proteins from cry genes of Bacillus thuringiensis. Microbiol Rev. 2010;2WO2010043928 A1:242–255. [Google Scholar]
- Faizal A, Geelen D. Agroinfiltration of intact leaves as a method for the transient and stable transformation of saponin producing Maesa lanceolate. Plant Cell Rep. 2012;31:1517–1526. doi: 10.1007/s00299-012-1266-4. [DOI] [PubMed] [Google Scholar]
- Fernandez-Da Silva R, Menendez Yuffa A (2003) Transient gene expression in secondary somatic embryos from coffee tissues electroporated with the gene gus and bar. EJB [online]. 6(1). http://www.ejbiotechnology.info/content/vol6/issue1/full/6/. ISSN 0717–3458
- Fister AS, Shi Z, Zhang Y, Helliwell E, Maximova S, Guiltinan M. Protocol: transient expression system for functional genomics in the tropical tree Theobroma cacao L. Plant Methods. 2016;12:19. doi: 10.1186/s13007-016-0119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka T, Choi YE, Kusano T, Sano H. Transgenic plant of coffee Coffea canephora from embryogenic callus via Agrobacterium tumefaciens mediated transformation. Plant Cell Rep. 1999;19(2):106–110. doi: 10.1007/s002990050719. [DOI] [PubMed] [Google Scholar]
- Ivamoto ST, Reis JO, Domingues DS, dos Santos TB, de Oliveira FF, Pot D, et al. Transcriptome analysis of leaves, flowers and fruits perisperm of Coffea arabica L. reveals the differential expression of genes involved in raffinose biosynthesis. PLoS One. 2017;12(1):e0169595. doi: 10.1371/journal.pone.0169595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5(4):387–405. doi: 10.1007/BF02667740. [DOI] [Google Scholar]
- Koscianska E, Kalantidis K, Wypijewsji K, Sadowski J, Tabler M. Analysis of RNA silencing in agroinfiltrated leaves of Nicotiana benthamiana and Nicotiana tabacum. Plant Mol Biol. 2005;59:647–661. doi: 10.1007/s11103-005-0668-x. [DOI] [PubMed] [Google Scholar]
- Kumar V, Satyanarayana KV, Sarala Itty S, Indu EP, Giridhar P, Chandrashekar A, Ravishankar GA. Stable transformation and direct regeneration in Coffea canephora P ex. Fr. by Agrobacterium rhizogenes mediated transformation without hairy-root phenotype. Plant Cell Rep. 2006;25(3):214–222. doi: 10.1007/s00299-005-0045-x. [DOI] [PubMed] [Google Scholar]
- Kumar V, Kumar P, Chattopadhyay T. A simple method for transient expression of reporter gene in Brinjal leaves through agroinfiltration. Int J Curr Microbiol App Sci. 2017;6(3):317–323. doi: 10.20546/ijcmas.2017.603.035. [DOI] [Google Scholar]
- Leroy T, Henry AM, Royer M, Altosaar I, Frutos R, Duris D, Philippe R. Genetically modified coffee plants expressing the Bacillus thuringiensis cry1Ac gene for resistance to leaf miner. Plant Cell Rep. 2000;19(4):382–389. doi: 10.1007/s002990050744. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Fukuzawa N, Matsumura T. A simple agroinfiltration method for transient gene expression in plant leaf discs. J Biosci Bioeng. 2016;122(3):351–356. doi: 10.1016/j.jbiosc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Mendez-Lopez I, Basurto-Ros R, Ibarra JE. Bacillus thuringiensis serovar israelensis is highly toxic to the coffee berry borer, Hypothenemus hampei Ferr. (Coleoptera: Scolytidae. ). FEMS Microbiol Lett. 2003;226:73–77. doi: 10.1016/S0378-1097(03)00557-3. [DOI] [PubMed] [Google Scholar]
- Mishra MK, Slater A. Recent advances in the genetic transformation of coffee. Biotechnol Res Int. 2012;2012:1–17. doi: 10.1155/2012/580857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M, Sreenath H, Srinivasan CS (2002) Agrobacterium-mediated transformation of coffee: an assessment of factors affecting gene transfer efficiency. In: Proceedings of the 15th Plantation Crop Symposium Sreedharan K, Vinod Kumar PK, Jayarama, Chulaki BM (eds), pp 251–256
- Mishra M, Sreenath H, Jayarama A, McCormac A, Devi S, Elliott M, y otros (2008) Two critical factors: agrobacterium strain and antibiotics selection regime improve the production of transgenic coffee plants. In: Proceedings of the 22nd International Association for Coffee Science (ASIC’08), pp 843–850
- Ogita S, Uefuji H, Choi Y, Hatanaka T, Ogawa M, Yamaguchi Y, Koizumi N, Sano H. Genetic modification of coffee plants. J Plant Biotech. 2002;3:91–94. [Google Scholar]
- Pratap D, Kashikar A, Mukherjee S. Molecular characterization and infectivity of a Tomato leaf curl New Delhi virus variant associated with newly emerging yellow mosaic disease of eggplant in India. Virol J. 2011;8:305. doi: 10.1186/1743-422X-8-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS (1997–2016) ImageJ. U. S. National Institutes of Health, Bethesda, Maryland, USA. https://imagej.nih.gov/ij/
- Ribas AF, Kobayashi AK, Pereira LFP, Vieira LGE. Genetic transformation of Coffea canephora by particle bombardment. Biol Plant. 2005;49(4):493–497. doi: 10.1007/s10535-005-0038-1. [DOI] [Google Scholar]
- Ribas AF, Dechamp E, Champion A, Bertrand B, Combes MC, Verdeil JL, Lapeyre F, Lashermes P, Etienne H. Agrobacterium-mediated genetic transformation of Coffea arabica (L.) is greatly enhanced by using established embryogenic callus cultures. BMC Plant. 2011;11:92. doi: 10.1186/1471-2229-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosillo AG, Acuña JR, Gaitan AL, Peña M. Optimized DNA delivery into Coffea arabica suspension culture cells by particle bombardment. Plant Cell Tiss Org Cult. 2003;74(1):45–49. doi: 10.1023/A:1023314128543. [DOI] [Google Scholar]
- Shah KH, Almaghrabi B, Bohlmann H. Comparison of Expression Vectors for Transient Expression of Recombinant Proteins in Plants. Plant Mol Biol Report. 2013;31:1529–1538. doi: 10.1007/s11105-013-0614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, Eriksson M. A simple and efficient transient transformation for hybrid aspen (Populus tremula x P. tremuloides) Plant Methods. 2012;8:30. doi: 10.1186/1746-4811-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boxtel JH, Berthouly M, Carrasco C, Dufour M, Eskes A. Transient expression of β-glucoronidase following biolistic delivery of foreing DNA into coffee tissues. Plant Cell Rep. 1995;14(12):748–752. doi: 10.1007/BF00232915. [DOI] [PubMed] [Google Scholar]
- Van Deynze A, Hulse-Kemp Amanda C, Dario, Medrano JF (2017) Update on sequencing of the Coffea arabica variety, Geisha. In: Plant and animal genome conference, San Diego, California, 12–18 Jan 2017. http://app.core-apps.com/pag-2017/abstract/1a72fbe5da697beb0993e254b2c68756. Accessed 7 July 2018
- Wroblewski T, Tomczak A, Michelmore R. Optimization of Agrobacterium mediated transient expression assays for lettuce, tomato and Arabidopsis. Plant Biotech J. 2005;3:259–273. doi: 10.1111/j.1467-7652.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li R, Qi M. In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J. 2000;22(6):543–551. doi: 10.1046/j.1365-313x.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- Yasmin A, Debener T. Transient gene expression in rose petals via Agrobacterium infiltration. Plant Cell Tiss Organ Cult. 2010;102:245–250. doi: 10.1007/s11240-010-9728-2. [DOI] [Google Scholar]
- Yuyama PM, Reis JO, Ivamoto ST, Domingues D, Carazzolle M, Guimarães Pereira GA, Pierre C, Leroy T, Pereira LFP. Transcriptome analysis in Coffea eugenioides, an Arabica coffee ancestor, reveals differentially expressed genes in leaves and fruits. Mol Genet Genom. 2016;291:323–336. doi: 10.1007/s00438-015-1111-x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Tan Z, Wen X, Wang Y. An improved syringe agroinfiltration protocol to enhance transformation efficiency by combinative use of 5-azacytidine, ascorbate acid and tween-20. Plants. 2017;6:9. doi: 10.3390/plants6010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottini M, Barizza E, Costa A, Formentin E, Ruberti C, Carimi F, Lo Schiavo F. Agroinfiltration of grapevine leaves for fast transient assays of gene expression and for long-term production of stable transformed cells. Plant Cell Rep. 2008;27:845–853. doi: 10.1007/s00299-008-0510-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.