Abstract
Study Objectives:
Understanding nightmares (NM) and disturbing dreams (DD) in posttraumatic stress disorder (PTSD) has been limited by the unpredictability of these events and their nonappearance in the sleep laboratory. This study used intensive, longitudinal, ambulatory methods to predict morning reports of NM/DD in veterans in whom chronic, severe PTSD was diagnosed.
Methods:
Participants were 31 male United States military veterans engaged in residential treatment for PTSD and participating in a service animal training intervention. Participants slept on mattress actigraphs and provided reports of momentary mood, as well as morning NM/DD reports, for up to 6 weeks. Mattress actigraphy provided sleep-period heart rate and respiratory sinus arrhythmia (RSA), and an actigraphic estimate of sleep efficiency. On one night, a respiratory event index (REI) was obtained using an ambulatory system.
Results:
A total of 468 morning reports were obtained, of which 282 endorsed NM/DD during the prior night, and 186 did not. After accounting for multiple predictors, only elevated REI and lower prior-night sleep RSA predicted morning endorsement of NM/DD. These two predictors did not interact.
Conclusions:
Elevated REI and lower sleep period RSA were independently predictive of NM/DD. The former result is consistent with studies showing that sleep-disordered breathing (SDB) is a factor in NM/DD, and that continuous positive airway pressure (CPAP) can reduce these symptoms in patients with comorbid PTSD and SDB. The latter result implicates dysregulated arousal modulation during sleep in trauma-related NM/DD. It is consistent with findings that NM/DD are reported in patients without SDB and can persist in patients with comorbid PTSD and SDB even when CPAP successfully remediates SDB.
Citation:
Miller KE, Jamison AL, Gala S, Woodward SH. Two independent predictors of nightmares in posttraumatic stress disorder. J Clin Sleep Med. 2018;14(11):1921–1927.
Keywords: ambulatory measurement, nightmares, posttraumatic stress disorder, psychophysiology, sleep
BRIEF SUMMARY
Current Knowledge/Study Rationale: No previous studies have examined self-reported and objective predictors of trauma-related disturbed dreaming over many nights within persons in whom posttraumatic stress disorder has been diagnosed. The current study used two intensive, naturalistic data acquisition methods, daily ecological momentary assessments, and nightly mattress actigraphy data, along with estimation of sleep-disordered breathing.
Study Impact: These data revealed that elevated respiratory event index and lower prior-night sleep respiratory sinus arrhythmia independently predicted disturbed dreaming reports. These results provide new insights into the dynamic autonomic concomitants of disturbed dreaming, while also confirming prior associations with sleep-disordered breathing..
INTRODUCTION
Nightmares (NM) and disturbing dreams (DD) that impair sleep are among the most frequently reported symptoms of posttraumatic stress disorder (PTSD),1 but their cause and precise nature remain elusive.2 Not only are they self-reported events that are impossible to observe directly; they are rarely reported during sleep laboratory monitoring, leaving their psychophysiological correlates largely unknown.3 It seems reasonable to propose that NM/DD, along with the other intrusive PTSD symptoms, originate in and are maintained by hyperarousal-driven, overconsolidation of trauma memories.4 This idea is supported, in part, by the replicative nature of some trauma-related NM5–7; however, there is limited empirical support for this proposition. Two recent reports in which ambulatory sleep recording was used failed to observe elevated heart rates prior to NM awakenings, though both observed elevated heart rates after NM awakenings.8,9 In fact, Woodward et al.9 found that sleep heart rate prior to NM awakenings tended to be lower than prior to non-NM awakenings. It is also notable that both studies found that trauma-related DD emerged in both rapid eye movement (REM) and non-rapid eye movement (NREM) sleep.
In this study, we sought to add to the literature on NM/DD in PTSD by collecting a large body of morning reports endorsing the presence/absence of a NM/DD during the prior night, and combining these reports with predictors including prior-night mattress actigraphy yielding heart rate, respiratory sinus arrhythmia, and an actigraphic estimate of sleep efficiency, prior-day momentary endorsements of negative mood, and an assessment of sleep-disordered breathing (SDB). Mattress actigraphy, described in greater detail in the next paragraphs, is similar to wrist actigraphy in that it depends solely on accelerometers; however, because the accelerometers are stationed under the thorax, they can also transduce movements of the chest associated with cardiac contractions, respiratory movements, and snoring.10 Mattress actigraphy is a completely passive measurement method that can be continued indefinitely, and so is well suited to the study of sleep events that occur infrequently and/or do not emerge in the laboratory. Respiratory sinus arrhythmia (RSA), a putative noninvasive index of parasympathetic tone,11 has garnered attention as a potential transdiagnostic biomarker for emotion regulation, with low resting RSA during emotion elicitation tasks observed across several psychopathologies.12,13 Studies also show RSA to be attenuated in PTSD samples during laboratory-induced emotion challenges14 and prior to or during trauma reminders.15,16 Assessing RSA during sleep offers additional protections from sources of artifact such as movement and speech, as well as affording long recording periods promoting reliable estimates. In healthy participants, presleep stress results in attenuation of sleep RSA.17 In our past research, sleep RSA magnitude has been found to be lower among participants with PTSD compared control groups without PTSD.18
In our prior effort to record perinightmare sleep outside of the laboratory, persons with SDB were excluded.9 Since that time, interest has grown in the possible overlap of nightmare phenomena with SDB independent of the panic arousals sometimes associated with severe SDB. In otherwise healthy samples, SDB has been linked to greater dream recall, particularly dreams with more emotional content,19 and with nightmare reports.20 Treatment of SDB with continuous positive airway pressure (CPAP) is associated with decreases in nightmares.21 Accordingly, in this study, persons were not excluded for SDB.
Instead, a validated ambulatory methodology (ApneaLink Air, ResMed, San Diego, California, United States)22 was used to estimate the respiratory event index (REI), the standard measure of SDB for home sleep tests, on a single night in the usual sleeping environment.
Among potential predictors of NM/DD, an additional candidate is prior-day negative affect.23 A recent ecological momentary assessment (EMA) in a sample of patients with PTSD found that elevated diurnal PTSD symptoms predicted NM/ DD reports the following morning.24 The current study used this method as well, administering select subsets of the Positive and Negative Affect Scale (PANAS)25 during the day.
This investigation is part of a larger study examining the effect of canine companionship on multiple aspects of PTSD in veterans undergoing residential treatment. Canine companionship has been postulated to mitigate NM/DD by increasing self-reported safety during sleep.26 Participants in this study provided early training and socialization to young service canines provided by a nonprofit organization (Paws for Purple Hearts) under the supervision of a professional trainer. The canines were exclusively Labrador and Golden Retrievers bred to achieve the highest levels of aid to mobility-impaired veterans. Participants alternated custody of the service canine with another veteran. On “canine-plus” days, the custodial trainer remained with the canine at virtually all times, including at night. On “canine-minus” days, participants had little contact with the canine, day or night.
METHODS
Participants
All participants provided written informed consent in accordance with the procedures of the Stanford/VA Palo Alto Health Care System (VAPAHCS) Human Research Protection Program. Inclusion criteria were engagement in residential PTSD treatment at the Trauma Recovery Program of the VAPAHCS, relative behavioral stability, and no known fall risk. Exclusion criteria included acute somatic disease, psychosis or mania, greater than mild traumatic brain injury, and medication with beta-adrenergic antagonists.
Procedures
Psychiatric diagnoses were obtained via the Clinician Administered PTSD Scale for DSM-5 (CAPS-5)27 and the Structured Clinical Interview for DSM-5.28 Severity of traumatic brain injury (TBI) was assessed using the Department of Defense TBI screening questionnaire, which indicates good concurrent validity with clinical interviews.29 A range of self-report psychometrics were also collected, as will be discussed in future reports.
Ecological Momentary Assessment
Morning reports, momentary mood reports, and evening reports, were obtained using diary app (esmi, Senti, Inc., San Francisco, California, United States) running on an Apple iPod that participants kept nearby at all times. Participants were alerted 7 times each day, once in the morning, once during each of five, 3-hour intervals spanning the period between 7:00 am and 9:00 pm, and once after 9:00 pm. The response mode typically involved moving a slider in relation to anchors such as “never…rarely…frequently” or “no… yes.” A weekly monetary bonus ($5) was provided to participants scaled to the rate at which they responded to alerts. Morning reports included the following two dichotomous-response (yes/no) questions presented in order: “Did you have a nightmare related to traumatic experiences?” followed by “Did you have any other disturbing dreams?” Any combination of positive endorsements of these questions defined a NM/DD-positive night. Nightmares and other distressing dreams were combined into one variable as there were few nightmare reports that were reported absent a concurrent disturbed dreaming report (see Discussion). The five intraday EMA reports included nine items drawn quasi-randomly from the PANAS25 with the requirement that four or five items from the positive affect and negative affect factors were always included. Participants rated the extent to which they experienced each of the presented emotions on a five-point scale ranging from “not at all” to “extremely.” The median score from all available negative affect items (eg, distressed, upset, ashamed, irritable) was used to form a daily negative mood score.
Mattress Actigraphy
Participants slept on twin beds in the residential treatment facility, in rooms they shared with no to three other veterans. Mattress actigraphy was continuously recorded, with gaps in the data associated with brief absences, as on weekends or with rare equipment failures. Mattress actigraphy employed a cotton-covered latex foam mattress “topper” approximately 1 inch in depth into which two microelectromechanical accelerometers (Silicon Designs, Issaquah, Washington, United States, Model 2210 2 g, bandwidth: 0–300 Hz) were embedded in the thorax region. Accelerometer signals were routed to a bedside personal computer hosting a data acquisition card (Measurement Computing 1408-FS, sampling rate, 600 Hz, amplitude resolution, 14-bits). Custom software managed the nighttime collection and daytime processing of accelerometer signals into per 30-second time-series of heart rate, RSA, respiratory parameters such as rate, rate variability, amplitude variability, and parameters of body movement. Later, as in conventional actigraphy, intended sleep periods (hereafter, sleep periods) were manually determined by judges blind to all other study variables. Unlike conventional actigraphy, this demarcation employed additional cues indicative of sleep intention other than low movement, including regular respiration movement signals, and prominent kinetocardiogram (KCG), both of which are indicative of lying posture and behavioral quiescence. Within the borders of the sleep periods, subepochs characterized by high respiratory regularity and/ or high KCG signal-to-noise ratio were automatically detected. Per night sleep efficiency was subsequently calculated as the ratio of the aggregate duration of such quiescent periods divided by the duration of the sleep period.
As detailed in Woodward et al.10 continuous recording of the KCG allows estimation of the timing of ventricular contractions for the calculation of heart rate and heart rate variability. The cardiac cycle is associated with stereotypic “precordial” movements detectable by sensitive accelerometers in contact with the thorax. The most prominent of these, sometimes labeled the “MC wave,” peaks approximately 100 msec after the R wave.30 The method used here for detecting MC waves has been designed to accommodate the highly variable conformation of the precordial movement complex, as compared to the QRS complex, over persons and over time within persons. It begins with frequency transformation of a rectified, low-pass-filtered version of the KCG signal to obtain a gross estimate of event timing. This timing information is used to adaptively segment the signal, with the segments then input into an iterative process leading to an optimal ensemble average of the precordial complex for that epoch. This ensemble average is then processed over the raw signal to obtain the timings of peak cross-covariances. Last, the resulting timings are subjected to quality checks prior to being retained. For retained epochs, conventional Matlab-based frequency-domain (pwelch) estimation of heart rate variability was performed, involving (1) (Hamming) windowing, (2) discrete Fourier transformation, and (3) postwindowing power renormalization. RSA-band (0.15–0.4 Hz) power magnitudes were square-root transformed to enhance normality. Though a relatively short, 30-second epoch length was used, subsequent analyses were restricted to all-night medians calculated over epochs meeting strict inclusion criteria. Such all-night sleep RSA estimates have been shown to be highly correlated with those based on electrocardiography.10 Respiratory movements employed for estimation of sleep periods were obtained by simple band-pass filtering of the raw accelerometer signal.
REI Estimation
REI was assessed ambulatorily using the ApneaLink Air system. This system uses thoracic and abdominal respiratory bands, nasal cannula, and an oximetry finger probe. Setup and recording occurred in participants' regular sleep quarters. The default settings of the ApneaLink software for apneas and hypopneas were used. These define an obstructive apnea as an 80% to 100% reduction in airflow associated with respiratory effort ≥ 10 seconds, and an obstructive hypopnea as a 50% to 80% reduction in airflow associated with respiratory effort ≥ 10 seconds. The software calculates REI from the number of apneas and hypopneas per hour of device recording time. This device has been validated against PSG and determined to demonstrate good sensitivity and specificity in quantifying REI.31
Prazosin Use
Because of its potential for attenuating nightmares in veterans,32 stable prazosin prescriptions were carefully tracked by reference to the medical chart.
Analytical Plan
All analyses were conducted using R, version 3.3.1.33 To accommodate missing data, multiple imputation was implemented via the Multiple Imputation by Chained Equations package in R MICE34; according to the assumption that they were either missing-at-random, or missing-completely-at-random. Mixed-effects binary logistic regressions were employed using glmer to account for nested data.35 A series of models that employed rationally derived subsets of predictors (night-associated, day-associated, canine accompaniment) were tested against the full model including all predictors. A random intercept was specified for each participant embedded in 1 of 10 canines to account for possible within-canine and within-participant clustering of observations and to adjust for otherwise unmeasured participant-level traits. The dependent variable was morning NM/DD reports (binary outcome). Night-associated predictors were REI (between-subjects), all-night RSA, and actigraphic sleep efficiency (within subjects). Daytime predictors were days since admission (between subjects), negative mood, and prior-morning NM/DD report (within subjects). To limit the number of variables included in the full model, as recommended when employing mixed-effects binary logistic regressions, prazosin prescription and weight (in pounds) were not further analyzed after being shown to not account for significant variance in NM/DD incidence when tested as main effects (odds ratio [OR] = 1.25, P = .60; OR = 0.95, P = .83, respectively). Model fits were assessed through the Akaike information criterion (AIC), Bayesian information criterion (BIC), and log-likelihood. Lower values of AIC and BIC, and higher values of LL were used to select the optimal model.
RESULTS
Participants were 31 male United States military veterans, primarily of the Iraq and Afghanistan wars, aged 25–66 (mean = 41.23, standard deviation [SD] = 11.86 years). Participants self-identified ethnicity from a list including white (52%); Hispanic, white (17%); Pacific Islander (10%); African-American, not Hispanic (7%); American-Indian/Alaskan (7%); and other (7%). All military service branches except the Coast Guard were represented, with most participants having served in the Army (67%). All participants met criteria for PTSD. The average CAPS-5 total severity score was 38.58 (SD = 7.74). Consistent with inpatient PTSD treatment status, 58% also met criteria for current major depressive disorder (MDD) and 84% for lifetime MDD. Sixty-five percent met criteria for current (past 12 months) alcohol use disorder (AUD) and 32% for lifetime AUD. Thirty-nine percent met criteria for a current (past 12 months) substance use disorder (SUD) and 61% for lifetime SUD. Eleven participants (35.48%) had stable prazosin prescriptions. The mean estimated REI was 8.79 (SD = 7.34) events/h, with 39% having REI < 5 events/h, 45% having REI = 5–15 events/h, and 13% having REI > 15 events/h. The mean weight of the sample in pounds was 205.20 (SD = 29.38), with the average body mass index 29.37 (SD = 4.71) kg/m2.
Missingness Model
The behavior sample obtained from the 31 participants included 886 nights of actigraphic sleep efficiency estimates, 867 all-night sleep heart rate and RSA estimates, 775 prior-day negative mood estimates (each a median of 1 to 5 reports), 545 reports of ± NM/DD from the prior morning, and 468 morning NM/DD reports endorsing the presence (282) or absence (186) of prior-night NM/DD. In particular, the response category with the most missingness, morning NM/DD reports (47.18%), was modeled as a function of the predictor set and found to be inversely associated with canine presence and no other predictor (OR = 3.25, P < .001). This result indicated that the inclusion of canine presence as a predictor in the imputation of missing values would attenuate non-response bias. MICE was employed to iteratively cross-impute the following variables, not all of which were subsequently analyzed for this report: prior-day pain, prior-day negative mood, prior-day positive mood, in-bed time, out-of-bed time, minutes of acti-graphic quiescence, sleep heart rate, sleep RSA, sleep respiratory frequency, seconds of snoring (per epoch), morning NM/ DD report. The imputation method for all continuous variables was predictive mean matching (ie, restricted to observed values based on linear regression models), whereas it was logistic for the binary NM/DD report variable.
Nightmare/Disturbed Dreaming Reports Model
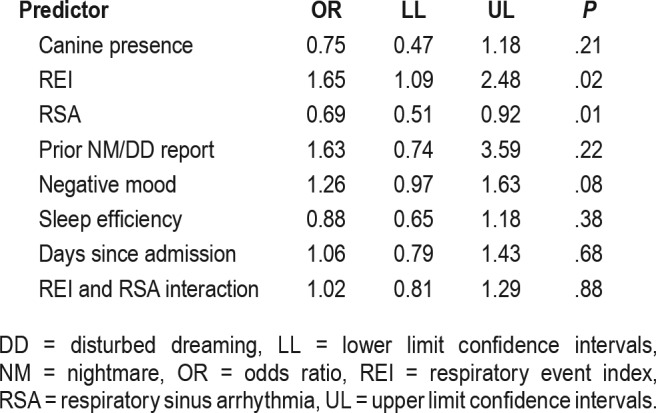
Fifty mixed effects binary logistic regression models were subsequently computed and their parameters pooled.35 Table 1 presents the fit indices for the alternative models of morning NM/DD reports. Model 1, the full model including all night-associated, day-associated, and canine presence variables, was significantly different only from model 2, which omitted the night-associated variables (F4, 2111 = 2.66, P = .03). No other model contrasts were significant and the full model fit was not significantly different from that of the model containing only the nocturnal variables (F5,689 = 1.57, P = .17). Employing the full model in order to control for all predictors, a greater likelihood of a morning NM/DD report was associated with elevated REI (OR = 1.65, P = .02), and lower sleep-period RSA (OR = 0.69, P = .01). The two-way interaction between REI and RSA did not approach significance (P = .88). There was a marginal effect for prior-day negative mood (OR = 1.32, P = .08), with higher scores predicting greater likelihood of a morning nightmare report. No associations were observed between morning reports of NM/DD actigraphic sleep efficiency, prior-morning NM/DD report, days since admission, or canine presence (Table 2; all Ps ≥ .21).
Table 1.
Median goodness-of-fit statistics across the 50 imputed data sets for each model and model comparisons.
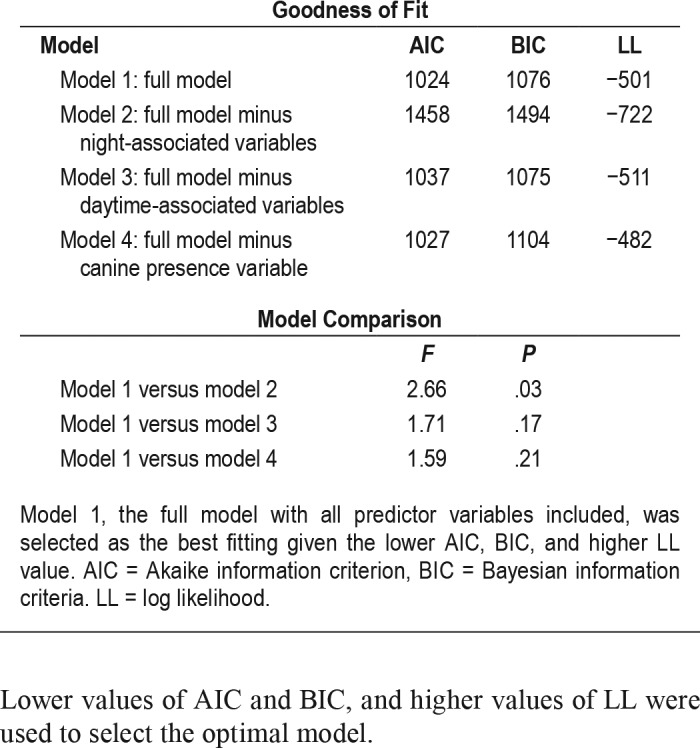
Table 2.
Predictors of disturbed dreaming morning reports.
DISCUSSION
The current study is the first to use a within-subject, intensive, longitudinal design to examine predictors of morning reports of NM/DD in persons with PTSD. Two variables independently predicted morning reports of NM/DD. One was elevated REI, and the other, attenuated sleep period RSA magnitude. The finding that elevated REI is associated with morning NM/DD reports aligns with the work of Krakow et al.36 and others21,37 who have shown that treating patients with comorbid PTSD and SDB with CPAP, can reduce NM/DD reports by up to 50% in patients who are adherent. Collectively, these studies imply that both observational studies and clinical trials in the area of PTSD nightmares include quantification of SDB. Certain key populations, such as veterans, have exhibited large secular increases in overweight,38 the leading risk factor for SDB. Uncontrolled SDB may attenuate the effect of some therapeutic intervention in this domain,39 but the study by Sweetman et al.40 shows that treatment for insomnia can be effective in the presence of comorbid sleep apnea. This situation is further complicated by the fact that low CPAP adherence rates are repeatedly observed among patients with PTSD,41,42 and few evidence-based interventions exist for promoting CPAP adherence.43 In this context it is interesting to note that nightly adherence and SDB data are provided by many CPAP systems. It is possible that morning NM/DD reports may be combined with these data to strengthen CPAP adherence interventions for patients with comorbid PTSD and SDB.21 Objective evidence that NM/DD are more likely to occur when CPAP is not worn may be a persuasive strategy to improve adherence.
Successful CPAP treatment in patients with comorbid PTSD and SDB may leave a substantial residuum of nightmares.37 El-Solh et al.21 reported “a ceiling effect” limiting the effect of CPAP on nightmares in PTSD. Such findings are consistent with the observation made here of a second independent predictor of morning NM/DD reports, attenuated sleep period RSA. Woodward et al.18 also found lower sleep period RSA in persons in whom PTSD was diagnosed as a result of civilian trauma relative to a control sample, suggesting that NM/ DD and lower RSA are concurrent manifestations of episodic worsening of PTSD sleep disturbance. It is also possible that there is a causal relationship between lower sleep period RSA and NM/DD. In a theoretical review, Gillie and Thayer44 argued that resting RSA indexes frontal inhibitory control over the central autonomic network's mediation of responses to negative emotional cues and memories. Their framework offers an important new perspective on NM/DD, suggesting that these symptoms, like other intrusive symptoms of PTSD, flow from the failure of a regulatory system that manages the adaptive allocation of cognitive resources to biologically significant cues (eg, threat cues). The failure of cognitive control, manifested as exaggerated attention to negative emotional memories, may act at the point of recollection and reporting of nocturnal mentation, regardless of the actual aggregate content of prior nocturnal mentation. The same framework may also accommodate evidence that dyscontrolled recollections of trauma-related nightmares can have toxic effects on mood that feed forward throughout the subsequent day. Further research will be necessary to verify whether the proposed relationships among intrusive symptoms, cognitive control, and basal RSA (and other objective measures), offer new clarity regarding this hard-to-quantify PTSD domain. Research appears warranted to determine whether interventions that increase RSA can attenuate nightmares and other symptoms of PTSD. To date, only two studies have used heart rate variability biofeedback in small samples of individuals who have received a diagnosis of PTSD,45,46 illustrating significant improvements in RSA and modest reductions in depression and global PTSD symptoms.
Despite widespread anecdotal reports from veterans regarding the mitigating effect of canine presence on nightmare reports, no such effect was observed in this study. Rather than reducing the frequency of nightmares, canine presence may reduce associated distress, extended arousal, and/or time awake following a nightmare experience; factors not captured by these data. Prior-day report of NM/DD, negative mood, and sleep efficiency also failed to predict morning reports of NM/ DD in this study. Although negative mood was close to significance (P = .08), these results are in contrast to prior work emphasizing the influence of diurnal mood symptoms on NM/ DD reports, and the cascading effects of a previous night of poor sleep.23,24
This study has important limitations. It was conducted on a male veteran sample with chronic military-related PTSD, and may have limited generalizability to other populations. Second, the residential treatment context afforded sleep scheduling consistency and constrained substance use, television-watching, and other sleep-relevant behaviors. It is unknown how these results may translate to the home sleep environment. Similarly, the influence of the roommates' concurrent sleep (eg, snoring patterns, nightmare reports, sleep schedule) is also unknown. In addition, NM/DD reports were based on morning self-reports, and therefore possibly hampered by the reliance on participants' imperfect memories of nocturnal events. The simplified binary outcome measures used also disregarded the intranight frequency and severity of these nocturnal events and so could not capture potentially important distinctions between nightmares versus disturbing dreams. That said, only small differences in emotional intensity between disturbing dreams and nightmares have been reported,47 suggesting that these phenomenon fall on a continuum. Finally, the few studies that have attempted to correlate trauma-related nightmare reports with objective sleep physiology have reported divergent nightmare appearance rates,8,9 leading to reasonable suspicion regarding how perfectly the objects of their studies overlap.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This research was supported by Contract W81XWH-15-2-0005 to Steven H. Woodward, PhD, from the Defense Health Program, Military Operational Medical Research Program, and US Army Medical Research and Materiel Command. One of the technologies used in this work was invented by Steven H. Woodward, PhD; however, the associated patent rights are the property of Stanford University and the Department of Veterans Affairs. The other authors report no conflicts of interest. The views expressed here are the authors' and do not necessarily represent the views of the Department of Veterans Affairs or the Department of Defense.
ACKNOWLEDGMENTS
This research would not have been possible without the support of the following persons and institutions, Ronald L. Hoover, PhD, Portfolio Manager, DHP/ MOMRP/AMRMC, Carly I. Kiselycznyk, PhD, Science Officer, CDMRP, the Palo Alto Veterans Institute for Research, the National Center for PTSD, the Trauma Recovery Programs at the Veterans Affairs Palo Alto Health Care System, Paws for Purple Hearts (Sandra Carson, VAPAHCS/Lead Trainer), and the Veterans who participated. Part of the writing of this manuscript was supported by the Department of Veterans Affairs, Office of Academic Affiliations, and the Fellowship in PTSD Research and Treatment at the Dissemination and Training Division of the National Center for PTSD. The authors thank Ned Arsenault and Sarah Righi for their help in assessing participants, and Maggi Mackintosh for statistical assistance.
ABBREVIATIONS
- AIC
Akaike information criterion
- AUD
alcohol use disorder
- BIC
Bayesian information criterion
- CAPS-5
Clinician-Administered PTSD Scale
- CPAP
continuous positive airway pressure
- DD
disturbing dreams
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- EMA
ecological momentary assessment
- Hz
hertz
- KCG
kinetocardiogram
- MDD
major depressive disorder
- MICE
multiple imputation by chained equations
- NM
nightmares
- NREM
non-rapid eye movement
- OR
odds ratio
- PANAS
Positive and Negative Affect Scale
- PTSD
posttraumatic stress disorder
- REI
respiratory event index
- REM
rapid eye movement
- RSA
respiratory sinus arrhythmia
- SDB
sleep-disordered breathing
- SUD
substance use disorder
- TBI
traumatic brain injury
- VAPAHCS
VA Palo Alto Health Care System
REFERENCES
- 1.Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169–184. doi: 10.1016/j.smrv.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Phelps AJ, Forbes D, Creamer M. Understanding posttraumatic nightmares: an empirical and conceptual review. Clin Psychol Rev. 2008;28(2):338–355. doi: 10.1016/j.cpr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Woodward SH, Arsenault NJ, Murray C, Bliwise DL. Laboratory sleep correlates of nightmare complaint in PTSD inpatients. Biol Psychiatry. 2000;48(11):1081–1087. doi: 10.1016/s0006-3223(00)00917-3. [DOI] [PubMed] [Google Scholar]
- 4.Cahill L. The neurobiology of emotionally influenced memory implications for understanding traumatic memory. Ann NY Acad Sci. 1997;821:238–246. doi: 10.1111/j.1749-6632.1997.tb48283.x. [DOI] [PubMed] [Google Scholar]
- 5.Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697–707. doi: 10.1176/ajp.146.6.697. [DOI] [PubMed] [Google Scholar]
- 6.van der Kolk B, Blitz R, Burr W, Sherry S, Hartmann E. Nightmares and trauma: a comparison of nightmares after combat with lifelong nightmares in veterans. Am J Psychiatry. 1984;141:187–190. doi: 10.1176/ajp.141.2.187. [DOI] [PubMed] [Google Scholar]
- 7.Rothbaum BO, Mellman TA. Dreams and exposure therapy in PTSD. J Trauma Stress. 2001;14(2):481–490. doi: 10.1023/A:1011104521887. [DOI] [PubMed] [Google Scholar]
- 8.Phelps AJ, Kanaan RAA, Worsnop C, Redston S, Ralph N, Forbes D. An ambulatory polysomnography study of the post-traumatic nightmares of post-traumatic stress disorder. Sleep. 2018;41(1) doi: 10.1093/sleep/zsx188. [DOI] [PubMed] [Google Scholar]
- 9.Woodward SH, Michel G, Santerre C. The Psychophysiology of PTSD Nightmares. In: Vermetten E, Germain A, Neylan TC, editors. Sleep and Combat-Related Post Traumatic Stress Disorder. New York, NY: Springer; 2018. pp. 233–242. [Google Scholar]
- 10.Woodward SH, Arsenault NJ, Voelker K, et al. Estimating heart rate and RSA from the mattress-recorded kinetocardiogram. Psychophysiology. 2007;44(4):635–638. doi: 10.1111/j.1469-8986.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- 11.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beauchaine TP. Respiratory sinus arrhythmia: a transdiagnostic biomarker of emotion dysregulation and psychopathology. Curr Opin Psychol. 2015;3:43–47. doi: 10.1016/j.copsyc.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol. 2007;74(2):263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Sahar T, Shalev AY, Porges SW. Vagal modulation of responses to mental challenge in posttraumatic stress disorder. Biol Psychiatry. 2001;49(7):637–643. doi: 10.1016/s0006-3223(00)01045-3. [DOI] [PubMed] [Google Scholar]
- 15.Sack M, Hopper JW, Lamprecht F. Low respiratory sinus arrhythmia and prolonged psychophysiological arousal in posttraumatic stress disorder: heart rate dynamics and individual differences in arousal regulation. Biol Psychiatry. 2004;55(3):284–290. doi: 10.1016/s0006-3223(03)00677-2. [DOI] [PubMed] [Google Scholar]
- 16.Hopper JW, Spinazzola J, Simpson WB, van der Kolk BA. Preliminary evidence of parasympathetic influence on basal heart rate in posttraumatic stress disorder. J Psychosom Res. 2006;60(1):83–90. doi: 10.1016/j.jpsychores.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Hall M, Vasko R, Buysse D, et al. Acute stress affects heart rate variability during sleep. Psychosom Med. 2004;66(1):56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- 18.Woodward SH, Arsenault NJ, Voelker K, et al. Autonomic activation during sleep in posttraumatic stress disorder and panic: a mattress actigraphic study. Biol Psychiatry. 2009;66(1):41–46. doi: 10.1016/j.biopsych.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher S, Lewis KE, Bartle I, Ghosal R, Davies L, Blagrove M. Emotional content of dreams in obstructive sleep apnea hypopnea syndrome patients and sleepy snorers attending a sleep-disordered breathing clinic. J Clin Sleep Med. 2011;7(1):69–74. [PMC free article] [PubMed] [Google Scholar]
- 20.BaHammam AS, Al-Shimemeri SA, Salama RI, Sharif MM. Clinical and polysomnographic characteristics and response to continuous positive airway pressure therapy in obstructive sleep apnea patients with nightmares. Sleep Med. 2013;14(2):149–154. doi: 10.1016/j.sleep.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 21.El-Solh AA, Vermont L, Homish GG, Kufel T. The effect of continuous positive airway pressure on post-traumatic stress disorder symptoms in veterans with post-traumatic stress disorder and obstructive sleep apnea: a prospective study. Sleep Med. 2017;33:145–150. doi: 10.1016/j.sleep.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Crowley KE, Rajaratnam SM, Shea SA, et al. Evaluation of a single-channel nasal pressure device to assess obstructive sleep apnea risk in laboratory and home environments. J Clin Sleep Med. 2013;9(2):109–116. doi: 10.5664/jcsm.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin R, Fireman G, Spendlove S, Pope A. The relative contribution of affect load and affect distress as predictors of disturbed dreaming. Behav Sleep Med. 2011;9(3):173–183. doi: 10.1080/15402002.2011.583905. [DOI] [PubMed] [Google Scholar]
- 24.Short NA, Allan NP, Stentz L, Portero AK, Schmidt NB. Predictors of insomnia symptoms and nightmares among individuals with post-traumatic stress disorder: an ecological momentary assessment study. J Sleep Res. 2018;27(1):64–72. doi: 10.1111/jsr.12589. [DOI] [PubMed] [Google Scholar]
- 25.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 26.Stern SL, Donahue DA, Allison S, et al. Potential benefits of canine companionship for military veterans with posttraumatic stress disorder (PTSD) Soc Anim. 2013;21:568–581. [Google Scholar]
- 27.Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, Keane TM. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) 2013. Interview available from the National Center for PTSD at www.ptsd.va.gov. [DOI] [PMC free article] [PubMed]
- 28.First MB, Williams JBW, Karg RS, Spitzer RL. Structured Clinical Interview for DSM-5 Disorders (SCID-5-RV), Research Version. Arlington, VA: American Psychiatric Publishing; 2014. [Google Scholar]
- 29.Schwab KA, Ivins B, Cramer G, et al. Screening for traumatic brain injury in troops returning from deployment in Afghanistan and Iraq: initial investigation of the usefulness of a short screening tool for traumatic brain injury. J Head Trauma Rehabil. 2007;22(6):377–389. doi: 10.1097/01.HTR.0000300233.98242.87. [DOI] [PubMed] [Google Scholar]
- 30.Inan OT, Migeotte PF, Park KS, et al. Ballistocardiography and seismocardiography: a review of recent advances. IEEE J Biomed Health Inform. 2015;19(4):1414–1427. doi: 10.1109/JBHI.2014.2361732. [DOI] [PubMed] [Google Scholar]
- 31.Crowley KE, Rajaratnam SM, Shea SA, Epstein LJ, Czeisler CA, Lockley SW. Evaluation of a single-channel nasal pressure device to assess obstructive sleep apnea risk in laboratory and home environments. J Clin Sleep Med. 2013;9(2):109–116. doi: 10.5664/jcsm.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raskind MA, Thompson C, Petrie EC, et al. Prazosin reduces nightmares in combat veterans with posttraumatic stress disorder. J Clin Psychiatry. 2002;63(7):565–568. doi: 10.4088/jcp.v63n0705. [DOI] [PubMed] [Google Scholar]
- 33.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2016. [Google Scholar]
- 34.van Buuren S, Groothuis-Oudshoorn K. Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 35.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 36.Krakow BJ, Ulibarri VA, Moore BA, McIver ND. Posttraumatic stress disorder and sleep-disordered breathing: a review of comorbidity research. Sleep Med Rev. 2015;24:37–45. doi: 10.1016/j.smrv.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA) J Clin Sleep Med. 2014;10(6):631–636. doi: 10.5664/jcsm.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rush T, LeardMann CA, Crum-Cianflone NF. Obesity and associated adverse health outcomes among US military members and veterans: findings from the millennium cohort study. Obesity (Silver Spring) 2016;24(7):1582–1589. doi: 10.1002/oby.21513. [DOI] [PubMed] [Google Scholar]
- 39.Mesa F, Dickstein BD, Wooten VD, Chard KM. Response to cognitive processing therapy in veterans with and without obstructive sleep apnea. J Trauma Stress. 2017;30(6):646–655. doi: 10.1002/jts.22245. [DOI] [PubMed] [Google Scholar]
- 40.Sweetman A, Lack L, Lambert S, Gradisar M, Harris J. Does comorbid obstructive sleep apnea impair the effectiveness of cognitive and behavioral therapy for insomnia? Sleep Med. 2017;39:38–46. doi: 10.1016/j.sleep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Collen JF, Lettieri CJ, Hoffman M. The impact of posttraumatic stress disorder on CPAP adherence in patients with obstructive sleep apnea. J Clin Sleep Med. 2012;8(6):667–672. doi: 10.5664/jcsm.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Solh AA, Ayyar L, Akinnusi M, Relia S, Akinnusi O. Positive airway pressure adherence in veterans with posttraumatic stress disorder. Sleep. 2010;33(11):1495–1500. doi: 10.1093/sleep/33.11.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wozniak DR, Lasserson TJ, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2014;(1):CD007736. doi: 10.1002/14651858.CD007736.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Gillie BL, Thayer JF. Individual differences in resting heart rate variability and cognitive control in posttraumatic stress disorder. Front Psychol. 2014;5:758. doi: 10.3389/fpsyg.2014.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan G, Dao TK, Farmer L, Sutherland RJ, Gevirtz R. Heart rate variability (HRV) and posttraumatic stress disorder (PTSD): a pilot study. Appl Psychophysiol Biofeedback. 2011;36(1):27–35. doi: 10.1007/s10484-010-9141-y. [DOI] [PubMed] [Google Scholar]
- 46.Zucker TL, Samuelson KW, Muench F, Greenberg MA, Gevirtz RN. The effects of respiratory sinus arrhythmia biofeedback on heart rate variability and posttraumatic stress disorder symptoms: a pilot study. Appl Psychophysiol Biofeedback. 2009;34(2):135–143. doi: 10.1007/s10484-009-9085-2. [DOI] [PubMed] [Google Scholar]
- 47.Hasler BP, Germain A. Correlates and treatments of nightmares in adults. Sleep Med Clin. 2009;4(4):507–517. doi: 10.1016/j.jsmc.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


