Abstract
Study Objectives:
Although both sleep-disordered breathing (SDB) and smoking are associated with cardiovascular disease (CVD), the potential for an interactive effect on CVD risk has not been explored. Our objective was to determine if smoking-related risk for CVD rises with greater SDB severity.
Methods:
Polysomnography and smoking history were obtained in 3,852 men and women in the Sleep Heart Health Study without baseline CVD. Fine-Gray proportional hazard models accounting for competing risk were used to calculate risk of incident CVD associated with SDB severity (defined by clinical cutoffs of the apnea-hypopnea index), smoking status (never, former, and current) and their interaction adjusting for potential confounders.
Results:
Over a mean (standard deviation) follow-up period of 10.3 (3.4) years, there were 694 incident CVD events. We found a significant three-way interaction of sex, current smoking, and moderate to severe SDB (P = .039) in the adjusted proportional hazards model. In adjusted analyses, women who were current smokers with moderate to severe SDB had a hazard ratio for incident CVD of 3.5 (95% confidence interval 1.6–8.0) relative to women who were nonsmokers without SDB. No such difference in CVD risk was observed in men or women of other strata of smoking and SDB.
Conclusions:
In women, smoking-related risk for CVD is significantly higher among individuals with moderate to severe SDB.
Citation:
Donovan LM, Feemster LC, Billings ME, Spece LJ, Griffith MF, Rise PJ, Parsons EC, Palen BN, O'Hearn D, Redline S, Au DH, Kapur VK. Risk of cardiovascular disease related to smoking is greater among women with sleep-disordered breathing. J Clin Sleep Med. 2018;14(11):1929–1935.
Keywords: cardiovascular disease, sleep-disordered breathing, smoking
BRIEF SUMMARY
Current Knowledge/Study Rationale: Small cross-sectional studies suggest effect modification between smoking and sleep-disordered breathing in markers of cardiovascular risk, but it is unclear whether smoking augments the risk of clinically significant cardiovascular disease posed by sleep-disordered breathing.
Study Impact: The current study finds evidence that smoking increases the risks of incident cardiovascular disease associated with moderate to severe sleep-disordered breathing among women but not men in the Sleep Heart Health Study. These results indicate that smoking and sleep-disordered breathing may interact to increase risks of cardiovascular events, such as heart attacks and strokes, among women. This subgroup may benefit from further research to evaluate the mechanisms of this interaction.
INTRODUCTION
Sleep-disordered breathing (SDB) and smoking are each associated with increased risk for cardiovascular disease (CVD).1–4 SDB increases risk for coronary heart disease and cerebrovascular disease mediated via multiple effects including endothelial dysfunction, increased sympathetic tone, augmented inflammation, and adverse metabolic effects involving glucose and lipid metabolism.5 Similarly, tobacco smoke predisposes patients to atherosclerosis and CVD, at least in part by causing endothelial dysfunction.3
As SDB and smoking individually cause endothelial dys-function, it is not surprising that, among individuals without clinical CVD, individuals with moderate to severe SDB and concurrent smoking had the greatest levels of endothelial dysfunction, as measured by peripheral arterial tonometry, compared to those with other intersections of SDB and smoking status.6 Further work has also shown that relative to the isolated exposure of smoking or SDB, those with combined exposure to smoking and SDB had augmentation of CVD risk factors such as adverse lipid profiles, C-reactive protein levels, and insulin resistance, with analyses demonstrating evidence of effect modification between smoking and SDB.7,8 These cross-sectional studies, however, do not address risk of incident disease, and two of these three studies focused exclusively on men.8
In order to evaluate whether SDB modifies the association between smoking and incident CVD, we analyzed long-term outcomes in the Sleep Heart Health Study (SHHS) among individuals without baseline CVD. In light of known differences by sex in cardiovascular risk associated with SDB in prior analyses,2,9,10 we explored effect modification by sex. Knowledge of which patients are at particularly high risk for the development of CVD can help prioritize groups for large-scale interventions.
METHODS
The SHHS was a nationwide prospective cohort study that recruited 6,441 individuals from 7 existing cohorts in order to evaluate CVD outcomes related to SDB.11 Individuals were included in the SHHS if they were not being treated for SDB and were older than 40 years. Data for the current analysis come from the Sleep Heart Health cohorts represented in the National Sleep Research Resource12 (NSRR, sleepdata.org) which includes data for 5,804 of the 6,441 individuals; 1 of the 7 parent cohorts, Strong Heart Study, did not provide data to the NSRR because of data sovereignty issues. Follow-up CVD data from a second cohort was not included in NSRR (n = 760) or included in prior SHHS longitudinal analyses9,10 because of concerns about data quality. We also excluded individuals with a history of baseline CVD (n = 626) or heart failure (n = 97), as heart failure substantially overlaps with CVD. Finally, we excluded individuals missing baseline smoking status (n = 14), follow-up CVD event data (n = 17), or with one or more missing covariates (total n = 438, of whom 362 were only missing lipids). Our final analytical cohort included 3,852 individuals (Figure 1). The use of the de-identified data in this analysis was approved by the National Sleep Research Resource, Brigham and Women's Hospital Institutional Review Board (# 2011P000161).
Figure 1. Flow of patients from SHHS parent cohort to the current sample.
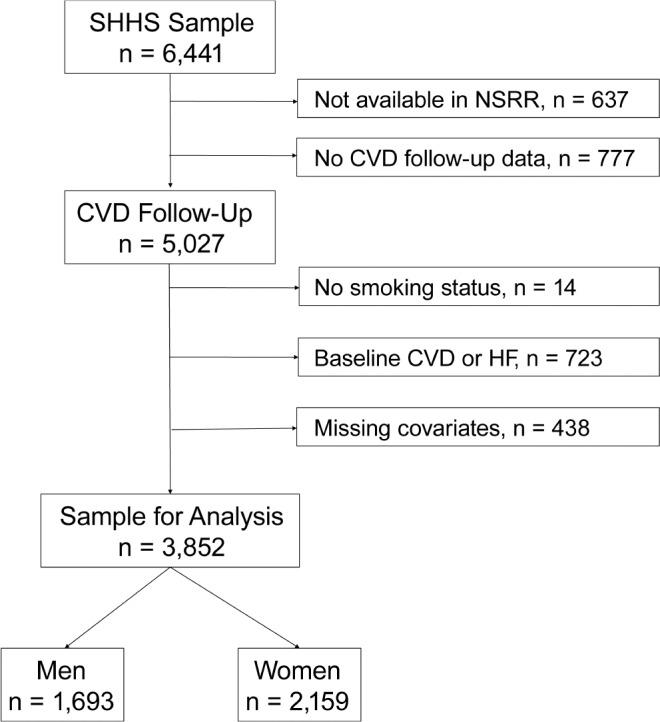
Covariates found to be missing included body mass index, blood pressure, and lipids (total cholesterol and high-density lipoprotein). CVD = cardiovascular disease, HF = heart failure, NSRR = National Sleep Research Resource, SHHS = Sleep Heart Health Study.
Participants underwent in-home polysomnography at the time of smoking history and baseline CVD risk assessment using methods described elsewhere.13 Apneas were defined as absence of airflow for at least 10 seconds, hypopneas were defined as at least a 30% reduction in airflow with an accompanied 4% drop in oxygen saturation by pulse oximetry, and apneas were characterized as either obstructive or central based on the presence or absence of respiratory muscle effort. All apneas and hypopneas were added together and divided by sleep duration in hours to create an apnea-hypopnea index (AHI). We categorized SDB severity according to the clinical cutoffs of AHI: < 5 events/h (no disease), 5 to < 15 events/h (mild disease), and ≥ 15 events/h (moderate to severe). Because only 89 women had severe SDB, moderate and severe SDB categories were collapsed into a single stratum in order to ensure that a sufficient number of smokers and nonsmokers were present in each SDB subgroup. The average as well as nadir (minimum) oxygen saturations during sleep were recorded. Spirometry was also obtained in a subset of individuals. Smoking history was obtained during an in-person interview that included questions regarding current smoking status and number of lifetime pack-years smoked. For our analyses, we classified individuals as current, former, or never smokers. Former smokers were considered never smokers if they reported less than one pack-year smoking history.
Our main outcome of interest was incident CVD. Incident CVD events were defined as the first occurrence of myocardial infarction, coronary artery bypass graft, coronary revascularization (including coronary artery bypass surgery), stroke, or death due to cardiovascular disease. Trained abstractors in the parent cohorts adjudicated each incident CVD event by thorough review of medical records.9
At baseline, SHHS participants completed questionnaires and underwent in-depth interviews regarding health history, current health status, and demographics. During in-person interviews participants were queried regarding physician diagnoses of CVD, heart failure, and diabetes mellitus as well as medication use. We defined diabetes as either participant-reported physician diagnosis of diabetes, or participant-reported use of an oral hypoglycemic drug or insulin. Study staff measured blood pressure, height, and weight. Blood samples were also collected for lipid levels at select parent examinations.9
Statistical Analysis
We present baseline data stratified by sex and SDB severity. In light of the competing risk of death without CVD, we modeled survival using a competing risk regression according to the proportional hazards model created by Fine and Gray that accounts for the subdistribution of a competing risk.14,15 To assess whether an interaction among sex, smoking status, and SDB was present, we used Fine-Gray proportional hazard models that included sex, SDB severity modeled as a categorical variable with common clinical cutoff points (no SDB AHI < 5 events/h; mild SDB AHI 5 to < 15 events/h; moderate to severe SDB, AHI ≥ 15 events/h), smoking status (never, current, and former), and the interaction term of sex × SDB severity × smoking status. To ease exposition, we also estimated the sex-specific risk at each intersection of smoking and SDB severity, by constructing sex-specific Fine-Gray proportional hazards models that included categories representing each strata of smoking status and SDB severity as independent variables. Never smokers with no SDB served as the referent group. The proportional hazards assumption was met in all models.16 To evaluate the cumulative exposure to cigarettes as ascertained at baseline, we also created proportional hazards models to evaluate the risk associated with every 10 pack-years of smoking history with models adjusted for the same aforementioned potential confounders. We adjusted each Fine-Gray proportional hazards model for age, race, body mass index (BMI), total and high-density cholesterol, systolic and diastolic blood pressure (SBP and DBP), and diabetes. With further sensitivity analyses, we repeated the initial three-way interaction model as a Cox proportional hazards model where competing risks were not considered. We also performed separate sensitivity analyses where lipids were not included as covariates and blood pressure was accounted for by the presence or absence of hypertension by the traditional definition of SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, or use of an antihypertensive medication17 in lieu of measured values.18–22 Given the known relationship between smoking and lung disease,23 we performed analyses to determine whether our findings existed independent of the potential mediators of hypoxic burden or impaired lung function. To do so, we performed sensitivity analyses that included average and nadir oxygen saturation, and percent predicted forced expiratory volume in 1 second as covariates. For all analyses, we used STATA version 14.2 (College Station, Texas, United States).
RESULTS
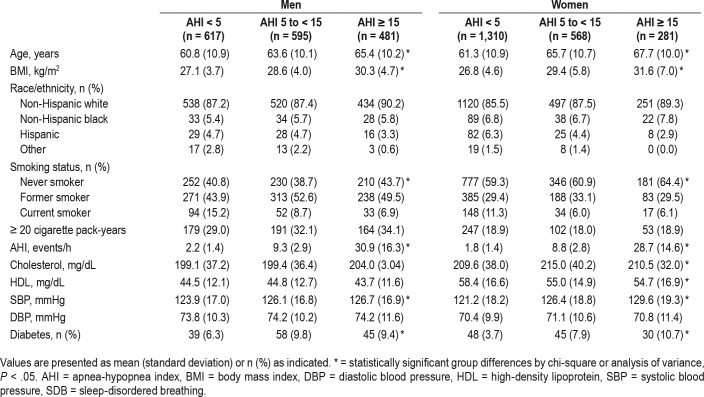
At baseline, this sample of 1,693 men and 2,159 women had a mean age of 63.2 years (standard deviation 10.8 years), with 1,996 never smokers, 1,478 former smokers, and 378 current smokers. Half of our sample had no SDB (n = 1,927, 50.0%), and of the 1,925 participants who had SDB, 1,163 (60.4%) had mild SDB, and 834 (39.6%) had moderate to severe SDB. The baseline statistics are presented stratified by sex and SDB severity in Table 1. Regardless of sex, those with greater severity of SDB tended to be older, with higher BMI, lower levels of education, and greater cholesterol levels. At the highest stratum of SDB severity, there was a lower proportion of current smokers (men, 6.9% current smokers with moderate-severe SDB versus 15.2% with no SDB; women, 6.1% current smokers with moderate-severe SDB versus 11.3% with no SDB). The proportion of participants with at least a 20 pack-year smoking history was similar across SDB strata.
Table 1.
Baseline characteristics of sample stratified by sex and SDB severity.
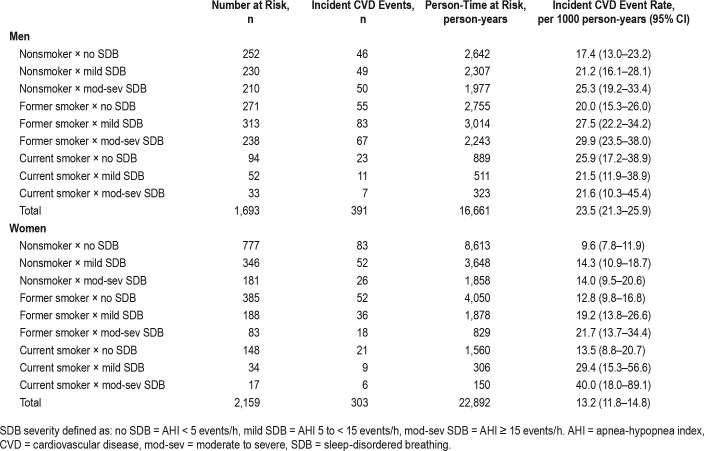
Over an average follow-up period of 10.3 (3.4) years, there were 694 incident CVD events, with 23.1% (n = 391) of men and 14.0% (n = 303) of women experiencing an incident CVD event. These events included 170 nonfatal myocardial infarctions, 73 fatal myocardial infarctions, 170 nonfatal strokes, 42 fatal strokes, and 239 coronary revascularization procedures. Additionally, 12.2% of men (n = 206) and 9.8% of women (n = 212) experienced a competing event of non-CVD related death. Unadjusted rates for incident CVD events for each intersection of smoking status and clinical cutoff point of SDB are presented in Table 2. Women with moderate to severe SDB who were current smokers had the highest rate of CVD (40.0 events per 1000 person-years, 95% confidence interval [CI] 18.0–89.1).
Table 2.
Rate of incident cardiovascular disease in each intersection of smoking status and sleep-disordered breathing severity by sex.
In a competing risks regression model assessing the three-way interaction of smoking status, SDB severity (defined by clinical cutoffs), and sex, the main effect of sex was found to be significant in both the unadjusted (hazard ratio [HR] for female sex 0.55, 95% CI 0.39–0.80) and adjusted (HR 0.49, 95% CI 0.33–0.71) models (Table S1 in the supplemental material, model 1). No interactions were significant in the unadjusted model, but the interaction of female sex, current smoking, and moderate to severe SDB (HR 4.30, 95% CI 1.08–17.18) was significant in the adjusted model. In sensitivity analyses, although point estimates and CIs varied, the interaction of female sex, current smoking, and moderate to severe SDB consistently had the highest point-estimate of any interaction term, including the model that incorporated average and nadir oxygen saturation (Table S1, models 2 through 6).
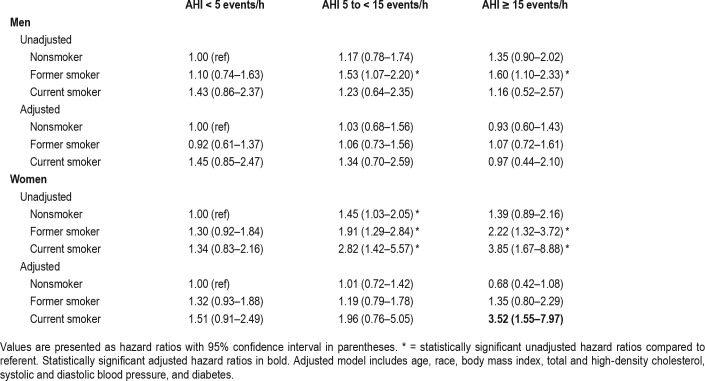
In light of a significant three-way interaction, we then explored the relative magnitude of risk by sex. We evaluated sexspecific risk for incident CVD at different smoking and SDB severity by clinical cutpoint. In Table 3, from separate models in men and women, we present unadjusted and adjusted HRs relative to nonsmokers without SDB. Among men, in the adjusted model, no significant association between SDB and CVD was found in any stratum. In contrast among women, only current smokers with moderate to severe SDB had a greater risk of incident CVD than nonsmokers without SDB in the adjusted model (HR of 3.52, 95% CI 1.55–7.97, Table 3).
Table 3.
Risk of cardiovascular disease event by interaction of smoking status and sleep-disordered breathing severity stratified by sex.
To evaluate the risk of CVD by cumulative smoking history, we evaluated risk related to cumulative pack years in sex-specific strata of SDB severity. As outlined in Figure 2, the greatest incidence of CVD related to cumulative smoking history was observed among women with moderate to severe SDB (HR 1.28, 95% CI 1.12 to 1.48 per 10 additional pack years).
Figure 2. Risk of CVD related to smoking history by SDB status.
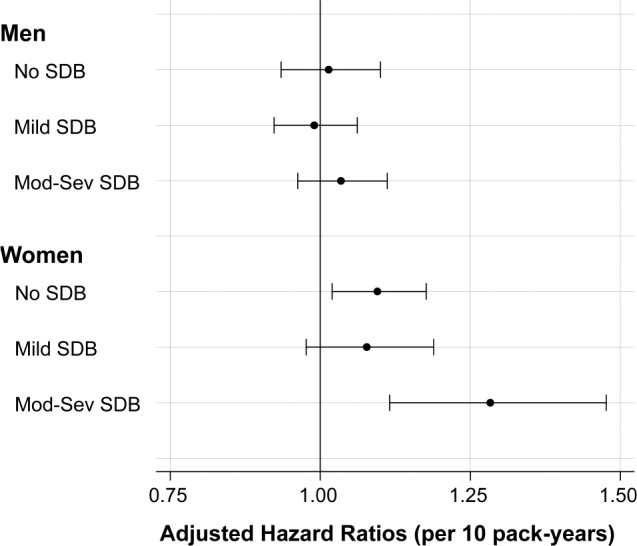
Forest plot indicates hazard ratio and 95% confidence interval for CVD associated with each 10 pack-years of smoking history in models stratified by sex SDB severity. Models were adjusted for age, race, body mass index, blood pressure, lipids, and diabetes status. CVD = cardiovascular disease, Mod-Sev = moderate to severe, SDB = sleep-disordered breathing.
Finally, we further explored whether measures of overnight oxygenation (average and nadir oxygen saturation during sleep) influenced the primary findings. We explored whether oxygen saturation values during sleep varied within strata of SDB severity by smoking status. There were no consistent differences in these values across smoking strata, especially among those with moderate to severe SDB (Figure S1 and Figure S2). Furthermore, inclusion of these variables as covariates in the primary model did not materially alter the significant sex × SDB × smoking interaction (Table S1, model 5).
DISCUSSION
Although overall prevalence of SDB and incidence of CVD was higher among men, we found that risk of incident CVD associated with current smoking was greater with moderate to severe SDB only in women. The sex-specific nature of our findings may relate to differences between men and women in the relationship between SDB and endothelial dysfunction. Prior work among older adults found that SDB was associated with greater endothelial dysfunction in women than men, a finding that may be related to sex-specific mechanisms in endothelial responses to stress and changes in blood flow.24,25 Furthermore, because SDB tends to develop after menopause, women are exposed to SDB at an older age than men, when rates of other CVD risk factors are increasing.26
In contrast to the relationship between sex and SDB related endothelial dysfunction, contradictory evidence exists with regard to sex differences in the association of SDB with CVD. Prior research from the SHHS, based on a follow-up period of approximately 8 years, demonstrated a relationship between SDB and incident coronary heart disease, heart failure, and stroke in men but not women.9,10 However, the Wisconsin Sleep Cohort Study found the opposite scenario with women tending to have greater risk for coronary heart disease related to SDB than men.2 The disparate results between these two groups have been attributed to the younger age group in the Wisconsin Sleep Cohort, which could capture SDB-related incident disease among individuals in middle age.2 The Wisconsin Sleep Cohort Study also followed participants over a longer period of time, 24 years. Furthermore, 14-year follow-up from of one of the subcohorts within SHHS, the Atherosclerosis Risk in Communities study, demonstrated a stronger association between high-sensitivity troponin, a marker of early cardiac injury, and SDB among women compared to men.27,28 It is possible that this longer duration of outcome assessment is necessary to fully capture the greater SDB-related CVD risk among women.
Our findings persisted after controlling for several potential confounders and possible mediators, including diabetes, hypertension, lipids, and intermittent hypoxemia, suggesting that the mechanism of our disparate sex-related findings occurs outside of these pathways and may be related to direct effects of SDB and smoking on the endothelium. However, we cannot exclude the possibility of unmeasured confounding. For example, adverse health behaviors associated with CVD such as inactivity could have been concentrated among individuals with a significant smoking history and SDB,29,30 and it is possible that these unmeasured confounders could be differentially distributed between men and women. For instance, we did not assess lung function over time, and women may be more susceptible to the deleterious effects of smoking on lung function.23 It is also worth noting that there were few women in our sample with moderate to severe SDB who were currently smoking within the cohort, and this could have allowed a small number of CVD events to bias our results. This concern is mitigated by the fact that our findings were robust and persisted in multiple sensitivity analyses, including analyses of cigarette pack- years as a continuous variable among all women with moderate to severe SDB.
This work has several limitations. The reliance on self-reported smoking history rather than an objective and more continuous marker of exposure such as urine cotinine increases the risk of exposure misclassification.31 This misclassification would likely bias toward a null finding, and would serve to blunt our ability to ascertain effect modification within other strata. Another limitation is the dearth of younger individuals in our cohort. As the disparate results of the Wisconsin Sleep Cohort and SHHS suggest, age plays a major role in the CVD risks of SDB, and therefore analysis of these findings in a younger cohort would be recommended. The study also has a number of strengths, including robust long-term follow-up with comprehensive ascertainment of CVD outcomes in multiple cohorts, a large sample size, and assessment of SDB with the gold standard, polysomnography.
This analysis presents evidence of an important sex-specific interaction between smoking and SDB in the risk of CVD. Such an interaction suggests that women who are current smokers and who have SDB are at particularly high risk for incident CVD. Future work evaluating the sex-specific effects of SDB treatment among individuals of different smoking status may yield additional insights into the possible mechanisms of our findings. For now, women who are current smokers may derive benefit from testing and therapy for SDB in addition to smoking cessation counseling to reduce their risk for CVD.
DISCLOSURE STATEMENT
This study was funded by NIH grants R24HL114473-02, T32HL007287-38, F32 HL140685-01, U01HL53916, U01HL53931, U01HL53934, U01HL53937, U01HL53938, U01HL53940, U01HL53941, U01HL64360. Dr. Donovan's work is funded in part by the ASPIRE (Academic Sleep Pulmonary Integrated REsearch/ Clinical) Fellowship. Study staff was supported by VA Puget Sound Health Services Research & Development. The funding sources were not involved in the design, conduction, or analysis of this project. Dr. Au reports personal fees from Novartis for service on a data monitoring committee, personal fees from American Board of Internal Medicine for service on the exam writing committee, and personal fees from Annals of the American Thoracic Society for service as a deputy editor, outside the submitted work. Dr. Billings reports nonfinancial support in the form of CPAP devices for research donated from Philips Respironics, outside the submitted work. Dr. Donovan reports grant support from NIH NHLBI T32HL007287-38, F32 HL140685-01, and the ATS ASPIRE (Academic Sleep Pulmonary Integrated REsearch/Clinical) Fellowship during the conduct of the study. Dr. Griffith reports grant support from NIH NHLBI T32HL007287-38, during the conduct of the study. Dr. Spece reports grant support from NIH NHLBI T32HL007287-38 and F32HL142125-01, during the conduct of the study. Dr. Feemster reports grants from NIH K23 HL111116, grants from American Lung Association, grants from Veteran's Health Administration, outside the submitted work. Dr. Redline reports grant support from the NIH, including R24HL114473 and R35HL135818, during the conduction of the study, and grant support from Jazz Pharmaceuticals and Beckman Coultier and personal fees from the Annals of the American Thoracic Society for work outside the submitted work. Dr. Kapur, Dr. O'Hearn, Dr. Palen, Dr. Parsons, and Mr. Rise report no conflicts of interest. The views expressed here are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
ACKNOWLEDGMENTS
The authors acknowledge and thank all of the investigators and staff involved in the Sleep Heart Health Study and the National Sleep Research Resource; this analysis would not have been possible without them.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- HR
hazard ratio
- NSRR
National Sleep Research Resource
- SBP
systolic blood pressure
- SDB
sleep-disordered breathing
- SHHS
Sleep Heart Health Study
REFERENCES
- 1.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 2.Hla KM, Young T, Hagen EW, et al. Coronary heart disease incidence in sleep disordered breathing: the Wisconsin Sleep Cohort Study. Sleep. 2015;38(5):677–684. doi: 10.5665/sleep.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 4.Strohl KP, Redline S. Recognition of obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154(2 Pt 1):279–289. doi: 10.1164/ajrccm.154.2.8756795. [DOI] [PubMed] [Google Scholar]
- 5.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569–576. doi: 10.1016/j.jacc.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lui MM, Mak JC, Lai AY, et al. The impact of obstructive sleep apnea and tobacco smoking on endothelial function. Respiration. 2016;91(2):124–131. doi: 10.1159/000443527. [DOI] [PubMed] [Google Scholar]
- 7.Lavie L, Lavie P. Smoking interacts with sleep apnea to increase cardiovascular risk. Sleep Med. 2008;9(3):247–253. doi: 10.1016/j.sleep.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Zhu H, Xu H, Chen R, et al. Smoking, obstructive sleep apnea syndrome and their combined effects on metabolic parameters: evidence from a large cross-sectional study. Sci Rep. 2017;7(1):8851. doi: 10.1038/s41598-017-08930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 12.Dean DA, 2nd, Goldberger AL, Mueller R, et al. Scaling up scientific discovery in sleep medicine: the National Sleep Research Resource. Sleep. 2016;39(5):1151–1164. doi: 10.5665/sleep.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21(7):759–767. [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 15.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grambsch PM, Therneua TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 17.Egan BM, Zhao Y. Different definitions of prevalent hypertension impact: the clinical epidemiology of hypertension and attainment of healthy people goals. J Clin Hypertens. 2013;15(3):154–161. doi: 10.1111/jch.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Ceron E, Barquiel B, Bezos AM, et al. Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. A randomized clinical trial. Am J Respir Crit Care Med. 2016;194(4):476–485. doi: 10.1164/rccm.201510-1942OC. [DOI] [PubMed] [Google Scholar]
- 19.Roche F, Pepin JL, Achour-Crawford E, et al. At 68 years, unrecognised sleep apnoea is associated with elevated ambulatory blood pressure. Eur Respir J. 2012;40(3):649–656. doi: 10.1183/09031936.00162710. [DOI] [PubMed] [Google Scholar]
- 20.Narkiewicz K, van de Borne PJ, Hausberg M, et al. Cigarette smoking increases sympathetic outflow in humans. Circulation. 1998;98(6):528–534. doi: 10.1161/01.cir.98.6.528. [DOI] [PubMed] [Google Scholar]
- 21.Borgel J, Sanner BM, Bittlinsky A, et al. Obstructive sleep apnoea and its therapy influence high-density lipoprotein cholesterol serum levels. Eur Respir J. 2006;27(1):121–127. doi: 10.1183/09031936.06.00131304. [DOI] [PubMed] [Google Scholar]
- 22.Craig WY, Palomaki GE, Haddow JE. Cigarette smoking and serum lipid and lipoprotein concentrations: an analysis of published data. BMJ. 1989;298(6676):784–788. doi: 10.1136/bmj.298.6676.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X, Weiss ST, Rijcken B, Schouten JP. Smoking, changes in smoking habits, and rate of decline in FEV1: new insight into gender differences. Eur Respir J. 1994;7(6):1056–1061. [PubMed] [Google Scholar]
- 24.Faulx MD, Larkin EK, Hoit BD, Aylor JE, Wright AT, Redline S. Sex influences endothelial function in sleep-disordered breathing. Sleep. 2004;27(6):1113–1120. doi: 10.1093/sleep/27.6.1113. [DOI] [PubMed] [Google Scholar]
- 25.Mirer AG, Young T, Palta M, Benca RM, Rasmuson A, Peppard PE. Sleep-disordered breathing and the menopausal transition among participants in the Sleep in Midlife Women Study. Menopause. 2017;24(2):157–162. doi: 10.1097/GME.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virdis A, Ghiadoni L, Sudano I, et al. Endothelial function in hypertension: role of gender. J Hypertens Suppl. 2002;20(2):S11–S16. [PubMed] [Google Scholar]
- 27.Querejeta Roca G, Redline S, Punjabi N, et al. Sleep apnea is associated with subclinical myocardial injury in the community. The ARIC-SHHS study. Am J Respir Crit Care Med. 2013;188(12):1460–1465. doi: 10.1164/rccm.201309-1572OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roca GQ, Redline S, Claggett B, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the Atherosclerosis Risk in Communities-Sleep Heart Health Study. Circulation. 2015;132(14):1329–1337. doi: 10.1161/CIRCULATIONAHA.115.016985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue-Choi M, Liao LM, Reyes-Guzman C, Hartge P, Caporaso N, Freedman ND. Association of long-term, low-intensity smoking with all-cause and cause-specific mortality in the National Institutes of Health-AARP Diet and Health Study. JAMA Intern Med. 2017;177(1):87–95. doi: 10.1001/jamainternmed.2016.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Silva RP, Martinez D, Pedroso MM, et al. Exercise, occupational activity, and risk of sleep apnea: a cross-sectional study. J Clin Sleep Med. 2017;13(2):197–204. doi: 10.5664/jcsm.6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray RP, Connett JE, Lauger GG, Voelker HT. Error in smoking measures: effects of intervention on relations of cotinine and carbon monoxide to self-reported smoking. The Lung Health Study Research Group. Am J Public Health. 1993;83(9):1251–1257. doi: 10.2105/ajph.83.9.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.