Abstract
Purpose
Lung cancer is one of the most common cancers worldwide and in Thailand. We characterize and forecast region-specific patterns of lung cancer incidence by histology and sex.
Methods
We analyzed lung cancer incidence trends in Thailand by histology (adenocarcinoma [AdC]; squamous cell carcinoma [SCC]; and large-cell, small-cell, and other carcinomas) from 1990 to 2014 in four cancer registries in three regions (north, Chiang Mai Province and Lampang Province; northeast: Khon Kaen Province; south: Songkhla Province). Annual percent change (APC) was calculated to quantify the incidence rate trends using joinpoint regression. Age-period-cohort models were used to examine the temporal trends of AdC and SCC by age, calendar year, and birth cohort. We projected the incidence of AdC and SCC up to 2030 using three independent approaches: joinpoint, age-period-cohort, and Nordpred models.
Results
AdC incidence significantly increased from 1990 to 2012 in Chiang Mai males (APC, 1.3%), Songkhla males from 2004 to 2014 (APC, 2.5%), Songkhla females from 1990 to 2014 (APC, 5.9%), and Khon Kaen females from 2005 to 2014 (APC, 3.1%). Conversely, SCC incidence significantly decreased from 1990 to 2012 in Chiang Mai males and females (APC, −1.2% and −4.8%, respectively), Lampang males and females from 1993 to 2014 (APC, −5.4% and −5.2%, respectively), and Songkhla females from 1990 to 2014 (APC, −2.1%). In general, trends of AdC and SCC correlated more with birth cohort than with calendar year. Three projection models suggested that incidence rates of AdC in Songkhla may continue to increase until 2030.
Conclusion
Temporal trends of lung cancer by histology varied among regions in Thailand. Reduction of lung cancer incidence in Thailand likely will require prevention strategies tailored to each specific region.
INTRODUCTION
Worldwide, lung cancer is one of the most common cancers. In 2012, an estimated 1.8 million patients are newly diagnosed, 58% of whom live in less developed regions.1 Specifically, lung cancer in Thailand was ranked as one of the most common cancers in males and females (32.0 and 10.1 per 100,000, respectively).2 Lung cancer mortality rates have already peaked in many high-income countries but are still on the rise in low- and middle-income countries (LMICs), including Thailand.3 This partly reflects the various stages of the tobacco epidemic, with smoking prevalence declining for many years in high-income countries but still on the rise and just recently decreasing in many LMICs.
Cigarette smoking is the main cause of lung cancer. In Thailand, smoking prevalence has decreased in the past 20 years from 60% to 39% in males and 5% to 2.1% in females between 1991 and 2014.4,5 Overall lung cancer incidence in contrast has been increasing in Thailand since the 1990s, which likely reflects, in part, the lag between exposure and cancer onset.6
Lung cancer histology can be classified into two major categories: small-cell lung cancer (SCLC) and non–small-cell lung cancer (NSCLC). Adenocarcinoma (AdC), squamous cell carcinoma (SCC), and large-cell carcinoma (LCC) are the main NSCLC subtypes. Smoking increases the risk of all histologies, but the degree of the association varies. SCC and SCLC are affected more profoundly by smoking than other histologies.7 SCLC is unique because it usually does not occur in never-smokers.8,9 Besides smoking, other factors such as secondhand smoke,10 cooking fumes,11 genetic predisposition,12 hormones,13 occupational exposures,14 and household radon15 contribute lung cancer risk.
The aim of this study was to better understand the trends of lung cancer incidence by histology and sex in various regions in Thailand. We first analyzed trends of age-adjusted lung cancer rates using joinpoint regression. We then investigated the temporal trends of AdC and SCC using age-period-cohort models. Finally, we used three projection methods to forecast lung cancer incidence rates by histology until 2030.
METHODS
Cancer Registries and Case Ascertainment
Lung cancer data were extracted from the Chiang Mai (1990 to 2012), Lampang (1993 to 2014), Khon Kaen (1990 to 2014), and Songkhla (1990 to 2014) cancer registries. These registries were chosen on the basis of their locations (Appendix Fig A1) and high-quality data. The population in each region in 2014 was 1.6 million in Chiang Mai, 1.7 million in Khon Kaen, 0.7 million in Lampang, and 1.5 million in Songkhla. Case ascertainment in each registry has been estimated to be > 70%.16
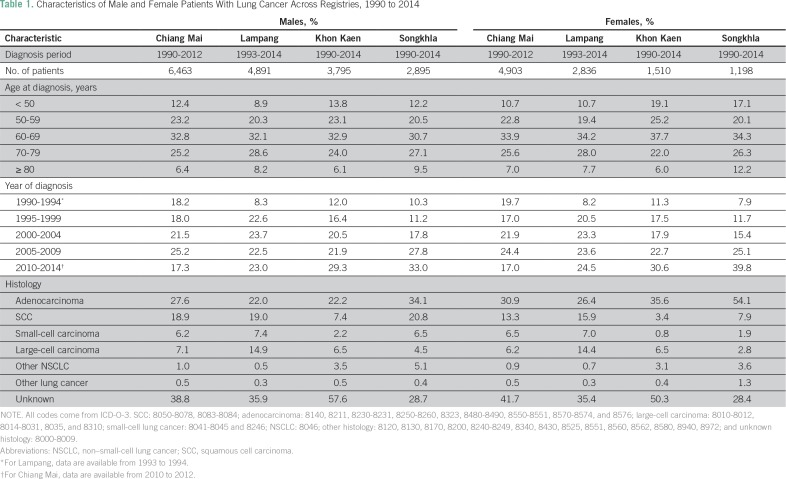
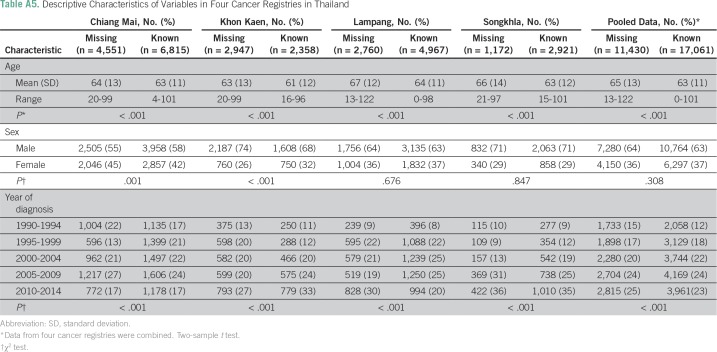
Diagnoses were obtained using the International Classification of Disease, Tenth Revision, codes C33 and C34. Patient information included age, sex, year of diagnosis, and histology. Population denominators were estimated from population censuses conducted by the National Statistical Office in 1990, 2000, and 2010.17,18 Intercensus populations were estimated using a log-linear function between two consecutive censuses. The populations beyond 2010 to 2030 were estimated and reported by the Office of the National Economic and Social Development Board.19 Age-adjusted lung cancer incidence was standardized to the Segi world population.20 Diagnoses were classified by histology on the basis of International Classification of Diseases for Oncology, third edition, code. Table 1 lists the distribution of patients with cancer by sex, age, period, histology, and registry. Missing histology was substantial across registries at approximately 30% to 50%, depending on the registry. We thus conducted multiple imputation using the R package MICE (version 3.2.0; https://cran.r-project.org) to complete the missing information. Details about the multiple imputation procedure and its appropriateness are provided in the Appendix.
Table 1.
Characteristics of Male and Female Patients With Lung Cancer Across Registries, 1990 to 2014
Because AdC and SCC are the most common types of carcinoma in all registries, our analyses primarily focused on these histologies. The main analyses were conducted using the imputed data, but we performed sensitivity analyses with complete patient data to evaluate the effect of imputation on trend assessment. This research was approved by the research ethics committee of the Faculty of Medicine, Prince of Songkla University (approval #58-013-18-1).
Trend Analysis
Joinpoint.
To identify significant changes in trends of age-adjusted lung cancer rates, we performed joinpoint regression analysis using the statistical software Joinpoint version 4.0.1.21 Joinpoint regression identifies the annual percent change (APC) of incidence rates in each statistically significant trend interval. Because of sparse data, we restricted the analysis to five data points as the minimum number of observations between two joinpoints. The average APC of incidence rates for the past 10 years was estimated for comparison across demographic and histology groups.
Age-period-cohort analysis.
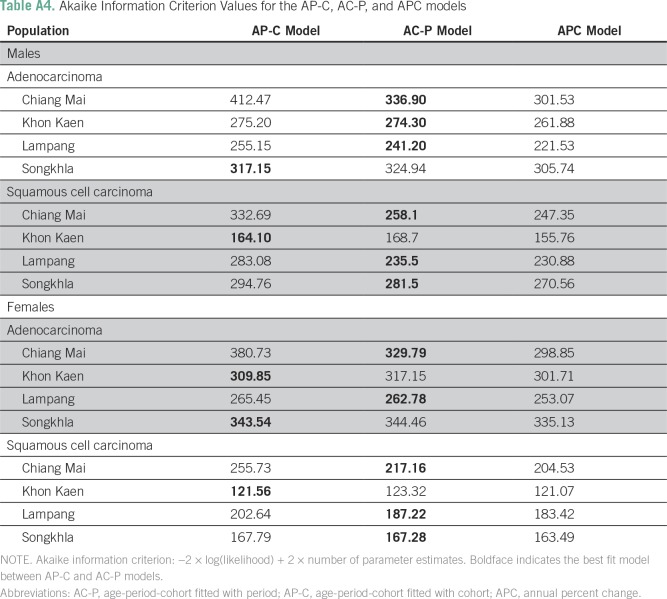
Age-period-cohort models were used to estimate the separate effects of age, period (calendar year), and cohort (birth cohort) on lung cancer incidence.22 This assumes that the incidence rates follow a Poisson distribution with mean equal to the product of age, period, and cohort effects. To deal with the identifiability issue of age-period-cohort models,22 we fitted the models with either cohort (AP-C) or period (AC-P) constrained to be zero on average with zero slope. The best-fitted models were determined on the basis of the Akaike information criterion. Analyses were performed using the R package Epi.23 We anchored the 1940 birth cohort and 2000 calendar year as reference. The AP-C and AC-P models were fitted to AdC and SCC data in each registry separately by sex.
Projections
We projected lung cancer incidence by histology in four registries until 2030 using three different approaches: joinpoint, age-period-cohort, and Nordpred models.24
Joinpoint.
Because recent trends are likely to be the best predictors of future cancer incidence, this projection was obtained by carrying forward the APC estimate from the last joinpoint period to future years.25 For projections, we attenuated (reduced) the drift in the joinpoint model by 8% each year after the period of observation. For instance, detrending by 8% annually attenuates the linear trend by 1 − (1 − 0.08)k, where k = 1, 2…, N. The average attenuation rate for the first 5 years is 21.6%, which corresponds to the first 5-year attenuation rate in the Nordpred model.
Age-period-cohort.
We used AP-C and AC-P models for age-period-cohort projections. For the AC-P approach, the estimated cohort effects were projected to 2030 while the age and period effects were kept constant. For the AP-C approach, the estimated period effects were projected to 2030 while the age and cohort effects remained constant. A similar detrending approach as for the joinpoint projections was applied to attenuate the linear cohort or period effects trend when projecting these into the future.
Nordpred.
We used the R package Nordpred to project lung cancer incidence.24 Using the calibrated age-period-cohort model, we computed incidence rates for each 5-year age-group and 5-year interval. The estimated trends on the basis of the observed data were extrapolated to four separate periods until 2030. To avoid potential overestimation of patients with lung cancer, a power function in Nordpred was used to attenuate the linear trend by 0%, 21.6%, 48.3%, 65.9%, and 77.6% for the corresponding five periods: 2012 to 2013, 2014 to 2018, 2019 to 2023, 2024 to 2028, and 2029 to 2030.
RESULTS
Trends
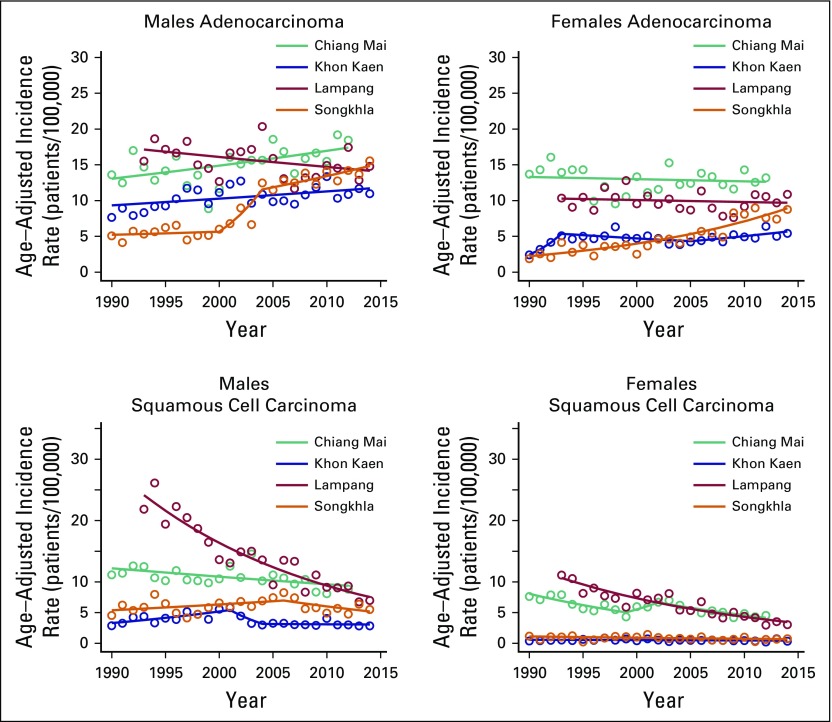
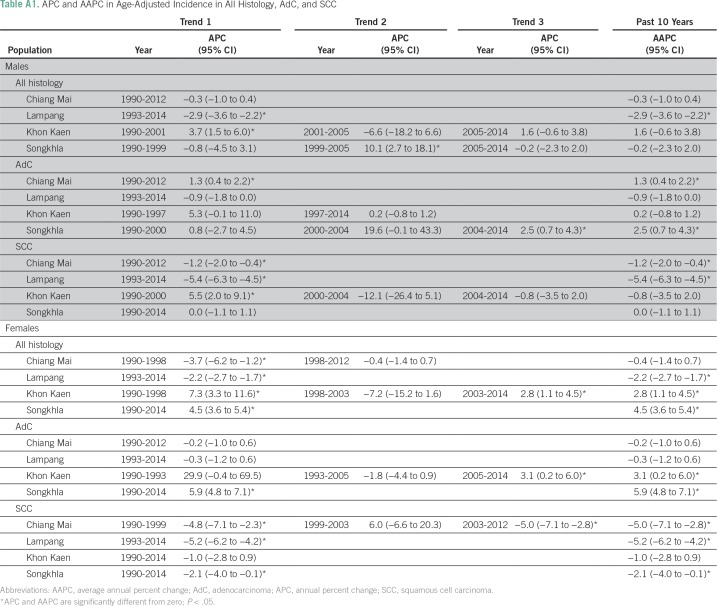
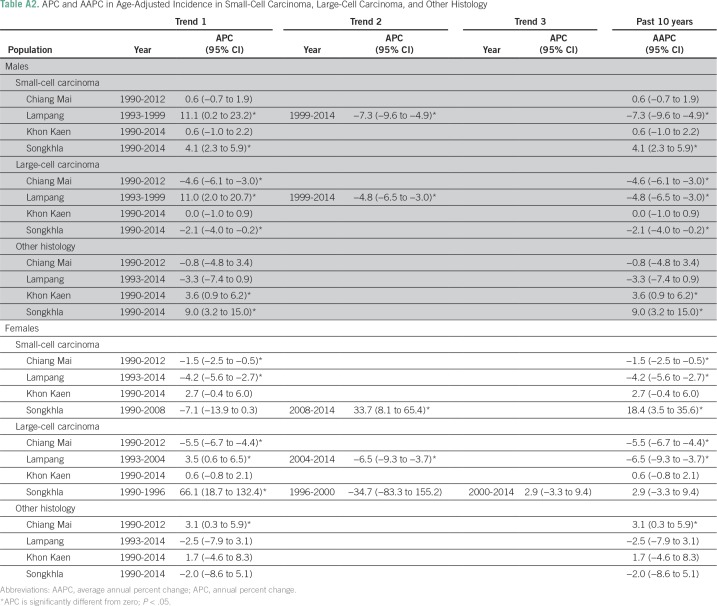
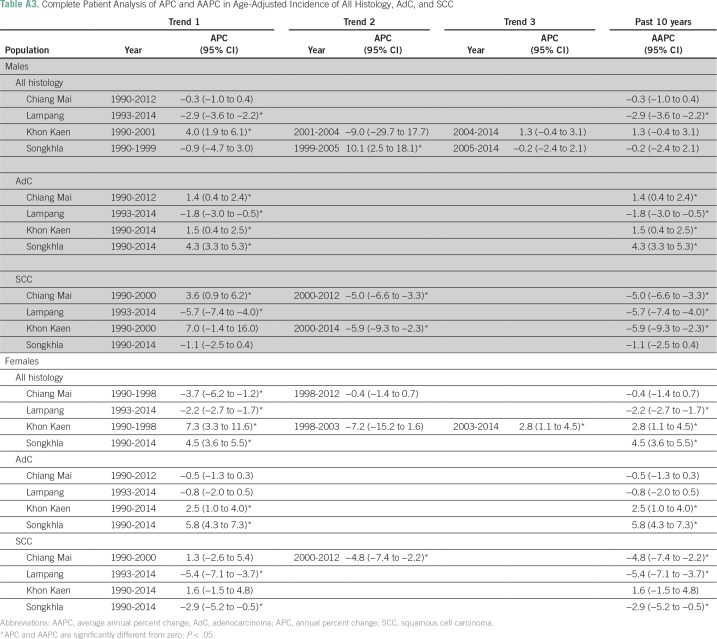
Figure 1 shows lung cancer trends by registry, sex, and histology from the joinpoint analysis, and the corresponding APC estimates are listed in Appendix Tables A1 and A2. Of the four registries, Chiang Mai had the highest AdC incidence rates in both males and females (12.5 and 13.3 patients per 100,000, respectively) in 1990. AdC incidence significantly increased from 1990 to 2012 in Chiang Mai males (APC, 1.3%; 95% CI, 0.4% to 2.2%), Songkhla males from 2004 to 2014 (APC, 2.5%; 95% CI, 0.7% to 4.3%), Songkhla females from 1990 to 2014 (APC, 5.9%; 95% CI, 4.8% to 7.1%), and Khon Kaen females from 2005 to 2014 (APC, 3.1%; 95% CI, 0.2% to 6.0%). SCLC, SCC, and LCC rates have been decreasing (or nonincreasing) in all registries, except for SCLC in Songkhla, but the number of patients is limited. As a sensitivity analysis, we also conducted a complete case analysis using joinpoint regression, and found that the main conclusions are unchanged (Appendix Table A3). Pairwise parallel tests in the joinpoint analysis show that in general, trends are similar for females but different for males by region (data not shown).
Fig 1.
Histology-specific age-adjusted incidence trends of lung cancer per 100,000 population in adenocarcinoma and squamous cell carcinoma. The lines represent the joinpoint model predictions and the circles the observed rates in the data.
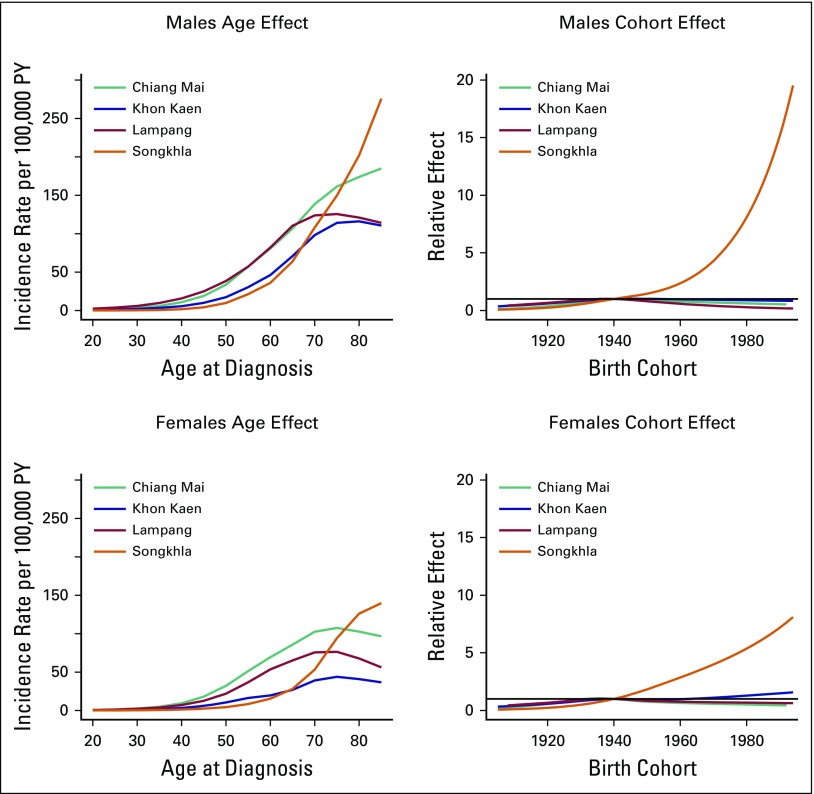
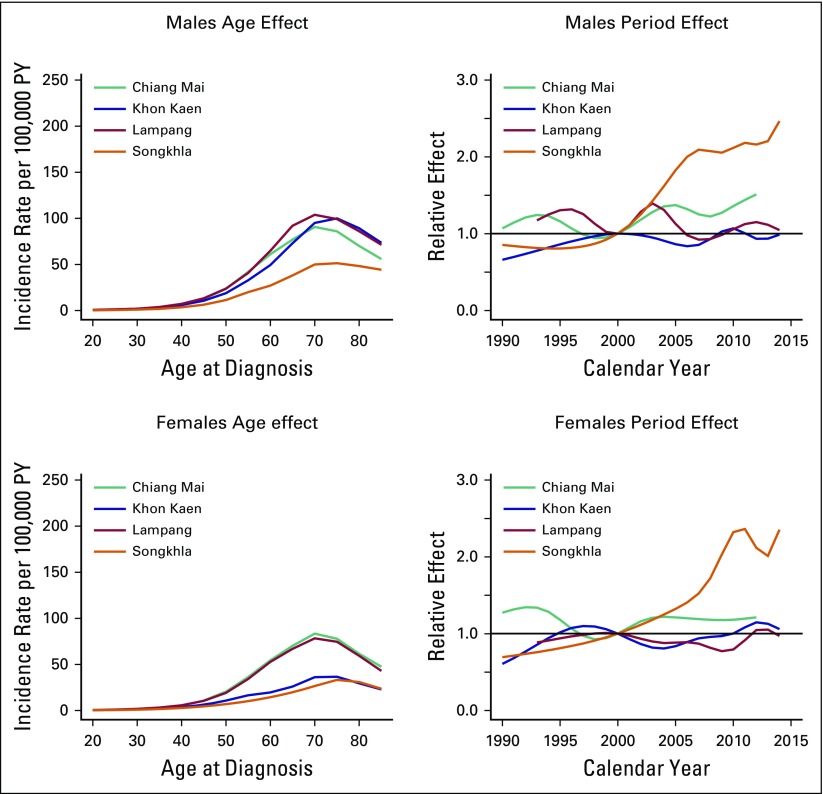
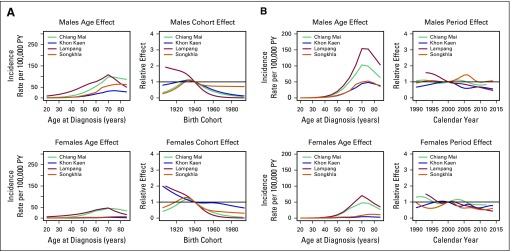
Age-Period-Cohort Analysis
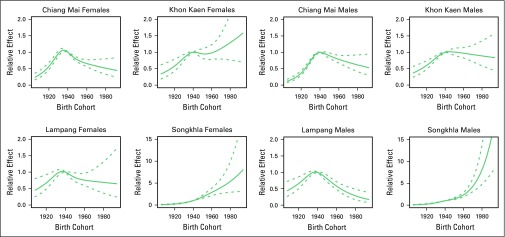
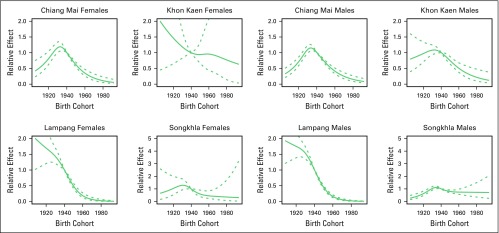
For males, the AC-P model, which gives predominance to cohort rather than to period, fit best for all registries except SCC in Khon Kaen and AdC in Songkhla. For females, the AC-P model fit best for AdC and SCC in Chiang Mai and Lampang (Appendix Table A4). Figures 2 and 3 show estimated age and cohort effects from the AC-P models and age and period effects from the AP-C models for AdC. Appendix Figures A2 and A3 shows similar values for SCC. The AdC period effects increased from 2000 to 2010 for both Chiang Mai and Songkhla males and females, whereas the effects were relatively flat for others. We also observed an increase in the cohort effects for Songkhla males and females, whereas others have remained constant. Conversely, for SCC, the cohort effects were lower in recent birth cohorts for both males and females in all regions. Appendix Figures A4 and A5 show birth cohort effects of AdC and SCC with 95% CIs by sex and registry.
Fig 2.
Age-period-cohort trend analysis fitted with period for adenocarcinoma in males and females. PY, person-years.
Fig 3.
Age-period-cohort trend analysis fitted with cohort for adenocarcinoma in males and females. PY, person-years.
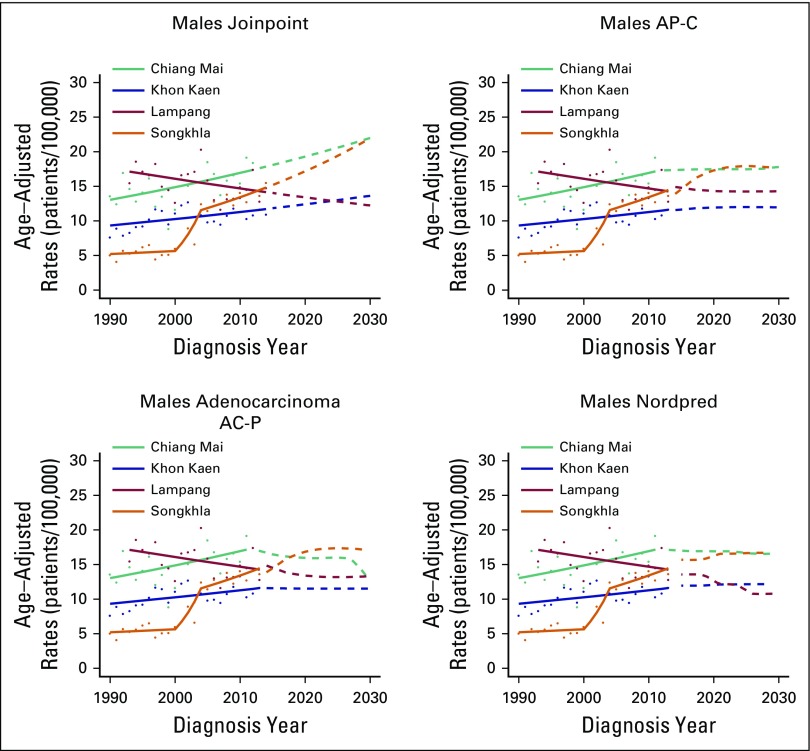
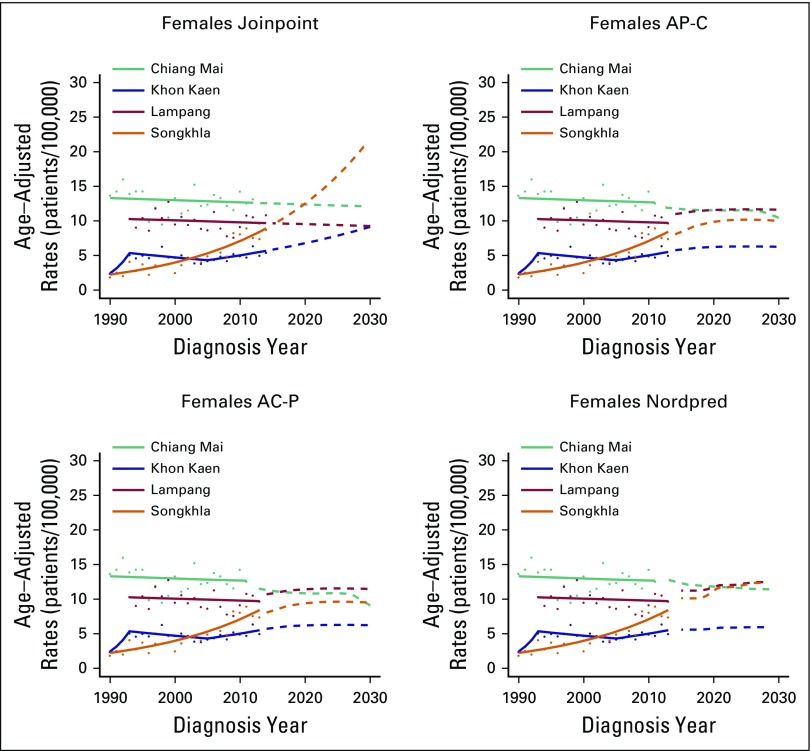
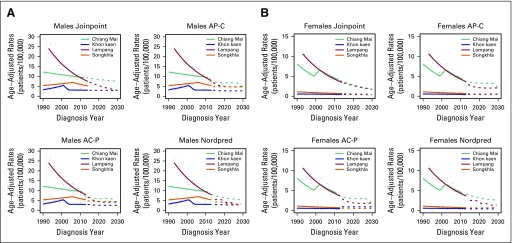
Projections
Figures 4 and 5 show projected incidence rates of AdC by region and sex. On the basis of the joinpoint approach for AdC, incidence rates are projected to increase to 20.89 patients per 100,000 for Chiang Mai males and to 20.59 and 19.25 per 100,000 for Songkhla males and females, respectively, in 2030. Both AP-C and AC-P models project that the incidence rates for Songkhla males in 2030 will reach to 17.6 and 17.1 patients per 100,000, respectively, and for Songkhla females to 10.0 and 9.6 per 100,000, respectively. The Nordpred model projects that incidence rates of AdC will continue to increase to 16.7 and 12.3 patients per 100,000 for Songkhla males and females, respectively. Conversely, the rates of SCC are projected to decrease over the years to reach fewer than five patients per 100,000 in 2030 for both males and females in all regions (Appendix Fig A6).
Fig 4.
Age-adjusted incidence rates of adenocarcinoma in males until 2030 using three projection models: Joinpoint, age-period-cohort, and Nordpred. The solid lines represent the model predictions, the dots the observed rates, and the dashed lines the model projections. AC-P, age-period-cohort trend analysis fitted with period; AP-C, age-period-cohort trend analysis fitted with cohort.
Fig 5.
Age-adjusted incidence rates of adenocarcinoma in females until 2030 using three projection models: Joinpoint, age-period-cohort, and Nordpred. The solid lines represent the model predictions, the dots the observed rates, and the dashed lines the model projections. AC-P, age-period-cohort trend analysis fitted with period; AP-C, age-period-cohort trend analysis fitted with cohort.
DISCUSSION
We investigated lung cancer incidence trends in Thailand by region, histology, and sex using joinpoint and age-period-cohort models. We also projected lung cancer rates until 2030 by using three approaches: joinpoint, age-period-cohort, and Nordpred. To our knowledge, this study is the first to examine lung cancer trends in Thailand by comparing incidence in multiple registries by histology. Overall, AdC rates were stable or increased moderately for both males and females in Chiang Mai and Lampang, whereas SCC, LCC, and SCLC rates generally have decreased. Similar patterns were observed in Songkhla and Khon Kaen but with larger increases for AdC. Projections suggest that the larger burden of AdC in Songkhla and lesser burden in Chiang Mai and Khon Kaen will continue until 2030.
The decrease in incidence of SCC, LCC, and SCLC can be partly explained by reductions in smoking. Since 1993, Thailand has implemented strong tobacco control policies, including taxation, packaging and labeling, advertising bans, and smoke-free public areas, which have resulted in a decrease in smoking prevalence from 32.0% in 1991 to 21.2% in 2007.4,26 However, smoking patterns vary by sex and region, which might explain some of the regional differences in cancer rates. According to the 2011 Thailand Global Adult Tobacco Survey, the south has the highest number of daily smokers (29.9%), and Bangkok has the lowest (18.1%).27 In males, smoking prevalence is highest in the south (a decrease from 60.9% in 1994 to 49.9% in 2007) and lowest in Bangkok (a decrease from 45.7% in 1991 to 26.9% in 2007). For females, smoking prevalence is highest in the north (a decrease from 12.5% in 1991 to 5% in 2007) and lowest in the northeast (a decrease from 2.1% in 1991 to 0.7% in 2007).28
Conversely, for AdC, we found increasing or nondecreasing trends across regions and sex. Similar patterns, particularly for females, have been found in some high-income Asian regions, including Osaka (Japan), Hong Kong, and Tianjin (China), and the underlying causes are the topic of much debate.29-34 With regard to trends by period and cohort, in Chiang Mai and Lampang, the trend of AdC was better explained by birth cohort effects, whereas in Khon Kaen and Songkhla, both period and birth cohort effects were important. Some of these differences also might be related to variations in smoking patterns by region as described in the Results. In addition, misclassification of HIV/AIDS-related malignancies as pulmonary cancers might have occurred in the 1990s as a result of the stigma associated with an HIV/AIDS diagnosis in Thailand.35 Chiang Mai and Lampang were more affected by the HIV/AIDS epidemic35; therefore, AdC incidence could have been artificially inflated in the 1990s and early 2000s in these provinces. Moreover, the increasing trends of AdC in Khon Kaen and Songkhla could be related to faster improvements in diagnosis and better case ascertainment36,37 and histologic classification in these registries as a result of histology-specific treatment guidelines38 because they are considered to have better data quality than the others.17-20
The relative shift from SCC, SCLC, and LCC to AdC may be explained by other factors. First, some researchers have hypothesized that the change in the composition of cigarettes to low-tar filtered cigarettes could change the prevalence of certain histologic types of lung cancer.39-42 With newer filtered cigarettes, smokers might have to inhale more deeply, which may lead to more peripheral tumors, such as AdC.43 Tobacco companies have increased the composition of tobacco-specific N-nitrosamines, which are AdC inducers, and decreased the polycyclic aromatic hydrocarbons.44 Future studies are needed to examine the composition of cigarettes used by the Thai population, especially with the increasing use of hand-rolled cigarettes.45,46
Second, there might be changes in exposure to environmental carcinogens. Studies have suggested that outdoor air pollution (particulate matter size of 2.5 μm [PM2.5] and PM10) is a relevant contributor to AdC risk.47 As Thailand undergoes globalization, we speculate that air pollution from vehicle emissions, biomass burning, and transboundary haze in rural and border areas could increase AdC incidence. Particularly, agricultural burning and forest fires in Chiang Mai have been major sources of high levels of PM10.48
This study has some limitations. First, we imputed missing histology using multiple imputation with available covariates in the registries (age, sex, year of diagnosis, and registry). Diagnostic analyses suggest that there is enough information in the available data to impute histology. However, other important variables that are correlated with histology, such as tumor stage and grade, were not available in all registries. Second, the age-period-cohort models have an inherent nonidentifiability issue that prevents the unique estimation of period or cohort effects. We resolved this problem by fitting models with either zero-slope period or zero-slope cohort effects.49 The relative fit of these models gives an assessment of whether period or cohort is better correlated with lung cancer incidence. Smoking is largely a cohort-related behavior,50 with most users beginning smoking as young adults and carrying out the behavior through adulthood. However, we cannot exclude the possibility of some underlying period-based trends as a result of improvements in registry quality with time and increasing awareness of the health hazards of smoking supported by the implementation of emerging tobacco control laws. Moreover, our conclusions and projections could be affected by uncertainty in the estimation of period or cohort trends, particularly for smaller registries like Khon Kaen (Appendix Figs A4 and A5). Third, the registry data do not have information on biomarkers51 or environmental and behavioral risk factors. Thus, examination of the causal relationship between these factors and observed lung cancer trends is difficult. Furthermore, many lung cancer diagnoses were extracted from death certificates in which histology information usually is not available. Although some patients’ tumor biopsy samples were available, pathologist misclassification of histology types is also possible, particularly as a result of the changes in diagnostic criteria for histopathologic diagnosis and coding practice in the past few decades, with a transition from classification of AdC and SCC as nonspecific NSCLC to more-specific codes.52,53
This study has several strengths. It is the first to our knowledge to examine the histology-specific lung cancer trends by age, period, and birth cohort in multiple regions in Thailand. The trend analysis was based on data from four population-based cancer registries, which have good quality with high completeness and validity (with the exception of histology).16 The age-period-cohort approach allowed us to examine the influence of age, period, and birth cohort on the changing trends of lung cancer incidence by histology. Moreover, we used three alternative projection approaches, which are based on different aspects of lung cancer incidence (age-adjusted rate, APC, and period and cohort trends). All projected consistent trends for lung cancer incidence by histology in future years.
Our projections can serve as a basis for assessing the future burden of lung cancer in Thailand and other LMICs. Given the current reductions in smoking prevalence, SCC incidence is expected to continue to decrease in the next decades (which is partially captured in our projections by the decreasing trends by birth cohort). Conversely, the AdC trends vary by region, either remaining constant or continuing to increase. Future studies should focus on characterizing the time-varying effect of smoking on lung cancer by histology, improving the projections by explicitly incorporating the relationship between smoking patterns and lung cancer rates,26,54 and understanding the effect of other nonsmoking lung cancer risk factors. The projected changes in histology distribution in future years will have major implications for prognosis and treatment options. In the United States, there are no differences in treatment options by histology for patients diagnosed with lung cancer at either stage I or stage II. However, for patients with either stage III or stage IV lung cancer, targeted therapy has been administered given their tumor histology.36,37 For instance, patients with AdC tend to have epidermal growth factor receptor mutations and anaplastic lymphoma kinase rearrangements, and treatments that target these genetic changes have been shown to improve patients’ prognosis.55 In Thailand, there seems to be a high prevalence of epidermal growth factor receptor in AdC tumors56; thus, additional increases in AdC rates, as projected here, could have important implications for resource and treatment planning in Thailand.
We found changes in lung cancer rates by histology in Thailand, with some differences by sex and geographic region. This highlights the need for regional surveillance systems for both risk factors and cancer to evaluate how nontobacco risk factors in synergy with cigarette smoking will shape future lung cancer rates in different areas. Lung cancer prevention and control policymakers should consider these dynamic patterns in lung cancer incidence when assessing potential interventions and planning health resource allocations.
Appendix
Cancer Registries
The Chiang Mai registry was established in 1983 and collects data on patients with cancer from all provincial hospitals in Chiang Mai province (International Association of Cancer Registries [IACR]: http://www.iacr.com.fr/index.php?option=com_comprofiler&task=userprofile&user=990&Itemid=498). The Lampang registry collects data from cancer centers and public and private hospitals in Lampang province in northern Thailand (IACR: http://www.iacr.com.fr/index.php?option=com_comprofiler&task=userprofile&user=992&Itemid=498). The Khon Kaen registry was established in 1985 at Khon Kaen University and collects data from all provincial hospitals (IACR: http://www.iacr.com.fr/index.php?option=com_comprofiler&task=userprofile&user=991&Itemid=498). The Songkhla registry is a population-based provincial registry located at the Prince of Songkla University in southern Thailand (IACR: http://www.iacr.com.fr/index.php?option=com_comprofiler&task=userprofile&user=994&Itemid=498). All registries provide data for the International Agency for Research on Cancer’s Cancer Incidence in Five Continents repository (Curado et al: IARC Sci Publ 2007).
Missing Histology Analysis
Missing histology was substantial across registries, with approximately 30% to 50%, depending on the registry. We thus conducted a descriptive analysis and evaluated Little’s missing completely at random test (Little RJA: J Am Stat Assoc 83:1198-1202, 1988) to explore whether the histology data are missing completely at random using the R package BaylorEdPsych. We then imputed the missing histology through the multiple imputation approach (using the R package MICE) using the available covariates in all registries: age, sex, year of diagnosis, and registry (Sriplung et al: BMC Cancer 16:389, 2016; R Development Core Team: http://www.R-project.org).
Our analysis shows that the distributions of age and year of diagnosis are different between patients with missing and nonmissing histology in all cancer registries (Table A5). In addition, the sex distribution is different only in the Chiang Mai and Khon Kaen registries.
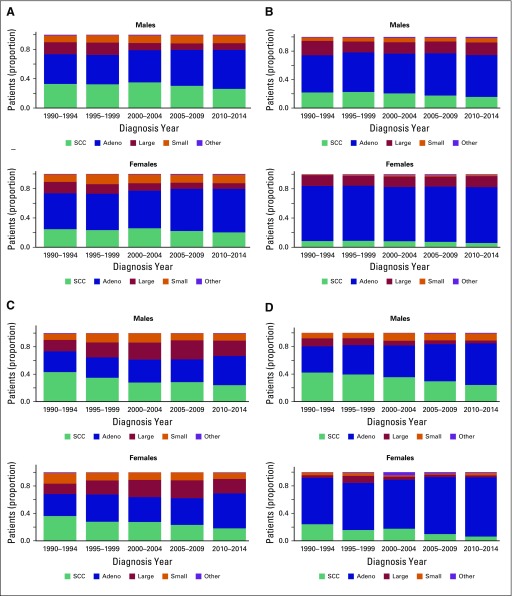
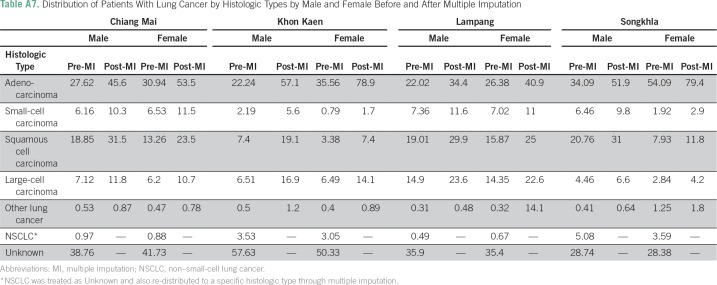
The results from the Little’s missing completely at random test indicate that the histology data are not missing completely at random in all cancer registries, which suggests that missing histology can be imputed (Table A6). Table A7 lists the distribution of patients with lung cancer by histology in each registry before and after imputation, and Figure A2 shows the distribution stratified by year of diagnosis in the imputed data. Pairwise parallel tests in joinpoint show that the trend of adenocarcinoma is similar among complete cases and imputed data for both males and females in all regions, except for Songkhla males. On the other hand, the trend of squamous cell carcinoma is similar between the two data sets only for Khon Kaen males and Lampang females and males (data not shown), although the long-term trends are consistent.
Fig A1.
Map of Thailand with four registries: Chiang Mai (red), Lampang (black), Khon Kaen (blue), and Songkhla (green).
Fig A2.
Distribution of patients with lung cancer by histologic type over diagnosis year for males and females in all registries. (A) Chiang Mai. (B) Khon Kaen. (C) Lampang. (D) Songkhla. Adeno, adenocarcinoma; Large, Large-cell carcinoma; Other, other histology; SCC, squamous cell carcinoma; Small, small-cell carcinoma.
Fig A3.
Age-period-cohort trend analysis for squamous cell carcinoma in males and females. (A) Age-period-cohort model fitted with period. (B) Age-period-cohort model fitted with cohort. PY, person-years.
Fig A4.
Cohort effect of adenocarcinoma and 95% CIs by sex and registry. The solid line represents cohort effect, and the dashed lines represent 95% CIs.
Fig A5.
Cohort effect of squamous cell carcinoma and 95% CIs by sex and registry. The solid line represents cohort effect, and the dashed lines represent 95% CIs.
Fig A6.
Age-adjusted incidence rates of squamous cell carcinoma until 2030 using three projection models: joinpoint analysis, age-period-cohort, and Nordpred by sex. (A) Males. (B) Females. The solid lines represent the model predictions, the dots the observed rates, and the dashed lines the model projections. AC-P, age-period-cohort fitted with period; AP-C, age-period-cohort fitted with cohort.
Table A1.
APC and AAPC in Age-Adjusted Incidence in All Histology, AdC, and SCC
Table A2.
APC and AAPC in Age-Adjusted Incidence in Small-Cell Carcinoma, Large-Cell Carcinoma, and Other Histology
Table A3.
Complete Patient Analysis of APC and AAPC in Age-Adjusted Incidence of All Histology, AdC, and SCC
Table A4.
Akaike Information Criterion Values for the AP-C, AC-P, and APC models
Table A5.
Descriptive Characteristics of Variables in Four Cancer Registries in Thailand
Table A6.
Summary of the Little’s MCAR Test for Missing Data on Histology in Four Cancer Registries in Thailand
Table A7.
Distribution of Patients With Lung Cancer by Histologic Types by Male and Female Before and After Multiple Imputation
Footnotes
Supported by the National Science and Technology Development Agency (P-10-10307 to H.S.) and the University of Michigan Center for South East Asian Studies (to J.J. and R.M.).
AUTHOR CONTRIBUTIONS
Conception and design: Joanne T. Chang, Hutcha Sriplung, Seesai Yeesoonsang, Rafael Meza
Financial support: Karnchana Daoprasert, Rafael Meza
Administrative support: Surichai Bilheem, Karnchana Daoprasert, Rafael Meza
Provision of study materials or patients: Hutcha Sriplung
Collection and assembly of data: Joanne T. Chang, Hutcha Sriplung, Surichai Bilheem, Imjai Chitapanarux, Donsuk Pongnikorn, Karnchana Daoprasert, Patravoot Vatanasapt, Krittika Suwanrungruang, Rafael Meza
Data analysis and interpretation: Joanne T. Chang, Jihyoun Jeon, Hutcha Sriplung, Seesai Yeesoonsang, Laura Rozek, Donsuk Pongnikorn, Rafael Meza
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Joanne T. Chang
No relationship to disclose
Jihyoun Jeon
No relationship to disclose
Hutcha Sriplung
No relationship to disclose
Seesai Yeesoonsang
No relationship to disclose
Surichai Bilheem
No relationship to disclose
Laura Rozek
No relationship to disclose
Imjai Chitapanarux
No relationship to disclose
Donsuk Pongnikorn
No relationship to disclose
Karnchana Daoprasert
No relationship to disclose
Patravoot Vatanasapt
No relationship to disclose
Krittika Suwanrungruang
No relationship to disclose
Rafael Meza
No relationship to disclose
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Eriksen M, Mackay J, Schluger NW, et al. The Tobacco Atlas. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 3.Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 4.WHO Prevalence of tobacco use in adults, Thailand. http://www.searo.who.int/thailand/areas/tobaccoprevalence/en
- 5.WHO . WHO Report on the Global Tobacco Epidemic, 2015: Country Profile Thailand. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 6.Khuhaprema T, Attasara P, Sriplung H, et al. Cancer in Thailand Volume VII, 2007-2009. 2013 http://www.nci.go.th/th/File_download/Nci Cancer Registry/Cancer in thailand_VII.pdf
- 7.Pesch B, Kendzia B, Gustavsson P, et al. Cigarette smoking and lung cancer--relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer. 2012;131:1210–1219. doi: 10.1002/ijc.27339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynder EL, Muscat JE. The changing epidemiology of smoking and lung cancer histology. Environ Health Perspect. 1995;103:143–148. doi: 10.1289/ehp.95103s8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stellman SD, Muscat JE, Thompson S, et al. Risk of squamous cell carcinoma and adenocarcinoma of the lung in relation to lifetime filter cigarette smoking. Cancer. 1997;80:382–388. doi: 10.1002/(sici)1097-0142(19970801)80:3<382::aid-cncr5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services The Health consequences of smoking—50 years of progress: A report of the Surgeon General, 2014. https://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html
- 11.Ko YC, Cheng LS, Lee CH, et al. Chinese food cooking and lung cancer in women nonsmokers. Am J Epidemiol. 2000;151:140–147. doi: 10.1093/oxfordjournals.aje.a010181. [DOI] [PubMed] [Google Scholar]
- 12.Kirsch-Volders M, Bonassi S, Herceg Z, et al. Gender-related differences in response to mutagens and carcinogens. Mutagenesis. 2010;25:213–221. doi: 10.1093/mutage/geq008. [DOI] [PubMed] [Google Scholar]
- 13.Weiss JM, Lacey JV, Jr, Shu XO, et al. Menstrual and reproductive factors in association with lung cancer in female lifetime nonsmokers. Am J Epidemiol. 2008;168:1319–1325. doi: 10.1093/aje/kwn257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam WK, White NW, Chan-Yeung MM. Lung cancer epidemiology and risk factors in Asia and Africa. Int J Tuberc Lung Dis. 2004;8:1045–1057. [PubMed] [Google Scholar]
- 15.Alberg AJ, Ford JG, Samet JM, et al. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 16.Suwanrungruang K, Sriplung H, Attasara P, et al. Quality of case ascertainment in cancer registries: A proposal for a virtual three-source capture-recapture technique. Asian Pac J Cancer Prev. 2011;12:173–178. [PubMed] [Google Scholar]
- 17.National Statistical Office 2000 Population and Housing Census. Bangkok. 2002 http://web.nso.go.th/en/census/poph/cen_poph.htm
- 18.National Statistical Office 2010 Population and Housing Census. Bangkok. 2012 http://web.nso.go.th/en/census/poph/2010/cen_poph_10_bkk.htm
- 19.Office of the National Economic and Social Development Board . Population Projections for Thailand 2010-2040. Bangkok, Thailand: Office of the National Economic and Social Development Board; 2013. [Google Scholar]
- 20.Bray F, Guilloux A, Sankila R, et al. Practical implications of imposing a new world standard population. Cancer Causes Control. 2002;13:175–182. doi: 10.1023/a:1014344519276. [DOI] [PubMed] [Google Scholar]
- 21. National Cancer Institute Division of Cancer Control & Population Sciences: Joinpoint Regression Program version 4.1.1.4, released February 19, 2015 (software program). Bethesda, MD, National Cancer Institute, 2015.
- 22.Holford TR, Zhang Z, McKay LA. Estimating age, period and cohort effects using the multistage model for cancer. Stat Med. 1994;13:23–41. doi: 10.1002/sim.4780130105. [DOI] [PubMed] [Google Scholar]
- 23.R Development Core Team The R Project for Statistical Computing. http://www.R-project.org
- 24.Møller B, Fekjaer H, Hakulinen T, et al. Prediction of cancer incidence in the Nordic countries: Empirical comparison of different approaches. Stat Med. 2003;22:2751–2766. doi: 10.1002/sim.1481. [DOI] [PubMed] [Google Scholar]
- 25.Mistry M, Parkin DM, Ahmad AS, et al. Cancer incidence in the United Kingdom: Projections to the year 2030. Br J Cancer. 2011;105:1795–1803. doi: 10.1038/bjc.2011.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy DT, Benjakul S, Ross H, et al. The role of tobacco control policies in reducing smoking and deaths in a middle income nation: Results from the Thailand SimSmoke simulation model. Tob Control. 2008;17:53–59. doi: 10.1136/tc.2007.022319. [DOI] [PubMed] [Google Scholar]
- 27.Ministry of Public Health . Executive summary: Global Adult Tobacco Survey: 2011 GATS, Thailand. Bangkok, Thailand: Thammasat University Press; 2012. [Google Scholar]
- 28.Tobacco Control Research and Knowledge Management Center . Thailand Tobacco Control Policies. Bangkok, Thailand: Tobacco Control Research and Knowledge Management Center; 2008. [Google Scholar]
- 29.Kinoshita FL, Ito Y, Nakayama T. Trends in lung cancer incidence rates by histological type in 1975-2008: A population-based study in Osaka, Japan. J Epidemiol. 2016;26:579–586. doi: 10.2188/jea.JE20150257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong J, Xu F, He M, et al. The incidence of lung cancer by histological type: A population-based study in Tianjin, China during 1981-2005. Respirology. 2014;19:1222–1228. doi: 10.1111/resp.12373. [DOI] [PubMed] [Google Scholar]
- 31.Au JSK, Mang OWK, Foo W, et al. Time trends of lung cancer incidence by histologic types and smoking prevalence in Hong Kong 1983-2000. Lung Cancer. 2004;45:143–152. doi: 10.1016/j.lungcan.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Meza R, Meernik C, Jeon J, et al. Lung cancer incidence trends by gender, race and histology in the United States, 1973-2010. PLoS One. 2015;10:e0121323. doi: 10.1371/journal.pone.0121323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen KY, Chang CH, Yu CJ, et al. Distribution according to histologic type and outcome by gender and age group in Taiwanese patients with lung carcinoma. Cancer. 2005;103:2566–2574. doi: 10.1002/cncr.21087. [DOI] [PubMed] [Google Scholar]
- 34.Park JY, Jang SH. Epidemiology of lung cancer in Korea: Recent trends. Tuberc Respir Dis (Seoul) 2016;79:58–69. doi: 10.4046/trd.2016.79.2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sriplung H, Parkin DM. Trends in the incidence of acquired immunodeficiency syndrome-related malignancies in Thailand. Cancer. 2004;101:2660–2666. doi: 10.1002/cncr.20622. [DOI] [PubMed] [Google Scholar]
- 36.Socinski MA, Evans T, Gettinger S, et al. Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e341S–e368S. doi: 10.1378/chest.12-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e314A–e340S. doi: 10.1378/chest.12-2360. [DOI] [PubMed] [Google Scholar]
- 38.Kulesza P, Ramchandran K, Patel JD. Emerging concepts in the pathology and molecular biology of advanced non-small cell lung cancer. Am J Clin Pathol. 2011;136:228–238. doi: 10.1309/AJCPO66OIRULFNLZ. [DOI] [PubMed] [Google Scholar]
- 39.Thun MJ, Hannan LM, Adams-Campbell LL, et al. Lung cancer occurrence in never-smokers: An analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5:e185. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen F, Cole P, Bina WF. Time trend and geographic patterns of lung adenocarcinoma in the United States, 1973-2002. Cancer Epidemiol Biomarkers Prev. 2007;16:2724–2729. doi: 10.1158/1055-9965.EPI-07-0455. [DOI] [PubMed] [Google Scholar]
- 41.Wen W, Shu XO, Gao YT, et al. Environmental tobacco smoke and mortality in Chinese women who have never smoked: Prospective cohort study. BMJ. 2006;333:376. doi: 10.1136/bmj.38834.522894.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devesa SS, Bray F, Vizcaino AP, et al. International lung cancer trends by histologic type: Male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–299. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 43.Burns DM, Anderson CM, Gray N. Do changes in cigarette design influence the rise in adenocarcinoma of the lung? Cancer Causes Control. 2011;22:13–22. doi: 10.1007/s10552-010-9660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hecht SS. Cigarette smoking and lung cancer: Chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3:461–469. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- 45.Young D, Yong HH, Borland R, et al. Prevalence and correlates of roll-your-own smoking in Thailand and Malaysia: Findings of the ITC-South East Asia Survey. Nicotine Tob Res. 2008;10:907–915. doi: 10.1080/14622200802027172. [DOI] [PubMed] [Google Scholar]
- 46.Sirichotiratana N, Techatraisakdi C, Rahman K, et al. Prevalence of smoking and other smoking-related behaviors reported by the Global Youth Tobacco Survey (GYTS) in Thailand. BMC Public Health. 2008;8(suppl 1):S3. doi: 10.1186/1471-2458-8-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raaschou-Nielsen O, Andersen ZJ, Beelen R, et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) Lancet Oncol. 2013;14:813–822. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 48.Vichit-Vadakan N, Vajanapoom N. Health impact from air pollution in Thailand: Current and future challenges. Environ Health Perspect. 2011;119:A197–A198. doi: 10.1289/ehp.1103728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carstensen B, Plummer M, Esa L, et al. Epi: A Package for Statistical Analysis in Epidemiology. R package version 1.1.67. https://CRAN.R-project.org/package=Epi
- 50.Holford TR, Levy DT, McKay LA, et al. Patterns of birth cohort-specific smoking histories, 1965-2009. Am J Prev Med. 2014;46:e31–e37. doi: 10.1016/j.amepre.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Virani S, Sriplung H, Rozek LS, et al. Escalating burden of breast cancer in southern Thailand: Analysis of 1990-2010 incidence and prediction of future trends. Cancer Epidemiol. 2014;38:235–243. doi: 10.1016/j.canep.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Thompson CA, Waldhör T, Schernhammer ES, et al. Smoking and lung cancer: Current trends in Austria. Wien Klin Wochenschr. 2012;124:493–499. doi: 10.1007/s00508-012-0207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu M, Feuer EJ, Cronin KA, et al. Use of multiple imputation to correct for bias in lung cancer incidence trends by histologic subtype. Cancer Epidemiol Biomarkers Prev. 2014;23:1546–1558. doi: 10.1158/1055-9965.EPI-14-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moolgavkar SH, Holford TR, Levy DT, et al. Impact of reduced tobacco smoking on lung cancer mortality in the United States during 1975-2000. J Natl Cancer Inst. 2012;104:541–548. doi: 10.1093/jnci/djs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: Current standards and the promise of the future. Transl Lung Cancer Res. 2015;4:36–54. doi: 10.3978/j.issn.2218-6751.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sriuranpong V, Chantranuwat C, Huapai N, et al. High frequency of mutation of epidermal growth factor receptor in lung adenocarcinoma in Thailand. Cancer Lett. 2006;239:292–297. doi: 10.1016/j.canlet.2005.08.029. [DOI] [PubMed] [Google Scholar]