Abstract
Purpose
The introduction of tyrosine kinase inhibitors has transformed the care of patients with chronic myeloid leukemia, with survival approaching that of healthy individuals. Current-day challenges in chronic myeloid leukemia care include adherence to tyrosine kinase inhibitor therapy. We studied adherence from resource-constrained settings and tried to analyze the factors responsible for nonadherence in these individuals. We also correlated adherence to current molecular status.
Patients and Methods
This was a single-center, cross-sectional, observational study from north India. It consisted of a questionnaire-based survey in which a one-to-one interview technique was used by trained nursing staff administering the Modified Morisky Adherence Scale (MMAS-9) questionnaire. Adherence was also measured on the basis of physician’s assessment. JMP 13.0.0 was used for statistical analysis.
Results
A total of 333 patients with a median age of 42 years were included in the study. The median BCR-ABL/ABL ratio (IS) was 0.175 (0.0 to 98.0). The mean MMAS-9 score was 11 ± 2. Adherence was seen in 54.95% on the basis of MMAS-9, whereas physician’s assessment reported adherence in 90.39% of patients. Using the χ2 test, no relationship was found between the two assessment techniques. There was a significant relationship between major molecular response status and adherence by physician’s assessment and MMAS-9 (P < .001). Bivariate analysis by logistic fit showed a good relation between the MMAS-9 score and the BCR-ABL/ABL ratio (IS), χ2 (1,220) = 135.45 (P < .001). On multivariate analysis, enrolment in the Novartis Oncology Access program (a patient assistance program) was significantly associated with adherence (P = .012).
Conclusion
This study highlights the lack of adherence in real-world settings and the various factors responsible. Such studies are important from a public health services perspective in various settings around the world because they may lead to corrective action being taken at the institutional level.
INTRODUCTION
Chronic myeloid leukemia (CML) has become one of the most curable malignancies since the introduction of tyrosine kinase inhibitors (TKI), the first of which was imatinib, introduced in early 2000, with mathematical models suggesting survival equivalent to that of normal individuals. The major challenge since the introduction of imatinib for CML treatment has been adherence to therapy. Studies have clearly shown an association between decreased compliance and adherence and decreased cytogenetic and molecular remission and, in turn, increased relapses, progression, and resistance.1,2 It is important to differentiate between the terms adherence and compliance.3 The WHO defines adherence as an extent to which an individual’s behavior taking medication corresponds with the recommendations of health care provider.4 There is no ideal method for evaluating adherence, because the interaction and interplay among different factors leading to nonadherence differ in different populations. Few studies have addressed adherence issues in real-world settings.5-9 We thus aimed to study adherence to imatinib therapy in patients with CML from north India in a cross-sectional manner using the treating physician’s assessment and the validated nine-item Morisky Medication Adherence Scale (MMAS).10 The other objectives of the study were to correlate adherence with molecular remission status, to identify factors specific to tertiary care centers in India, and to evaluate the quality of life (QoL) of patients with CML who are receiving continuous TKI therapy.
PATIENTS AND METHODS
This was a cross-sectional, single-center study performed in north India. It was a questionnaire-based survey in which a one-to-one interview technique was used. All patients attending a patient awareness program conducted on the occasion of CML day (September 22, 2015) were included in the survey after providing informed consent. A total of 1,100 patients attended the daylong event, of which 454 random patients were assessed by the MMAS on the same day; 333 patients finally finished the physician assessment and were enrolled in the outpatient department by appointment.
Adherence was assessed using the MMAS and as recorded by physicians in their case records. The physician’s compliance assessment was determined on the basis of the treating physician’s perception, taking into account the patient’s clinical and laboratory evaluation, CML molecular remission status, and interviews with patients and relatives during clinical visits. When a physician was not fluent in a patient’s native language, an interpreter was used.
The questionnaire and interviews were conducted in three languages (ie, English and two local languages [Hindi, and Punjabi for patients unable to understand English]; Data Supplement). The first part of the questionnaire was designed on the basis of a validated scoring system, the nine-item MMAS (MMAS-9), used for all continuous medication and which has been used in other similar studies of patients with CML who are receiving imatinib.7,8,11-13 This questionnaire is composed of nine questions that are based on four themes involving forgetfulness, negligence, interruption of drug intake after clinical improvement, and restart of drug intake when symptoms worsen.10,14,15 In MMAS-9, adherence behavior, rather than dose intake, is explored, and the response categories are yes or no for questions 1 to 8; question 9 uses a five-point graded response. The summary score ranges from 1 to 13, and higher scores reflect better adherence. In the study, good adherence was defined as a Morisky score of ≥11. The second part of the questionnaire was designed to evaluate predefined factors that were identified as influencing drug and treatment adherence in Indian settings on the basis of a pilot study conducted at our center and on a literature review.7-9 These factors included degree of social support, knowledge of disease and treatment, accessibility to treating clinic, and the receiving of free drugs, as well as factors specific to tertiary care centers in India (particularly various hurdles in obtaining physician consultations at these centers).16,17 The third part of the questionnaire included questions on QoL.
Interviews
The interviews were conducted by a group of four independent nurses. These nurses were fluent in the three above-mentioned languages. The nursing staff was trained in conducting this questionnaire in four hourly sessions twice a week for 2 weeks; training also included a pilot run in the CML clinics, with 10 patients each, in the week before CML day. All pilot questionnaires were conducted in front of the principal investigator and in the presence of the remaining three nursing staff to modulate the patterns of interviews and to ensure homogeneity. In case of any discrepancy on the final day during interviews, the principal investigator was readily available to clarify issues. This was performed to lower interobserver variability and to raise the internal consistency and reliability.
The results of the questionnaire and the interviews were stored anonymously, and patient identities were replaced by code numbers. Only the principal investigator (UY) had access to the code. The treating physicians were blinded to the individual results.
Treatment Characteristics
Interpretation of the current disease remission status was attained from patient records available from the central disease database. The management of CML at this institute was based on the prevalent national and National Comprehensive Cancer Network guidelines at the time of therapy.
Statistics
JMP version 13.0.0 (SW) was used for statistical analysis. The Mann-Whitney U test was used to compare Morisky scores in patient groups with major molecular response (MMR) with those without MMR, and in patients who were enrolled in a Novartis Oncology Access program (NOA) with those who were not taking part in the program. Descriptive statistics were provided for questions with categorical variables, and scores were summarized using frequency distributions and percentages. Quantitative variables were summarized using n, mean ± SD, and median (range). Patients’ responses to the questionnaire were compared with the treating physicians’ clinical assessments of the patients’ adherence. We also correlated adherence using the last available molecular response status for patients with evaluable reports.
RESULTS
Basic Information
We studied a total of 333 patients in a cross-sectional study. The mean age of the study cohort was 42.94 ± 12.39 years (median, 42 years; range, 12 to 83 years). There was a male predominance (59%; Data Supplement). A total of 62% of patients were enrolled in an NOA program (Data Supplement). All patients in the study cohort were receiving imatinib therapy (as the preferred TKI), with doses ranging from 200 to 800 mg (Data Supplement). The median duration of imatinib therapy to evaluation of remission status at the time of the study was 991 days (mean ± SD, 1,277.2 ± 1,107.4 days; range, 42 to 4,964 days). Molecular remission status was available in 220 patients. The median BCR-ABL/ABL ratio (IS) was 0.175 (mean ± SD, 4.363 ± 13.3; range, 0.0 to 98.0). The number of patients who were in MMR is illustrated in the Data Supplement.
Adherence
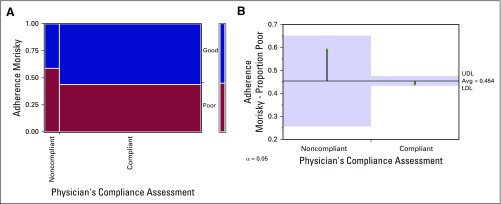
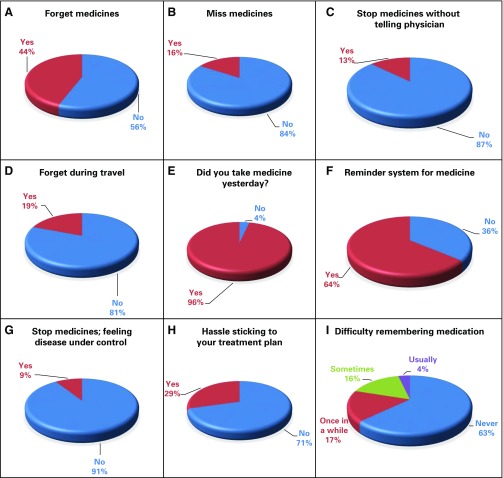
The mean MMAS-9 score was 11 ± 2 (median, 11; range, 5 to 13). On the basis of this score, 54.95% of the patients (n = 183) had good adherence to TKI therapy and 45.05% of the patients (n = 150) had poor adherence. Adherence on the basis of physician’s compliance assessment revealed that 90.39% (n = 207) were compliant to therapy and that a mere 9.61% (n = 22) were not compliant. A χ2 test was performed, and no relationship was found between the two assessment techniques; χ2 (1,229) = 1.836; P = .17). Among the patients who were compliant according to physician’s assessment, 92.8% were also compliant by MMAS. However, among the patients who were noncompliant, MMAS-9 revealed poor adherence in only 59%, thus indicating over-reporting of compliance by the physicians. The relation between the two techniques is illustrated in Figure 1 and Table 1. The responses to the first nine questions in the MMAS are depicted in Figures 2A–2I.
Fig 1.
(A) Mosaic plot showing the relation of the adherence assessment by physicians with the Morisky Medication Adherence Scale. (B) Analysis of means of proportion between the two techniques by the Cochrane Armitage trend test, P = .1754. LDL, lower decision limit; UDL, upper decision limit.
Table 1.
Relation of Adherence Assessment by Physicians to MMAS
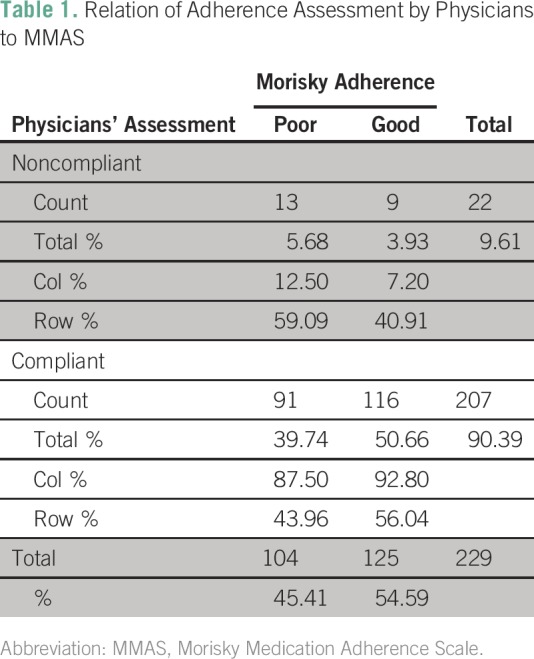
Fig 2.
(A–I) Responses by the patients to the questions in the Morisky Medication Adherence Scale.
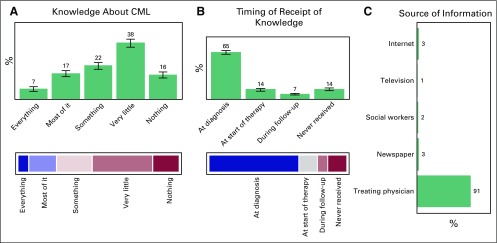
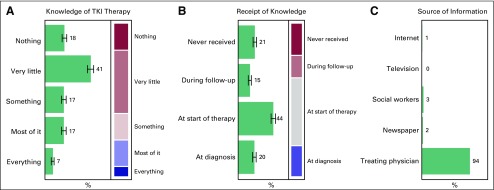
Knowledge
Data were obtained about the receipt knowledge regarding CML and TKI therapy, and the source and timing of that information. The results for both the aspects are not different and are depicted in Figures 3 and 4, respectively.
Fig 3.
Trend of study cohort for (A) knowledge about chronic myeloid leukemia (CML), (B) timing of receipt of knowledge, and (C) source of information for this acquired knowledge.
Fig 4.
Trend of study cohort for (A) knowledge about tyrosine kinase inhibitor (TKI) therapy, (B) timing of receipt of knowledge, and (C) source of information for this acquired knowledge.
QoL
As part of this questionnaire, we also studied the basic tenets of the impact of disease and therapy on QoL; 33% (n = 112) experienced no change, 28.2% (n = 94) experienced mild change, 21.9% (n = 73) experienced moderate change, and 16.2% (n = 54) experienced a great change in daily life. Fifty-four percent of patients reported no change in social situation after the diagnosis of CML when compared with previous years. On inquiring about the change in daily life (family, work, spare time) caused by the diagnosis of CML, TKI therapy, regular investigations, and physician visits, 34% of patients reported no change, whereas 28%, 22%, and 16% of patients reported mild, moderate, and significant change, respectively. Thirty percent and 35% of patients reported patient participation in therapeutic decision making at all times and most of the time, respectively, whereas 35% of the patients reported being left out of the therapeutic decision making.
Tertiary Institute–Specific Issues
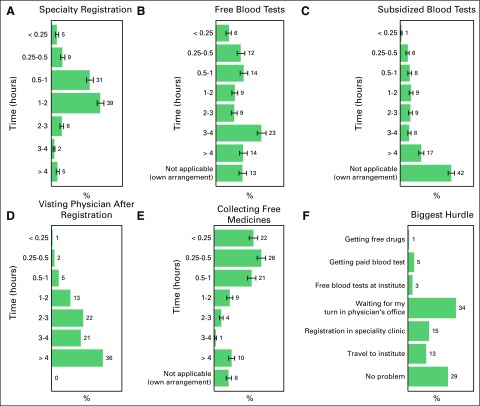
Eighty-two percent of the patients visit this hospital, a tertiary care center, for consultation and medical care from > 50 km away (20%: 50 to 100 km; 30%: 100 to 200 km; 21%: 200 to 300 km; 8%: 300 to 400 km; 2%: 400 to 500 km; and 1% > 500 km). The greatest hurdle for the patient in visiting his or her physician is depicted in Figure 5A. The details of the time taken to visit a physician, to get free or subsidized tests, for specialty registration, and to collect free medicines are illustrated in Figures 5B–5F.
Fig 5.
Time taken by the patients at the tertiary care center for (A) specialty registration, (B) free blood tests, (C) subsidized blood tests, (D) visiting physician after registration, and (E) collecting free medicines. (F) Biggest hurdle in getting treatment at the tertiary care center as reported by the patients.
Relation of Adherence to Molecular Responses
A χ2 test was performed, and a relationship was found between MMR status and adherence by physician’s assessment, χ2 (1, 220) = 25.79 (P < .001), and Fisher’s exact two-tail t test (P < .001). Similar χ2 analysis found no relationship between MMR status and adherence according to MMAS-9, χ2 (1, 220) = 168.95 (P < .001) and Fisher’s exact two-tail t test (P < .001). Bivariate analysis by logistic fit showed a good relationship χ2 (1, 220) = 135.45 (P < .001) between the MMAS-9 score and the BCR-ABL/ABL ratio (IS) (Data Supplement).
Factors Associated With Adherence
Using a regression fit model, we assessed various factors influencing adherence. The factors analyzed included age, sex, duration of treatment, frequency and dose of treatment, educational qualification, monthly income, enrolment in patient assistance program, social support, knowledge about medicine and disease, concomitant drug burden, tertiary institute–specific factors (as mentioned previously in the text), and polypharmacy. On univariate analysis, monthly income, enrolment in an NOA program, knowledge about medicine, and disease statistically influenced adherence, but on multivariate analysis, only enrolment in an NOA program was statistically significant (P = .012).
DISCUSSION
CML is one of the most common forms of adult leukemia in Indian settings, with a reported incidence of 30% to 60% of all leukemias.18,19 The age-adjusted incidence, as reported in the Mumbai Cancer Registry, is 0.71 in men and 0.53 in women.20,21 Population studies in India are extremely heterogeneous, because of the heterogeneity in medical care and the sociodemographic profile of the clientele; thus, none of the studies independently reflects the real epidemiology of CML status in India. Patients of a lower socioeconomic status preferentially approach government-sponsored tertiary care centers (such as ours), and patients of a higher socioeconomic status prefer private tertiary care units. The impact of economic status (as assessed by monthly income) is blunted by the enrolment of patients with CML who cannot afford private tertiary care units and who enroll in patient assistance programs (with the provision of free medication). These programs have a nationwide coverage of 60% to 80%.22,23
Adherence to chemotherapy is a crucial factor in the overall survival of patients with cancer. Adherence to a chemotherapeutic agent is limited mostly by the agent’s toxicity and, in resource-limited settings, by cost. It is perceived that patients with cancer generally have high adherence to therapy because treatment generally affects their survival. It is important to ensure patients’ adherence and to take corrective actions to avoid intentional or unintentional nonadherence, to avoid poor outcomes and survival. Our study is a step forward in this direction; we attempted to identify adherence issues in a real-world setting (specifically India) with its extremely high load of CML cases constituting 75% of the world’s CML case load. In addition, 62% of our patients were receiving free medication, which precludes cost as an important factor in nonadherence. Imatinib, a first-generation TKI, still remains the backbone of therapy in such real-world settings, owing to the prohibitive cost (Data Supplement) of second-line TKIs (nilotinib, dasatinib) and the unavailability of third-line TKIs (bosutinib, ponatinib). Identifying the relevant factors can go a long way toward improving survival in developing countries, where the availability and affordability of second-line TKIs are limited. Such studies also help increase adherence by educating patients during interviews. These issues are more important in our country, with the median age of CLR onset a decade younger (32 to 42 years), requiring TKI for a prolonged proportion of life span.18,19,24 In addition, in real-world settings, patients younger than 30 years can be considered for early hematopoietic stem cell transplant (particularly during first remission after relapse) to avoid the development of complex kinase domain mutations and subjecting them to lifelong costly second-line TKI therapy with a high financial burden.
Long-term adherence to imatinib is poor across the world, and studies have shown it to clearly influence patient outcome.2,22 Two large studies, from Belgium1 and the United Kingdom,2 have objectively measured adherence. Only 14.2% had perfect adherence in the Belgian study, whereas the median adherence by microelectronic monitoring systems was 98% in the study in the United Kingdom. According to the study in the United Kingdom, the most common reason for intentional nonadherence was the adverse effects secondary to imatinib, and the most common reason for unintentional nonadherence was forgetfulness.25 Few Indian studies have attempted to study adherence.8,9,22 The above-mentioned studies measured adherence in different ways, thus hindering a clear comparison or meta-analysis and making it difficult to identify the clear implicative factors associated with imatinib-receiving behavior. The major reason for these drawbacks were the challenges involved in measuring adherence and the different methods used, which each have their own advantages and disadvantages.26-28 We assessed adherence on the basis of MMAS-9, which has been used by few other studies in a CML setting, owing to the ease of using this tool when considering the educational status of our clientele.8,11,12 Only two studies have used MMAS for assessing adherence in patients with CML in Indian settings, with reported adherence of 45% and 75%, respectively.7,8 In our study, adherence on the basis of physician’s assessment was higher than that on the basis of MMAS-9 (90.39 v 54.95%). This difference in adherence could be a result of an erroneous physician assessment owing to the short face-to-face time available per patient in the physician’s office (patient load: 80 to 90 patients with CML per day).
Our study could have been limited by the Hawthorne effect (as for any self-reported questionnaire) and by recall bias. Most studies that have used these questionnaires tried to overestimate the rate of adherence because patients are unwilling to admit nonadherence; in addition, such studies are subjective. To minimize these biases, we used trained nursing staff who supervised the filling out of the questionnaire. This is important in a country like India, where most of the clientele have extremely poor literacy (median of sixth grade in our study) and because the interpretation of a given question can be different between different individuals, particularly when conducted as multilingual questionnaires. The purpose of the multilingual MMAS was to avoid communication gaps for the nursing staff who administered the instrument.
In our study, 14% of patients reported never having received information on their disease; this group primarily included those patients who began receiving therapy at a peripheral institute and then were referred to tertiary care centers for follow-up. At our institute we make an effort to counsel patients and their primary care givers at the time of diagnosis and initiation of therapy. For those patients with knowledge about the disease, we inquired about the source of that information. A total of 91% (n = 273) received information from their treating physician, 3% (n = 9) received it from the newspaper or the Internet, 2% (n = 6) received it from a social worker, and 1% (n = 3) received it from television. Social workers currently constitute only 2% of the sources of information; it would be better to employ full-time, dedicated health care workers on the treatment team. A total of 53.78% of patients had suboptimal knowledge about the disease. Social and print media constituted only 4% of the sources of information; this can be explored for imparting knowledge about the disease in the future. This low percentage of newspapers and Internet as sources of information also highlights the low education status in our society. The patient’s initiative, curiosity, and inquisitiveness in understanding the disease is meager in our society, with the complete responsibility from curing to counseling resting on the treating physician. Currently, physicians are overburdened in real-world settings because of physician and health care worker scarcity and they hardly find any time to deal with the various facets of management (ie, treating, counseling, imparting disease information, answering patient queries, and assessing adherence). The use of audiovisual aids, which can be a major boon in patient education in such resource-constrained settings, would allow the burden to be shared with the physician and would result in long-term benefits in adherence to therapy. This particular study was conducted at a tertiary care center, which is not a true representative of the real-world situation (there are more peripheral centers); these tertiary care centers are thus striving for much-needed resources.
Various reasons, such as global health status and depression, have been reported as reasons for lack of adherence to TKI in real-world settings.7,8 Another reason for lack of adherence in our society is the heavy reliance on the complex alternative or complimentary medicine system. These aspects were not formally assessed in our study and should be analyzed in future studies. In individuals who cannot overcome the above-mentioned factors, early hematopoietic stem cell transplantation is an alternative viable option. In our study, enrolment in an NOA program was the only factor that influenced adherence positively in multivariate analysis. The reason for this could be multifactorial, and could include the provision of free medication, close follow-up of these patients, routine feedback, and the return of empty containers for refill. The problems that are raised here are unfortunately not limited to the low- and middle-income countries.29-32 High-income countries also have issues with compliance, not just by patients, but also by physicians who frequently do not monitor their patients.31,33
An important limitation of this study was the lack of objective methods to assess adherence, such as measuring imatinib plasma concentration or any alternative measures as was performed in the study in the United Kingdom, where the investigators electronically recorded the daily intake of imatinib by monitoring the number of times the medication bottles were opened.2 Because this was a cross-sectional analysis, another important limitation was the evaluation of survival between the groups; this requires a prospective long-term study.
This study highlights the lack of adherence in real-world settings and also highlights the various factors responsible. Such studies are important in various settings around world and from a public health services perspective, if corrective action is to be taken at an institutional level.
ACKNOWLEDGMENT
We thank K.K. Parathan, MD, from the Postgraduate Institute of Medical Education and Research for help provided in preparing the cost table for tyrosine kinase inhibitors.
AUTHOR CONTRIBUTIONS
Conception and design: Uday Yanamandra, Pankaj Malhotra, K.K. Sahu
Financial support: Pankaj Malhotra
Administrative support: Uday Yanamandra, K.K. Sahu, Yanamandra Sushma, Pooja Chauhan, Jasmeen Gill, Deepika Rikhi, Alka Khadwal, Gaurav Prakash, Deepesh Lad, Vikas Suri, Savita Kumari, Neelam Varma
Provision of study materials or patients: Uday Yanamandra, Gaurav Prakash, Subhash Varma
Collection and assembly of data: Uday Yanamandra, K.K. Sahu, Yanamandra Sushma, Neha Saini, Pooja Chauhan, Jasmeen Gill, Deepika Rikhi, Alka Khadwal, Gaurav Prakash, Deepesh Lad, Vikas Suri, Savita Kumari, Neelam Varma
Data analysis and interpretation: Uday Yanamandra, Subhash Varma
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Uday Yanamandra
No relationships to disclose
Pankaj Malhotra
No relationships to disclose
K.K. Sahu
No relationships to disclose
Yanamandra Sushma
No relationships to disclose
Neha Saini
No relationships to disclose
Pooja Chauhan
No relationships to disclose
Jasmeen Gill
No relationships to disclose
Deepika Rikhi
No relationships to disclose
Alka Khadwal
No relationships to disclose
Gaurav Prakash
No relationships to disclose
Deepesh Lad
No relationships to disclose
Vikas Suri
No relationships to disclose
Savita Kumari
No relationships to disclose
Neelam Varma
No relationships to disclose
Subhash Varma
No relationships to disclose
REFERENCES
- 1.Noens L, van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: The ADAGIO study. Blood. 2009;113:5401–5411. doi: 10.1182/blood-2008-12-196543. [DOI] [PubMed] [Google Scholar]
- 2.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–2388. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilson HH. Adherence or compliance? Changes in terminology. Ann Pharmacother. 2004;38:161–162. doi: 10.1345/aph.1D207. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization: Adherence to Long-Term Therapies. Evidence for Action. Geneva, Switzerland, World Health Organization, 2003. [Google Scholar]
- 5.Efficace F, Baccarani M, Rosti G, et al. Investigating factors associated with adherence behaviour in patients with chronic myeloid leukemia: An observational patient-centered outcome study. Br J Cancer. 2012;107:904–909. doi: 10.1038/bjc.2012.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharf G, Hoffmann V, Bombaci F, et al: Non-adherence in chronic myeloid leukemia: Results of a global survey of 2546 CML patients in 79 countries. Haematologica 98:453, 2013 (abstr 104) [Google Scholar]
- 7.Unnikrishnan R, Veeraiah S, Mani S, et al. Comprehensive evaluation of adherence to therapy, its associations, and its implications in patients with chronic myeloid leukemia receiving imatinib. Clin Lymphoma Myeloma Leuk. 2016;16:366–371.e3. doi: 10.1016/j.clml.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 8.Kapoor J, Agrawal N, Ahmed R, et al. Factors influencing adherence to imatinib in Indian chronic myeloid leukemia patients: A cross-sectional study Mediterr J Hematol Infect Dis 7e2015013, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganta RR, Nasaka S, Gundeti S. Impact of imatinib adherence on the cytogenetic response in pediatric chronic myeloid leukemia - chronic phase. Indian J Pediatr. 2016;83:1009–1012. doi: 10.1007/s12098-015-2007-9. [DOI] [PubMed] [Google Scholar]
- 10.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kekäle M, Peltoniemi M, Airaksinen M. Patient-reported adverse drug reactions and their influence on adherence and quality of life of chronic myeloid leukemia patients on per oral tyrosine kinase inhibitor treatment. Patient Prefer Adherence. 2015;9:1733–1740. doi: 10.2147/PPA.S92125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kekäle M, Talvensaari K, Koskenvesa P, et al. Chronic myeloid leukemia patients’ adherence to peroral tyrosine kinase inhibitors compared with adherence as estimated by their physicians. Patient Prefer Adherence. 2014;8:1619–1627. doi: 10.2147/PPA.S70712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Södergård B, Halvarsson M, Lindbäck S, et al. Differences in adherence and motivation to HIV therapy--two independent assessments in 1998 and 2002. Pharm World Sci. 2006;28:248–256. doi: 10.1007/s11096-006-9036-4. [DOI] [PubMed] [Google Scholar]
- 14.Södergård B, Halvarsson M, Tully MP, et al. Adherence to treatment in Swedish HIV-infected patients. J Clin Pharm Ther. 2006;31:605–616. doi: 10.1111/j.1365-2710.2006.00782.x. [DOI] [PubMed] [Google Scholar]
- 15.Morisky DE, Ang A, Krousel-Wood M, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Kjellgren KI, Ahlner J, Säljö R. Taking antihypertensive medication--controlling or co-operating with patients? Int J Cardiol. 1995;47:257–268. doi: 10.1016/0167-5273(94)02203-u. [DOI] [PubMed] [Google Scholar]
- 17.Kjellgren KI, Ahlner J, Dahlöf B, et al. Patients’ and physicians’ assessment of risks associated with hypertension and benefits from treatment. J Cardiovasc Risk. 1998;5:161–166. [PubMed] [Google Scholar]
- 18. doi: 10.1200/JGO.2015.002667. Ganesan P, Kumar L: Chronic myeloid leukemia in India. J Glob Oncol 3:647-71, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bansal S, Prabhash K, Parikh P. Chronic myeloid leukemia data from India. Indian J Med Paediatr Oncol. 2013;34:154–158. doi: 10.4103/0971-5851.123711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dikshit RP, Nagrani R, Yeole B, et al. Changing trends of chronic myeloid leukemia in greater Mumbai, India over a period of 30 years. Indian J Med Paediatr Oncol. 2011;32:96–100. doi: 10.4103/0971-5851.89792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Au WY, Caguioa PB, Chuah C, et al. Chronic myeloid leukemia in Asia. Int J Hematol. 2009;89:14–23. doi: 10.1007/s12185-008-0230-0. [DOI] [PubMed] [Google Scholar]
- 22.Ganesan P, Sagar TG, Dubashi B, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol. 2011;86:471–474. doi: 10.1002/ajh.22019. [DOI] [PubMed] [Google Scholar]
- 23. The Max Foundation: Glivec International Patient Assistance Program (GIPAP). https://www.themaxfoundation.org/what/treatment/glivec-international-patient-assistance-program-gipap/
- 24.Malhotra P, Varma N, Varma S. A short report on chronic myeloid leukemia from Post Graduate Institute of Medical Education and Research, Chandigarh. Indian J Med Paediatr Oncol. 2013;34:186–188. doi: 10.4103/0971-5851.123728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eliasson L, Clifford S, Barber N, et al. Exploring chronic myeloid leukemia patients’ reasons for not adhering to the oral anticancer drug imatinib as prescribed. Leuk Res. 2011;35:626–630. doi: 10.1016/j.leukres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 27.Partridge AH, Avorn J, Wang PS, et al. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652–661. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 28.Breccia M, Efficace F, Alimena G. Imatinib treatment in chronic myelogenous leukemia: What have we learned so far? Cancer Lett. 2011;300:115–121. doi: 10.1016/j.canlet.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Darkow T, Henk HJ, Thomas SK, et al. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: A retrospective analysis among managed care patients with chronic myelogenous leukaemia. Pharmacoeconomics. 2007;25:481–496. doi: 10.2165/00019053-200725060-00004. [DOI] [PubMed] [Google Scholar]
- 30. Feng W, Henk H, Thomas S, et al: Compliance and persistency with imatinib. J Clin Oncol 24:6038, 2006 (suppl 18)
- 31.Guérin A, Chen L, Dea K, et al. Association between regular molecular monitoring and tyrosine kinase inhibitor therapy adherence in chronic myelogenous leukemia in the chronic phase. Curr Med Res Opin. 2014;30:1345–1352. doi: 10.1185/03007995.2014.904281. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg SL. Monitoring chronic myeloid leukemia in the real world: Gaps and opportunities. Clin Lymphoma Myeloma Leuk. 2015;15:711–714. doi: 10.1016/j.clml.2015.08.088. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg SL, Akard LP, Dugan MJ, et al. Barriers to physician adherence to evidence-based monitoring guidelines in chronic myelogenous leukemia. J Oncol Pract. 2015;11:e398–e404. doi: 10.1200/JOP.2014.001099. [DOI] [PubMed] [Google Scholar]