Abstract
Purpose
Data on prostate cancer (PCa) treatment in Africa remains under-reported. We present a review of the management of PCa at the cancer center of the largest tertiary referral facility in Ghana, with emphasis on curative treatment.
Methods
We retrospectively reviewed data on 1,074 patients seen at the National Center for Radiotherapy and Nuclear Medicine from 2003 to 2016. Patient and disease characteristics at presentation are presented using descriptive statistics. The χ2 and Fisher’s exact tests and Mann-Whitney U test were used to analyze differences between categorical and continuous variables, respectively. Methods of survival analysis were used to evaluate the relative risk of biochemical disease-free survival (bDFS).
Results
Seventy percent of the study population presented with localized disease. High-risk disease presentation accounted for 64.4% of these patients. Only 57.6% of patients with localized disease received curative radiotherapy. The 5-year overall survival for the curative cohort was 96% (interquartile range, 93% to 98%). The 5-year bDFS rates for low-, intermediate-, and high-risk groups were 95%, 70%, and 48%, respectively. Both Gleason score and pretreatment prostate-specific antigen were significant predictors for bDFS in multivariable analysis.
Conclusion
We show that the majority of patients with PCa have locally advanced disease at the time of presentation for radiotherapy. bDFS was significantly better for low- and intermediate-risk than for high-risk disease. These data emphasize the dire need to re-evaluate screening and patient education of PCa in regions of the world with high incidence and mortality as well as the need for improved access to care and treatment delivery.
INTRODUCTION
Prostate cancer (PCa) is the second most commonly diagnosed cancer and the fifth leading cause of cancer-related mortality in men worldwide.1 The incidence of PCa is highest in Australia, New Zealand, North America, and western and northern Europe likely because of the widespread use of prostate-specific antigen (PSA) screening and subsequent biopsies in these regions. PCa has been reported as the most commonly diagnosed cancer among men in West Africa and is near uniformly lethal, albeit at a lower incidence compared with western populations.1 However, more-recent population-based epidemiologic and prevalence studies have suggested that PCa is largely under-reported in Africa because of a lack of screening and robust cancer registries and may have incidence rates comparable to western populations.2-4 Consistent with this finding, men of African descent from regions with more-reliable registry data are reported to have higher incidence and mortality rates.5 The relatively high mortality rate among African men has been attributed to a number of factors, including poverty, inadequate health care systems and budgets, and genetic differences.6
Currently, data on PCa from African countries, including Ghana, with regard to risk factors, presentation, treatment, and outcomes are lacking. This article attempts to increase our understanding of the burden of PCa, stage at presentation, and clinical outcomes by focusing on patients who present to the National Center for Radiotherapy and Nuclear Medicine (NCRNM) of the Korle Bu Teaching Hospital (KBTH) in Accra, the largest tertiary referral center in Ghana.
METHODS
Patient Selection
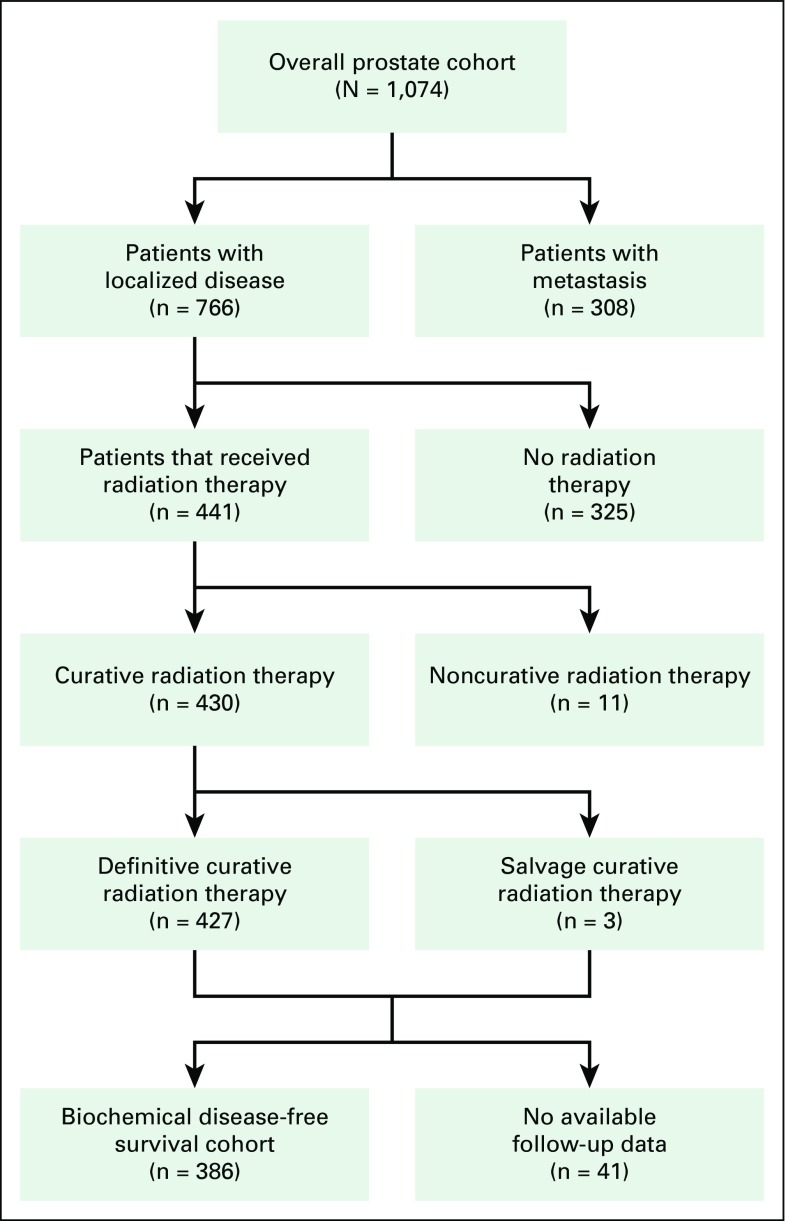
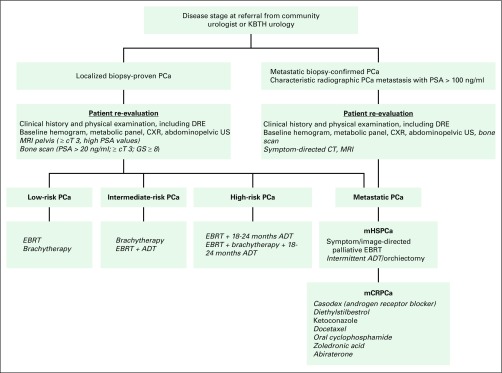
Charts of all patients with PCa referred to the NCRNM from January 2003 to December 2016 (14 years) were reviewed upon receipt of institutional review board approval from KBTH. A total of 1,074 patients had medical records available for review and were included in the study (Fig 1). Seven hundred sixty-six patients (71.3%) in the study cohort presented with localized disease after initial clinical and metastatic work-up, which included bone scans. These patients, including eight referred postoperatively, were eligible for curative radiation therapy. Curative radiation consisted of brachytherapy, external beam radiation therapy (EBRT), or a combination of both modalities. Androgen deprivation therapy (ADT) was offered with radiation treatment on the basis of patient PCa risk profile per National Comprehensive Cancer Network (NCCN) guidelines (Fig 2). Follow-up information, including post-treatment PSA levels, for patients who received radiation of at least 60 Gy and had histologic confirmation of adenocarcinoma of the prostate, a documented tumor stage, and a Gleason score (GS) was reviewed. Of the 766 patients eligible for curative treatment, only 430 received radiation therapy with curative intent. Three of these patients were postoperative referrals. After curative treatment, 386 patients (89.8%) had follow-up information available in their medical records (referred to as the biochemical disease-free survival [bDFS] cohort). Patients with metastatic disease or who did not receive curative radiation treatment were excluded from the bDFS cohort. Qualifying bDFS events were death as a result of any cause or biochemical recurrence and were defined from treatment completion. Patients who did not have an event were censored at the end of the follow-up period. All patients were staged according to the American Joint Committee on Cancer staging system, sixth or seventh edition, depending on the era of their treatment.7,8 Patients with localized disease were stratified into low-, intermediate-, and high-risk groups per NCCN guidelines (version 2, 2014).9
Fig 1.
Patient selection diagram.
Fig 2.
Diagnostic and treatment pathways for prostate cancer. Cost of italicized items may be access limiting. cT, clinical tumor stage; CT, computed tomography; CXR, chest x-ray; DRE, digital rectal examinations; EBRT, external beam radiotherapy; KBTH, Korle Bu Teaching Hospital; mCRPCa, metastatic castrate-resistant prostate cancer; mHSPCa, metastatic hormone-sensitive prostate cancer; MRI, magnetic resonance imaging; PCa, prostate cancer; PSA, prostate-specific antigen; US, ultrasound.
Treatment Details
EBRT.
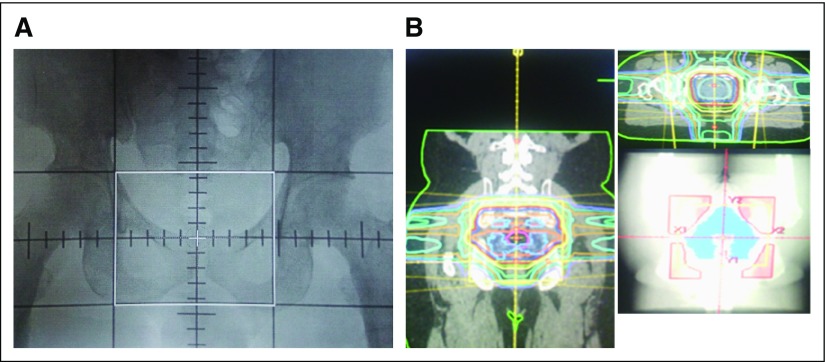
Treatment details were previously described by Yamoah et al.10 Briefly, all EBRT was delivered with a cobalt-60 teletherapy unit (Co-60). A four-field box technique guided by computed tomography scans was used with two parallel-opposed anterior-posterior beams and two parallel-opposed lateral beams with custom blocking for patients treated before 2008. The mid-pubic arch, 2 cm lateral to the pelvic inlet, mid-S2 to S3 intervertebral space, lower sacro-iliac joint, and 1 cm superior to the ischial tuberosity served as anterior, lateral, posterior, superior, and inferior borders of the treatment field, respectively (Fig 3A) . Prescribed doses ranged from 68 to 70 Gy in 34 to 35 daily fractions of 2 Gy each over 7 weeks. In 2008, three-dimensional conformal radiation treatment planning became available and was used to deliver a total prescribed planning target volume dose of 74 Gy in 37 daily fractions (1.8 to 2 Gy per fraction) over 7.5 weeks. The clinical target volume comprised the prostate with or without the entire seminal vesicles on the basis of the risk profile. Three-dimensional conformal radiation treatment plans were generated with Prowess Panther Treatment Planning System version 4.6 software (Radiology Oncology Systems, San Diego, CA; Fig 3B). Multiple fields (four to six), wedges, and customized cerrobend blocks were used to optimize dose distribution to the planning target volume and to meet constraints for organs at risk using quantitative analysis of normal tissue effects in the clinic for the bladder, small bowel, rectum, and femoral heads.11 All treatment plans were approved at weekly quality assurance meetings, and portal images were taken on the Co-60 before treatment start and weekly to ensure appropriate patient set-up to plan.
Fig 3.
(A) Two-dimensional plan simulation film of 0° field (borders marked in white) and (B) 2 × 2 view of three-dimensional conformal radiotherapy plan.
Low-dose-rate brachytherapy.
Real-time transrectal ultrasound-based planning with 125I implant sources (Bard Medical Division, Covington, GA) was used for low-dose-rate (LDR) brachytherapy to the prostate.12 Low-risk and favorable intermediate-risk patients were prescribed 160 Gy to 90% isodose line (before American Association of Physicists in Medicine Task Group No. 43 formalism), whereas high-risk patients received 110 Gy to the prostate followed by EBRT of 45 Gy to the pelvis 2 months after brachytherapy.
ADT.
ADT was not recommended for low-risk patients. Patients with moderate to high intermediate- and high-risk were considered candidates for combined ADT and radiotherapy. Additional ADT was based on risk category, with high-risk patients receiving up to 24 months in total. Gonadotropin-releasing hormone agonists (goserelin acetate or leuprolide acetate) were the drugs used for ADT. A nonsteroidal anti-androgen (bicalutamide) usually was given for at least 2 weeks before administration of the first dose of gonadotropin-releasing hormone agonist in patients at risk for the flare phenomenon.13 A few patients chose orchiectomy to circumvent the high cost of medical castration.
Follow-Up
After completion of treatment, patients were followed with physical examination and serial PSA screenings every 3 to 6 months for the first 2 years or every 6 to 12 months on the basis of their risk profile. Biochemical failure was defined using the Phoenix definition (PSA nadir + 2.0 ng/mL).14 Patients lost to follow-up were reached by telephone when feasible to update survival data.
Statistical Analysis
Clinicopathologic and demographic characteristics of the study cohort were described using descriptive statistics. The χ2 and Fisher’s exact tests and Mann-Whitney U test were used to assess the association between NCCN-defined risk criteria and various patient- and treatment-specific factors. Methods of survival analysis also were used to calculate the risk of bDFS on the basis of important clinical characteristics. Both unadjusted and adjusted Cox proportional hazards regression models were used to obtain associated hazard ratios (HRs) for the bDFS end point. Variables used in univariable analysis and multivariable analysis were clinical stage, GS, PSA category, age at presentation, and radiation therapy delivery modality. In addition, Kaplan-Meier method was used to evaluate 5-year bDFS differences, and log-rank test P value was used to assess the difference within the strata of comparison groups. Finally, HRs and 95% CIs were reported. Analyses were performed using SAS 9.4 statistical software (SAS Institute, Cary, NC).
RESULTS
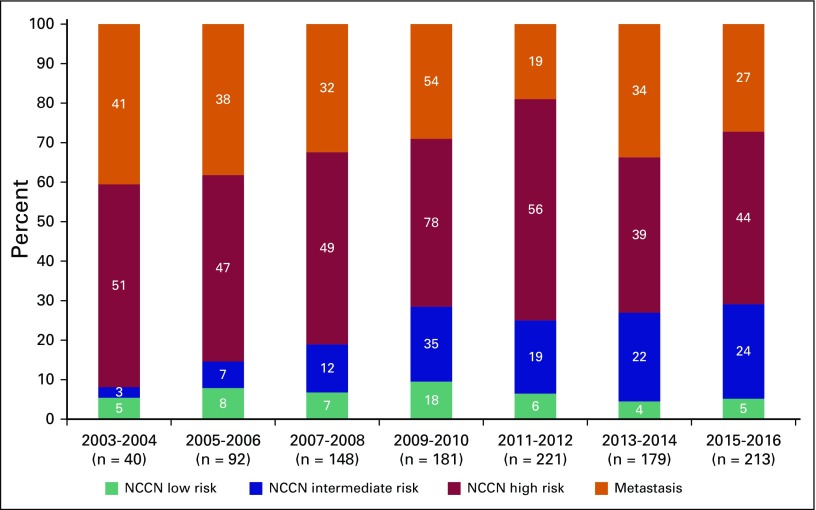
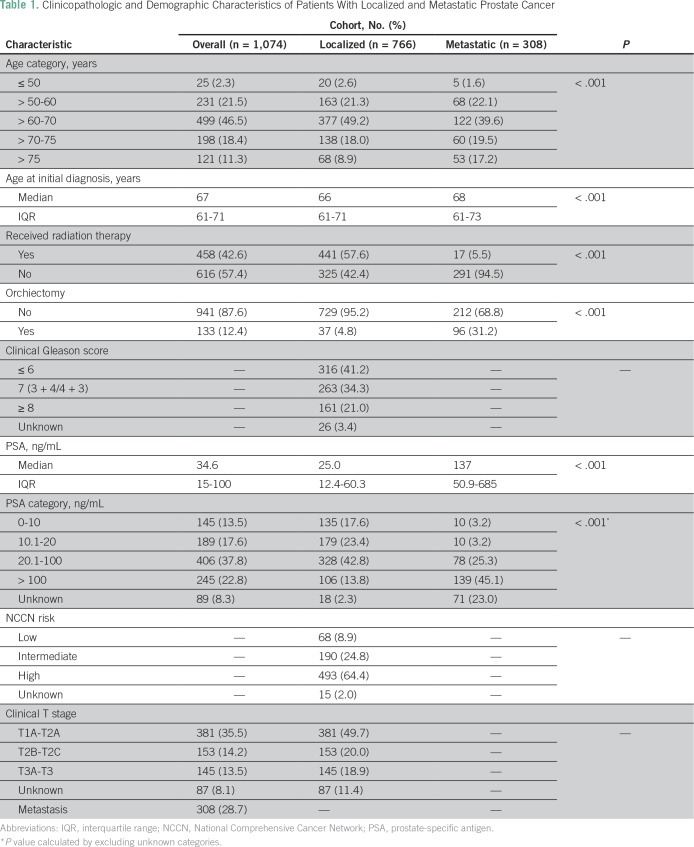
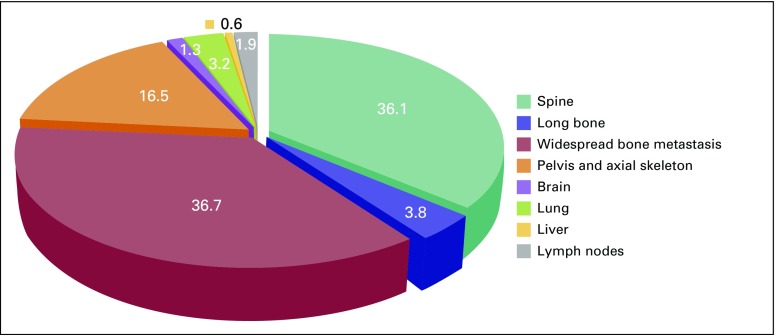
A total of 1,074 patients with PCa were analyzed to determine their disease characteristics and treatment patterns. An upward trend was found in the number of referrals to NCRNM over a 14-year period (Fig 5). The median follow-up in this study was 43 months (interquartile range [IQR], 21 to 81 months); 71.3% of the study population had clinically localized disease, whereas the remaining 28.7% presented with metastasis (Table 1; Fig 4). Median age at presentation was higher for patients with metastasis than for those with localized disease (68 v 66 years; P < .01). In addition, a higher proportion of patients with metastatic PCa (31.2%) received orchiectomy compared with patients with localized disease (4.8%). Median pretreatment PSA for the localized cohort was 25 ng/mL. A significantly large proportion of the localized cohort had high-risk disease (64.4%) compared with that with intermediate-risk (24.8%) and low-risk (8.9%) disease (Table 1).
Fig 5.
Distribution of metastatic disease by site.
Table 1.
Clinicopathologic and Demographic Characteristics of Patients With Localized and Metastatic Prostate Cancer
Fig 4.
Patterns of prostate cancer presentation at the National Center for Radiotherapy and Nuclear Medicine.
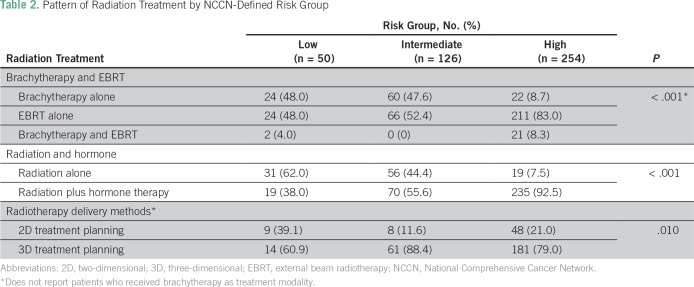
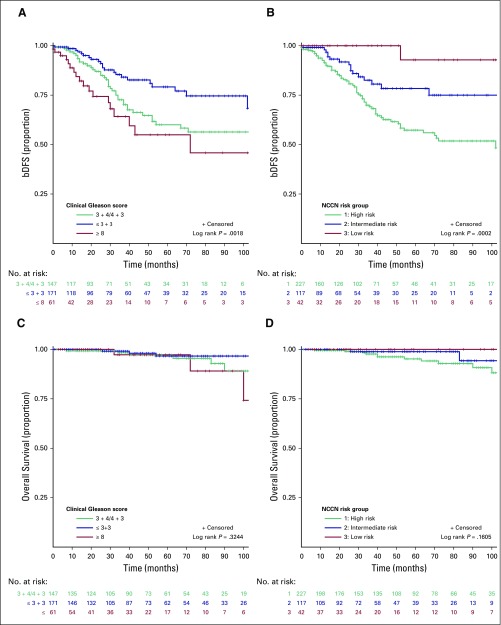
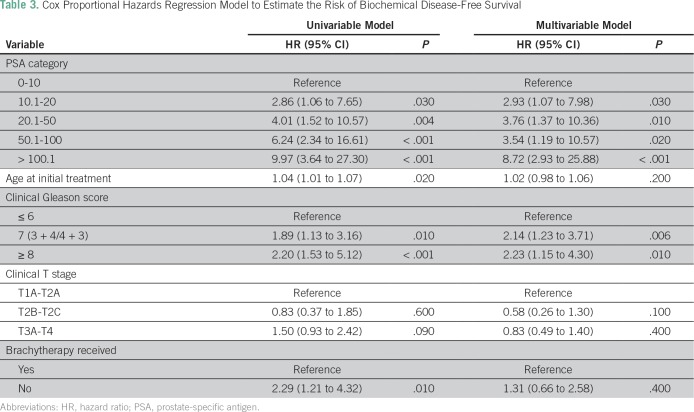
Among the patients with localized disease, 430 received radiotherapy with curative intent. In this cohort of curatively treated patients, categorical analysis between treatment modality and NCCN risk criteria showed that most high-risk patients (82.9%) were treated with EBRT and ADT (Table 2). In contrast, a greater proportion of low- and intermediate-risk patients received brachytherapy compared with high-risk patients. In addition, a significant difference was found in the use of hormone therapy in combination with radiotherapy among high-risk patients (92.9%) compared with intermediate-risk (55.6%) and low-risk (36.7%) patients (P < .001; Table 2). The median time to complete EBRT was 59 days (IQR, 55 to 66 days) for a 7.5-week course of EBRT. In the Kaplan-Meier analysis, the 5-year overall survival rate was 96% (IQR, 93% to 98%), whereas the bDFS rate within the strata of NCCN-defined low-, intermediate-, and high-risk patients was 95%, 70%, and 48%, respectively (P = .01; Fig 6). Compared with patients with a GS of 6, a significantly lower bDFS was found in the multivariable Cox proportional hazards model for patients with a GS of 7 (HR, 1.85; 95% CI, 1.03 to 3.30; P = .03) and GS ≥ 8 (HR, 3.91; 95% CI, 1.96 to 7.79; P < .001; Table 3).
Table 2.
Pattern of Radiation Treatment by NCCN-Defined Risk Group
Fig 6.
Kaplan-Meier curves of biochemical disease-free survival (bDFS) by (A) clinical Gleason score and (B) National Comprehensive Cancer Network (NCCN) risk group and of overall survival by (C) clinical Gleason score and (D) and NCCN risk group.
Table 3.
Cox Proportional Hazards Regression Model to Estimate the Risk of Biochemical Disease-Free Survival
DISCUSSION
In our institution-based review of > 1,000 patients with PCa, the median age at presentation with localized disease was 66 years and 68 years for those with metastatic disease. Most patients had high-risk or metastatic disease (74.6% of all patients). The median PSA at presentation with localized disease was 25 ng/mL, and the use of brachytherapy was strikingly low among patients who presented with high-risk disease. Orchiectomy frequently was used for the purposes of androgen suppression among patients who presented with metastatic disease.
PCa remains a significant global public health concern, with improvement in treatment outcomes raising concerns about overtreatment in patients with indolent disease who will not benefit from therapy but rather, would suffer long-term adverse effects as a result of therapy.15 The prevalence of PCa is considered low in Ghana; however, researchers have questioned this assertion.2,4,16 In a population-based screening study, Hsing et al4 showed that prevalence rates among men in Accra, Ghana, are comparable with US black men. This discrepancy likely is due to the inaccurate population-based estimates in GLOBOCAN, particularly on the African continent where resources for rigorous data reporting are limited.
The NCRNM saw an increasing number of PCa referrals, with a significantly larger proportion of patients presenting with either high-risk disease or metastasis, during the period under review. These increases may reflect increased awareness of PCa, with more patients seeking treatment at the center. The greatest benefit of this increased awareness was evident in the number of patients who presented with intermediate-risk disease. However, the proportion of patients with high-risk and metastatic disease seen during this period remained steady (Fig 5). SEER data on PCa indicate a downward trend in incidence and mortality in the United States since 2003, with a median age of 66 years and 91% with locoregional and 5% with metastatic disease at presentation for the period of 2007 to 2013.17 Although these data share striking similarities with regard to age at disease presentation, the Ghanaian cohort had a fivefold excess of metastatic disease. Whether this finding is related to disease biology or other factors requires additional research. Data from a genome-wide association study reported that single nucleotide polymorphism 5q31.3 was associated with aggressive PCa subtypes in Ghanaian patients, which suggests that disease biology plays a role in aggressive PCa in men of African descent.18 The findings from the current study show that patients present with PCa at a similar age in both the United States and Ghana, yet that Ghanaian patients often had more-advanced disease brings into focus the potential benefit for early detection through PCa screening programs for men in Ghana. Ghana has launched a cancer control program to reverse this trend in disease presentation, with an emphasis on opportunistic screening for PCa.19 The issue of PCa screening has been met with much controversy over the past decade. PCa screening offered a significant PCa survival benefit of 21% and 44% in two trials conducted in Europe, whereas a contemporaneous trial in the United States reported no benefit.20-22 However, a recent review that adjusted for the effect of PCa screening contamination in the control arm of the US trial found screening to offer similar survival benefit in the American trial as that in the larger European prospective study.21,23 The scope of PCa screening programs in at-risk populations needs to be redefined in light of these findings.
Specific challenges exist in treatment delivery. The first is cost burden. A large proportion of the patients who were candidates for curative radiation therapy did not return for treatment after their first visit or after initiating ADT. Patients pay out of pocket for screening, diagnosis, and treatment for PCa. A review of health care financing in Ghana indicates a favorable effect of universal and comprehensive health insurance schemes to limit out-of-pocket payment for health services on access to care.24 In the absence of insurance schemes that cover the entire cancer care continuum, patients often wait until their symptoms worsen before presenting to health care facilities for diagnosis and treatment.25 This phenomenon explains the high proportion of patients who presented with symptomatic metastatic disease and who overwhelmingly opted to have orchiectomy (a less-expensive option) rather than ADT. Conversely, medical castration was overwhelmingly used to achieve androgen suppression among patients with localized disease (Table 1).
The curative radiation treatment regimen with or without hormonal therapy closely adhered to the recommended NCCN risk-adapted treatment guidelines. Most patients with NCCN-defined high-risk status received a combination of hormone therapy and EBRT. Nine patients classified as having NCCN high-risk disease received brachytherapy. These patients only had borderline high-risk PSA values or a focus GS of 5 and were assessed to have low-volume disease confined to the prostate. This reflects the occasional practice of individualized treatment decisions on the basis of unique patient characteristics. Indication for ADT in low-risk patients is to downsize large prostate volumes to 30% to 40% before LDR in consonance with American Brachytherapy Society guidelines for transrectal ultrasound–guided LDR.26-28
The second challenge is radiation dose escalation. The median EBRT treatment time of 59 days for a 7.5-week schedule is commendable with consideration of delays that may have been occasioned by acute toxicity and downtime on a single treatment unit. The bDFS rate for high-risk patients, mainly those who underwent EBRT, at 5 years (48%) seems inferior to that reported for a high-risk cohort (57% at 5 years) in a dose escalation trial that treated patients in the escalation arm to 74 Gy over 7.5 weeks, with a shorter course of ADT (3 to 6 months) given in the neoadjuvant setting.29,30 Although we cannot draw a direct comparison between these two studies, heavier disease burden in our cohort, as evidenced by higher median pretreatment PSA (25 v 12.8 ng/mL) and a lower achievable median dose of 72 Gy with Co-60 technology, may account for this observation. PSA at presentation, clinical T stage, and GS were predictive of bDFS on univariable analysis; however, only GS and PSA at presentation remained significant on multivariable analysis. Brachytherapy did not predict bDFS, even among the high-risk subset, which likely is due to the low proportion of high-risk patients who received brachytherapy coupled with a short follow-up period, particularly for brachytherapy-treated patients. This finding also speaks to the high attrition rate of surveillance programs after treatment completion, which made the true measurement of the role of predictors of disease outcomes in our study challenging.
Significant infrastructural improvement at NCRNM has occurred in recent years. New 100-cm source-to-axis distance Co-60, conventional simulator, and high-dose-rate Co-60 source brachytherapy units are operational. In addition, a 6-MV teletherapy unit capable of image-guided and arc therapy is near commissioning. Improved conformal radiation treatments and dose escalation with arc and intensity-modulated radiation therapy capabilities are envisaged with these upgrades and expected to translate to durable disease control and patient survival. However, these benefits can only materialize if patients present early for treatment.31,32
This work has some limitations. First, it was a retrospective study from a referral center subject to selection for patients with access to facilities with expertise to make a PCa diagnosis and referral for treatment. Second, cost and distance may have prevented some patients from accessing the center. Third, predominantly grade 2 with some grade 3 genitourinary late treatment–related adverse effects were documented among 10.2% of treated patients, with no report of GI toxicity. However, this rate may not be representative of all toxicity because it was not collected in a structured format, and a significant number of patients were lost to follow-up. Finally, incomplete data at the time of review accounted for unknown entries in the data summary tables. Despite these limitations, this study offers an insight into PCa at presentation and treatment outcomes in this patient population.
Significant improvement in delivering standard-of-care treatment to patients with PCa at NCRNM has been achieved over the past decade. However, much more needs to be done to increase early PCa detection and treatment, remove barriers to access, maintain an institutional registry to better inform PCa policy, and explore the full potential of the center to further improve PCa outcomes in Ghana. The acquisition of modern therapy units offers these patients the opportunity to achieve more-durable disease control. Prospective and collaborative studies should be vigorously pursued to better understand the biology and outcomes of available standards of care in this understudied population of patients with PCa.
AUTHOR CONTRIBUTIONS
Conception and design: Joel Yarney, Shivanshu Awasthi, Verna Vanderpuye, Puja S. Venkat, Angelina K. Fink, Kosj Yamoah
Financial support: Kosj Yamoah
Administrative support: Kosj Yamoah
Provision of study material or patients: Samuel N.A. Tagoe, Kosj Yamoah
Collection and assembly of data: Francis A. Asamoah, Joel Yarney, Verna Vanderpuye, Puja S. Venkat, Arash O. Naghavi, Afua Abrahams, James E. Mensah, Samuel N.A. Tagoe, Kosj Yamoah
Data analysis and interpretation: Francis A. Asamoah, Joel Yarney, Shivanshu Awasthi, Verna Vanderpuye, Arash O. Naghavi, Evans Sasu, James E Mensah, Peter A.S. Johnstone, Kosj Yamoah
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Francis A. Asamoah
No relationship to disclose
Joel Yarney
Honoraria: Roche
Speakers’ Bureau: Johnson & Johnson
Travel, Accommodations, Expenses: Roche
Shivanshu Awasthi
No relationship to disclose
Verna Vanderpuye
No relationship to disclose
Puja S. Venkat
No relationship to disclose
Angelina K. Fink
Consulting or Advisory Role: WellMation (I)
Arash O. Naghavi
No relationship to disclose
Afua Abrahams
No relationship to disclose
James E. Mensah
No relationship to disclose
Evans Sasu
Employment: Medical Imaging Ghana
Honoraria: National Center for Radiotherapy and Nuclear Medicine
Samuel N.A. Tagoe
No relationship to disclose
Peter A.S. Johnstone
Travel, Accommodations, Expenses: ViewRay
Kosj Yamoah
No relationship to disclose
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Rebbeck TR, Devesa SS, Chang B-L, et al. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of African descent. Prostate Cancer. 2013;2013:560857. doi: 10.1155/2013/560857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung CC, Ciampa J, Yeager M, et al. Fine mapping of a region of chromosome 11q13 reveals multiple independent loci associated with risk of prostate cancer. Hum Mol Genet. 2011;20:2869–2878. doi: 10.1093/hmg/ddr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsing AW, Yeboah E, Biritwum R, et al. High prevalence of screen detected prostate cancer in West Africans: Implications for racial disparity of prostate cancer. J Urol. 2014;192:730–735. doi: 10.1016/j.juro.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 6.Adebamowo CA, Akarolo-Anthony S. Cancer in Africa: Opportunities for collaborative research and training. Afr J Med Sci. 2009;38(suppl 2):5–13. [PubMed] [Google Scholar]
- 7.Schmoll HJ. F.L. Greene, D.L. Page, I.D. Fleming et al. (eds). AJCC Cancer Staging Manual, 6th edition. Ann Oncol. 2003;14:345–346. [Google Scholar]
- 8.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 9.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010;8:145. doi: 10.6004/jnccn.2010.0010. [DOI] [PubMed] [Google Scholar]
- 10.Yamoah K, Beecham K, Hegarty SE, et al. Early results of prostate cancer radiation therapy: An analysis with emphasis on research strategies to improve treatment delivery and outcomes. BMC Cancer. 2013;13:23. doi: 10.1186/1471-2407-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mensah JE, Yarney J, Vanderpuye V, et al. Prostate brachytherapy in Ghana: Our initial experience. J Contemp Brachytherapy. 2016;8:379–385. doi: 10.5114/jcb.2016.62972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 14.Roach M, III, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65:1046–1055. doi: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith DS, Bullock AD, Catalona WJ, et al. Racial differences in a prostate cancer screening study. J Urol. 1996;156:1366–1369. [PubMed] [Google Scholar]
- 17.National Cancer Institute. Surveillance, Epidemiology, and End Results Program Cancer Stat Facts: Prostate Cancer. 2016 https://seer.cancer.gov/statfacts/html/prost.html
- 18.Cook MB, Wang Z, Yeboah ED, et al. A genome-wide association study of prostate cancer in West African men. Hum Genet. 2014;133:509–521. doi: 10.1007/s00439-013-1387-z. [Erratum: Hum Genet 133:523, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghana News Agency Five-year national strategy for cancer control launched. 2015 http://www.ghananewsagency.org/health/five-year-national-strategy-for-cancer-control-launched--85403
- 20.Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–732. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–2035. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andriole GL, Crawford ED, Grubb RL, III, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: Mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med. 2017;167:449–455. doi: 10.7326/M16-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asenso-Okyere WK, Anum A, Osei-Akoto I, et al. Cost recovery in Ghana: Are there any changes in health care seeking behaviour? Health Policy Plan. 1998;13:181–188. doi: 10.1093/heapol/13.2.181. [DOI] [PubMed] [Google Scholar]
- 25.Fenny AP, Asante FA, Enemark U, et al. Treatment-seeking behaviour and social health insurance in Africa: The case of Ghana under the National Health Insurance Scheme. Glob J Health Sci. 2014;7:296–314. doi: 10.5539/gjhs.v7n1p296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petit JH, Gluck C, Kiger WS, III, et al. Androgen deprivation-mediated cytoreduction before interstitial brachytherapy for prostate cancer does not abrogate the elevated risk of urinary morbidity associated with larger initial prostate volume. Brachytherapy. 2007;6:267–271. doi: 10.1016/j.brachy.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Davis BJ, Horwitz EM, Lee WR, et al. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy. 2012;11:6–19. doi: 10.1016/j.brachy.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Solhjem MC, Davis BJ, Pisansky TM, et al. Prostate volume before and after permanent prostate brachytherapy in patients receiving neoadjuvant androgen suppression. Cancer J. 2004;10:343–348. doi: 10.1097/00130404-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: Long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464–473. doi: 10.1016/S1470-2045(14)70040-3. [DOI] [PubMed] [Google Scholar]
- 30.Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: First results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8:475–487. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 31.Cooperberg MR, Lubeck DP, Mehta SS, et al. Time trends in clinical risk stratification for prostate cancer: Implications for outcomes (data from CaPSURE) J Urol. 2003;170:S21–S25. doi: 10.1097/01.ju.0000095025.03331.c6. discussion S26-S27. [DOI] [PubMed] [Google Scholar]
- 32.Cooperberg MR, Broering JM, Litwin MS, et al. The contemporary management of prostate cancer in the United States: Lessons from the Cancer of the Prostate Strategic Urologic Research Endeavor (CapSURE), a national disease registry. J Urol. 2004;171:1393–1401. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]