Abstract
Study Objectives:
A home sleep apnea test (HSAT) is an acceptable alternative to polysomnography (PSG) for the diagnosis of obstructive sleep apnea (OSA) in patients with high pretest probability without certain comorbidities, such as severe pulmonary disease, congestive heart failure, or neuromuscular weakness. Current guidelines recommend repeat in-laborataory PSG in those with an initial negative PSG and high clinical suspicion for OSA. This retrospective study evaluated predictors of OSA on HSAT in patients who had a negative PSG.
Methods:
Electronic medical records were reviewed on 206 patients who underwent an in-laboratory PSG followed by HSAT at the Baylor Scott and White Sleep Institute. Of these patients, 141 were included in the study. Clinical patient characteristics, PSG data, and HSAT data were obtained.
Results:
A total of 141 patients had a negative PSG and underwent a subsequent HSAT. Of these patients, 83.7% had a positive diagnosis on HSAT, as defined by respiratory event index greater than or equal to 5 events/h, using the 4% oxygen desaturation criteria, (64.5% mild, 17.7% moderate, 1.4% severe) and 16.3% had a negative HSAT. Older age and hypertension predicted the diagnosis of OSA made on HSAT in patients with an initial negative PSG.
Conclusions:
This retrospective study illustrates that there are patients for whom PSG gave a false-negative study. Patients who had negative PSG and positive HSAT are more likely to be older and have the diagnosis of hypertension. Sleep physicians may consider repeat testing with HSAT in patients with a negative PSG and clinical symptoms of OSA.
Commentary:
A commentary on this article appears in this issue on page 1839.
Citation:
Lipatov K, Hayek A, Ghamande S, Boethel C, Chen W, Jones S. Predictors of obstructive sleep apnea on home sleep apnea test after a negative attended polysomnography. J Clin Sleep Med. 2018;14(11):1889–1894.
Keywords: home sleep apnea test, obstructive sleep apnea, polysomnography
BRIEF SUMMARY
Current Knowledge/Study Rationale: A home sleep apnea test (HSAT) can be an effective tool for the diagnosis of obstructive sleep apnea (OSA); however, its use in repeat testing after a negative comprehensive in-laboratory test with polysomnography (PSG) has not been previously studied. Understanding factors associated with the diagnosis of OSA on HSAT after a negative PSG will help improve clinical judgement and decrease cost.
Study Impact: This study suggests that the use of an HSAT to repeat testing in older patients with a history of hypertension may be a reasonable option compared to repeat in-laboratory PSG. A prospective study is needed to further evaluate the use of HSAT after negative PSG.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep-related disorder with multiple negative health effects that continues to rise in prevalence.1,2 It has been shown to be a risk factor in cardiovascular disease, hypertension, stroke, and cognitive decline and is associated with increased mortality.3–5 Polysomnography (PSG) is considered the gold standard for the evaluation of OSA. A home sleep apnea test (HSAT) is an acceptable alternative to PSG in appropriate patient populations that do not have certain comorbidities such as severe pulmonary disease, congestive heart failure, or neuromuscular disease.6 The American Academy of Sleep Medicine (AASM) 2017 guidelines outlines the use of HSAT in patients with a high probability of a diagnosis.7 Studies have shown, however, that data loss with the use of the HSAT can be as high as 18%.8 As such, guidelines state that if there is a high pretest probability of at least moderate OSA, an attended PSG should be completed if the HSAT is negative or “technically inadequate.”6 However, the PSG is not without its limitations.
Currently, the guidelines recommend a repeat in-laboratory PSG for the diagnosis of OSA if the initial PSG is negative and there is still clinical suspicion for OSA; however, the evidence to support this recommendation is considered to be weak.7 This was based on observational studies that looked at the incidence of first-night effect and night-to-night variability that showed there were patients who had considerable night-to-night variability in which their apnea-hypopnea index (AHI) crossed the threshold of the diagnosis cutoffs that would in turn change their management.9–12 With the time, inconvenience, and costs that come with repeat in-laboratory testing, our objective was to evaluate the utility of HSAT after a negative PSG and a high clinical suspicion for OSA. We hypothesized that when a sleep physician ordered a HSAT after negative PSG, it would more likely change the sleep diagnosis, and our aim was to identify the clinical and PSG characteristics more commonly seen in that particular group.
METHODS
Study Population
This was a retrospective study of 206 consecutive patients who had undergone an attended PSG and subsequent HSAT within the 3-year period between February 2012 and January 2015 at Baylor Scott and White Sleep Institute, a 14-bed sleep center with 7 sleep physicians who perform 4,000 attended PSG tests and 1,000 home tests annually. Exclusion criteria included children younger than 18 years and those without an initial negative diagnostic PSG. No therapeutic intervention was performed between the initial PSG and subsequent HSAT. Clinical data and data from both the PSG and HSAT for each patient was obtained. Demographics including age, sex, and race were recorded. Certain comorbid conditions including hypertension, coronary artery disease (CAD), stable congestive heart failure (CHF), and type 2 diabetes mellitus were recorded, along with specific treatments: oral appliances, sleep apnea management, or weight loss surgeries. Medication history was recorded, specifically the use of sedatives and any medication that may cause respiratory depression, such as narcotics. Anthropometric measurements included body mass index (BMI) and neck circumference. Excessive daytime sleepiness was measured using the Epworth Sleepiness Scale (ESS).
Polysomnography
All patients underwent a full in-laboratory PSG at the Baylor Scott and White Sleep Institute in accordance with American Academy of Sleep Medicine (AASM) guidelines using Compumedics Profusion 3 software (Abbotsford, Victoria, Australia). Standard 10–20 electroencephalogram, electrocardiogram, electromyography of the chin and anterior tibialis muscle, electrooculography, and pulse oximetry monitoring were applied. Oral and nasal airflow were measured with a thermister and nasal cannula. Patients' respiratory efforts were measured with plethysmography bands at the chest and abdomen including summation channel. Sleep staging was classified into awake, non-rapid eye movement sleep with stages N1, N2, N3, and rapid eye movement (REM) sleep (stage R sleep). Episodes of apnea were defined as decrease in airflow by ≥ 90% for ≥ 10 seconds and episodes of hypopnea were defined as decrease in airflow by ≥ 30% for ≥ 10 seconds along with a decrease in oxygen saturation of ≥ 4% in accordance with AASM guidelines. Apnea episodes were further classified into obstructive, central, or mixed according to the presence or absence of breathing efforts with thoracoabdominal paradox. The AHI was determined by the frequency of these events per hour during the patients' sleep time. Both the REM AHI and central AHI were recorded. Other PSG data included sleep efficiency, number of awakenings, sleep latency, total sleep time, time to wake after sleep onset, time spent with oxygenation < 90% (PSG 90%), % of sleep spent in REM sleep (REM%), minutes spent in REM sleep, REM AHI, time spent in the supine position, and the oxygen desaturation index (PSG ODI).
Home Sleep Apnea Tests
The HSAT was ordered by a sleep provider based on their clinical assessment following the negative PSG. There were a total of three different Type III devices used during the time of enrollment: ApneaLink (ResMed, Bella Vista, Sydney, Australia), Stardust II (Philips Respironics, Murrysville, Pennsylvania, United States), and Embletta (Flaga Group hf, Reykjavik, Iceland). Each patient was instructed on the use of the device and techniques for troubleshooting in person as to ensure understanding and answer any questions directly. Only a single night was recorded. The tests were considered to be satisfactory if there was a minimum of 6 hours of recording time along with a minimum of 4 hours (75%) of technically adequate oximetry and flow data obtained during the recording time. Variables collected from the HSAT included recording time, time spent with an oxygen saturation < 90%, the respiratory event index (REI) and ODI. Apnea and hypopnea were scored using the 4% criteria. The studies were scored by two sleep technologists, both of whom had no prior knowledge of the patient's history or previous studies. Interpreting physicians and ordering physicians were not necessarily the same person. Patients did not undergo therapeutic intervention between the PSG and the HSAT.
Statistical Analysis
All data were imported into SAS 9.4 (SAS Institute, Cary, North Carolina, United States) for analysis. Pearson chi-square test or Fisher exact test were used for comparisons of categorical variables by groups. Two sample t tests were used for comparison of the means of normally distributed variables. Wilcoxon rank-sum tests were used for comparison of the medians of non-normally distributed variables. Weighted kappa values were used to explore the level of agreement between the severity of PSG AHI and the HSAT REI. All statistical tests were two-sided and a test resulting in a value of P ≤ .05 was considered statistically significant. There were no multiplicity adjustments, or adjustments for multiple comparisons.
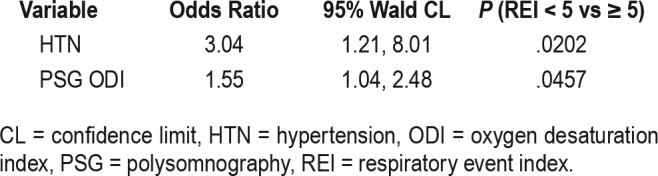
Univariate logistic regression models were implemented to select the possible predictors. A value of P = .10 was used to evaluate possible predictors in the final model. Regression diagnostics on the residuals and c statistics were used to assess the model. A multivariate logistic regression model was fit to the data. The Hosmer-Lemeshow test was used to assess fitness of data. Candidate predictors were the following variables: hypertension, age, PSG ODI, PSG AHI, PSG 90%. Diabetes could not be included in the model because all patients who had an HSAT REI < 5 events/h did not have diabetes. Due to the potential multicollinearity issue, only hypertension, age, and PSG ODI were included in the final model. The backward, forward, and stepwise model selection procedures were used to find the predictors in the final model. The binary outcome was evaluated by multivariate logistic regression, adjusted for hypertension and PSG ODI; P valves were .0202 and .0457, respectively. The c statistic was equal to .7065, indicating that the model predicts about 70.6% of the variability in the model. The Hosmer-Leme-show test indicates a good fit to the data. The study was Institutional Review Board (IRB) approved and consent was waived due to the retrospective, observational nature of the study (IRB 160029).
RESULTS
A total of 206 patients underwent an HSAT after an initial PSG. Of those patients, 10 were excluded for age younger than 18 years, 30 were excluded for insufficient data from the electronic medical record, and 25 were excluded for a positive PSG as defined as an AHI ≥ 5 events/h. A total of 141 patient charts were then further analyzed (Figure 1).
Figure 1. Study inclusion flow chart.
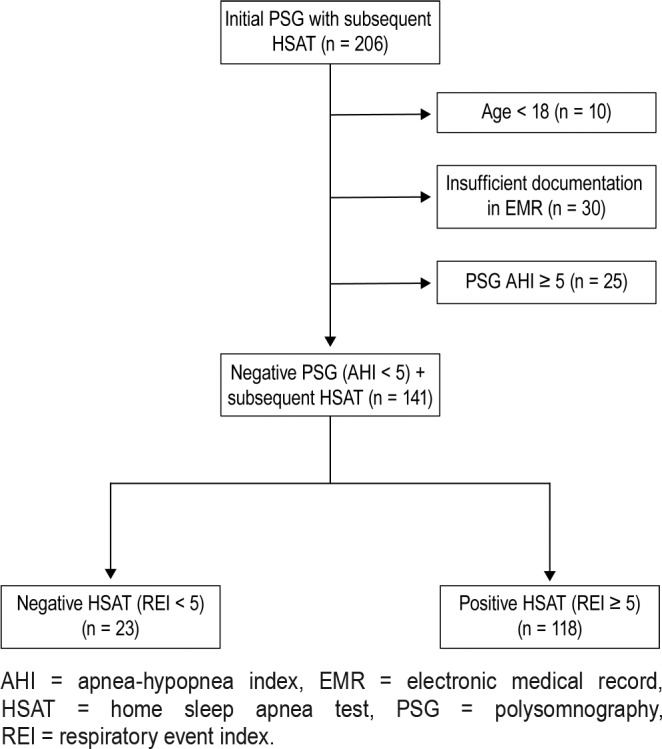
AHI = apnea-hypopnea index, EMR = electronic medical record, HSAT = home sleep apnea test, PSG = polysomnography, REI = respiratory event index.
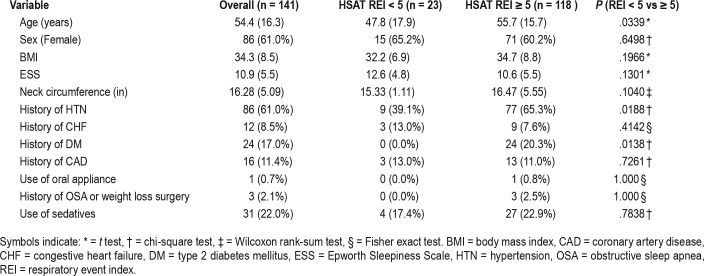
The average age was 54.4 ± 16.3 years. A total of 86 patients (61%) were female. Average BMI was 34.3 ± 8.5 kg/m2 and the average ESS score was 10.9 ± 5.5. In our study population, 118 patients (83.7%) had a subsequently positive HSAT (false-negative group). Of these, 91 patients (64.5%) received a diagnosis of mild OSA, 25 patients (17.7%) received a diagnosis of moderate OSA, and 2 patients (1.4%) received a diagnosis of severe OSA. Only 23 patients (16.3%) had a confirmatory negative diagnosis on HSAT (true negative group). Patients in the false-negative group more commonly had hypertension, diabetes mellitus, a higher PSG AHI (but less than 5 events/h), higher HSAT ODI, and were older (Table 1). The time between the PSG and the HSAT ranged from 1 to 1,043 days with a mean of 84.7 days and a median of 17 days.
Table 1.
Patient characteristics.
In analysis of clinical data between the two groups, there was no association between sex, comorbidities of CHF or CAD, use of oral appliances, sedatives, or a history of weight loss surgery. In the false-negative group (positive HSAT with REI ≥ 5 events/h), compared to the true negative group (negative HSAT with REI < 5 events/h), there was a higher incidence of hypertension 54.61% versus 6.38% (P = .0188) and type 2 diabetes mellitus 17% versus 0% (P = .0138). Average age was slightly older in the false-negative group at 55.7 years compared to 47.8 years in the other group (P = .0339). The BMI, ESS, and neck circumference were comparable between the two groups (Table 1).
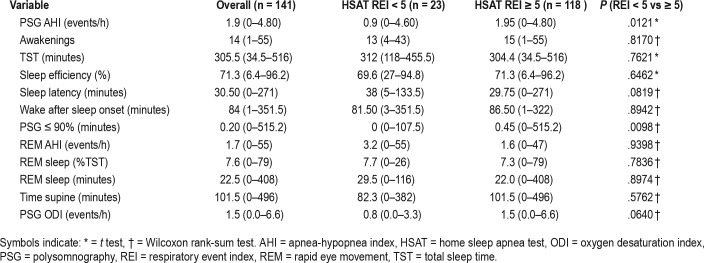
Analyzing polysomnographic data between the two groups, there was no statistical significance in the PSG REM AHI, REM%, or time spent supine. Sleep efficiency, number of awakenings, PSG latency, and PSG wake after sleep onset were also similar between the two groups. There was statistical significance in the PSG AHI (1.95 versus 0.90 events/h, P = .0121), but as they all had an AHI < 5 events/h per protocol, this is not considered to be clinically significant. The time in minutes spent with an oxygen saturation of ≤ 90% during the PSG (PSG 90%) was significant as those with a negative study did not have any desaturations (0.45 versus 0, P = .0098). Although there was no difference in the PSG ODI between the two groups, the HSAT ODI was higher in the false-negative group (8 versus 2.20 events/h, P < .0001) (Table 2).
Table 2.
Sleep variables.
All HSAT devices except the ApneaLink recorded time spent supine. Overall, patients spent more time supine while at home (231.5 minutes on HSAT versus 101.5 minutes on PSG). The false negative group had an average increase of 145.2 minutes spent supine from PSG to HSAT. The true negative group had an average increase of 130.9 minutes. The central apnea index on the HSAT devices were minimal with an average of 0.5 events/h across both groups (Table 3). Further logistic regression was performed (Table 4 and Table 5). The c statistic was equal to .706, indicating that the model predicts about 70.6% of the variability in the model. Most variables were not considered statistically significant except for hypertension. The odds of having an HSAT REI ≥ 5 events/h in patients with hypertension were 3.03 times the odds of patients without hypertension.
Table 3.
HSAT variables.
Table 4.
Univariate regression.
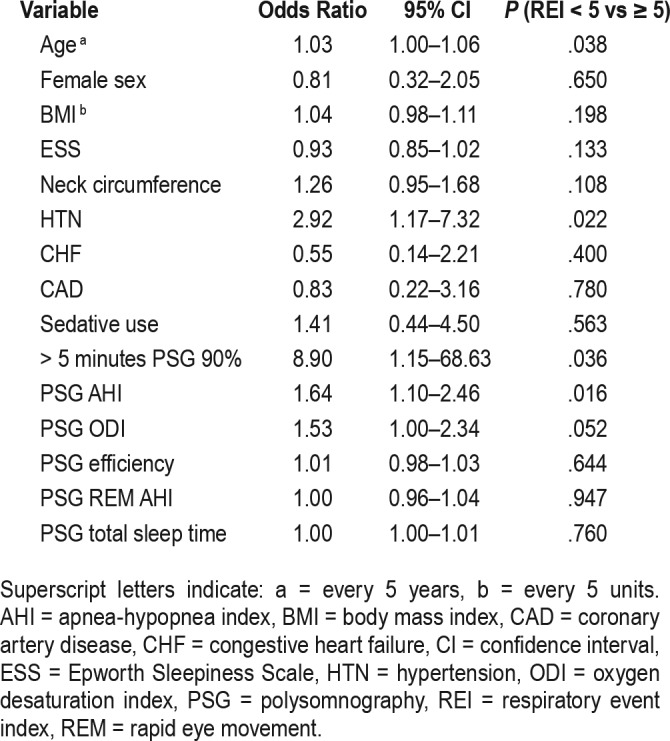
Table 5.
Multivariate regression.
Only approximately 20 of the 118 patients (17%) did not have documentation of treatment, whereas 98 patients (83%) were prescribed a positive airway pressure device.
DISCUSSION
In our cohort of patients, we found that when a sleep physician ordered an HSAT based on clinical suspicion after a negative PSG, OSA was diagnosed 83.7% of the time. The diagnosis of OSA included all severities, from mild to moderate to severe, with most patients having a diagnosis of mild OSA. The current guideline recommendation of a second in-laboratory PSG following a negative initial PSG is based on weak data.7 Our research effort is a discovery-phase study of clinical practice patterns of sleep study ordering. First, we found the follow-up study of HSAT to be useful in detecting OSA in those with a high pre-test clinical probability of OSA. Second, the patients with higher probability of having OSA tended to be older and had hypertension. Hypertension was also noted to triple the odds of having OSA on HSAT. The definition of OSA was based on a cutoff of 5 events/h, however, this does not indicate if the patients actually had OSA. In addition to the ESS score, symptoms were not assessed in this study. Most patients with a positive HSAT were prescribed treatment after repeat testing. The clinical significance of mild OSA is unclear and at this time, effectiveness of positive airway pressure as treatment in mild OSA remains controversial.13 Last, confounders such as the use of sleep aids, oral appliances, or weight loss surgery were similar in both groups; however, the total numbers were very low. Interestingly, the BMI, neck circumference, and ESS score were comparable between the two groups. Most sleep data such as sleep efficiency and REM AHI were not statistically significant. We speculate the increased representation of women in our study with negative PSG tests (61%) might be random. Older literature looking at negative PSG tests reflects a predominantly young population with average age of approximately 44 years. Between the two studies, one had 73% males, and the other had 55% females.14,15
There are several limitations to this study. This was only an observational, retrospective single-study review. A negative PSG with high pretest probability of OSA would certainly prompt discussions between providers and patients about the technical features of the overnight study that could have led to less than optimal sleep, including supine sleep, and therefore detection of OSA. The rationale for the HSAT as a second study was to improve sleep efficiency and supine sleep, as well as having more REM sleep to determine whether OSA would be detected in a more natural sleep environment. However, at our institution, there is no set protocol for second testing, and no specific instructions are provided for the HSAT such as spending more time supine while testing. The variability in the clinical decision reasoning for ordering the HSAT was not always mentioned. There are cost advantages to the insurance payers with HSAT, as well as convenience to the patients.16,17 At our center, the factors that played a role included insurance preference, patient preference, and clinical judgment. There was also variability in the time frame between the original PSG and the HSAT, so confounders, such as weight change between the studies, are possible, but not assessed. That raises the question as to why not order the HSAT as the first sleep study. Each sleep provider group follows a sleep study ordering pattern based on patient preferences, insurer mandates, as well as institution requirements, that cannot be generalized across regions or states. We estimate our ordering pattern of a PSG as the initial test mirrors that of many sleep laboratories in the country. Of note, our sleep laboratory follows the 4% desaturation rule for scoring hypopneas as mandated by the Centers for Medicare & Medicaid Services. It would be interesting to speculate the effect of using a 3% desaturation criteria on our study. We would expect to see less HSAT ordered due to higher percentage of positive PSG tests using the 3% criteria based on prior studies.18,19
Multiple HSAT type III devices were used. We are unaware of any major differences that would cause one device to be more prone to an REI ≥ 5 events/h. The variety in devices increases generalizability of our results. Outside of the neck circumference, data on gross airway assessment, such as the Mallampati score, was not analyzed due to incomplete documentation. It would be difficult to assess for differences in sleep deprivation between the two studies; however, the sleep efficiency in PSG between the two groups were similar. Although the first- night effect is not expected for the HSAT, night-to-night variability in REI on the HSAT might account for the positive HSAT, especially in those with mild OSA. BMI, comorbidities, and level of sleepiness did not predict the night-to-night variability in one prospective study where at least 2 nights of home testing was carried out in 84 subjects after a positive PSG. Notably, the REI on HSAT was higher than the AHI on PSG in those with mild OSA on PSG (Δ6.6, P < .0001), but not in those with moderate or severe OSA on PSG.20 In our study, 64.5% of our positive HSATs (false-negative group) had mild OSA. Our results reflect similar “overestimation bias” previously reported in patients with mild OSA on HSAT.20 Although postarousal central apneas can lead to false elevation of the REI on HSAT, the average central apnea index on HSAT in our study was 0.5 events/h, which is unlikely to have influenced the overall REI on HSAT. The possibility of “overscoring” bias was minimized by the use of two sleep technologists, who were only given the raw data from the HSAT to score. The PSG tests are scored by a separate set of sleep technicians.
PSG remains the gold standard in the diagnosis of OSA. HSAT is most recommended for use in patients with a high pretest probability of OSA and without comorbidities such as severe pulmonary disease, congestive heart failure, or neuro-muscular disease. There have been several studies that demonstrate similar continuous positive airway pressure adherence and outcomes in protocols utilizing either PSG or HSAT.21,22 Due to the time and costs associated with repeating an attended PSG along with the incidence of night-to-night variability,9–12 a subsequent HSAT may be a more reasonable option in patients with clinical features of OSA with a negative PSG.
OSA is a common sleep-related disorder that has been shown to be a risk factor in many different comorbid conditions such as stroke and cardiovascular disease.3,4 With the increasing prevalence and underdiagnosis of OSA, a single in-laboratory PSG may be insufficient to accurately assess disease, particularly patients with mild OSA, where night-to-night variability may increase the possibility of underdiagnosis or misdiagnosis. PSG, although comprehensive, is not without limitations. This study invokes a question about current recommendations for repeat testing after a negative PSG. Our study suggests repeat testing using HSAT in older patients with hypertension be considered if initial PSG is negative for OSA and there is a high clinical suspicion. Future randomized studies looking into the validity of HSAT after a negative PSG are needed.
DISCLOSURE STATEMENT
Institution where work was performed: Baylor Scott and White Sleep Institute All authors have seen and approve the manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CAD
coronary artery disease
- CHF
congestive heart failure
- ESS
Epworth Sleepiness Scale
- HSAT
home sleep apnea test
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- PSG 90%
time spent with oxygen saturation < 90% during polysomnography
- REI
respiratory event index
- REM
rapid eye movement
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shamsuzzaman ASM, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290(14):1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 4.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 5.Bawden FC, Oliveira CA, Caramelli P. Impact of obstructive sleep apnea on cognitive performance. Arq Neuropsiquiatr. 2011;69(4):585–589. doi: 10.1590/s0004-282x2011000500003. [DOI] [PubMed] [Google Scholar]
- 6.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med. 2007;3(7):737–747. [PMC free article] [PubMed] [Google Scholar]
- 7.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;1313(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flemons WW, Littner MR, Rowley J, et al. Home diagnosis of sleep apnea: a systematic review of the literature. Chest. 2003;124(4):1543–1579. doi: 10.1378/chest.124.4.1543. [DOI] [PubMed] [Google Scholar]
- 9.Ma J, Zhang C, Zhang J, et al. Prospective study of first night effect on 2-night polysomnographic parameters in adult Chinese snorers with suspected obstructive sleep apnea hypopnea syndrome. Chin Med J (Engl) 2011;124(24):4127–4131. [PubMed] [Google Scholar]
- 10.Gouveris H, Selivanova O, Bausmer U, Goepel B, Mann W. First-night-effect on polysomnographic respiratory sleep parameters in patients with sleep-disordered breathing and upper airway pathology. Eur Arch Otorhinolaryngol. 2010;267(9):1449–1453. doi: 10.1007/s00405-010-1205-3. [DOI] [PubMed] [Google Scholar]
- 11.Selwa LM, Marzec ML, Chervin RD, et al. Sleep staging and respiratory events in refractory epilepsy patients: Is there a first night effect? Epilepsia. 2008;49(12):2063–2068. doi: 10.1111/j.1528-1167.2008.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmadi N, Shapiro GK, Chung SA, Shapiro CM. Clinical diagnosis of sleep apnea based on single night of polysomnography vs. two nights of polysomnography. Sleep Breath. 2009;13(3):221–226. doi: 10.1007/s11325-008-0234-2. [DOI] [PubMed] [Google Scholar]
- 13.Littner MR. Mild obstructive sleep apnea syndrome should not be treated. Con. J Clin Sleep Med. 2007;3(3):263–264. [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer TJ, Eveloff SE, Kline LR, Millman RP. One negative polysomnogram does not exclude obstructive sleep apnea. Chest. 1993;103(3):756–760. doi: 10.1378/chest.103.3.756. [DOI] [PubMed] [Google Scholar]
- 15.Dean RJ, Chaudhary BA. Negative polysomnogram in patients with obstructive sleep apnea syndrome. Chest. 1992;10(1):105–108. doi: 10.1378/chest.101.1.105. [DOI] [PubMed] [Google Scholar]
- 16.Kim RD, Kapur VK, Redline-Bruch J, et al. An economic evaluation of home versus laboratory-based diagnosis of obstructive sleep apnea. Sleep. 2015;38(7):1027–1037. doi: 10.5665/sleep.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hui DS, Ng SS, To KW, et al. A randomized controlled trial of an ambulatory approach versus the hospital-based approach in managing suspected obstructive sleep apnea syndrome. Sci Rep. 2017;8:45901. doi: 10.1038/srep45901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruehland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32(2):150–157. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campos-Rodriguez F, Martínez-García MA, Reyes-Nuñez N, Selma-Ferrer MJ, Punjabi NM, Farre R. Impact of different hypopnea definitions on obstructive sleep apnea severity and cardiovascular mortality risk in women and elderly individuals. Sleep Med. 2016;27-28:54–58. doi: 10.1016/j.sleep.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Prasad B, Usmani S, Steffen AD, et al. Short-term variability in apneahypopnea index during extended home portable monitoring. J Clin Sleep Med. 2016;12(6):855–863. doi: 10.5664/jcsm.5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skomro RP, Gjevre J, Reid J, et al. Outcomes of home-based diagnosis and treatment of obstructive sleep apnea. Chest. 2010;138(2):257–263. doi: 10.1378/chest.09-0577. [DOI] [PubMed] [Google Scholar]
- 22.Rosen CL, Auckley D, Benca R, et al. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep. 2012;35(6):757–767. doi: 10.5665/sleep.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]