Abstract
Study Objectives:
Obstructive sleep apnea (OSA) and hypertension are independent risk factors of cardiovascular morbidities. This study aims to investigate the relationship between OSA, blood pressure (BP) control, and myocardial injury in patients with difficult-to-control hypertension.
Methods:
Patients with hypertension who required three or more medications were prospectively recruited at a tertiary referral center. In-laboratory polysomnography, followed by blood tests for fasting glucose, glycated hemoglobin, lipids, high-sensitivity troponin I (hsTnI), B-type natriuretic peptide (BNP), C-reactive protein, and advanced oxidation protein products were performed. After polysomnography, 24-hour ambulatory BP monitoring was arranged.
Results:
A total of 98 participants were analyzed, with mean age 51 ± 9 years and body mass index 30 ± 5 kg/m2. Previously undiagnosed severe OSA (apneahypopnea index [AHI] ≥ 30 events/h) was present in 51 patients (52%). hsTnI was negatively correlated with nocturnal dip in systolic BP (r = −.205, P = .048). After controlling for confounders, including BP control, AHI and oxygen desaturation index (ODI) were positively correlated with hsTnI (r = .282, P = .009 and r = .279, P = .010, respectively) and C-reactive protein (r = .302, P = .005 and r = .285, P = .008, respectively), but not with BNP or advanced oxidation protein products. Age, ODI, and loss of nocturnal systolic BP dip were significant determinants of hsTnI level (β = .225, P = .022; β = .293, P = .003; and β = −.215, P = .029; R2 = .151). Age, female sex, 24-hour mean diastolic BP, and metabolic syndrome, but not indices of apnea severity, were predictors of BNP level.
Conclusions:
Unrecognized severe OSA was common in patients with difficult-to-control hypertension, and OSA severity was associated with myocardial injury, independent of BP control with medications.
Clinical Trial Registration:
Registry: ClinicalTrials.gov, Title: A Cross-sectional Study of the Occurrence and Effect of Obstructive Sleep Apnea in Subjects With Resistant Hypertension, Identifier: NCT00843583, URL: https://clinicaltrials.gov/ct2/show/NCT00843583
Citation:
Lui MM, Tse HF, Mak JC, Lam DC, Chan CW, Chong PW, Ip MS. Untreated obstructive sleep apnea is associated with myocardial injury independent of blood pressure control in hypertension. J Clin Sleep Med. 2018;14(11):1841–1847.
Keywords: brain natriuretic peptide, hypertension, obstructive sleep apnea, troponin
BRIEF SUMMARY
Current Knowledge/Study Rationale: Individuals with hypertension requiring multiple medications have a high cardiovascular risk, and they commonly have concomitant obstructive sleep apnea (OSA). Evidence supporting the association between OSA and myocardial damage in difficult-to-control hypertension is lacking.
Study Impact: This is the first study substantiating that low-grade myocardial injury is present in patients with OSA and difficult-to-control hypertension, irrespective of blood pressure control with medications. Frequent intermittent hypoxia, aging, and loss of nocturnal dip in systolic blood pressure were predictors of higher high-sensitivity troponin level. The current study affirms the high prevalence of severe OSA in difficult-to-control hypertension, and emphasizes that in addition to controlling blood pressure with medications, it is also necessary to target the control of OSA to mitigate cardiovascular risk.
INTRODUCTION
Obstructive sleep apnea (OSA) and hypertension are highly prevalent diseases worldwide and both are linked to a series of cardiovascular diseases, mortality, and tremendous economic loss.1–3 OSA is common in patients with resistant hypertension, with prevalence rates ranging from 71% to 85%.4–7 Hypertension is known to lead to end- organ damage, but the effect of concomitant OSA on end-organ injury has not been well investigated.7
Lately, the causal relationship between OSA and coronary heart disease (CHD) is strengthened with more evidence.1 Interplay of multiple mechanisms, including heightened sympathetic activity, activated inflammation, oxidative stress, and impaired vascular repair in OSA, has been implicated to explain the association.8,9 Troponin I is widely used as a sensitive and specific marker of myocardial injury in acute coronary syndromes, and recent availability of high-sensitivity troponin I (hsTnI) assay allows its detection in the general population and in patients with stable CHD.10 Several studies have investigated the relationship between troponin level and OSA severity in stable patients, but results have been conflicting.11–17 Another biomarker, B-type natriuretic peptide (BNP), has been used for diagnosis and prognostication of heart failure.18 Most studies did not find a significant association between OSA severity and BNP levels. C-reactive protein (CRP) and advanced oxidation protein products, indicating systemic inflammation and oxidative stress respectively, were also investigated in the current study.
Given the advent of many potent antihypertensive drugs, blood pressure (BP) control could be achieved more effectively, and theoretically end-organ injury due to high BP should be mitigated. We hypothesize that untreated OSA would confer independent myocardial injury in these patients, even if BP control is achieved with medications. This study aims to investigate the prevalence of untreated OSA and its association with myocardial injury, regardless of BP control, in a clinic-based Chinese sample with difficult-to-control hypertension—defined as requiring three or more antihypertensive medications.
METHODS
Participant Recruitment
Medical records of Chinese patients (age 18 years or older) attending the Hypertension Clinic, Department of Medicine, Queen Mary Hospital, were screened for eligibility. Those who were on three or more antihypertensive medications for BP control were selected and interviewed by the investigators between February 2009 and February 2014. They were invited for overnight attended polysomnography (PSG) at the Ho Ting Sik Sleep Disorder Center of Queen Mary Hospital. Types of antihypertensive medication included angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, beta blockers, calcium-channel blockers, diuretics, alpha blockers, and central-acting agents. Metabolic syndrome was defined when three of the criteria were met: elevated waist circumference (≥ 85 cm for Chinese men, ≥ 80 cm for Chinese women), elevated triglyceride level (≥ 1.7 mmol/L) or on medication, reduced HDL or on medication (1.0 mmol/L in men, 1.3 mmol/L in women) elevated BP or on medication (systolic ≥ 130 mmHg and/or diastolic ≥ 85 mmHg), elevated fasting glucose level (≥ 100 mg/dL) or on medication.19 All participants fulfilled the criteria on BP, and the presence of two additional criteria would define metabolic syndrome. BP goal referenced the recommendation from the Eighth Joint National Committee.20 Because most of the study participants were younger than 60 years and all were at high cardiovascular risk, the universal BP goal of systolic BP < 140 mmHg and diastolic BP < 90 mmHg were adopted. Resistant hypertension was defined as BP that remained above goal despite the concurrent use of three antihypertensive medications, or using more than three medications.21
Exclusion criteria were: moderate renal impairment (glomerular filtration rate < 30 mL/min/m2), presence of other causes of secondary hypertension, known history of OSA with or without treatment, uncontrolled congestive heart failure, regular use of drugs that affect BP (such as nonsteroidal anti-inflammatory drugs or steroid), chronic drinker (alcohol intake > 3 times per week), and noncompliance with antihypertensive medications (by interview).
All recruited participants underwent the investigations described in the following paragraphs. The study was approved by the Institutional Review Board of the University of Hong Kong and Hospital Authority Hong Kong (IRB ref: UW08-421). All participants gave written informed consent. The study was registered at ClinicalTrials.gov (NCT00843583).
Questionnaire, Anthropometric and Ambulatory Blood Pressure Measurements
All participants filled in a sleep questionnaire inquiring about smoking exposure, drinking status, and Epworth Sleepiness Scale (ESS) score. History of CHD with or without interventions, heart failure, and ventricular hypertrophy were determined via questionnaire and medical records. Body mass index (kg/m2) was calculated, and waist circumference was measured at a level halfway between the lower rib margin and the iliac crest. Ambulatory BP was measured by Spacelab Ultralite monitor (Spacelabs Heathcare, Issaqua, Washington, United States) with appropriate cuff sizes, and the measurements were taken every 30 minutes in daytime (7:00 am to 11:00 pm) and every hour at bedtime (11:00 pm to 7:00 am).22 The nocturnal decrease in BP (dips in circadian rhythm) was calculated by the following formula23:
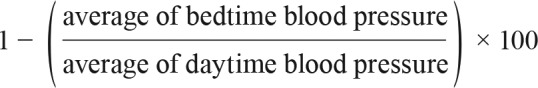 |
Polysomnography
Participants underwent an overnight 16-channel PSG (Alice 5 Diagnostics System; Respironics, Inc., Murrysville, Pennsylvania, United States), which consisted of recording for electroencephalography, electrooculography, electromyography, electrocardiography, nasal pressure transducer, thermistor for nasal airflow, thoracic and abdominal impedance belts, pulse oximetry, tracheal microphone for snoring, and sensors for leg and sleep position. Polysomnographic recordings were manually scored by a qualified technologist. Apnea was scored when there was a drop in the peak signal excursion by ≥ 90% of baseline for ≥ 10 seconds using an oronasal thermal sensor. Hypopnea was scored when the peak signal excursions drop by ≥ 30% of baseline for ≥ 10 seconds using nasal pressure in association with either ≥ 3% arterial oxygen desaturation or an arousal.24 OSA was defined by apnea-hypopnea index (AHI) ≥ 5 events/h, whereas AHI ≥ 30 events/h indicated severe OSA.
Blood Sampling for Biomarker Assays
Blood sample was collected on the next morning after fasting for 8 hours. Biochemical analyses for lipid, glucose, and HbA1c were performed. Glomerular filtration rate was calculated with the Modified Diet in Renal Disease Equation using serum creatinine levels. Serum/plasma was stored at −80°C. hsTnI was measured by commercially available chemilumines-cent microparticle immunoassay kits (Architect i1000SR AbbottW, Paris, France), of which the lower level of detection is 1.2 ng/L. The 99th percentile values of serum hsTnI in the Chinese community-dwelling male and female participants are 8.5 and 7.6 ng/L, respectively.25 CRP (Diagnostic Systems Laboratories Inc., Webster, Texas, United States) and BNP (Architect i1000SR AbbottW) were measured by enzyme-linked immunosorbent assay kits. The detectable limit of BNP was above 10 pg/mL. The value below the limit would be considered as undetectable, and was converted to 0.5 for regression analysis. Advanced oxidation protein products (AOPP) was measured in duplicate using a microplate reader by spectrophotometric detection method. All assays were performed according to manufacturer's instruction.
Statistics
Variables not in normal distribution were log-transformed for parametric tests. Pearson correlation was used to examine for any linear association between continuous variables without adjustment and their residuals after adjustment for covariates. Stepwise multivariate regression analysis with forward selection was performed with hsTnI and BNP as the dependent variable. Potential determinants of hsTnI and BNP were entered as independent variables. For two-group analysis, participants were divided into two groups according to their OSA severity: those without severe OSA (AHI < 30 events/h) and those with severe OSA (AHI ≥ 30 events/h), and the means/medians were compared using the t test or Mann-Whitney U test. Chi-square test was used for categorical data. Statistical significance was defined by values of P < .05. Statistical analysis was performed using a commercial software package (SPSS version 20, IBM Corp., Armonk, New York, United States).
RESULTS
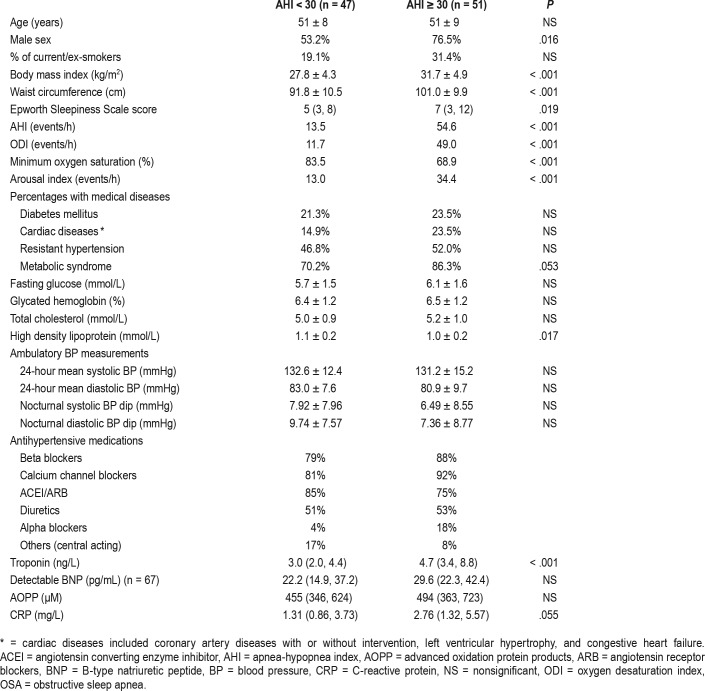
A total of 786 outpatient medical records were screened for eligibility. Of these, 50 individuals (16 were on 3 or more antihypertensive medications) with prior history of OSA were excluded. We found 116 individuals who fulfilled the inclusion criteria and attended clinic visits. They were interviewed by the investigators, and 108 consented to the study and had undergone PSG. Six were excluded from further analysis due to inadequate total sleep time (less than 200 minutes), three were excluded due to obesity hypoventilation or central sleep apnea, and one withdrew due to refusal of 24-hour ambulatory BP measurement. Thus, 98 participants were analyzed, of whom 64 (64.3%) were men. The median age was 52 years (inter-quartile range 45, 58). Twenty-six participants (27%) had BP above goal (uncontrolled BP), whereas 48 (50%) fulfilled the definition of resistant hypertension. Seventy-seven participants (79%) fulfilled the diagnostic criteria of metabolic syndrome. The prevalence of undiagnosed OSA (AHI ≥ 5 events/h) in this cohort was 90.8% (n = 89): 17.3% (17/98) had mild OSA, 21.4% (21/98) had moderate OSA, and 52% (51/98) had severe OSA. Among those with severe OSA (n = 51), 30 (59%) had an ESS score < 10, indicating that presence of OSA was poorly reflected by degree of sleepiness as assessed by ESS.
No direct correlations were found between polysomno-graphic variables and BP variables (24-hour mean systolic or diastolic BP, mean nocturnal systolic or diastolic BP, and nocturnal fall in BP), with or without adjustment for confounding factors. Presence of severe OSA or OSA severity indices was not associated with nocturnal BP decrease, even after adjustment for the number of antihypertensive medications (Table 1).
Table 1.
Demographic and clinical characteristics according to OSA severity.
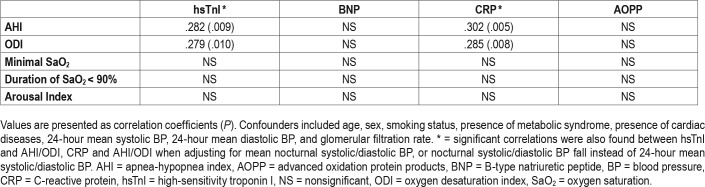
Nearly all participants (99%) had detectable hsTnI. Twenty-eight percent of the group with severe OSA and 8.5% in the group without severe OSA had higher hsTnI level than the 99th percentile values from the community-dwelling control patients in a previous study (chi-square, P = .014).25 The presence of severe OSA was associated with higher level of hsTnI (P < .001) (Table 1). On crude correlation analysis, AHI was positively correlated with hsTnI and CRP levels (r = .295, P = .003 and r = .218, P = .033, respectively). Similar positive correlation was demonstrated between oxygen desaturation index (ODI) and hsTnI and CRP (r = .298, P = .003 and r = .233, P = .022, respectively). After controlling for potential confounders, both AHI and ODI remained positively correlated with hsTnI and CRP (Table 2). No significant correlation was found between arousal index, time with oxygen saturation < 90% or minimal oxygen saturation, and hsTnI/CRP. There was no correlation between any sleep indices and BNP or AOPP levels, with or without adjustment for confounders (Table 2).
Table 2.
Pearson partial correlation analyses between sleep parameters and cardiovascular biomarkers with adjustment for confounders.
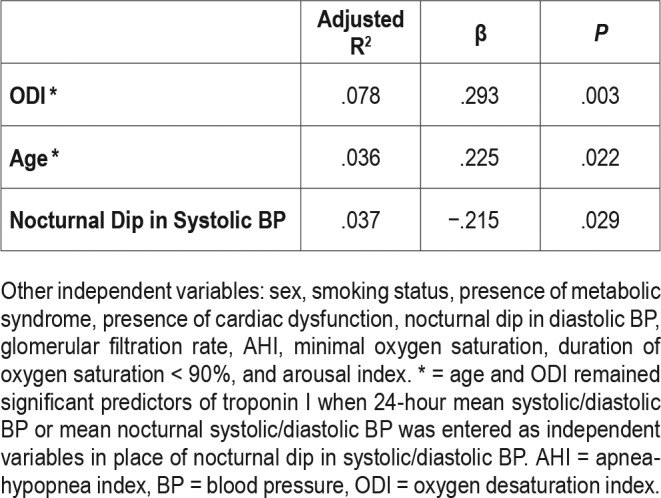
On multivariate stepwise linear regression model, ODI, age and loss of nocturnal systolic BP decrease were found to be determinants of hsTnI level (R2 = .151, β = .293, P = .003; β = .225, P = .022; and β = −.215, P = .029, respectively) (Table 3).
Table 3.
Multivariate stepwise linear regression model with troponin I as dependent variable (n = 98, adjusted R2 = .115).
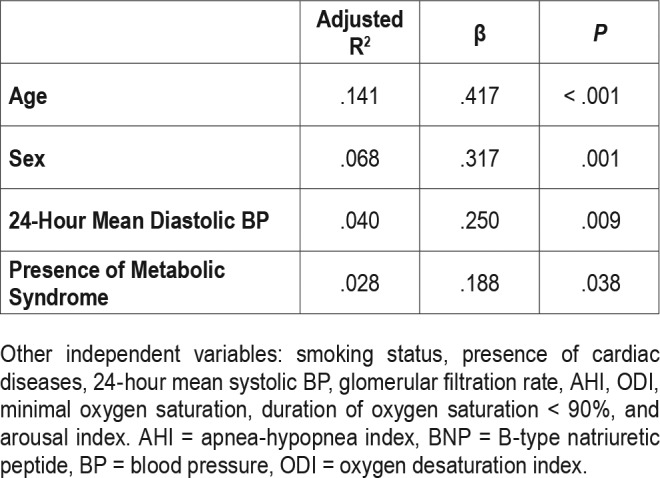
Among the 98 participants, 67 (68.4%) had detectable BNP level (> 10 pg/mL). Age, female sex, 24-hour mean diastolic BP, and presence of metabolic syndrome, but not sleep parameters, were the predictors of BNP level (Table 4).
Table 4.
Multivariate stepwise linear regression model with BNP as dependent variable (n = 98, adjusted R2 = .277).
DISCUSSION
Our study highlights the high prevalence of undiagnosed OSA (90.8% with AHI ≥ 5 events/h) in a Chinese clinic cohort with hypertension requiring three or more antihypertensive medications, which is in line with previous reports on other ethnic groups. Most (73%) of the cohort achieved BP pressure control using multiple medications, and there was no association between indices of OSA severity and BP readings on 24-hour ambulatory monitoring. However, for the first time, it is demonstrated that there is a linear correlation between OSA severity and troponin I level independent of BP control in these participants who have a high cardiovascular risk at baseline. Severity of intermittent hypoxia (ODI), age, and loss of nocturnal systolic BP decrease are the predictors of troponin level.
The importance of OSA in difficult-to-control hypertension is underrecognized. Only 6.4% of the clinic cohort (50/786) had known OSA prior to the study, which is similar to the rate (< 10%) reported in a large retrospective cohort study in California.26 Hypertension afflicts 20% to 25% of the general population in Hong Kong, and among which about 12% has resistant hypertension.27,28 In the presence of OSA, the concomitant presence of hypertension that requires multiple medications, compared to simple hypertension, has been shown to confer higher risks of ischemic heart disease (hazard ratio 1.24) and congestive heart failure (hazard ratio 1.43) in a retrospective study.26 The advent of hsTnI assays has allowed the detection of subclinical myocardial insults in the general population or patients in stable cardiovascular state.29 Higher hsTnI level has been associated with structural heart disease, risk of cardiovascular events, and mortality.30–32 However, studies on the relationship between troponin and OSA reported conflicting findings. Several studies on participants with stable CHD did not find any association between OSA and troponin levels,11,17,33,34 whereas other studies on participants with either stable CHD or heart failure reported that AHI was independently associated with higher troponin level.35–37 The contradictory findings can partially be explained by variations in troponin assays using either high-sensitivity troponin T (hsTnT) or hsTnI, which differ in molecular sizes and kinetics, epitopes, and detection antibodies.29 The study by Hall and coworkers highlighted the discordance in detection rates by hsTnT versus hsTnI assays.11 Similarly, Randby and coworkers did not find any association between hsTnT and OSA, given the suboptimal detection rate of 43% by hsTnT assay in a community-dwelling cohort in Norway.12 Subsequently, the same group repeated the analyses of the cohort using a hsTnI assay, and resulted in an improved detection rate of 62% and a significant association between hsTnI level and indices of OSA severity.14 In the current study, both the detection rate (99%) and the median hsTnI level are higher compared to the study in Norway, which is possibly related to the higher baseline cardiovascular risk in the current cohort, with 80% having metabolic syndrome and 90% having OSA. Our center has previously conducted a prospective case-control study using the hsTnI assay, and hsTnI has been shown to predict major adverse cardiovascular events, heart failure, and cardiovascular mortality in patients with diabetes mellitus.25 The baseline median hsTnI level in participants with diabetes mellitus (4.8, 3.2–8.4 ng/L) was significantly higher than that of the control patients from the community (2.9, 2.2–3.9 ng/L).25 The current study demonstrates that in difficult-to-control hypertension, the concomitant presence of severe OSA is associated with higher hsTnI than those without severe OSA, and there is a dose-response relationship between severity of OSA and hsTnI level.
Several large-scale longitudinal community-based studies have demonstrated the prognostic value of baseline troponin levels in predicting adverse cardiovascular outcomes in patients with OSA. In a community-based study including participants free of CHD or heart failure at baseline, hsTnI level was related to mortality risk and incident heart failure in all OSA categories after 12-year median follow-up.15 Another community study also concluded that the presence of OSA conferred a higher risk of incident heart failure or death in women after follow-up for 14 years, and the association was significantly affected by baseline troponin level.13 The finding implies that low-grade myocardial injury in the general population with OSA could be linked to adverse cardiovascular outcomes and mortality. The current study has found a positive association between hsTnI levels and indices of OSA severity (AHI, ODI) in participants with difficult-to-control hypertension. Whether the higher hsTnI level has any prognostic value in this specific group of patients would require further longitudinal research. Other than age and ODI, loss in nocturnal systolic BP decrease is found to be a predictor of hsTnI level in the study. This is in line with the finding from previous literature, and could provide a plausible mechanism linking to myocardial injury. In a subgroup of the Wisconsin Sleep Cohort Study, increasing severity of OSA at baseline showed a dose-dependent effect on the development of nondipping systolic BP after a median follow-up for 7.2 years.38 Loss of circadian BP changes in participants with hypertension (nondippers) is linked to greater end-organ damage and worse cardiovascular outcomes compared to nocturnal dippers.39
CRP is also an established predictor of cardiovascular disease,40 and recent literature has advocated the benefit of anti-inflammatory therapy in atherosclerotic disease.41 The current study finds a significant correlation between CRP and indices of OSA severity that supports the role of inflammation in vascular pathogenesis in OSA.
BNP and the degradation product N-terminal prohormone BNP (NT-proBNP) aid in the diagnosis and monitoring of heart failure.42,43 Several studies have investigated the effect of OSA on BNP levels, with conflicting results. A community-dwelling study in Sweden has found a dose-dependent relationship between severity of OSA and BNP level the next morning.44 However, no association was found between OSA and BNP/NT-proBNP/pro-BNP in several studies,17,34,45,46 including a large community-based study.47 Similarly, no definite conclusion can be drawn from the limited studies on the effect of continuous positive airway pressure therapy on NT-proBNP level.33,48 In the current study, indices of OSA severity were not the determinants of BNP in participants with difficult-to-control hypertension, which could be explained in part by the optimally controlled BP in more than half of the study participants and exclusion of those in fluid overload.
There are several limitations of our study. First, the study is cross-sectional in design, which precludes investigation of any casual relationships between OSA and the biomarkers. Although meticulous adjustment for potential confounders has been undertaken in the multivariate analyses, residual confounding is plausible due to the complicated medical backgrounds of the participants. The study is observational in nature and does not include coronary investigations per study protocol. Presence of cardiac diseases confirmed by workups with or without interventions is retrieved from medical records.
In summary, our study demonstrates that in participants who remain on multiple drugs for hypertension, occurrence and importance of OSA is underrecognized. The severity of OSA is independently associated with higher levels of hsTnI, suggesting that subclinical heart injury may be present despite control of BP with medications. The exact significance of low-grade myocardial injury on clinically relevant outcomes still needs to be clarified, especially given the literature on possible protective effect from intermittent hypoxia by enhancing vascular repair mechanisms.49 The findings of the current study call for more longitudinal studies on prognostic value of troponin in predicting cardiovascular outcomes in patients with OSA, severe hypertension, or both conditions. Further exploration on the effects of continuous positive airway pressure therapy on cardiovascular outcomes stratified by baseline troponin levels should be pursued.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Ms. Choi Siu-Ling and all the staff in the sleep laboratory for coordination of investigations; Mr. Kelvin Lau for project coordination and data entry; and Ms. Michelle Cheong for manual scoring of polysomnography.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AOPP
advanced oxidation protein products
- BNP
B-type natriuretic peptide
- BP
blood pressure
- CHD
coronary heart disease
- CRP
C-reactive protein
- ESS
Epworth Sleepiness Scale
- hsTnI
high-sensitivity troponin I
- hsTnT
high-sensitivity troponin T
- NT-proBNP
N-terminal prohormone B-type natriuretic peptide
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PSG
polysomnography
REFERENCES
- 1.Pafili K, Steiropoulos P, Papanas N. The relationship between obstructive sleep apnoea and coronary heart disease. Curr Opin Cardiol. 2015;30(4):439–446. doi: 10.1097/HCO.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 2.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118(10):1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 3.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10(4):355–362. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goncalves SC, Martinez D, Gus M, et al. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest. 2007;132(6):1858–1862. doi: 10.1378/chest.07-1170. [DOI] [PubMed] [Google Scholar]
- 5.Ruttanaumpawan P, Nopmaneejumruslers C, Logan AG, Lazarescu A, Qian I, Bradley TD. Association between refractory hypertension and obstructive sleep apnea. J Hypertens. 2009;27(7):1439–1445. doi: 10.1097/HJH.0b013e32832af679. [DOI] [PubMed] [Google Scholar]
- 6.Muxfeldt ES, Margallo VS, Guimaraes GM, Salles GF. Prevalence and associated factors of obstructive sleep apnea in patients with resistant hypertension. Am J Hypertens. 2014;27(8):1069–1078. doi: 10.1093/ajh/hpu023. [DOI] [PubMed] [Google Scholar]
- 7.Torres G, Sanchez-de-la-Torre M, Barbe F. Relationship between OSA and hypertension. Chest. 2015;148(3):824–832. doi: 10.1378/chest.15-0136. [DOI] [PubMed] [Google Scholar]
- 8.Ayas NT, Taylor CM, Laher I. Cardiovascular consequences of obstructive sleep apnea. Curr Opin Cardiol. 2016;31(6):599–605. doi: 10.1097/HCO.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 9.Garvey JF, Taylor CT, McNicholas WT. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J. 2009;33(5):1195–1205. doi: 10.1183/09031936.00111208. [DOI] [PubMed] [Google Scholar]
- 10.Shemisa K, Bhatt A, Cheeran D, Neeland IJ. Novel biomarkers of subclinical cardiac dysfunction in the general population. Curr Heart Fail Rep. 2017;14(4):301–310. doi: 10.1007/s11897-017-0342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall TS, Herrscher T, Jarolim P, et al. Obstructive sleep apnea: no independent association to troponins. Sleep Breath. 2014;18(2):351–358. doi: 10.1007/s11325-013-0892-6. [DOI] [PubMed] [Google Scholar]
- 12.Randby A, Namtvedt SK, Einvik G, et al. Obstructive sleep apnea is associated with increased high-sensitivity cardiac troponin T levels. Chest. 2012;142(3):639–646. doi: 10.1378/chest.11-1779. [DOI] [PubMed] [Google Scholar]
- 13.Roca GQ, Redline S, Claggett B, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the Atherosclerosis Risk in Communities-Sleep Heart Health study. Circulation. 2015;132(14):1329–1337. doi: 10.1161/CIRCULATIONAHA.115.016985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Einvik G, Rosjo H, Randby A, et al. Severity of obstructive sleep apnea is associated with cardiac troponin I concentrations in a community-based sample: data from the Akershus Sleep Apnea Project. Sleep. 2014;37(6):1111–1116. doi: 10.5665/sleep.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Querejeta Roca G, Redline S, Punjabi N, et al. Sleep apnea is associated with subclinical myocardial injury in the community. The ARIC-SHHS study. Am J Respir Crit Care Med. 2013;188(12):1460–1465. doi: 10.1164/rccm.201309-1572OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barcelo A, Esquinas C, Bauca JM, et al. Effect of CPAP treatment on plasma high sensitivity troponin levels in patients with obstructive sleep apnea. Respir Med. 2014;108(7):1060–1063. doi: 10.1016/j.rmed.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Valo M, Wons A, Moeller A, Teupe C. Markers of myocardial ischemia in patients with obstructive sleep apnea and coronary artery disease. Pulm Med. 2015;2015:621450. doi: 10.1155/2015/621450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masson S, Latini R, Anand IS, et al. Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (Valsartan Heart Failure Trial) J Am Coll Cardiol. 2008;52(12):997–1003. doi: 10.1016/j.jacc.2008.04.069. [DOI] [PubMed] [Google Scholar]
- 19.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 20.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 21.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117(25):e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 22.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1):142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 23.Sousa F, Neves J, Ferreira R, Polonia J, Bastos JM. 1B.05: In hypertension the change from a non-dipper to a dipper pattern is associated with a better cardiovascular prognosis than the persistence within the non-dipper pattern [abstract] J Hypertens. 2015;33(Suppl 1):e6. [Google Scholar]
- 24.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yiu KH, Lau KK, Zhao CT, et al. Predictive value of high-sensitivity troponin-I for future adverse cardiovascular outcome in stable patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2014;13:63. doi: 10.1186/1475-2840-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhandari SK, Shi J, Molnar MZ, et al. Comparisons of sleep apnoea rate and outcomes among patients with resistant and non-resistant hypertension. Respirology. 2016;21(8):1486–1492. doi: 10.1111/resp.12840. [DOI] [PubMed] [Google Scholar]
- 27.Cheung BM, Wat NM, Tso AW, et al. Association between raised blood pressure and dysglycemia in Hong Kong Chinese. Diabetes Care. 2008;31(9):1889–1891. doi: 10.2337/dc08-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao G, Chen C, Lin Q, et al. Prevalence, clinical characteristics and echocardiography parameters of non-resistant, resistant and refractory hypertension in Chinese. Postgrad Med. 2017;129(2):187–192. doi: 10.1080/00325481.2017.1272398. [DOI] [PubMed] [Google Scholar]
- 29.Apple FS, Steffen LM, Pearce LA, Murakami MM, Luepker RV. Increased cardiac troponin I as measured by a high-sensitivity assay is associated with high odds of cardiovascular death: the Minnesota Heart Survey. Clin Chem. 2012;58(5):930–935. doi: 10.1373/clinchem.2011.179176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304(22):2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omland T, de Lemos JA, Sabatine MS, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361(26):2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang WH, Wu Y, Nicholls SJ, et al. Subclinical myocardial necrosis and cardiovascular risk in stable patients undergoing elective cardiac evaluation. Arterioscler Thromb Vasc Biol. 2010;30(3):634–640. doi: 10.1161/ATVBAHA.109.201210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valo M, Wons A, Moeller A, Teupe C. Markers of myocardial ischemia in patients with coronary artery disease and obstructive sleep apnea: effect of continuous positive airway pressure therapy. Clin Cardiol. 2015;38(8):462–468. doi: 10.1002/clc.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maeder MT, Strobel W, Christ M, et al. Comprehensive biomarker profiling in patients with obstructive sleep apnea. Clin Biochem. 2015;48(4-5):340–346. doi: 10.1016/j.clinbiochem.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Inami T, Seino Y, Otsuka T, et al. Links between sleep disordered breathing, coronary atherosclerotic burden, and cardiac biomarkers in patients with stable coronary artery disease. J Cardiol. 2012;60(3):180–186. doi: 10.1016/j.jjcc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Yoshihisa A, Suzuki S, Yamaki T, et al. Impact of adaptive servo-ventilation on cardiovascular function and prognosis in heart failure patients with preserved left ventricular ejection fraction and sleep-disordered breathing. Eur J Heart Fail. 2013;15(5):543–550. doi: 10.1093/eurjhf/hfs197. [DOI] [PubMed] [Google Scholar]
- 37.Miyata M, Yoshihisa A, Yamauchi H, et al. Impact of sleep-disordered breathing on myocardial damage and metabolism in patients with chronic heart failure. Heart Vessels. 2015;30(3):318–324. doi: 10.1007/s00380-014-0479-6. [DOI] [PubMed] [Google Scholar]
- 38.Hla KM, Young T, Finn L, Peppard PE, Szklo-Coxe M, Stubbs M. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep. 2008;31(6):795–800. doi: 10.1093/sleep/31.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370(9594):1219–1229. doi: 10.1016/S0140-6736(07)61538-4. [DOI] [PubMed] [Google Scholar]
- 40.Wilson PW, Pencina M, Jacques P, Selhub J, D'Agostino R, Sr, O'Donnell CJ. C-reactive protein and reclassification of cardiovascular risk in the Framingham Heart Study. Circ Cardiovasc Qual Outcomes. 2008;1(2):92–97. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 42.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128(16):1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 43.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 44.Ljunggren M, Lindahl B, Theorell-Haglow J, Lindberg E. Association between obstructive sleep apnea and elevated levels of type B natriuretic peptide in a community-based sample of women. Sleep. 2012;35(11):1521–1527. doi: 10.5665/sleep.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hubner RH, El Mokhtari NE, Freitag S, et al. NT-proBNP is not elevated in patients with obstructive sleep apnoea. Respir Med. 2008;102(1):134–142. doi: 10.1016/j.rmed.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 46.Cifci N, Uyar M, Elbek O, Suyur H, Ekinci E. Impact of CPAP treatment on cardiac biomarkers and pro-BNP in obstructive sleep apnea syndrome. Sleep Breath. 2010;14(3):241–244. doi: 10.1007/s11325-009-0306-y. [DOI] [PubMed] [Google Scholar]
- 47.Patwardhan AA, Larson MG, Levy D, et al. Obstructive sleep apnea and plasma natriuretic peptide levels in a community-based sample. Sleep. 2006;29(10):1301–1306. doi: 10.1093/sleep/29.10.1301. [DOI] [PubMed] [Google Scholar]
- 48.Colish J, Walker JR, Elmayergi N, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;141(3):674–681. doi: 10.1378/chest.11-0615. [DOI] [PubMed] [Google Scholar]
- 49.Berger S, Aronson D, Lavie P, Lavie L. Endothelial progenitor cells in acute myocardial infarction and sleep-disordered breathing. Am J Respir Crit Care Med. 2013;187(1):90–98. doi: 10.1164/rccm.201206-1144OC. [DOI] [PubMed] [Google Scholar]