Abstract
Purpose
The understanding of patients with cancer of their condition and their wishes regarding care as they approach end of life (EoL) have been studied more in high-income countries than in low- and middle-income countries (LMICs).
Patients and Methods
Data were analyzed from a cohort study (N = 221) of patients with advanced cancer who were recruited from a palliative care center in Soweto, South Africa (LMIC), between May 2016 and June 2017. Patients were asked about their understanding of their illness, estimated life expectancy, EoL care communication, and EoL care preferences.
Results
Only 13 patients (5.9%) acknowledged that they were terminally ill; nine patients (4.1%) estimated accurately that they had months, not years, left to live. A total of 216 patients (97.7%) reported that they had not had an EoL care discussion with their physician, and 170 patients (76.9%) did not want to know their prognosis even if the doctor knew it. Most patients preferred comfort care (72.9%; n = 161) to life-extending care (14.0%; n = 31), and did not want to be kept alive using extreme measures (80.5%; n = 178) or have their doctors do everything possible to extend their lives (78.3%; n = 173). Finally, 127 patients (57.5%) preferred to die at home, and 51 (23.1%) preferred to die in the hospital. Most patients (81.0%; n = 179) had funeral plans.
Conclusion
South African patients demonstrated less awareness of the fact that they were terminally ill, were less likely to have discussed their prognosis with their doctor, and more strongly preferred comfort care to life-extending EoL care than US and other LMIC patients in prior research. These differences highlight the need for culturally appropriate, patient-centered EoL care for South African patients with advanced cancer as well as to determine individual preferences and needs in all EoL settings.
INTRODUCTION
United States–based studies indicate that patients who receive futile aggressive care at the end of life (EoL) have poorer survival,1 report lower quality of life,2-5 and incur higher costs than do other terminal patients.6 Moreover, receiving aggressive care at EoL is often contrary to patients’ preferences and values.5,7-11 As a result, in part, of these findings, health care policy makers in the United States have increasingly sought to ensure that EoL care is consistent with patient preferences.12,13 Critical to providing high-quality, patient-centered EoL care is ascertaining patients’ preferences for care and their understanding of their illness.14 Outside the United States, especially in low- and middle-income countries (LMICs), little is known about EoL care. Until recently, major causes of death in LMICs were trauma and infection, but death from chronic disease is increasingly common.15 Cancer incidence, in particular, is rising, and cancer survival remains poorer in LMICs than in high-income countries (HICs).16,17 Patients with terminal cancer in LMICs are unlikely to receive the kind of aggressive care that complicates EoL care in HICs; however, research on patients’ knowledge, preferences, and access to resources in such settings is sparse. Advancing the evidence base with respect to the EoL preferences of patients with advanced cancer, as well as EoL communication within international settings, is critical to the provision of ethical, culturally appropriate, high-quality, patient-centered EoL care.
South Africa is a middle-income sub-Saharan African country, but most of its population relies on the resource-constrained public health care system.18 Partly as a result of the lack of dedicated hospital-based palliative care centers within the public health care system, many patients with advanced illness are sent home to die without proper assessment or management of preferred or needed EoL care.18 The infrastructure with which to provide high-quality EoL care, such as symptom relief, is limited.18,19 The few studies of EoL care in sub-Saharan Africa are primarily qualitative, but they indicate that EoL care is predominantly provided in patients’ homes.20,21 These studies also reveal a lack of stigma and fear surrounding death20 that is common in Western settings, including in the United States.22 Elderly people who live in sub-Saharan Africa may be more accepting of death and view it as bringing peace and an end to suffering.20 These cultural differences, as well as differences in access to resources, may shape EoL care preferences and communication surrounding EoL care in a South African population living with advanced illness.
The limited research findings that are available in Africa reveal challenges to achieving informative and culturally sensitive communication around a terminal cancer diagnosis.23 Because of the need to improve our understanding of best-quality EoL care within a sub-Saharan African setting, the goal of the current study was to investigate the understanding of South African patients with advanced cancer of their illness, EoL care preferences, and EoL care communication. We examined patients’ terminal illness awareness, their preferences for the type of care received at EoL, and their current and preferred communication surrounding poor prognosis, seeking to understand what patient-centered and culturally appropriate EoL care might look like within a South African population of patients with advanced cancer.
PATIENTS AND METHODS
Study Sample and Procedure
Data were collected from a cohort of patients with advanced cancer and their informal, unpaid caregivers who were enrolled at the Wits Palliative Care Centre at Chris Hani Baragwanath Academic Hospital (CHBAH) in Soweto, South Africa, between May 2016 and June 2017. CHBAH is a public-sector hospital that serves mostly an urban black population within a lower socioeconomic level in South Africa. This study was modeled after the United States–based Coping with Cancer 1 and 2 cohort studies5,24 and included many of the same measures. The Coping with Cancer studies are prospective, multi-institutional cohort studies—funded by the National Cancer Institute and the National Institute of Mental Health—designed to evaluate the effect psychosocial influences among patients with advanced cancer and their informal caregivers on patients’ illness understanding, EoL care, and quality of death. The current study focuses only on patient-reported data.
The CHBAH Wits Palliative Care Centre represents one of the few dedicated hospital-based palliative care centers in South Africa. Patient eligibility criteria included diagnosis of a nonhematologic cancer (breast, prostate, upper and lower GI, lung, soft tissue sarcoma, or melanoma); age ≥ 18 years; interventional surgery, chemotherapy, or radiation therapy considered no longer appropriate for the patient; estimated life expectancy of ≤ 6 months (as determined by medical staff); and formal referral to the CHBAH Wits Palliative Care Center for EoL support and care. Patients were excluded from enrollment if they were unable to provide informed consent (n = 48), were too weak to take part in the study (n = 20), or unable to provide informed consent as a result of dying before enrollment (n = 35), being unconscious (n = 50), or not communicating (n = 69).
After confirming patients’ eligibility, trained research staff obtained informed consent for the study overall and for anonymized use of their HIV test results in the study. Patients who refused the latter consent or whose HIV status was unknown were not excluded from the study. The staff then administered face-to-face a baseline questionnaire in the patient’s preferred language (English, isiZulu, Sesotho, isiXhosa, or Setswana). All survey measures were conducted verbally to overcome literacy barriers, enhance data accuracy, and reduce the frequency of missing data.
Measures
Sociodemographic and clinical characteristics.
Patients self-reported age, sex, race, marital status, education, HIV status, primary cancer site, country of origin, language of interview, and access to running hot and cold water in their places of residence. HIV status and primary cancer site were confirmed through medical records.
Illness understanding.
Illness understanding was assessed using two questions that were used in prior research from the US Coping with Cancer studies24 to assess terminal illness acknowledgment and life expectancy. Patients were asked, “How would you describe your current health status?” Response options were as follows: relatively healthy (response 1); relatively healthy, but terminally ill (response 2); seriously ill, but not terminally ill (response 3); and seriously ill and terminally ill (response 4). Terminal illness acknowledgment was coded as “1” or “terminally ill” for responses of 2 and 4, and “0” or “not terminally ill” for responses of 1 and 3. Patients were also asked, “How long do you think you have left to live?” The following response options given: can estimate (response 1) and don’t know (response 2). Those patients who responded with option 1 were asked to estimate how many months or years they had left to live.
Communication with physician.
Patients were asked about their actual and preferred communication with physicians about how long they had left to live. To assess current communication, patients were asked, “Have the doctors talked with you about how much time you have left to live?” Response options included yes, specified time (response 1); yes, did not specify time (response 2); and no (response 3). To assess preferred communication, patients were asked, “If the doctor knew, would you want to know how long you have left to live?” Response options were yes (response 1), no (response 2), and do not know (response 3).
EoL care preferences.
We included four measures of EoL care preferences. First, patients were asked, “If you could choose, what would you prefer?” Response options were as follows: a course of treatment that focused on extending life as much as possible, even if it meant more pain and discomfort (life-extending care; response 1); or a plan of care that focused on relieving pain and discomfort as much as possible, even if that meant not living as long (comfort care; response 2). This measure was used in the Coping with Cancer 1 and 2 studies to assess preference for life-extending versus comfort care at EoL.5,25,26
To illuminate their preferences about specific treatments at EoL, patients were asked, “Would you want to be kept alive if it required you being on a breathing machine/kidney dialysis?” and “Would you want your doctors to do anything possible to keep you alive if you were going to die in a few days?” Response options for both questions were yes (response 1), no (response 2), and do not know (response 3). Finally, patients were asked, “If you were dying or at the end of your life, where would you most want to be?” Response options were as following: home (response 1), hospital (response 2), nursing home/step-down facility (response 3), inpatient hospice (response 4), or other (response 5).
Funeral plan.
South African patients were asked, “Do you have a funeral plan?” Response options were yes (response 1), no (response 2), and do not know (response 3).
ANALYTIC PROCEDURES
Descriptive statistics provided information about patient demographic and clinical characteristics as well as patients’ illness understanding, communication—both current and preferred—with their physician, EoL care preferences, and funeral plans. Means and standard deviations were used for continuous variables, and counts and percentages were used for categorical variables.
RESULTS
Demographic and Medical Characteristics
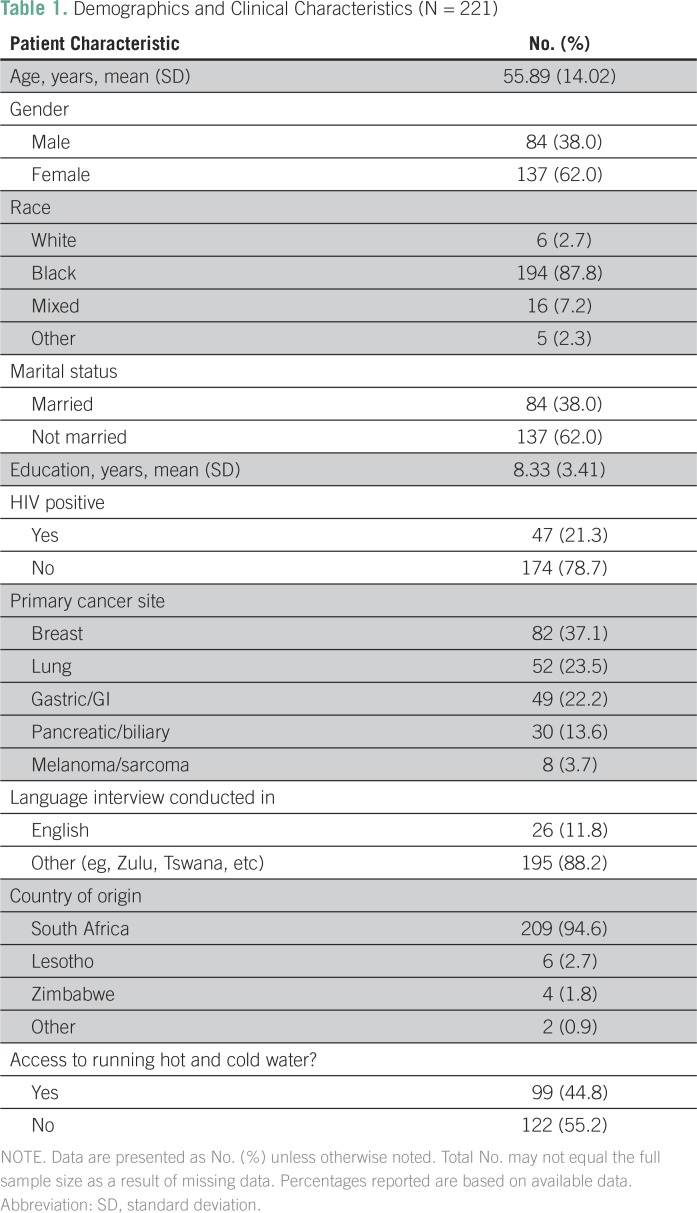
A total of 221 patients were recruited to the study and were included in the present analyses. Mean patient age was 55.89 years (standard deviation, 14.02). Patients were predominately black or of mixed race (95.0%), female (62.0%), and not married (62.0%), and they had a mean of 8.33 years of education (standard deviation, 3.41). Forty-seven patients (21.3%) were HIV positive (78.7%). Eighty-two patients (37.1%) had breast cancer, 52 (23.5%) lung cancer, 49 (22.2%) gastric cancer, 30 (13.5%) pancreatic or biliary cancer, and eight (3.6%) melanoma or sarcoma cancer sites (Table 1).
Table 1.
Demographics and Clinical Characteristics (N = 221)
Illness Understanding
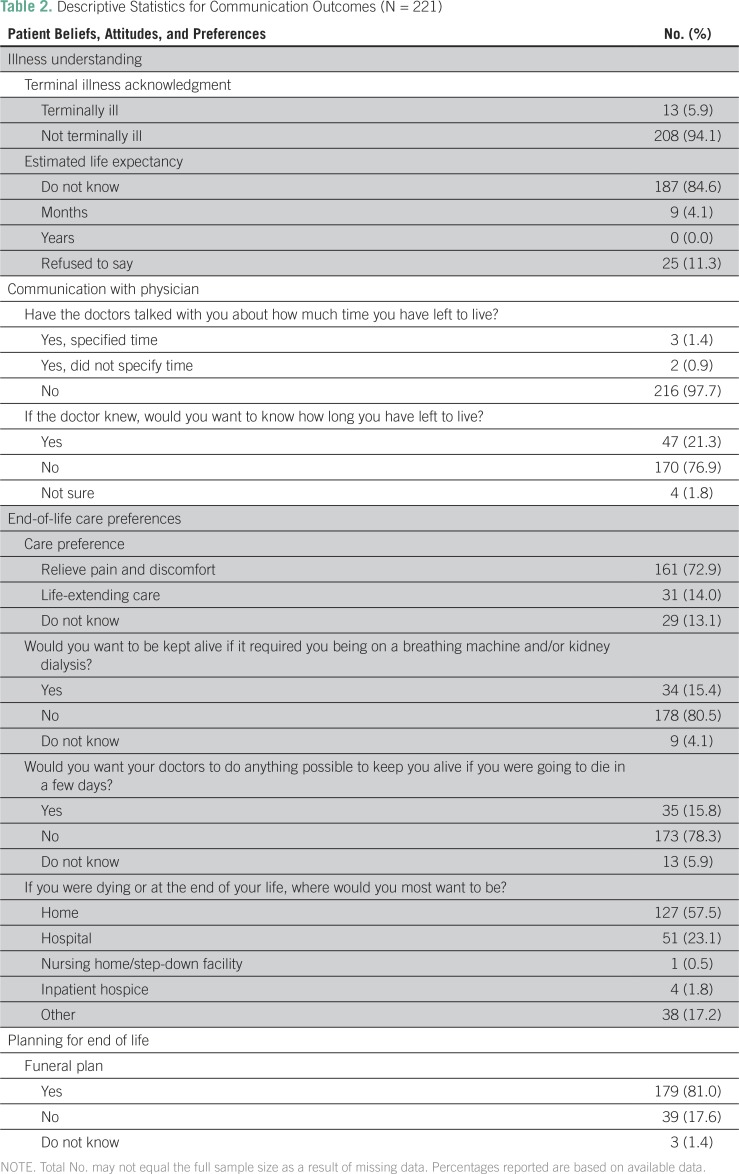
Only 13 patients (5.9%) acknowledged their terminal status; 187 patients (84.6%) stated that they could not estimate their life expectancy (Table 2).
Table 2.
Descriptive Statistics for Communication Outcomes (N = 221)
Communication With Physician
Only five patients (2.3%) reported that they had talked with their physicians about the amount of time they had left to live. Most patients (n = 190; 76.9%) reported that they would not want to know how long they had left to live, even if their doctors knew (Table 2).
EoL Care Preferences
A total of 161 patients (72.9%) reported wanting comfort care compared with 31 patients (14.0%) who wanted life-extending care. A total of 178 patients (80.5%) reported not wanting to be kept alive on a breathing machine or kidney dialysis, and 173 patients (78.3%) reported not wanting their doctor to do everything possible to keep them alive if they only had a few days left to live. One hundred twenty-seven patients (57.5%) reported preferring to die at home, whereas 51 patients (23.1%) preferred to die in the hospital (Table 2).
Funeral Plans
A total of 179 patients (81.0%) reported having made funeral plans or arrangements (Table 2).
DISCUSSION
The findings of our study illuminate the understanding of South African (LMIC) patients with advanced cancer of their illness and EoL care communication and care preferences. Only 6% of patients acknowledged their terminal status, a much smaller proportion than that observed in the United States–based Coping with Cancer studies,27 in which 39% of white patients, 27% of black patients, and 11% of Hispanic or Latino patients with advanced cancer reported being aware of their terminal illness status.27
This lack of terminal illness acknowledgment among our South African patients may be related to their stated preferences for not knowing their terminal illness status. Most patients indicated that they did not want to know how long they had left to live, even if their physician knew. Moreover, only 2.3% of South African patients reported that their physician had a conversation with them regarding the amount of time they had left to live compared with 37% of patients with advanced cancer in the United States2 and 60% in China.28 Nevertheless, nearly one quarter of South African patients did want to know how long they had left to live. Thus, the rates of South African patients in the current study who wanted to know their prognosis were lower than among patients with cancer who were surveyed in other LMICs. For instance, prior research has indicated that patients with advanced cancer from LMICs preferred to know their prognosis at much higher rates—60% of patients in Pakistan,29 92% of patients in Brazil,30 79% of patients in China,28 and 92% of patients in India.31 Given these findings, it is important to determine and accommodate patient preferences regarding prognostic information individually, as South African patients may tend to prefer not knowing their prognosis.
Most (72.9%), but not all, South African patients endorsed a preference for comfort care over life-extending care and did not want any extreme measures taken to extend their life. This finding seems to be consistent with prior observations that sub-Saharan Africans are more accepting of death20 than those in HICs.22 Resource constraints and a lack of awareness of options may also factor into this preference for comfort care.18 These findings differ from those reported in studies of other LMICs, with one study in Pakistan indicating that 90% of terminal patients wanted to receive all possible care until EoL.29
Most South African patients also prefer to die at home (57.5%), a proportion that is to that observed in Pakistan (42.0%),29 but smaller than that observed in US samples (85%).32 A larger proportion of South African patients (23.1%) than United States patients—for example, 0%32—reported a preference to die in the hospital. South African patients who preferred the hospital may have done so because of the high burden that home care of a patient with terminal cancer imposes on caregivers. This burden may be especially prominent among those with financial and social constraints, including a lack of access to such basic needs as indoor running hot and cold water and lack of access to and knowledge of how to administer pain medication and other comfort measures.20,21
Despite a preference not to know their prognosis and a lack of illness understanding, most South African patients had planned for death by making funeral plans. It should be noted that funeral plans, regardless of health status, are quite common in South Africa33—a notable difference with Western cultural norms, and particularly United States’ cultural norms. This may be a result of a sense that their illness has progressed or may be because of cultural norms surrounding death and dying; however, many people in the United States have life insurance, which originated and is still commonly used as a system by which to cover funeral and burial costs. Future research is needed to indicate the most culturally appropriate ways to ensure that patients are able to plan for both EoL care and potential mortality from advanced illness.
Our study has several unique strengths, including its use of validated measures to assess EoL care preferences among cohorts of patients with advanced cancer in the United States with estimated life expectancies of ≤ 6 months—that is, the Coping with Cancer 1 and 2 studies. As such, it has enabled us to compare our findings with those observed among US patients and to identify differences in EoL care and communication preferences between South African and US patients living with advanced cancers; however, our study also has some limitations. First, it reports on patients’ self-reported illness understanding, EoL care preferences, and EoL care current and preferred communication. Our study did not collect data on actual communication or care received. Future research should examine actual communication and care measures, and compare them with the preferences that were expressed by our patients. Second, our study recruited patients from a hospital system that has one of the few hospital-based palliative care centers in South Africa. Hence, our findings may not be generalizable to the broader South African or sub-Saharan African population without access to palliative care, nor can it examine the effects of palliative care on EoL outcomes because all patients were enrolled at the CHBAH Wits Palliative Care Centre. An additional strength and weakness was the administration of the survey by native speakers of non-English languages. Face-to-face interviews in the patients’ languages enabled the patients to understand the questions asked of them, but did not allow us to confirm the accuracy of the translations. Finally, our study did not address the role of resource constraints in shaping preferences. Future research should examine these issues.
In summary, findings from the current study provide critical insight into the preferences of South African patients with advanced cancer regarding EoL care and communication, and may generate hypotheses regarding EoL care preferences and communication within Sub-Saharan African LMICs. We found that these preferences varied drastically from those elicited among United States–based patients and even among other LMICs—for example, Pakistan, India, China, and Brazil. Care should be taken to ensure that prognoses are communicated and care provided to patients with advanced cancer in South Africa in ways that are culturally appropriate, ethical, and patient centered on the basis of South African patient preferences and values, not Western- or other LMIC-based values. To the extent possible, individual preferences, which may differ from those of a group, should also be taken into account. Future research should further examine how to implement high-quality EoL care given the resource constraints that are present in South Africa.18,19 Palliative care may by highly cost effective, given its ability to provide symptom relief34,35 and improve quality of life36 in this setting.
Footnotes
Supported by National Cancer Institute Grants No. K07-CA207580 (to M.J.S.), CA197730 (to H.G.P.), P30-CA13696 (to. M.J.S.), and R01CA192627 (to J.S.J., A.I.N., and M.J.), and by the South African Medical Research Council/University of Witwatersrand Common Epithelial Cancer Research Centre (led by P.R.).
AUTHOR CONTRIBUTIONS
Conception and design: Holly G. Prigerson, Alfred I. Neugut, Maureen Joffee, Charmaine Blanchard, Mpho Ratshikana-Moloko, Paul Ruff, Herbert Cubasch, Michelle Wong, Keletso Mmoledi
Financial support: Holly G. Prigerson, Paul Ruff, Alfred I. Neugut
Administrative support: Charmaine Blanchard, Maureen Joffe, Mpho Ratshikana-Moloko, Keletso Mmoledi, Paul Ruff, Michelle Wong, Herbert Cubasch
Provision of study materials or patients: Paul Ruff, Charmaine Blanchard, Maureen Joffee, Keletso Mmoledi, Michelle Wong
Collection and assembly of data: Chamaine Blanchard, Maureen Joffe, Paul Ruff, Mpho Ratshikana-Moloko, Keletso Mmoledi,Herbert Cubasch, Michelle Wong
Data analysis and interpretation: Megan Johnson Shen, Holly G. Prigerson, Jamila Amanfu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Megan Johnson Shen
No relationship to disclose
Holly G. Prigerson
No relationship to disclose
Mpho Ratshikana-Moloko
No relationship to disclose
Keletso Mmoledi
No relationship to disclose
Paul Ruff
Honoraria: Sanofi, Amgen, Roche, MSD Oncology, Bayer Schering Pharma, Merck Serono
Research Funding: Amgen (Inst), Sanofi (Inst), MSD Oncology (Inst), Janssen Oncology (Inst),Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Roche, Novartis, Merck Serono, Sanofi
Judith S. Jacobson
No relationship to disclose
Alfred I. Neugut
No relationship to disclose
Jamila Amanfu
No relationship to disclose
Herbert Cubasch
No relationship to disclose
Michelle Wong
No relationship to disclose
Maureen Joffe
No relationship to disclose
Charmaine Blanchard
Travel, Accommodations, Expenses: Janssen Pharmaceuticals
REFERENCES
- 1.Carr D. Racial differences in end-of-life planning: Why don’t blacks and Latinos prepare for the inevitable? Omega (Westport) 2011;63:1–20. doi: 10.2190/OM.63.1.a. [DOI] [PubMed] [Google Scholar]
- 2.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang B, Nilsson ME, Prigerson HG. Factors important to patients’ quality of life at the end of life. Arch Intern Med. 2012;172:1133–1142. doi: 10.1001/archinternmed.2012.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: Associations with end-of-life conversations. Arch Intern Med. 2009;169:480–488. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mack JW, Weeks JC, Wright AA, et al. End-of-life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28:1203–1208. doi: 10.1200/JCO.2009.25.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanchate A, Kronman AC, Young-Xu Y, et al. Racial and ethnic differences in end-of-life costs: Why do minorities cost more than whites? Arch Intern Med. 2009;169:493–501. doi: 10.1001/archinternmed.2008.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volandes AE, Ariza M, Abbo ED, et al. Overcoming educational barriers for advance care planning in Latinos with video images. J Palliat Med. 2008;11:700–706. doi: 10.1089/jpm.2007.0172. [DOI] [PubMed] [Google Scholar]
- 8.Blackhall LJ, Frank G, Murphy ST, et al. Ethnicity and attitudes towards life sustaining technology. Soc Sci Med. 1999;48:1779–1789. doi: 10.1016/s0277-9536(99)00077-5. [DOI] [PubMed] [Google Scholar]
- 9.Davis A. Ethics and ethnicity: End-of-life decisions in four ethnic groups of cancer patients. Med Law. 1996;15:429–432. [PubMed] [Google Scholar]
- 10.Duffy SA, Jackson FC, Schim SM, et al. Racial/ethnic preferences, sex preferences, and perceived discrimination related to end-of-life care. J Am Geriatr Soc. 2006;54:150–157. doi: 10.1111/j.1532-5415.2005.00526.x. [DOI] [PubMed] [Google Scholar]
- 11.Gutheil IA, Heyman JC. “They don’t want to hear us”: Hispanic elders and adult children speak about end-of-life planning. J Soc Work End Life Palliat Care. 2006;2:55–70. doi: 10.1300/J457v02n01_05. [DOI] [PubMed] [Google Scholar]
- 12.Institute of Medicine . Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academies Press; 2015. [PubMed] [Google Scholar]
- 13.Institute of Medicine Delivering high-quality cancer care: Charting a new course for a system in crisis. http://nationalacademies.org/hmd/~/media/Files/Report Files/2013/Quality-Cancer-Care/qualitycancercare_rb.pdf [PubMed]
- 14.Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709–1714. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 15.Abegunde DO, Mathers CD, Adam T, et al. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet. 2007;370:1929–1938. doi: 10.1016/S0140-6736(07)61696-1. [DOI] [PubMed] [Google Scholar]
- 16.Ott JJ, Ullrich A, Mascarenhas M, et al. Global cancer incidence and mortality caused by behavior and infection. J Public Health (Oxf) 2011;33:223–233. doi: 10.1093/pubmed/fdq076. [DOI] [PubMed] [Google Scholar]
- 17.Batouli A, Jahanshahi P, Gross CP, et al. The global cancer divide: Relationships between national healthcare resources and cancer outcomes in high-income vs. middle- and low-income countries. J Epidemiol Glob Health. 2014;4:115–124. doi: 10.1016/j.jegh.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleary J, Radbruch L, Torode J, et al. Next steps in access and availability of opioids for the treatment of cancer pain: Reaching the tipping point? Ann Oncol. 2013;24(suppl 11):xi60–xi64. doi: 10.1093/annonc/mdt504. [DOI] [PubMed] [Google Scholar]
- 19.Gwyther E. South Africa: The status of palliative care. J Pain Symptom Manage. 2002;24:236–238. doi: 10.1016/s0885-3924(02)00455-4. [DOI] [PubMed] [Google Scholar]
- 20.Gysels M, Pell C, Straus L, et al. End of life care in sub-Saharan Africa: A systematic review of the qualitative literature. BMC Palliat Care. 2011;10:6. doi: 10.1186/1472-684X-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harding R, Stewart K, Marconi K, et al. Current HIV/AIDS end-of-life care in sub-Saharan Africa: A survey of models, services, challenges and priorities. BMC Public Health. 2003;3:33. doi: 10.1186/1471-2458-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmermann C, Swami N, Krzyzanowska M, et al. Perceptions of palliative care among patients with advanced cancer and their caregivers. CMAJ. 2016;188:E217–E227. doi: 10.1503/cmaj.151171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris JJ, Shao J, Sugarman J. Disclosure of cancer diagnosis and prognosis in Northern Tanzania. Soc Sci Med. 2003;56:905–913. doi: 10.1016/s0277-9536(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 24.Epstein AS, Prigerson HG, O’Reilly EM, et al. Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J Clin Oncol. 2016;34:2398–2403. doi: 10.1200/JCO.2015.63.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrido MM, Harrington ST, Prigerson HG. End-of-life treatment preferences: A key to reducing ethnic/racial disparities in advance care planning? Cancer. 2014;120:3981–3986. doi: 10.1002/cncr.28970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balboni TA, Maciejewski PK, Balboni MJ, et al. Racial/ethnic differences in end-of-life (EoL) treatment preferences: The role of religious beliefs about care. J Clin Oncol. 2013;31(suppl):6529. [Google Scholar]
- 27.Smith AK, McCarthy EP, Paulk E, et al. Racial and ethnic differences in advance care planning among patients with cancer: Impact of terminal illness acknowledgment, religiousness, and treatment preferences. J Clin Oncol. 2008;26:4131–4137. doi: 10.1200/JCO.2007.14.8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai Q, Zhang Z, Lu X, et al. Attitudes towards palliative care among patients and health professionals in Henan, China. Prog Palliat Care. 2010;18:341–345. [Google Scholar]
- 29.Zafar W, Hafeez H, Jamshed A, et al. Preferences regarding disclosure of prognosis and end-of-life care: A survey of cancer patients with advanced disease in a lower-middle-income country. Palliat Med. 2016;30:661–673. doi: 10.1177/0269216315625810. [DOI] [PubMed] [Google Scholar]
- 30.Fumis RR, De Camargo B, Del Giglio A. Physician, patient and family attitudes regarding information on prognosis: A Brazilian survey. Ann Oncol. 2012;23:205–211. doi: 10.1093/annonc/mdr049. [DOI] [PubMed] [Google Scholar]
- 31.Laxmi S, Khan JA. Does the cancer patient want to know? Results from a study in an Indian tertiary cancer center. South Asian J Cancer. 2013;2:57–61. doi: 10.4103/2278-330X.110487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratner E, Norlander L, McSteen K. Death at home following a targeted advance-care planning process at home: The kitchen table discussion. J Am Geriatr Soc. 2001;49:778–781. doi: 10.1046/j.1532-5415.2001.49155.x. [DOI] [PubMed] [Google Scholar]
- 33.Case A, Garrib A, Menendez A, et al. Paying the piper: The high cost of funerals in South Africa. Econ Dev Cult Change. 2013;62:1–20. doi: 10.1086/671712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hearn J, Higginson IJ. Do specialist palliative care teams improve outcomes for cancer patients? A systematic literature review. Palliat Med. 1998;12:317–332. doi: 10.1191/026921698676226729. [DOI] [PubMed] [Google Scholar]
- 35.Sepúlveda C, Marlin A, Yoshida T, et al. Palliative care: The World Health Organization’s global perspective. J Pain Symptom Manage. 2002;24:91–96. doi: 10.1016/s0885-3924(02)00440-2. [DOI] [PubMed] [Google Scholar]
- 36.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The Project ENABLE II randomized controlled trial. JAMA. 2009;302:741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]