Abstract
Purpose
Because of the profound financial crisis that commenced in Greece in 2010, severe cuts in health care spending and other restriction measures led to significant delays in the reimbursement of novel antineoplastic agents. In 2011, the Hellenic Society of Medical Oncology initiated a program of early access to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors for the treatment of patients with advanced, EGFR-mutant non–small-cell lung cancer (NSCLC). We evaluated treatment patterns and clinical outcomes in patients with EGFR-mutant or wild-type disease treated at a large center in Greece throughout the period of financial crisis.
Patients and Methods
From 2011 through 2015, 252 patients with newly diagnosed advanced NSCLC were treated at the Department of Medical Oncology of the Papageorgiou Hospital, a tertiary cancer center in northern Greece. We retrospectively reviewed patient medical records to obtain clinicopathologic characteristics, EGFR mutation status, and follow-up data. The primary end point was time to treatment failure.
Results
Of the 198 evaluable patients, 25 (12%) had EGFR mutations. All patients with EGFR mutations except one received treatment with an EGFR tyrosine kinase inhibitor. Median times to treatment failure for patients with EGFR-mutant and wild-type disease were 15.8 and 7.1 months, respectively (hazard ratio, 0.58; 95% CI, 0.35 to 0.95; P = .031). There was no difference in overall survival between the two groups (P = .293). No deviation from treatment guidelines or discontinuation of treatment regimens occurred because of logistic reasons or drug shortages.
Conclusion
Despite restrictions in the reimbursement policy and accompanying controls in the use of high-cost medicines, the national program enabled treatment of patients with EGFR-mutant NSCLC according to established guidelines. Therefore, the clinical outcomes of such patients treated in Greece during the economic crisis were in accordance with international standards.
INTRODUCTION
Lung cancer remains a prominent public health care issue; it is the leading cause of cancer mortality worldwide.1,2 Non–small-cell lung cancer (NSCLC) accounts for 80% to 85% of all lung cancer cases.3 The incidence of its two main histopathologic subtypes has changed during the last years, following the evolution of smoking habits in both sexes. In men, the incidence of squamous cell carcinoma has decreased, whereas the incidence of adenocarcinoma has increased in both the United States and Europe. In women, the same trends have been observed in the United States; however, in Europe, the incidence of both histologic subtypes has increased.4
Genotyping studies have revealed molecular abnormalities in NSCLC, resulting in changes in patient management. Activating epidermal growth factor receptor (EGFR) mutations are found in approximately 10% to 15% of whites with lung adenocarcinoma and are more frequent in never-smokers, women, and those of East Asian ethnicity. EGFR mutations predict benefit from EGFR tyrosine kinase inhibitors (TKIs)5-7; specifically, EGFR TKIs confer significantly improved progression-free survival (PFS) compared with standard platinum-based chemotherapy in patients with EGFR mutations.8,9 The EGFR TKIs have become the treatment of choice for patients with advanced, EGFR-mutant NSCLC in the first-line setting, recommended by European Society for Medical Oncology guidelines with level I evidence since 2011,10 whereas chemotherapy has remained the gold standard for patients with tumors without targetable genetic alterations.11 The advent of immunotherapy has dramatically changed the treatment landscape; however, its actual place in the treatment algorithm remains to be elucidated.
The implementation of novel therapeutic strategies against NSCLC, such as molecular targeted agents and immunotherapy, has substantially increased the costs related to cancer care and challenged the reimbursement capacity of health care systems, especially in countries with weak economies. Greece is such a country; it entered a profound financial crisis in 2010, which has continued to date, and has been forced to follow a strict rescue program with unprecedented reforms and expense restrictions, including major cuts in health care and pharmaceutical costs. On the fiscal side, Greece has experienced as a result of the restriction policies the largest annual average reduction in health care and pharmaceutical expenditures of all countries in the Organisation for Economic Co-operation and Development.12 Of note, in this context, gefitinib, the first EGFR TKI to gain European Medicines Agency (EMA) approval, in July 2009, became refundable in Greece almost 5 years later, in 2014. To tackle this issue and assess the impact of the aforementioned trends, we performed a retrospective, observational, single-institute study in a tertiary cancer center to describe and analyze treatment patterns and clinical outcomes in Greek patients diagnosed with advanced NSCL, during the period of economic crisis, with a special focus on those with EGFR mutations.
PATIENTS AND METHODS
Patient Characteristics
We studied patients with newly diagnosed advanced NSCLC treated from January 2011 through December 2015 at the Department of Medical Oncology, Papageorgiou Hospital, in the Aristotle University School of Medicine (AUTH) in Thessaloniki, which covers a large area of northern Greece. We retrospectively reviewed patient medical records to obtain clinicopathologic characteristics, EGFR mutation status, and outcome data. Informed consent had been obtained at the time of diagnosis from all patients for the use of their medical records and biologic material for research purposes. All procedures were performed according to the principles of the Declaration of Helsinki and were approved by the ethics committee of the AUTH (A13064; July 16, 2010) and the scientific committee of the Hellenic Cooperative Oncology Group.
EGFR Status Assessment
Tumor tissue (formalin fixed, paraffin embedded) and/or cytologic (cell block) material was obtained at the time of diagnosis from either the primary tumor or a metastatic site, depending on availability. Molecular testing was performed in laboratories internationally certified for EGFR mutation testing; 70% of the tumors were analyzed in the AUTH Department of Pathology or Hellenic Foundation for Cancer Research/Hellenic Cooperative Oncology Group Laboratory of Molecular Oncology, and 30% were analyzed in private laboratories, as previously described.13 Details are provided in the Data Supplement.
Statistical Analyses
Categorical data were assessed using THE χ2 test, and continuous data were assessed with the nonparametric Mann-Whitney test. The primary end point of the study was time to treatment failure (TTF), defined as time in months from first-line treatment initiation to the date of radiographically or clinically observed disease progression. PFS was defined as time in months from first-line treatment initiation to the date of radiographically or clinically observed disease progression or death, whichever occurred first. Overall survival (OS) was defined as time in months from the date of initiation of treatment for metastatic NSCLC to the date of patient death or last contact. Patients alive were censored at the date of last contact. Kaplan-Meier curves and log-rank tests were used to compare survival distributions between groups of patients. Cox multivariable analysis was performed to identify independent variables associated with survival. Statistical significance was set at two-sided P = .05. Statistical analyses were performed with SPSS software (IBM SPSS Statistics for Windows [version 24.0]; IBM, Armonk, NY).
RESULTS
Patient Characteristics
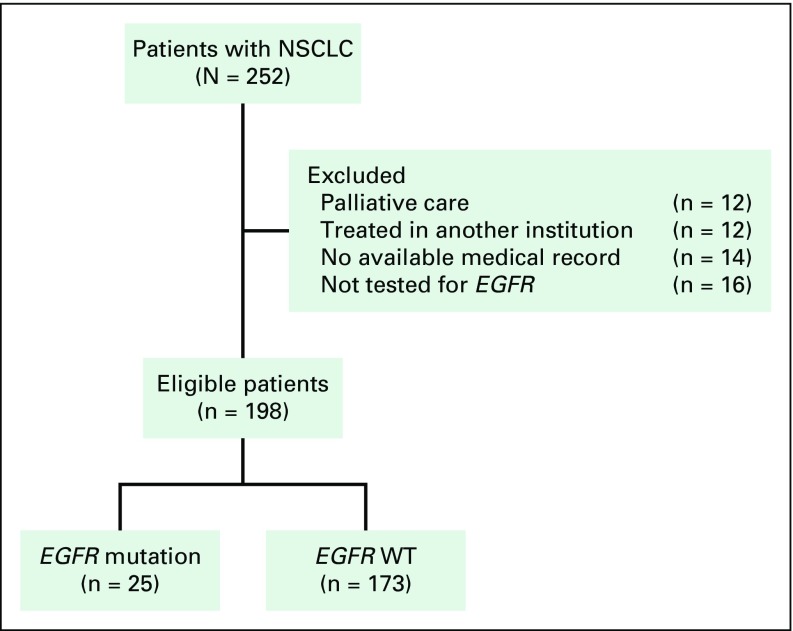
From January 2011 to December 2015, 252 patients were diagnosed with advanced NSCLC, of whom 228 (90.5%) received first-line treatment. Because of poor performance status and advanced disease, 12 patients received supportive care, whereas another 12 chose to be treated elsewhere. EGFR status was not available for 30 patients (lack of EGFR testing or medical record data; Fig 1).
Fig 1.
CONSORT diagram. NSCLC, non–small-cell lung cancer; WT, wild type.
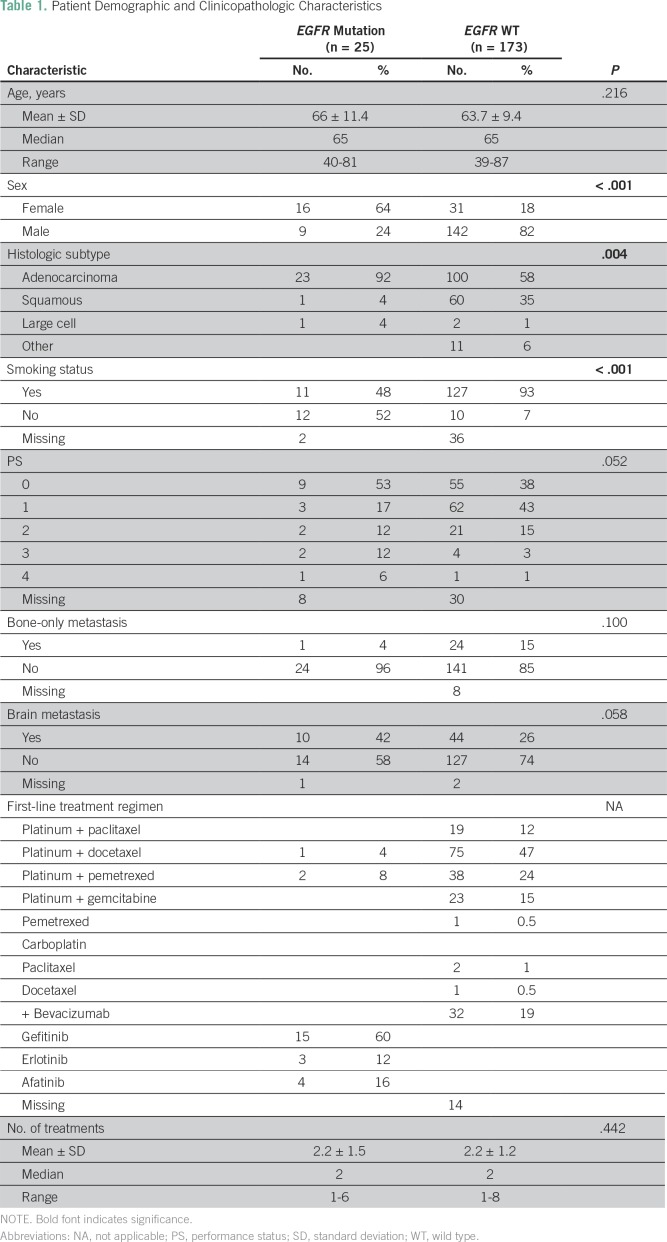
Patient clinical characteristics are listed in Table 1. Our study included 198 evaluable patients, 151 of whom were men; median age was 65 years. Twenty-five (12.6%) of the patient tumors harbored an EGFR mutation in exons 18 to 21. The most common mutation was p.E746_A750delELREA in exon 19 (44%), followed by the p.L858R point mutation in exon 21 (28%). The distribution and annotations of the identified mutations are shown in Figure 2.
Table 1.
Patient Demographic and Clinicopathologic Characteristics
Fig 2.
EGFR mutation distribution. Thirteen (52%) EGFR mutations were in frame deletions, 11 (44%) were substitutions, and one was an insertion (4%).
Patients with EGFR mutations were more likely to be women (64% v 18%; χ2 P < .001) and nonsmokers (48% v 7%; χ2 P < .001) compared with patients without EGFR mutations (EGFR wild type [WT]). They were also more likely to be diagnosed with lung adenocarcinoma (92% v 58%; χ2 P = .004). Performance status (PS) did not differ between patients with EGFR mutations versus WT (χ2 P = .052). Both groups received a median of two chemotherapy lines, with 15% of patients receiving ≥ four lines of treatment.
Treatments
All patients with EGFR mutations except three received an EGFR TKI as first-line treatment. Two patients who were initially treated with chemotherapy based on physician’s choice received an EGFR TKI after disease progression, for 3 (p.N771>GY, exon 20) and 24 months (L858R, exon 21), respectively. The third patient died before he could receive any additional treatment. Overall, 15 patients received treatment with gefitinib, five with erlotinib, and four with afatinib. Circulating tumor cell–free DNA analysis (liquid biopsy) was performed in four patients, two of whom tested positive for the T790M mutation. The tumors of both of these patients initially harbored a deletion in exon 19.
Among EGFR WT patients, 98% received platinum-based doublet chemotherapy in the first-line setting. Twelve patients received immunotherapy with an anti–programmed death 1 (PD1) checkpoint inhibitor as a subsequent therapy line, following pertinent EMA approvals. Notably, all patients received treatment until disease progression or unacceptable toxicity, whichever came first, and no treatment discontinuation occurred because of logistic reasons or drug shortages.
Patient Outcomes
Median follow-up for all patients in our study was 27 months. As expected, patients with good PS (0 or 1) had significantly increased OS, compared with patients with poor PS (≥ 2; hazard ratio [HR], 0.43; 95% CI, 0.26 to 0.72; P = .001; Figs 3A and 3B). TTF was longer for patients with good versus poor PS, but not at a statistically significant level (HR, 0.64; 95% CI, 0.40 to 1.03; P = .064). In univariable analysis, women had increased OS compared with men (HR, 0.62; 95% CI, 0.40 to 0.97; P = .038), but there was no difference in TTF between the two sexes. There was a trend toward increased OS and TTF for nonsmokers compared with smokers (HR, 0.53; 95% CI, 0.28 to 1.01; P = .055 and HR, 0.58; 95% CI, 0.33 to 1.00; P = .051, respectively). There was no significant difference in TTF (HR, 1.10; 95% CI, 0.93 to 1.30; P = .231) or OS (HR, 1.09; 95% CI, 0.90 to 1.34; P = .375) between EGFR WT patients who received different platinum doublets (Figs 3C and 3D). Bevacizumab was part of first-line treatment in 19% of patients. The addition of bevacizumab to first-line treatment resulted in improved TTF (HR, 0.64; 95% CI, 0.41 to 0.98; P = .042; Figs 3E and 3F) and a trend toward improved OS (HR, 0.64; 95% CI, 0.38 to 1.06; P = .084; Fig 3E and 3F). Median TTF and OS of patients who received immunotherapy as part of later treatment lines were 8 months and not yet reached, respectively.
Fig 3.
Patient outcomes. (A) Time to treatment failure (TTF) and (B) overall survival (OS) in patients with good and poor PS. (C) TTF and (D) OS in EGFR wild-type (WT) patients receiving different first-line platinum-based treatment regimens. (E) TTF and (F) OS in EGFR WT patients treated with platinum-based first-line therapy with or without bevacizumab. + indicates censored patients.
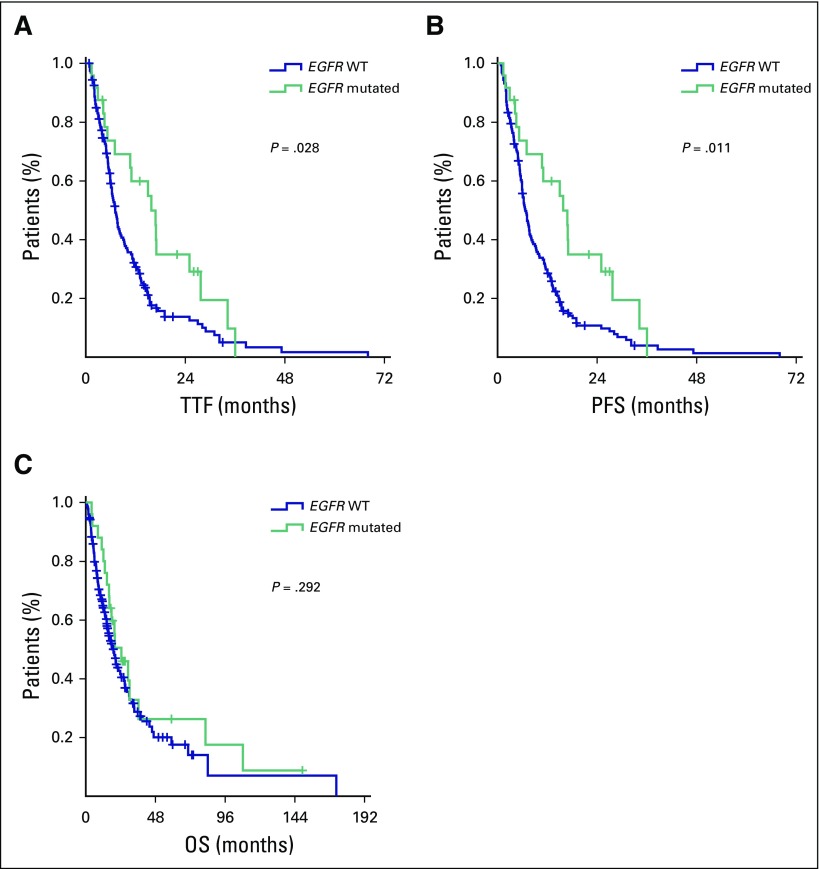
Regarding patients with and without oncogene-addicted NSCLC, we recorded 102 deaths among EGFR WT patients and 18 among those with EGFR mutations. Median TTF for those with EGFR mutations versus WT was 15.8 versus 7.1 months, respectively (HR, 0.58; 95% CI, 0.35 to 0.95; P = .031; Fig 4). Median PFS of those with EGFR mutations was also improved compared with EGFR WT patients (15.8 v 6.7 months, respectively; HR, 0.53; 95% CI, 0.33 to 0.87; P = .013; Fig 4). There was no significant difference in OS between the two groups (HR, 0.76; 95% CI, 0.46 to 1.27; P = .293; Fig 4). There was no difference in either OS (P = .337) or TTF (P = .560) between patients with EGFR mutations and WT patients with brain metastases. In a multivariable model encompassing PS (the only parameter associated with TTF in our patient cohort), EGFR status maintained its prognostic significance (HR, 0.51; 95% CI, 0.28 to 0.92; P = .025).
Fig 4.
Patient outcomes in patients with EGFR mutations and EGFR WT patients: (A) time to treatment failure (TTF), (B) progression-free survival (PFS), and (C) overall survival (OS). + indicates censored patients.
We did not identify any difference in survival outcomes between patients with tumors harboring EGFR exon 19 mutations versus patients with tumors with the exon 21 substitution mutation (TTF: HR, 1.07; 95% CI, 0.64 to 1.82; P = .789; OS: HR, 1.10; 95% CI, 0.65 to 1.86; P = .720). Two patients with exon 19, one with exon 21, and one with exon 18 EGFR mutations received afatinib. Because of the small number of patients, we did not address EGFR TKI efficiency.
DISCUSSION
This was a single-institute retrospective observational study of patients with newly diagnosed advanced NSCLC. To our knowledge, this is the first study reporting clinical outcomes in correlation with treatment regimens in Greek patients with oncogene-addicted NSCLC in the era of the financial crisis. We found that Greek patients with EGFR-mutant tumors diagnosed during this period had clinical outcomes consistent with those in other parts of the world reported in the literature.14-17
In 2010, Greece entered a deep economic crisis, which led to significant reduction in gross domestic product, coupled with large deficits and public debts. Significant deformities in the economy, public sector, and government made it impossible to borrow money from the international markets. Hence, in May 2010, the European Commission, European Central Bank, and International Monetary Fund, colloquially called the European troika, agreed with the Greek government to a 3-year financial aid program, outlined in a memorandum of understanding, which was subsequently extended with two additional programs ending in mid 2018.18,19 Greece agreed to undertake unprecedented reforms in health and pharmaceutical care sectors. Specifically, three stability programs were implemented from May 2010 to August 2018 to attain fiscal and structural reforms. One of the major areas of intervention was health care, where the reforms were aimed at rationalizing expenditure and modernizing the system. As a result, an unprecedented reform program was implemented in health service provision, pharmaceuticals, primary care, public health care insurance, and financing. Rationalization of pharmaceuticals was attempted through development of an e-prescription system, prospective and retrospective physician prescription controls, prescription restrictions with prior authorizations, protocol implementation, compulsory prescription by international nonproprietary name, positive and negative reimbursement lists, fixed budgets, external and internal reference systems, generic penetration support, negotiations, compulsory discounts, rebates, claw backs, and health technology assessment. During the 3-year period after the commencement of crisis, there was a freeze in the introduction of new products, followed by gradual controlled introduction thereafter. These reforms led to the largest reduction in health and pharmaceutical care spending among Organisation for Economic Co-operation and Development countries.12
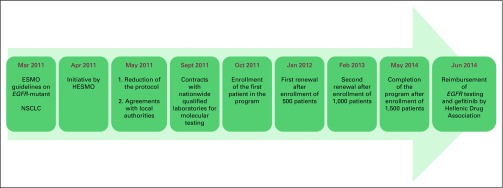
Because of economic restrictions, even though EGFR TKIs had received EMA approval and have represented the treatment of choice for patients with EGFR-mutant advanced NSCLC since 2011,10 they were not reimbursable in Greece until 2014. In light of their profound clinical benefit and to confront the drug shortage, in 2011, the Hellenic Society of Medical Oncology (HESMO) undertook the initiative to organize a program of early access to these drugs in conjunction with the label-owning pharmaceutical companies. To this purpose, HESMO collaborated with the Hellenic Drug Administration, Greek Ministry of Health, and Hellenic Association of Pharmaceutical Companies, as well as with public and private laboratories performing certified molecular testing, to elaborate on an early-access program that included both free EGFR testing and access to the three available EGFR TKIs: erlotinib, gefitinib, and afatinib. The timeline of this initiative is presented in Figure 5. The initial program included free testing for 500 patients but was renewed twice, in January 2012 and February 2013, resulting in 1,500 patients registered until 2014, when reimbursement for EGFR TKIs and EGFR testing was established in Greece. This initiative covered the needs of Greek patients during the first years of the financial crisis and ensured that all eligible patients had access to these pivotal and irreplaceable agents.
Fig 5.
Timeline of the initiative undertaken by the Hellenic Society for Medical Oncology (HESMO) to confront shortage of epidermal growth factor receptor tyrosine kinase inhibitors during the period of financial crisis in Greece. ESMO, European Society for Medical Oncology.
We identified EGFR mutations in 12.6% of the patients tested. These results are comparable to previously reported EGFR mutations in 10% to 15% of unselected white patients with advanced NSCLC.20,21 In one systematic review, Greece was one of the countries with the lowest EGFR mutation frequency among patients with NSCLC (8%) compared with other European countries.22 Others reported the presence of EGFR mutations in 15.8% of Greek patients with NSCLC.23 In the nationwide initiative by the HESMO, the overall incidence of EGFR mutations among the 1,500 patients was 10.1% (152 patients). This difference might be attributed to disparities in sample size and patient clinical characteristics or to adequacy of tissue material for molecular profiling, with the nationwide results probably being the most representative. A vast majority of the EGFR mutations were deletions in exon 19 and the L858R point mutations, as expected.24 Two of the seven less-common mutations identified in our patients’ tumors (exon 21 mutations p.V843I and p.K860E) were previously identified in Greek patients with lung cancer.25
Patients with EGFR mutations had improved TTF and PFS compared with EGFR WT patients, whereas OS was similar between the two groups, as previously reported for the white15,26 and Asian populations.14,16,17,27 Among patients with rare EGFR mutations (exon 21 p.R841P and p.K860E and exon 20 p.N771>GY), only the patient with the p.R841P mutation responded to the EGFR inhibitor. Probably because of the small number of patients with EGFR mutations, we did not identify any difference in survival outcomes between patients with exon 19 and 21 mutations, as previously reported.28 Because a majority of patients were diagnosed and treated earlier than 2015, data regarding T790M mutation status beyond progression during treatment with TKIs were available in only four patients, through a program for free testing organized by HESMO. Of note, patients were treated before the era of third-generation EGFR TKIs. Availability of these drugs might have further improved patient outcomes.
In EGFR WT patients of our cohort, median OS was higher compared with reports in clinical trials assessing standard first-line treatment regimens in advanced NSCLC.29 These results may be attributed to newer therapeutic approaches, such as the addition of bevacizumab to chemotherapy and use of maintenance therapy after initial platinum doublet chemotherapy in the patients of our cohort.30,31 Because of the small sample size, these results must be interpreted with caution.
We found a higher, although of borderline significance, incidence of brain metastases (BMs) in patients with EGFR mutations compared with WT. Published data on BM frequencies in patients with EGFR mutations and EGFR WT patients are contradictory.32-36 A large meta-analysis incorporating 22 studies with 8,152 patients revealed that EGFR mutations were associated with a significantly higher incidence of BMs and that those with EGFR mutations who presented with BMs had a longer BM-related OS.36
There are several limitations to our study. The major drawbacks are the retrospective nature of the study and inclusion of patients from only one institution, leading to a small number of patients with EGFR mutations in the final analysis. These limitations did not allow the assessment of outcome differences between patients with EGFR mutations in different exons or between different EGFR inhibitors or according to mutation.
Our results suggest that despite the economic crisis and restrictions in reimbursement policy, patients with EGFR mutations received the appropriate EMA-approved treatment according to established international guidelines. Collaboration of HESMO with the Hellenic regulatory agencies and the Hellenic Association of Pharmaceutical Companies ensured that there would be no treatment deviations or discontinuation because of logistic or economic reasons. Therefore, clinical outcomes of Greek patients with EGFR mutations were similar to the expected outcomes. With the introduction of novel treatment agents (ie, third-generation EGFR inhibitors and anti-PD1/PD1 ligand checkpoint inhibitors), additional studies are warranted to reevaluate outcome differences between patients with and without EGFR mutations, taking into account both the efficacy of novel agents and increased pharmaceutical costs.
ACKNOWLEDGMENT
We thank the Hellenic Society of Medical Oncology (HESMO) for the provision of indispensable information on the HESMO program of early access to epidermal growth factor receptor tyrosine kinase inhibitors. E.F. received a scholarship from the Hellenic Society of Medical Oncology (October 2017 to September 2018).
AUTHOR CONTRIBUTIONS
Conception and design: Elena Fountzilas, Sofia Levva
Provision of study material or patients: Vassiliki Kotoula, George Fountzilas
Collection and assembly of data: Elena Fountzilas, Sofia Levva, Genovefa Polychronidou, Vassiliki Kotoula, George Fountzilas
Data analysis and interpretation: Elena Fountzilas, Sofia Levva, Giannis Mountzios, Nikos Maniadakis, Vassiliki Kotoula, George Fountzilas
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Treating EGFR-Mutated Oncogene-Addicted Advanced Non–Small-Cell Lung Cancer in the Era of Economic Crisis in Greece: Challenges and Opportunities
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Elena Fountzilas
Honoraria: AstraZeneca (I)
Consulting or Advisory Role: Pfizer (I), Sanofi (I), Roche (I)
Sofia Levva
No relationship to disclose
Giannis Mountzios
No relationship to disclose
Genovefa Polychronidou
No relationship to disclose
Nikos Maniadakis
Honoraria: Abbott Pharmaceuticals, Roche, Amgen, Jansen, BMS, AstraZeneca, Genesis
Research Funding: Abbott Pharmaceuticals, Roche, Amgen, Jansen, BMS, AstraZeneca, Genesis
Travel, Accommodations, Expenses: Abbott Pharmaceuticals
Vassiliki Kotoula
No relationship to disclose
George Fountzilas
Honoraria: AstraZeneca
Consulting or Advisory Role: Pfizer, Roche, Sanofi
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Laversanne M, et al. Cancer incidence in five continents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int J Cancer. 2015;137:2060–2071. doi: 10.1002/ijc.29670. [DOI] [PubMed] [Google Scholar]
- 5.Zhang YL, Yuan JQ, Wang KF, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget. 2016;7:78985–78993. doi: 10.18632/oncotarget.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuette W, Schirmacher P, Eberhardt WE, et al. EGFR mutation status and first-line treatment in patients with stage III/IV non-small cell lung cancer in Germany: An observational study. Cancer Epidemiol Biomarkers Prev. 2015;24:1254–1261. doi: 10.1158/1055-9965.EPI-14-1149. [DOI] [PubMed] [Google Scholar]
- 7.Morgensztern D, Politi K, Herbst RS. EGFR mutations in non-small-cell lung cancer: Find, divide, and conquer. JAMA Oncol. 2015;1:146–148. doi: 10.1001/jamaoncol.2014.278. [DOI] [PubMed] [Google Scholar]
- 8.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non–small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–2449. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 9.Zhang WQ, Li T, Li H. Efficacy of EGFR tyrosine kinase inhibitors in non-small-cell lung cancer patients with/without EGFR-mutation: Evidence based on recent phase III randomized trials. Med Sci Monit. 2014;20:2666–2676. doi: 10.12659/MSM.892476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felip E, Gridelli C, Baas P, et al. Metastatic non-small-cell lung cancer: Consensus on pathology and molecular tests, first-line, second-line, and third-line therapy—1st ESMO Consensus Conference in Lung Cancer; Lugano 2010. Ann Oncol. 2011;22:1507–1519. doi: 10.1093/annonc/mdr150. [DOI] [PubMed] [Google Scholar]
- 11.Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v1–v27. doi: 10.1093/annonc/mdw326. [DOI] [PubMed] [Google Scholar]
- 12. Organisation for Economic Co-operation and Development: Health at a Glance 2017: OECD Indicators. Paris, France, OECD Publishing, 2017. [Google Scholar]
- 13.Levva S, Kotoula V, Kostopoulos I, et al. Prognostic evaluation of epidermal growth factor receptor (EGFR) genotype and phenotype parameters in triple-negative breast cancers. Cancer Genomics Proteomics. 2017;14:181–195. doi: 10.21873/cgp.20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 15.Douillard JY, Ostoros G, Cobo M, et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer. 2014;110:55–62. doi: 10.1038/bjc.2013.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 17.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 18. European Commission: The Economic Adjustment Programme for Greece, Occasional Papers, 2010. http://ec.europa.eu/economy_finance/publications/occasional_paper/2010/pdf/ocp61_en.pdf.
- 19. European Commission: The Second Economic Adjustment Programme for Greece, Occasional Papers, 2012. http://ec.europa.eu/economy_finance/publications/occasional_paper/2012/pdf/ocp94_en.pdf.
- 20.Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 21.Esteban E, Majem M, Martinez Aguillo M, et al. Prevalence of EGFR mutations in newly diagnosed locally advanced or metastatic non-small cell lung cancer Spanish patients and its association with histological subtypes and clinical features: The Spanish REASON study. Cancer Epidemiol. 2015;39:291–297. doi: 10.1016/j.canep.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Szumera-Ciećkiewicz A, Olszewski WT, Tysarowski A, et al. EGFR mutation testing on cytological and histological samples in non-small cell lung cancer: A Polish, single institution study and systematic review of European incidence. Int J Clin Exp Pathol. 2013;6:2800–2812. [PMC free article] [PubMed] [Google Scholar]
- 23.Papadopoulou E, Tsoulos N, Tsirigoti A, et al. Determination of EGFR and KRAS mutational status in Greek non-small-cell lung cancer patients. Oncol Lett. 2015;10:2176–2184. doi: 10.3892/ol.2015.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo A, Franchina T, Ricciardi GR, et al. A decade of EGFR inhibition in EGFR-mutated non small cell lung cancer (NSCLC): Old successes and future perspectives. Oncotarget. 2015;6:26814–26825. doi: 10.18632/oncotarget.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voutsina A, Koutsopoulos A, Kalykaki A, et al. Novel EGFR mutations in patients with non small cell lung cancer (NSCLC) and correlation with sensitivity to gefitinib. Proc Am Assoc Cancer Res. 2006;47 (abstr 5091) [Google Scholar]
- 26.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 27.Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002) Ann Oncol. 2013;24:54–59. doi: 10.1093/annonc/mds214. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Sheng J, Kang S, et al. Patients with exon 19 deletion were associated with longer progression-free survival compared to those with L858R mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: A meta-analysis. PLoS One. 2014;9:e107161. doi: 10.1371/journal.pone.0107161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemjabbar-Alaoui H, Hassan OU, Yang Y-W, et al. Lung cancer: Biology and treatment options. Biochim Biophys Acta. 2015;1856:189–210. doi: 10.1016/j.bbcan.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 31.Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non–small-cell lung cancer. J Clin Oncol. 2013;31:2895–2902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 32.Guan J, Chen M, Xiao N, et al. EGFR mutations are associated with higher incidence of distant metastases and smaller tumor size in patients with non-small-cell lung cancer based on PET/CT scan. Med Oncol. 2016;33:1. doi: 10.1007/s12032-015-0714-8. [DOI] [PubMed] [Google Scholar]
- 33.Hsu F, De Caluwe A, Anderson D, et al. EGFR mutation status on brain metastases from non-small cell lung cancer. Lung Cancer. 2016;96:101–107. doi: 10.1016/j.lungcan.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Xi KX, Wen YS, Zhu CM, et al. Tumor-stroma ratio (TSR) in non-small cell lung cancer (NSCLC) patients after lung resection is a prognostic factor for survival. J Thorac Dis. 2017;9:4017–4026. doi: 10.21037/jtd.2017.09.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B, Sun SZ, Yang M, et al. The correlation between EGFR mutation status and the risk of brain metastasis in patients with lung adenocarcinoma. J Neurooncol. 2015;124:79–85. doi: 10.1007/s11060-015-1776-3. [DOI] [PubMed] [Google Scholar]
- 36.Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro-oncol. 2010;12:1193–1199. doi: 10.1093/neuonc/noq076. [DOI] [PMC free article] [PubMed] [Google Scholar]