Abstract
Purpose
Middle-income countries like Brazil often have a dichotomous health care system in which patients may be treated in either public or private institutions that differ substantially in terms of level of access to diagnostic and therapeutic procedures.
Patients and Methods
This was a prospective, observational study to assess real-world data in 1,230 female patients with breast cancer who were treated in a private health care institution between 2012 and 2016 in Brazil.
Results
Breast cancer in these patients mostly was diagnosed at early (79.0% stages I or II) or locally advanced (16.1% stage III) stages. The primary tumor was resected in 89.0% of cases, most often through breast-conserving surgery (55.1%). Patients with locally advanced disease received more aggressive therapy (eg, higher rates of mastectomy, axillary dissection and chemotherapy use) than patients with early-stage disease. The estimated 2-year overall survival (OS) was 95.3%. Survival was significantly longer among patients with stage I or II disease (2-year OS, 97.9% and 97.5%, respectively) than those with stage III or IV disease (89.4% and 69.5%, respectively; P < .01). Tumor grade was also correlated with OS in the overall cohort (P = .05); triple-negative status was only prognostic for patients with stage III disease (P < .01).
Conclusion
The data provided aid understanding of the current scenario of breast cancer presentation and treatment in the Brazilian private health care system and may serve as a foundation to guide resource allocation. Our results reinforce the need to pursue adequate access to cancer care in low- and middle-income countries to optimize patient outcome.
INTRODUCTION
Breast cancer is the most common malignancy in women worldwide, accounting for 1.67 million new cases in 2012 (25% of all cancers).1 It is also a leading cause of cancer death, with an estimate of 522,000 deaths a year, 70% of which occur in low- and middle-income countries. In Brazil, the National Cancer Institute estimated a total of 206,000 new cancer cases among women in 2016, 28% of which (57,960) are primary breast malignancies.2 These numbers represent an incidence rate of 56 new cases per 100,000 women per year. The mortality rate in Brazil was estimated at 14.7 per 100,000 women in 2014, with a total of 14,622 deaths in the same year.3 It is estimated that 369,160 years of life lost were due to breast cancer in the country in 2014. The projections for 2017 are 392,356 years of life lost and 445,859 disability-adjusted life-years lost due to breast cancer.4,5
The Brazilian health care system is divided into private and public coverage.6 Approximately 23% of individuals have access to the private health system through health plans and insurances,7,8 which are either self-funded or provided by an employer. There is a higher concentration of health-insured individuals in the south and southeastern regions of the country.7 The dichotomy in health care highlights a substantial gap in terms of level of access to diagnostic and therapeutic procedures.9-11 Patients with private coverage often have access to international standards of care, similar to what is exercised in developed countries and supported by international guidelines.12 However, there is a lack of high-quality epidemiologic studies to address outcome data in this setting.
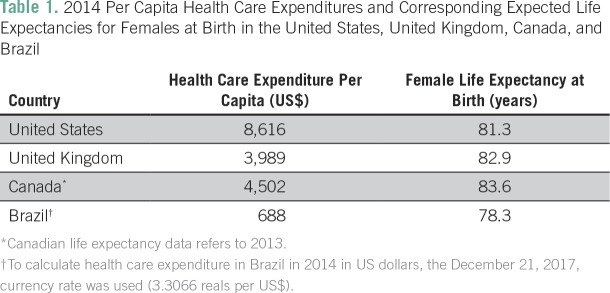
Although patients with private health insurance have access to most of the new technologies, the model of care in the private system is completely fragmented, which may lead to significant inefficiency and, consequently, to clinical outcomes that are not as good as one could predict and expect. Another critical point is that being a middle-income country, Brazil has fewer financial resources than high-income countries that are often used as references in terms of access and health care models. In the Brazilian private health care system, a private health insurance expenditure of US$1,100 per person per year is projected for 2017,13,14 with an additional out-of-pocket expenditure of US$46 billion dollars for the whole population. If we assume that the out-of-pocket spending has a distribution similar to the one that exists between the public and the private health insurance markets, the final values of health care expenditure per capita would be US$659 and US$1,691 in the public and private systems, respectively. The per capita health care spending projections for the United States, United Kingdom, and Canada for 2017 are US$10,800, US$4,512, and US$4,861, respectively.15-19 The critical point is that although the United Kingdom and Canada spend less money per capita to deliver health care, the life expectancy at birth is higher in these two countries than in the United States, which points to the question about the efficiency of different health care systems, especially the US system. Table 1 summarizes the 2014 per capita health care expenditures and the corresponding expected life expectancies for females at birth for those three countries and Brazil.17,20
Table 1.
2014 Per Capita Health Care Expenditures and Corresponding Expected Life Expectancies for Females at Birth in the United States, United Kingdom, Canada, and Brazil
It is critical to measure and understand the clinical benefit derived from new technologies and practices in the community setting to prioritize what should be adopted. Meaningful clinical outcomes, life expectancy, quality of life, and well-being are derived from a complex combination of access, efficiency, personal values, and comfort. In July 2012, COI Institute initiated a prospective study to assess real-world data from patients with breast cancer who were treated in a private health care institution in Brazil. The primary aim was to understand the value of the care being delivered to women with breast cancer who receive treatment and advice in our institution. Value can be described and evaluated as the clinical output derived from the financial input. The way we use our financial and human resources defines the efficiency of our care. This article focuses on the measurement and description of the clinical outcomes, as well as baseline characteristics of the patients and treatment choices. Herein, we present the first report of this study.
PATIENTS AND METHODS
Study Design
This is a prospective, observational study of patients with breast cancer treated in a private cancer care institution that comprises six units in Rio de Janeiro and surroundings. In June 2017, the number of citizens with private health insurance in the Rio de Janeiro metropolitan area, the main region our patients come from, was 4.4 million. Approximately 5,800 new patients are seen each year at Americas Oncologia, one-fourth of whom have breast cancer. Most cases are covered by one of the 87 health plans affiliated with Americas Oncologia. Approximately 33% of the new patients with breast cancer are referred to our service to receive only adjuvant radiotherapy (internal data base, 2017 data).
Eligible patients were at least 18 years old and had a histology-proven diagnosis of breast cancer between July 2012 and November 2016. For this analysis, only female patients with an invasive breast cancer were included. Patients with prior malignancies in the 5 years preceding the diagnosis of breast cancer (except for nonmelanoma skin cancer and cervical cancer) and patients who received first therapy (except for surgery) in other institutions were excluded. These criteria minimize the risk of selection biases that could affect the prognosis.
For data collection, an electronic clinical research form was created, including characteristics such as age, menopausal status, histologic subtype, staging at diagnosis, hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status, type of therapy, date of progression or recurrence, and date of death. The staging was defined according to the sixth edition of the American Joint Committee on Cancer.21 A subset of patients consented to respond to quality-of-life questionnaires, which will be the subject of a separate paper. The research form in this subset included some exclusive clinical data such as type of axillary approach (ie, sentinel lymph node and axillary dissection). When patients did not attend regular follow-up appointments or when clinical data were not found in medical records, patients or relatives were contacted by telephone to ensure that all information was annotated. Data quality was certified by regular monitoring. This study was approved by the local research ethics committee.
Statistical Methods
Overall survival (OS) was estimated using the Kaplan-Meier method and defined as the interval between date of diagnosis and death. For patients still alive or lost to follow-up, data were censored at the date of last contact. Survival outcomes are described at the 2-year time point. Variable analysis for survival was performed using the log-rank method and considered the following variables: age, staging, HR and HER2 status, tumor grade, menopausal status, and histologic subtype. Fisher exact test was used to compare the proportion of baseline characteristics (eg, type of surgery, axillary approach, and chemotherapy) according to disease stage. P ≤ .05 was considered statistically significant. Statistical analyses were performed using the statistical software SPSS, version 22.0 (IBM, Armonk, NY).
RESULTS
Baseline Characteristics
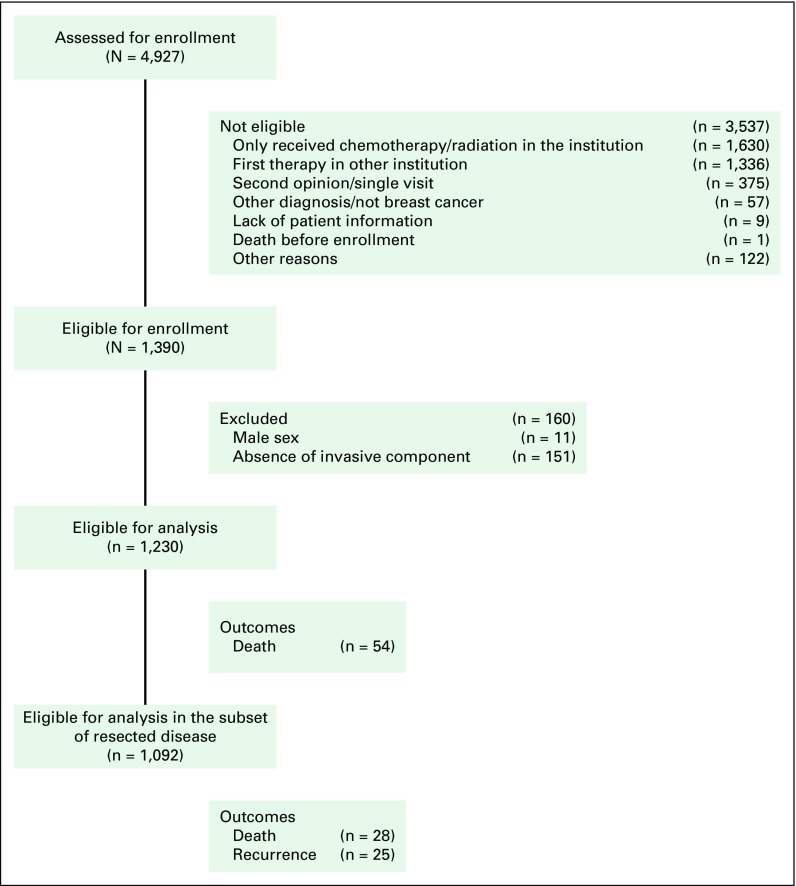
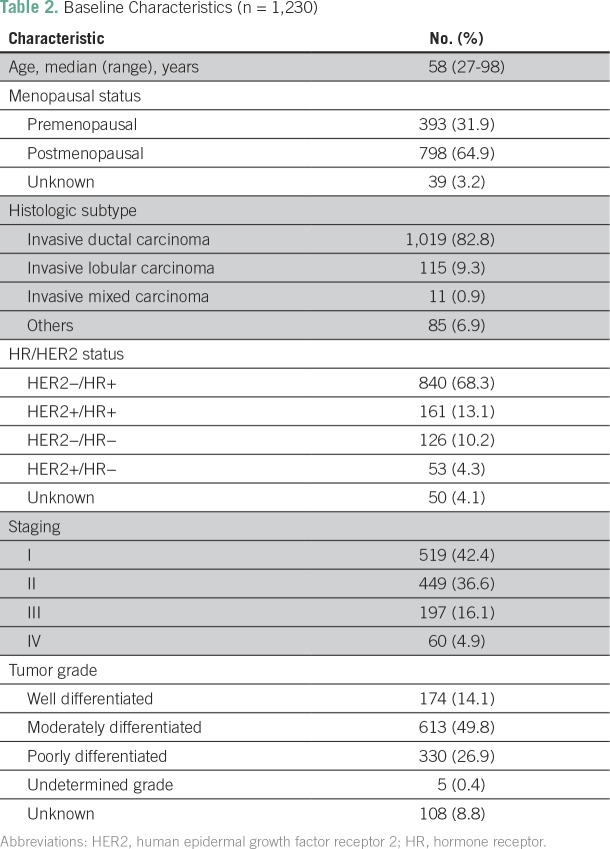
A total of 1,390 patients were enrolled (Fig 1), of whom 160 were excluded from this analysis (n = 11 due to male sex and n = 151 with exclusively in situ carcinoma). The final report comprises 1,230 female patients. The baseline characteristics are listed in Table 2 and reflect a cohort with predominantly early-stage (79.0% stages I or II) or locally advanced-stage (16.1% stage III) disease.
Fig 1.
Flow diagram of patient enrollment and analysis.
Table 2.
Baseline Characteristics (n = 1,230)
Treatment
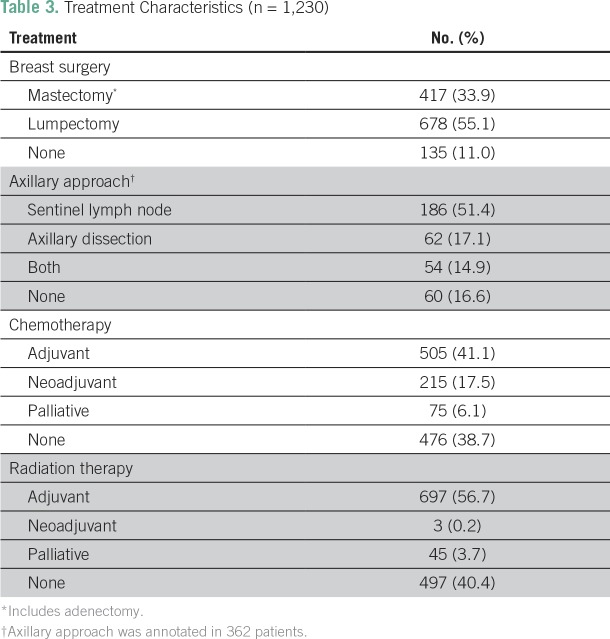
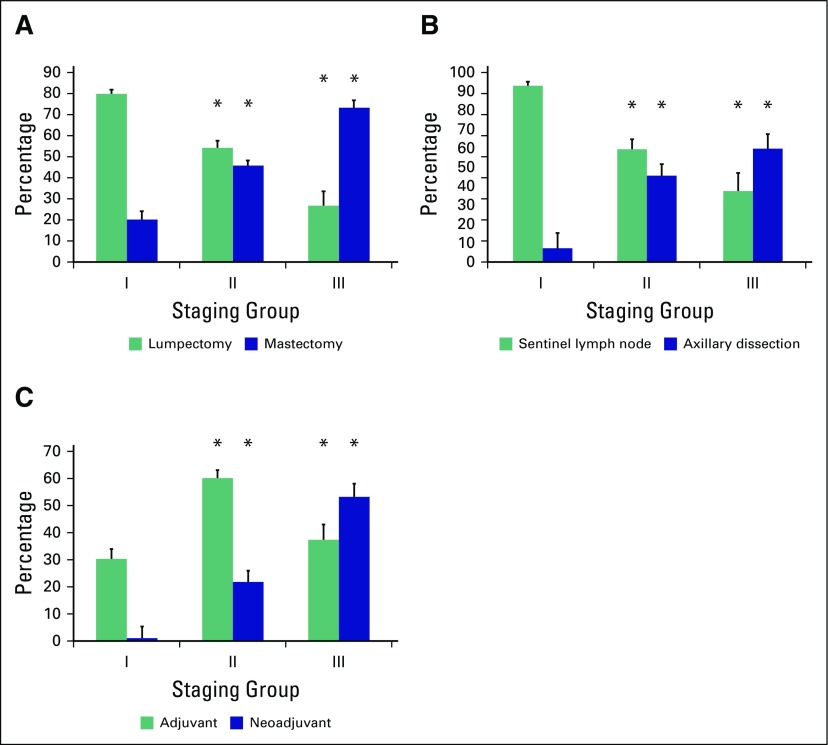
Treatment characteristics are listed in Table 3. The primary tumor was resected in 89.0% of cases, most often through breast-conserving surgery (55.1%). Axillary lymph nodes were assessed in 83.4% of cases, with 32.0% requiring complete axillary dissection. Chemotherapy was used in 61.3% of cases and radiation therapy in 59.6%. Patients with locally advanced disease received more aggressive therapy than did patients with early-stage disease (Fig 2). Among patients with early-stage disease, breast-conserving surgery was used more often than mastectomy (79.8% v 20.2% at stage I; 54.2% v 45.8% at stage II), whereas an opposite ratio was observed at stage III (26.8% v 73.2%, respectively). Sentinel lymph node was assessed in 92.9% of patients at stage I, 59.4% at stage II, and 37.3% at stage III. However, axillary dissection was recommended in only 7.1% of patients at stage I, but in 45.4% and 59.7% at stages II and III, respectively (P < .01). Patients with stage III disease also received more neoadjuvant chemotherapy (52.8%) than early stages (1.0% and 21.6% at stages I and II, respectively; P < .01). In contrast, adjuvant chemotherapy was recommended in 30.0%, 59.7%, and 37.1% of patients with stage I, II, and III disease, respectively.
Table 3.
Treatment Characteristics (n = 1,230)
Fig 2.
Percentage of patients by (A) type of surgery, (B) axillary approach, and (C) chemotherapy use according to disease stage. Patients with locally advanced disease (stage III) received more aggressive therapy than patients with early stage (stages I-II). (*)P ≤ .05; comparisons were made using each variable at stage I as the reference.
Outcomes
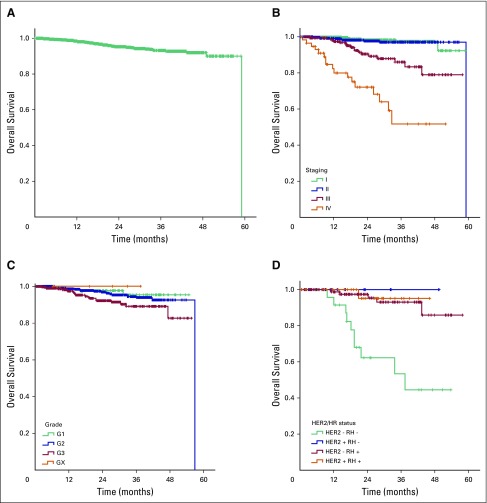
After a median follow-up of 22.5 months (95% CI, 21.09 to 23.90), 54 deaths (4.4%) were reported. The estimated 2-year OS was 95.3%, and median OS was not reached (Fig 3A). OS was significantly longer among patients with stage I or II disease (2-year OS, 97.9% and 97.5%, respectively) than those with stage III and IV disease (89.4% and 69.5%, respectively; P < .01; Fig 3B). Tumor grade was also correlated to OS in the overall cohort (P = .05; Fig 3C), whereas triple-negative status was only prognostic at stage III (P < .01; Fig 3D), because no events were detected in triple-negative cases at early stages. Age (P = .10), menopausal status (P = .74), and histologic subtype (P = .55) were not correlated with OS.
Fig 3.
Overall survival in the (A) total cohort, according to (B) disease stage, (C) grade of differentiation, and (D) HER2/HR status in locally advanced disease (stage III). HER2, human epidermal growth factor receptor 2; HR, hormone receptor.
Among patients with resected tumors, stages I through III (n = 1,092), 25 recurrences (2.3%) and 28 deaths (2.6%) were reported. In this analysis, tumor stage and HER2/HR status were significantly correlated with OS (P < .01 in both). Again, HER2/HR was a strong prognostic factor at stage III (P < .01). The 2-year disease-free survival was 95.6% at stages I and II and 67.0% at stage III (P < .01).
DISCUSSION
This study provides a unique data set of patients with breast cancer, with prospective data collection and follow-up, who were treated in the private health care system in Brazil. To our knowledge, this is the largest, most comprehensive, and best annotated study in this setting to date. Prior studies on breast cancer outcome were based on national22-25 or retrospective (chart review) institutional9,11,26,27 data sets. Each of these methods has important limitations that may invariably lead to bias, including, but not limited to, data absence or inaccuracy. Moreover, institutional data are most often generated from public or academic centers, which illustrates the lack of data from private care.
In our data set, most patients received their diagnosis at early or locally advanced stages of disease, which is presumably an advantage of private care. In a retrospective study published in 2014,11 data from 4,912 patients treated in different regions of Brazil were reviewed. The authors demonstrated that patients treated in private care more often presented with less advanced-stage disease, a strong factor of favorable prognosis.11 This assumption is further supported by a review of 87,969 Brazilian women with breast cancer using hospital cancer registries, mostly comprising public hospitals.24 In that series, only 58.9% presented with stage I or II disease, reflecting the later stages at diagnosis in the general population.24 These data highlight the enormous disparities in cancer care seen in middle-income countries like Brazil. The fact that most patients in our cohort were capable of receiving prompt diagnosis, coupled with proper and timely treatment, emphasizes the importance of access to care.
Our results support the knowledge that insured patients in middle-income countries are getting the same treatments and outcomes as patients treated in high-income nations, which is mostly a matter of access and efficiency. Treatment performed in our cohort reflects international recommendations12 and is in agreement with data from developed countries,16 with more aggressive interventions used at advanced stages.28 Most cases were surgically resected, which is in line with data reported by Leidke et al,11 but is in clear contrast to that of Medeiros et al,25 which were based on a hospital cancer registry. Because the Leidke et al study11 and the current study were carried out in reference cancer institutions, both studies may not adequately reflect the scenario in general hospitals throughout the country. Importantly, most breast procedures in our cohort were breast conserving, as opposed to data from Leidke et al.11 Our frequency of complete axillary dissection was also considerably lower than in the Leidke et al study,11 supporting the notion of earlier diagnosis and less aggressive therapy in the private care. The difference in results coming from the mentioned studies reflects the problem of access and efficiency that exist in heterogeneous, unequal, and continental countries like Brazil.
The outcomes reported herein illustrate the favorable prognosis when patients are diagnosed at early stages of breast cancer. The 2-year survival was similar to that described both in national11 and international series.16 Importantly, despite the easier access to therapeutic options, patients in the private care did not receive more therapy. On the contrary, most patients in our series received less aggressive surgery as well as less chemotherapy than indicated from data from those receiving public care, and the former had favorable survival and disease control. Altogether, our data emphasize the importance of early access and proper therapy to achieve better outcome.
This study provides data on the frequency of breast cancer subtypes according to HR and HER2 positivity. Due to limited access to adequate testing in the public health care (for HER2) and data annotation in prior retrospective studies, this information is missing in most published data sets in Brazil. In the Leidke et al study,11 the authors often relied on the annotation of hormone therapy to report HR status, which may have led to recall bias. Moreover, HER2 data were missing for 41% of patients in that study11 (21% in the subset of private care). To our knowledge, there is no comparison of triple-negative cases between private and public institutions in Brazil.
Several factors may have contributed to generate quality data in our study. The prospective data collection enabled real-time access to patients and physicians, thus minimizing recall bias and avoiding missing relevant data that could cause selection bias. Other important factors include the active search to minimize missing data and regular data monitoring to ensure adequate information. Indeed, the frequency of variables with missing data were relatively small. Nonetheless, it is still possible that relevant data have been missed. For instance, treatment applied in other institutions may not have been adequately annotated. The health plan coverage was considerably heterogeneous in this cohort, which makes our data set likely generalizable to the whole population with private health care coverage in Brazil and perhaps in other high- to middle-income countries. On the other hand, because Rio de Janeiro is one of the largest cities in the country, more specialized health care is often available. The patient characteristics and outcomes might not be the same in less-specialized centers in smaller cities.
Among the potential limitations to this study is the lack of a comparative group from public health care. However, there is no prospective collection of data performed in the public setting that could parallel the standards applied herein. For this reason, any formal comparison would have been biased and inferences could be unreliable. The follow-up time was relatively short to observe enough events in a cohort of predominantly HR-positive and with early-stage disease. As a prospective and ongoing study, data will be revisited to report updated survival within disease stages. There is also a need to deepen the analysis of specific procedures used and evaluate cost and impact on quality of life according to therapeutic interventions. Many interventions have a greater impact in advanced disease; therefore, more data will need to be collected from patients with stages III and IV disease to define which therapies are resulting in a meaningful benefit to patient outcome and quality of life. These aspects will be in the scope of future research on this data base. Despite the report of early diagnosis in this data set, the rate of diagnosis based on mammogram screening is not available.
In summary, our results reinforce the need to pursue adequate access to cancer care in middle-income countries like Brazil. Early diagnosis and adequate breast cancer treatment may lead to outcomes that are favorably similar to those described in high-income countries. The data provide an understanding of the current scenario of breast cancer presentation and treatment in the private health care system in Brazil. Our results may reflect the reality in other middle-income countries and may serve as a foundation to guide resource allocation.
AUTHOR CONTRIBUTIONS
Conception and Design: Alexandre Boukai, Aline C. Gonçalves, Monica Padoan, Perla Andrade, Thamires Almeida, Nelson Teich, Luiz H. Araujo
Administrative support: Perla Andrade, Natalia Carvalho, Luiz H. Araujo
Provision of study material or patients: Alexandre Boukai, Aline C. Gonçalves, Luiz H. Araujo
Collection and assembly of data: Alexandre Boukai, Aline C. Gonçalves, Perla Andrade, Natalia Carvalho, Flavio Lemos, Thamires Almeida, Luiz H. Araujo
Data analysis and interpretation: Alexandre Boukai, Aline C. Gonçalves, Perla Andrade, Flavio Lemos, Thamires Almeida, Jonas Salem, Maria F. D. Gauí, Nelson Teich, Luiz H. Araujo
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Alexandre Boukai
Consulting or Advisory Role: Genomic Health
Aline C. Gonçalves
No relationship to disclose
Mônica Padoan
No relationship to disclose
Perla Andrade
No relationship to disclose
Natalia Carvalho
No relationship to disclose
Flavio Lemos
No relationship to disclose
Thamires Almeida
No relationship to disclose
Jonas Salem
No relationship to disclose
Maria F. Gauí
No relationship to disclose
Nelson Teich
Employment: United Health Group
Stock and Other Ownership Interests: Clinicas Oncológicas Integradas
Honoraria: Roche, Novartis, AstraZeneca, Libbs
Consulting or Advisory Role: Libbs
Luiz H. Araujo
No relationship to disclose
REFERENCES
- 1.World Health Organization, International Agency for Research on Cancer Breast cancer: Estimated incidence, mortality and prevalence worldwide 2012. http://globocan.iarc.fr/old/FactSheets/cancers/breast-new.asp
- 2.Miller MC, Shuman AG, American Head and Neck Society’s Committee on Survivorship Survivorship in head and neck cancer: A primer. JAMA Otolaryngol Head Neck Surg. 2016;142:1002–1008. doi: 10.1001/jamaoto.2016.1615. [DOI] [PubMed] [Google Scholar]
- 3.Ost DE, Yeung SC, Tanoue LT, et al. Clinical and organizational factors in the initial evaluation of patients with lung cancer: Diagnosis and management of lung cancer. 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e121S–141S. doi: 10.1378/chest.12-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015. A systematic analysis for the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathers CD, Loncar D, World Health Organization Updated projections of global mortality and burden of disease, 2002-2030: data sources, methods and results. doi: 10.1371/journal.pmed.0030442. http://www.who.int/healthinfo/statistics/bodprojectionspaper.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Instituto Brasileiro de Geografia e Estatística . Escassez e fartura: Distribuição da oferta de equipamentos de diagnóstico por imagem no Brasil. Indicadores sociodemográficos e de saúde no Brasil: 2009] Rio de Janeiro, Brazil: Instituto Brasileiro de Geografia e Estatística; 2009. [Google Scholar]
- 7.Barros JA, Valladares G, Faria AR, et al. Early diagnosis of lung cancer: The great challenge. Epidemiological variables, clinical variables, staging and treatment [in Portuguese] J Bras Pneumol. 2006;32:221–227. [PubMed] [Google Scholar]
- 8.Agência Nacional de Saúde Suplementar General data. http://www.ans.gov.br/perfil-do-setor/dados-gerais
- 9.Kaliks RA, Pontes LB, Bognar CL, et al. Treatment of breast cancer patients from a public healthcare system in a private center: Costs of care for a pilot public-private partnership in oncology. Einstein (Sao Paulo) 2013;11:216–223. doi: 10.1590/S1679-45082013000200014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee BL, Liedke PE, Barrios CH, et al. Breast cancer in Brazil: Present status and future goals. Lancet Oncol. 2012;13:e95–e102. doi: 10.1016/S1470-2045(11)70323-0. [DOI] [PubMed] [Google Scholar]
- 11.Liedke PE, Finkelstein DM, Szymonifka J, et al. Outcomes of breast cancer in Brazil related to health care coverage: A retrospective cohort study. Cancer Epidemiol Biomarkers Prev. 2014;23:126–133. doi: 10.1158/1055-9965.EPI-13-0693. [DOI] [PubMed] [Google Scholar]
- 12.Morigi C. Highlights from the 15th St Gallen International Breast Cancer Conference 15-18 March, 2017, Vienna: Tailored treatments for patients with early breast cancer. Ecancermedicalscience. 2017;11:732. doi: 10.3332/ecancer.2017.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Instituto Brasileiro de Geografia e Estatística. 2016 https://biblioteca.ibge.gov.br/visualizacao/periodicos/2121/cnt_2016_4tri.pdf
- 14.Banco Central do Brasil: Focus. Relatório de Mercado. 2017 http://www.bcb.gov.br/pec/GCI/PORT/readout/readout.asp
- 15.Keehan SP, Stone DA, Poisal JA, et al. National Health Expenditure Projections, 2016-25: Price increases, aging push sector to 20 percent of economy. Health Aff (Millwood) 2017;36:553–563. doi: 10.1377/hlthaff.2016.1627. [DOI] [PubMed] [Google Scholar]
- 16.Canadian Institute for Health Information How much does Canada spend on health care? 2017. http://www.cihi.ca/en/how-much-does-canada-spend-on-health-care-2017
- 17.Organization for Economic Co-operation and Development Health expenditure and financing. http://stats.oecd.org/index.aspx?r=619633
- 18.ukpublicspending.co.uk Time series chart of public spending. https://www.ukpublicspending.co.uk/spending_chart_2012_2020UKm_17c1li111mcn_10t20t40t
- 19.Canadian Institute for Health Information Access data and reports. https://www.cihi.ca/en/access-data-reports/results?f%5B0%5D=field_primary_theme%3A2058
- 20.Organization for Economic Co-operation and Development Health care quality indicators. http://stats.oecd.org/BrandedView.aspx?oecd_bv_id=health-data-en&doi=data-00540-en
- 21.Singletary SE, Connolly JL. reast cancer staging: Working with the sixth edition of the AJCC Cancer Staging Manual. CA Cancer J Clin. 2006;56:37–47. doi: 10.3322/canjclin.56.1.37. [DOI] [PubMed] [Google Scholar]
- 22.Kluthcovsky AC, Faria TN, Carneiro FH, et al. Female breast cancer mortality in Brazil and its regions. Rev Assoc Med Bras (1992) 2014;60:387–393. doi: 10.1590/1806-9282.60.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Cecilio AP, Takakura ET, Jumes JJ, et al. Breast cancer in Brazil: Epidemiology and treatment challenges. Breast Cancer (Dove Med Press) 2015;7:43–49. doi: 10.2147/BCTT.S50361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abrahão KS, Bergmann A, Aguiar SS, et al. Determinants of advanced stage presentation of breast cancer in 87,969 Brazilian women. Maturitas. 2015;82:365–370. doi: 10.1016/j.maturitas.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Medeiros GC, Bergmann A, Aguiar SS, et al. Determinants of the time between breast cancer diagnosis and initiation of treatment in Brazilian women [in Portuguese] Cad Saude Publica. 2015;31:1269–1282. doi: 10.1590/0102-311X00048514. [DOI] [PubMed] [Google Scholar]
- 26.Balabram D, Turra CM, Gobbi H. Survival of patients with operable breast cancer (stages I-III) at a Brazilian public hospital--a closer look into cause-specific mortality. BMC Cancer. 2013;13:434. doi: 10.1186/1471-2407-13-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alves Soares Ferreira N, Melo Figueiredo de Carvalho S, Engrácia Valenti V, et al. Treatment delays among women with breast cancer in a low socio-economic status region in Brazil. BMC Womens Health. 2017;17:13. doi: 10.1186/s12905-016-0359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]