Abstract
Study Objectives:
No consensus exists regarding monitoring the initiation of positive airway pressure (PAP) by oximetry. A PAP device report may be insufficient to ensure a good therapeutic response in all patients. This study aimed to identify patients who would potentially benefit from oximetry monitoring during PAP initiation.
Methods:
PAP initiation was routinely monitored at home with an oximeter. Data were reviewed for all patients who underwent PAP initiation in 2015, including a baseline sleep study and PAP initiation data. Group A included patients with an apnea-hypopnea index as determined from the PAP device (AHIPAP) of < 5 events/h and a residual 3% oxygen desaturation index (ODI3) of ≥ 10 events/h. Group B included all remaining patients. Cases with a leak of over 24 L/min or with an oximetry recording time of < 1 hour were excluded. AHIPAP < 5 events/h and residual ODI3 < 10 events/h represented good PAP responses.
Results:
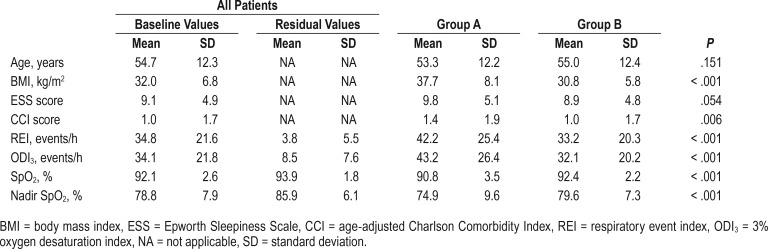
From 787 patients, 723 were included in this study. Among these, 158 had an AHIPAP of ≥ 5 events/h, whereas 565 had an AHIPAP of < 5 events/h. Group A consisted of 129 patients (18%). The sensitivity of the PAP device indicating a good PAP response reached 93.1%, with a specificity of 37.2%, a negative predictive value of 96.2%, and a positive predictive value of 23.9% using body mass index (BMI) ≥ 30 kg/m2 and baseline SpO2 < 92% as the cutoff points.
Conclusions:
Relying only on the PAP device parameter to evaluate therapeutic responses provided inconsistent results in one-fifth of cases. Thus, oximetry monitoring during PAP initiation is recommended when baseline SpO2 < 92% or when BMI ≥ 30 kg/m2. Otherwise, oximetry monitoring remains optional.
Citation:
Koivumäki V, Maasilta P, Bachour A. Oximetry monitoring recommended during pap initiation for sleep apnea in patients with obesity or nocturnal hypoxemia. J Clin Sleep Med. 2018;14(11):1859–1863.
Keywords: CPAP, evaluation, obstructive sleep apnea, oximetry, PAP initiation
BRIEF SUMMARY
Current Knowledge/Study Rationale: Polysomnography represents the gold standard for positive airway pressure (PAP) therapy initiation. This practice is rare and more often the therapy control relies on the self-monitoring of the PAP device.
Study Impact: Oximetry should be part of PAP initiation among patients with obesity and those with low oxygen values during sleep because relying only on PAP device monitoring is unreliable. Oximetry monitoring identified patients who need additional examination to ensure a good PAP therapy response.
INTRODUCTION
Continuous positive airway pressure (CPAP) is a standard treatment for patients with obstructive sleep apnea (OSA), a sleep-related breathing disorder characterized by full or partial occlusion of the upper airway during sleep. The gold standard for CPAP initiation occurs during polysomnography.1 Auto-titrating positive airway pressure (APAP) has been used for titration and OSA therapy, and appears to be as efficient as CPAP when following the application rules.2 Meanwhile, no recommendation exists for monitoring positive airway pressure (PAP) initiation other than via polysomnography. Some sleep centers continue to use in-laboratory PAP initiation monitored by polysomnography, whereas other centers depend primarily on PAP device values.3
PAP devices have undergone considerable technological advances recently, notably in the field of remote medicine. These new devices have reduced the cost of PAP initiation and allowed for the more efficient monitoring of therapy.3 By contrast, the use of polygraphy or oximetry during PAP initiation in remote medicine remains under development.
Although a PAP device has been validated for detecting the apnea-hypopnea index (AHI)4 its criteria for calculating hypopnea differs from recommendations from the American Academy of Sleep Medicine (AASM).5,6 Recently, Thomas and Bianchi7 presented remarkable findings, whereby PAP device algorithms missed the detection of respiratory events. Accordingly, the authors have concerns regarding the reliability of the PAP device in estimating AHI values. In essence, although scoring apnea without oximetry is possible, hypopneas rely on arousals or oximetry values. A PAP device alone provides none of these parameters.7
It is of utmost importance to simplify PAP initiation at home while maintaining good therapeutic control because an increasing emphasis has been placed on appropriate resource utilization—including efforts aimed at decreasing the cost of care—and to cope with the increasing number of patients seeking treatment for sleep disorders. Our clinical experience allows us to conclude that the apnea-hypopnea index reported by a PAP device (AHIPAP) is not trustworthy among a specific group of patients. Therefore, the PAP therapeutic response must be monitored using additional tools such as oximetry.
This study aims to identify groups of patients who may benefit most from the addition of oximetry in monitoring PAP initiation. Patients with normal AHIPAP but abnormal oximetry results manifested through a high 3% oxygen desaturation index (ODI3) were analyzed.
METHODS
Data were collected from all patients referred to our sleep unit for PAP initiation for OSA during 2015. The indication for PAP initiation was a respiratory event index (REI) of ≥ 5 events/h associated with significant daytime sleepiness or the presence of cardiovascular or metabolic comorbidity. Patients with a REI of ≥ 15 events/h were also recommended for PAP therapy regardless of their symptoms or comorbidity. Patients with a central sleep apnea or obesity hypoventilation syndrome were excluded. The sleep apnea diagnosis was confirmed by an outof-center type III sleep study and scoring was performed in accordance with prevailing AASM guidelines.8 Hypopnea was defined as a decrease in the nasal flow of at least 50% for at least 10 seconds or a decrease of at least 3% in the saturation.5
PAP therapy was initiated at home with an APAP device (ResMed, Sydney, Australia). An oximeter (Nonin Xpod, Nonin Medical Inc. Minneapolis, Minnesota, United States) was connected and data were synchronized with the PAP device. The pressure was set to start automatically from 4 cmH2O up to a maximum of 20 cmH2O.
Our routine practice included the addition of oximetry for all PAP initiation. Nevertheless, if the sleep nurse noticed on the day of initiation that the available number of oximeters was insufficient to satisfy the number of planned PAP initiations, he or she received instructions to prioritize patients with a lower baseline SpO2 value for an oximetry connection.
PAP data were collected from the ResScan program and validated visually as described previously.9 The PAP initiation period varied from 3 to 5 days. The day with the longest PAP usage was used for the analysis reported here. The program does not allow editing of its reported AHI or ODI values.
Oximetry data were visually checked and only oximetry data during “PAP On” were included. This is feasible as the ResScan program allows a simultaneous check of airflow, PAP pressure, and oximetry. The baseline SpO2 value represented the mean oximetry value during the cardiorespiratory sleep study (out-of-center type III sleep study).
Patients were excluded if they fulfilled one of the following: a recording time with an oximeter “when PAP was on” of less than 1 hour or a median air leak of more than 24 L/min. Although we considered an AHIPAP of < 5 events/h as normal, it is also reasonable to consider an ODI3 value of < 5 events/h as normal. When analyzing the data, we noticed a large overlap between the two groups. To reduce this overlap and increase the value of the abnormal ODI3, we considered an ODI3 value of ≥ 10 events/h as abnormal. We found no previous studies on this issue in the literature. A good PAP therapeutic response was indicated when AHIPAP < 5 events/h. Furthermore, a good PAP and oximetry therapeutic response was considered when AHIPAP < 5 events/h and residual ODI3 < 10 events/h. In our analysis, we divided our patients into two groups based on their AHIPAP values and on the residual ODI3 values. As such, group A consisted of patients with AHIPAP < 5 events/h and residual ODI3 ≥ 10 events/h. Group B included all the remaining patients.
We used the age-adjusted Charlson Comorbidity Index to evaluate comorbidity. This index consists of 19 medical conditions weighted 1 to 6 with total scores ranging from 0 to 37 (0 = no comorbidity).10
This study was approved by the Heart and Lung Center of the Helsinki University Hospital (code §10, 13 May 2016, permit number HUS/152/2016).
We used IBM's SPSS Statistics software package (IBM SPSS Statistics version 22.0, Armonk, New York, United States) to analyze our data. The t test or chi-square test was used when appropriate. A value of P < .05 was considered statistically significant. We used the receiver operating characteristic (ROC) curve analysis to test the appropriateness of the mean baseline SpO2 and body mass index (BMI) values as predictors. Correlation analyses were performed using the Pearson correlation.
RESULTS
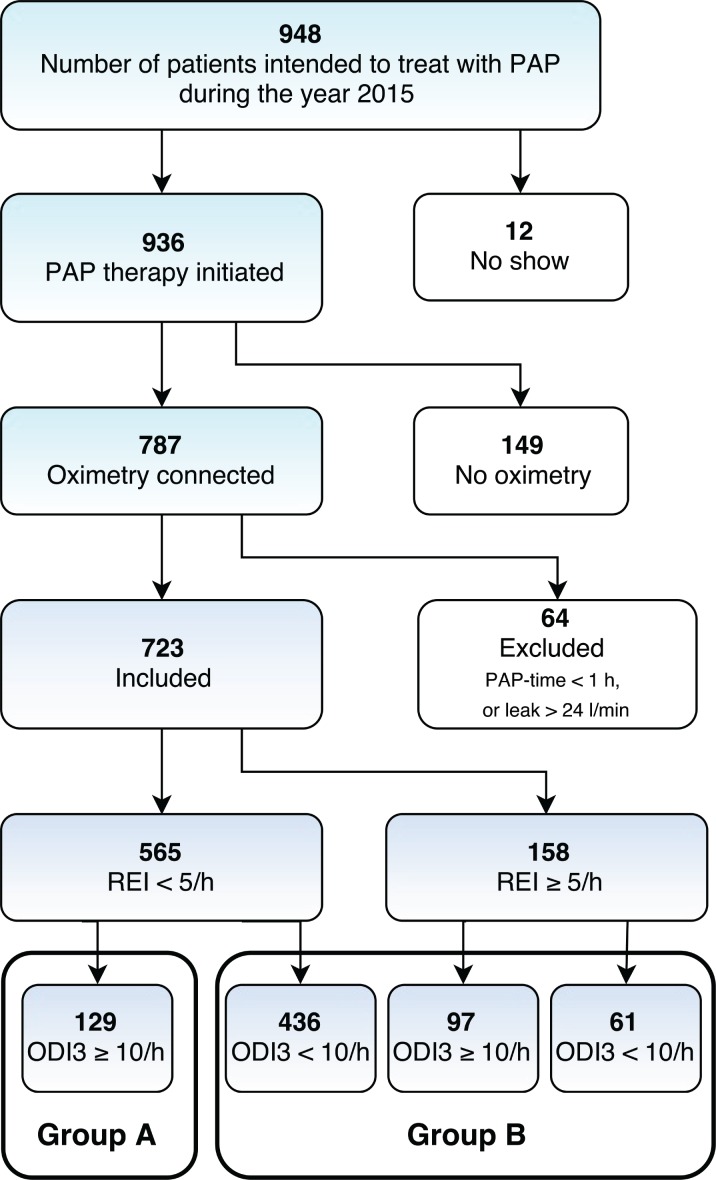
A total of 948 patients were scheduled to undergo PAP initiation (Figure 1). Among these, 12 were no-shows, and oximetry was not available for 149 patients. We analyzed data for a total of 787 patients who received oximetry during PAP initiation. Among these, 36 had a PAP time with oximetry of less than 1 hour and were excluded from the analysis. We also excluded 28 patients who presented with a median leak of more than 24 L/min. Thus, the final analysis included 723 patients; their PAP time was a mean of 7:10 hours:minutes, standard deviation (SD) 2:13. The characteristics of these patients are outlined in Table 1.
Figure 1. Flowchart of all patients.
ODI3 = 3% oxygen desaturation index, PAP = positive airway pressure, REI = respiratory event index.
Table 1.
Characteristics of patients and their respiratory parameters.
Among the 149 patients who underwent PAP initiation not connected to oximetry (no oximetry group), we found no difference from the oximetry group regarding their sex (c2 [1, n = 936] = 3.831, P = .060) or age (t934 = 0.812, P = .417). As expected, however, the no oximetry group exhibited a higher baseline SpO2 (93.0, SD 2.1 versus 92.1, SD 2.5; t856 = 4.187, P < .001), a lower baseline REI (26.0, SD 19.0 versus 34.9, SD 21.7; t924 = −4.635, P < .001), and a lower baseline ODI3 (23.4, SD 15.1 versus 34.2 SD 21.9; t868 = −5.414, P < .001) than the oximetry group.
A total of 565 patients had an AHIPAP of < 5 events/h, whereas 158 patients (22%) showed an AHIPAP of ≥ 5 events/h. Among patients with an AHIPAP of < 5 events/h, 129 patients had a residual ODI3 of ≥ 10 events/h (group A, 18% of all patients). Group A, therefore, included patients classified as having a good therapeutic response using the PAP device (false positive), but an abnormal residual ODI3.
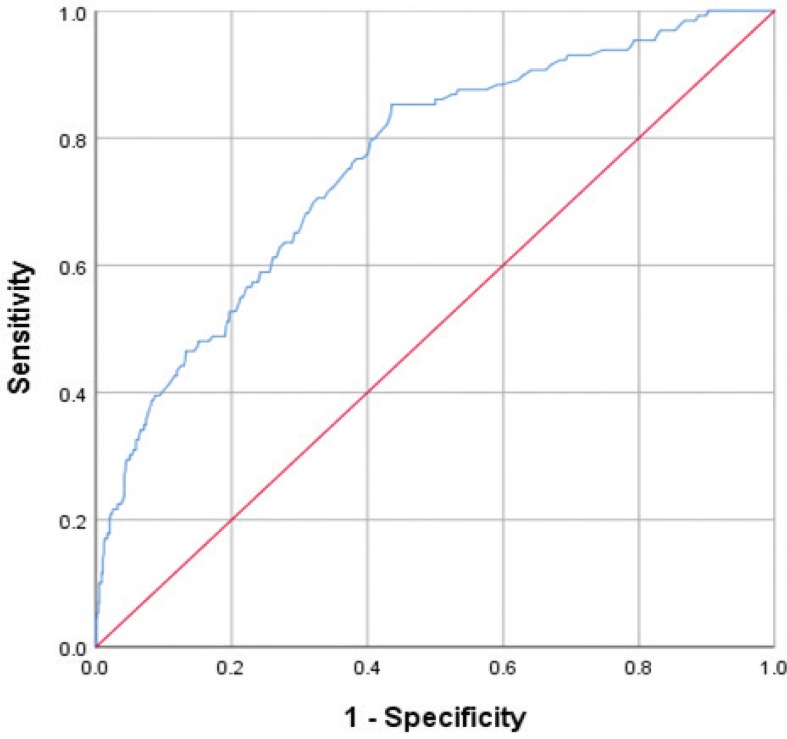
To evaluate the variation in baseline SpO2 and BMI against the capacity of the PAP device to indicate a good therapeutic response, we drew an ROC curve showing the point of equal sensitivity and specificity for SpO2 values at 92.05%, with an area under the curve (AUC) of 0.645. We completed the same analysis for BMI, finding a cutoff point of 32.75 kg/m2 with a corresponding AUC of 0.758 (Figure 2). Applying a BMI cutoff point of 30 kg/m2 or more and an SpO2 cutoff value of less than 92%, the sensitivity of the PAP device indicating a good therapeutic response stood at 93.1%, with a specificity of 37.2%, a negative predictive value (NPV) of 96.2%, and a positive predictive value of 23.9%. Using the abovementioned results, we chose a BMI cutoff point of 30 kg/m2 and SpO2 cutoff point of 92%.
Figure 2. Receiver operating characteristic curve.
Receiver operating characteristic curve analysis for the variation in the mean body mass index values among group A as a positive actual state, indicating a cutoff point of 32.75 kg/m2 with a corresponding area under the curve of 0.758. Thus, the PAP device provides less sensitivity in indicating a true therapeutic response among patients with obesity. PAP = positive airway pressure.
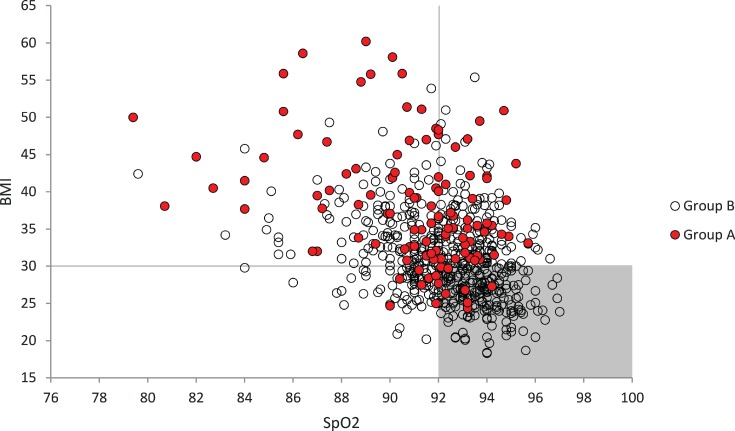
The distribution of patients in groups A and B based on their baseline SpO2 and BMI revealed an area where patients in group A (the shaded area defined by a BMI of < 30 kg/m2 and a sleep study SpO2 of ≥ 92%) appeared relatively rarely (Figure 3). In other words, the PAP device alone could be relatively trustworthy in identifying a good therapeutic response among patients with a BMI of < 30 kg/m2 and a baseline SpO2 of ≥ 92%.
Figure 3. Distribution of patients based on baseline SpO2 and BMI.
Group A patients (AHIPAP < 5 events/h and residual ODI3 ≥ 10 events/h), indicated in red, are proportionally scarce in the shaded area. Patients situated in the unshaded area benefit the most from the addition of oximetry during PAP initiation. The shaded area contains a total of 212 patients, 32% of the total number of patients (n = 664) and 8 patients belonging to group A, or 7% of group A (n = 116). The total number of patients here reached 664, since the baseline SpO2 values were missing for 59 patients. AHI = apnea-hypopnea index, BMI = body mass index, ODI 3 = 3% oxygen desaturation index, PAP = positive airway pressure.
DISCUSSION
The main finding of this study was that in 22% of PAP initiation cases the AHI reported by the PAP device was normal, whereas the oximetry revealed abnormal findings. These inconsistent results reported by the PAP device appeared more pronounced in patients with obesity and among patients with a baseline sleep study SpO2 < 92%. Therefore, we recommend connecting oximetry to PAP initiation among patients with obesity.
For patients without obesity with a concomitant SpO2 value of 92% or more, AHIPAP values were sufficiently reliable to evaluate the therapeutic response in 204 of 212 patients. Therefore, connecting oximetry during PAP initiation for these patients remains optional. In other words, applying the aforementioned cutoff points to choose whether to connect an oximetry or not during PAP initiation reveals that only 8 of 212 patients would have needed oximetry, but they did not have it (NPV values of 96.2%).
Ignoring the use of oximetry or other monitoring devices during PAP initiation may lead to a false finding that everything is going well when evaluating the PAP machine AHI values alone. As such, Thomas and Bianchi7 recently identified cases where the PAP device failed to recognize respiratory disturbances in patients with sleep apnea with a high loop gain or in patients under high doses of opiates. In this study, we had no patients under high doses of opiates.
Our results, however, agree with those from the study by Boyd et al.,11 who recently found that, although the hours of PAP use represent an accepted clinical metric to assess the adequacy of adherence to PAP, as a surrogate measure it does not directly quantify AHI over the entire sleep period. Therefore, it was proposed to use instead “effective AHI,” which represents the sum of all apneas and hypopneas during the time the patient uses PAP (“PAP On”) and when not using PAP (“PAP Off”) divided by the hours of total sleep time. Our study supports this conclusion. That is, the PAP therapeutic response is sometimes misleading and suboptimal when abnormal desatu-ration during PAP initiation remains undetected because oximetry is not used and the therapeutic response was mistakenly considered satisfactory.
This study carries some limitations. First, it is not a multi-center study. In addition, we intended to add oximetry to all our PAP initiations; however, 15% of our patients did not receive oximetry for technical reasons. Furthermore, we did not use the gold standard of polysomnography for PAP initiation because this study was conducted in a real-life clinical practice. Our sleep unit is part of the Helsinki University Hospital. Finally, all patients possessed general insurance coverage that covers all costs, and the hospital serves as a health care provider. Our study, however, does carry practical applications. Patients with ODI3 ≥ 10 events/h need additional examination to ensure a good PAP therapeutic response. In our sleep laboratory, we perform a cardiorespiratory study during PAP therapy to verify the findings of the oximetry and to exclude potential “spontaneous” desaturations that may occur in patients with OSA and which are not controlled by PAP. In addition, some patients may benefit from a higher fixed pressure whereas others may respond well to bilevel pressure. However, we did not have sufficient data here to generalize our recommendations. Our study was performed using only one type of device, and findings from other types of devices may differ.
CONCLUSIONS
Relying only on the PAP device parameter to evaluate therapeutic responses may lead to identifying therapeutic responses as falsely satisfactory in one-fifth of patients. To avoid this bias, we recommend systematically connecting oximetry during PAP initiation for all patients with a BMI of ≥ 30 kg/m2, and among patients with a mean SpO2 of less than 92% during their sleep study. The use of oximetry in other patients remains optional.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at the Sleep Unit, Heart and Lung Center, Helsinki University Hospital, University of Helsinki, Finland. This study was funded by the Helsinki University Special Fund (V1016SK001). The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AUC
area under the curve
- BMI
body mass index
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- NA
not applicable
- NPV
negative predictive value
- ODI3
3% oxygen desaturation index
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- REI
respiratory event index
- ROC
receiver operating characteristic
- SD
standard deviation
- SpO2
blood oxygen saturation
REFERENCES
- 1.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. [PMC free article] [PubMed] [Google Scholar]
- 2.Morgenthaler TI, Aurora RN, Brown T, et al. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep. 2008;31(1):141–147. doi: 10.1093/sleep/31.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anttalainen U, Melkko S, Hakko S, Laitinen T, Saaresranta T. Telemonitoring of CPAP therapy may save nursing time. Sleep Breath. 2016;20(4):1209–1215. doi: 10.1007/s11325-016-1337-9. [DOI] [PubMed] [Google Scholar]
- 4.Rees K, Wraith PK, Berthon-Jones M, Douglas NJ. Detection of apnoeas, hypopnoeas and arousals by the AutoSet in the sleep apnoea/hypopnoea syndrome. Eur Respir J. 1998;12(4):764–769. doi: 10.1183/09031936.98.12040764. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 6.Schwab RJ, Badr SM, Epstein LJ, et al. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med. 2013;188(5):613–620. doi: 10.1164/rccm.201307-1282ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas RJ, Bianchi MT. Urgent need to improve PAP management: the devil is in two (fixable) details. J Clin Sleep Med. 2017;13(5):657–664. doi: 10.5664/jcsm.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachour A, Virkkala JT, Maasilta PK. AutoCPAP initiation at home: optimal trial duration and cost-effectiveness. Sleep Med. 2007;8(7-8):704–710. doi: 10.1016/j.sleep.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004;4:94. doi: 10.1186/1471-2407-4-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd SB, Upender R, Walters AS, et al. Effective apnea-hypopnea index (“effective AHI”): a new measure of effectiveness for positive airway pressure therapy. Sleep. 2016;39(11):1961–1972. doi: 10.5665/sleep.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]