Abstract
Systemic autoinflammatory disorders (SAIDs) are inherited defects of innate immunity characterized by recurrent sterile inflammatory attacks involving skin, joints, serosal membranes, gastrointestinal tube, and other tissues, which recur with variable rhythmicity and display reactive amyloidosis as a potential long-term complication. Dysregulated inflammasome activity leading to overproduction of many proinflammatory cytokines, such as interleukin-1 (IL-1), and delayed shutdown of inflammation are considered crucial pathogenic keys in the vast majority of SAIDs. Progress of cellular biology has partially clarified the mechanisms behind monogenic SAIDs, such as familial Mediterranean fever, tumor necrosis factor receptor-associated periodic syndrome, cryopyrin-associated periodic syndrome, mevalonate kinase deficiency, hereditary pyogenic diseases, idiopathic granulomatous diseases and defects of the ubiquitin-proteasome pathway. Whereas, little is clarified for the polygenic SAIDs, such as periodic fever, aphthous stomatitis, pharyngitis, and cervical adenopathy (PFAPA) syndrome. The puzzle of symptomatic febrile attacks recurring over time in children requires evaluating the mixture of clinical data, inflammatory parameters in different disease phases, the therapeutic efficacy of specific drugs such as colchicine, corticosteroids or IL-1 antagonists, and genotype analysis in selected cases. The long-term history of periodic fevers should also need to rule out chronic infections and malignancies. This review is conceived as a practical template for proper classification of children with recurring fevers and includes tips useful for the diagnostic approach to SAIDs, focusing on the specific acute painful symptoms and hematologic manifestations encountered in childhood.
Keywords: Autoinflammatory disorder, Autoinflammation, Interleukin-1, Innovative biotechnologies, Recurrent fever, Personalized medicine, Child
Introduction
Innate immunity uses bactericidal weapons in both vascular and tissue compartments and has its main alarm system in the intracellular “inflammasome”, a multiprotein complex which regulates the clearance of any invading microbes via germ-line encoded pattern recognition receptors: all activated innate immune cells manifest their antibacterial activity through the inflammasome-mediated production of potent inflammatory cytokines to mount an appropriate response against microbial threats.
In addition to being crucial for pathogen recognition through the mammalian cysteine protease caspase-1, a central enzyme of innate immunity, which processes pro-interleukin-1 (IL-1)β into the mature proinflammatory cytokine IL-1β, the discovery of monogenic and polygenic disorders in which inflammasome activity and IL-1 release are deregulated highlights the essential importance of inflammasome-supervised human defences against infections.1 Different studies have demonstrated the existence of a functional hierarchy of proinflammatory cytokines in systemic autoinflammatory disorders (SAIDs), a heterogeneous cluster of rare genetically determined diseases involving innate immunity, mainly characterized by the recurrence of inflammatory flares affecting the skin, joints, serosal membranes, gastroenteric tube, central nervous system and other tissues, in which IL-1β is the most critical driver of inflammation at different levels.2,3 The term “autoinflammatory” describes the nearly spontaneous appearance of inflammation in the almost complete absence of autoreactive T lymphocytes and/or specific autoantibodies and no clear evidence of infectious triggers.4 All SAIDs are caused by impaired regulation in the production of proinflammatory cytokines, with IL-1β being the foremost implicated, which leads to a pathological delay in the shutdown of inflammatory responses.5 The group of SAIDs can be categorized in hereditary monogenic disorders and multifactorial polygenic disorders, encompassing an increasing number of conditions, such as periodic fever, aphthous stomatitis, pharyngitis and cervical adenopathy (PFAPA) syndrome, which is the most relevant cause of idiopathic recurrent fevers in childhood.6
From the historical understanding that monogenic periodic fevers are the prototype of pure SAIDs, our knowledge has now expanded to encompass a larger number of inflammatory diseases with autoinflammatory mechanisms and a presumed polygenic basis, such as Behçet’s disease, gout and idiopathic recurrent acute pericarditis. Further advances in genetics and molecular biology have also consented to classify the hereditary monogenic SAIDs more rationally, though they are characterized by quite similar clinical phenotypes during any flare with fever and varying signs of systemic and sometimes organ-specific inflammation. The group of SAIDs includes familial Mediterranean fever (FMF), tumor necrosis factor receptor-associated periodic syndrome (TRAPS), the family of cryopyrin-associated periodic syndrome (CAPS), which in turn encompasses familial cold urticaria syndrome (FCAS), Muckle-Wells syndrome (MWS) and chronic infantile neurological cutaneous and articular (CINCA) syndrome, mevalonate kinase deficiency (MKD), hereditary pyogenic diseases including pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) syndrome, Majeed syndrome (MS) and deficiency of the IL-1 receptor antagonist (DIRA), in addition to idiopathic granulomatous diseases with familiar presentation, such as Blau syndrome (BS), dysregulations in interferon (IFN) signaling and defects of the ubiquitin-proteasome pathway, such as OTULIN-related autoinflammatory syndrome (ORAS) and proteasome-associated autoinflammatory syndrome (PRAAS).
A descriptive summary of the monogenic SAIDs described in this review is listed in the Table 1. Some of these - namely FMF, MKD, MS, DIRA, ORAS, PRAAS - are transmitted by autosomal recessive inheritance, while the others - TRAPS, FCAS, MWS, CINCA syndrome, PAPA syndrome and BS - are autosomal dominant. The genes associated with these diseases have been sequentially identified from 1997 onwards and, with the exception of MKD, the majority of them encodes for proteins involved in the inflammasome activity or in the process of programmed cell death.7 In particular, inflammasomes, which have a characteristic structure consisting of a central scaffold and sensor protein (a Nod-like receptor or IFN-inducible protein), an adaptor protein ASC (an apoptosis-associated speck-like protein containing a CARD domain) and the effector protein caspase-1, modulate both synthesis and release of IL-1β; they work - under numerous different activation modes - as innate immunologic platforms assembled in the cytosol in response to the sensing of pathogens or cellular damage.8
Table 1.
Descriptive Summary of the Monogenic Systemic Autoinflammatory Disorders.
| Gene Locus | Protein | Inheritance | Prominent manifestations during flares and potential complications | Therapies | |
|---|---|---|---|---|---|
| FMF |
MEFV 16p13.3 |
Pyrin (marenostrin) | AR | fever, serositis, limb pain or transient frank arthritides, erysipelas-like eruption on the legs, other nonspecific skin manifestations (urticaria, angioedema, erythema nodosum, vitiligo), amyloidosis in nontreated or noncompliant patients | Colchicine, canakinumab, anakinra |
| TRAPS |
TNFRSF1A 12p13 |
Tumor necrosis factor receptor 1 (55 kD) | AD | fever, migrating severe muscle involvement, arthralgias or arthritides, serosal involvement, painful orbital edema, conjunctivitis, amyloidosis | Canakinumab, anakinra |
| FCAS |
NLRP3 1q44 |
Cryopyrin | AD | fever, cold-induced evanescent urticaria-like rash, conjunctivitis, arthralgias | Anakinra, rilonacept, canakinumab |
| MWS | fever, urticaria-like rash, conjunctivitis, episcleritis, arthralgias, neurosensorial deafness, amyloidosis | ||||
| CINCA s. | sporadic fever, persistent non-itchy urticaria-like rash, uveitis, papilledema, deforming arthritis involving large joints, aseptic chronic meningopathy, retinal dystrophy, neurosensorial deafness, amyloidosis | ||||
| MKD |
MVK 12q24 |
Mevalonate kinase | AR | fever, fatigue, miserable status, lymph node enlargement, vomiting, diarrhea, abdominal pain, arthralgia or arthritides, polymorphous skin rash, oral and/or genital aphthosis, spleenmegaly | Anti-inflammatory drugs, corticosteroids, anakinra |
| FCAS2 |
NLRP12 19q13.42 |
Monarch 1 | AD | fever (mainly induced by the exposure to cold), episodic and recurrent non-itchy urticaria-like rash, arthralgia, myalgia, headache, abdominal pain, sensorineural deafness | Anakinra, TNF-α inhibitors, IL-6 antagonists (tocilizumab) |
| PAPA s. |
PSTPIP1 15q24–25 |
CD2 antigen-binding protein 1 | AD | pyogenic sterile arthritis, pyoderma gangrenosum, severely disfiguring acne, skin abscesses, recurrent nonhealing sterile ulcers, risk of spoiling scars | Corticosteroids, infliximab, anakinra, immunosuppressive agents |
| MS |
LPIN2 18p11.31 |
Lipin 2 (phosphatidate phosphatase) | AR | recurrent multifocal osteomyelitis, dyserythropoietic anemia, neutrophilic dermatosis, growth failure | Corticosteroids, bisphosphonates, TNF-inhibitors, IL-1 antagonists (anakinra) |
| DIRA |
IL1RN 2q14.1 |
IL-1 receptor antagonist | AR | neonatal onset of sterile multifocal osteomyelitis, osteopenia, periostitis, pustulosis | Anakinra |
| BS |
NOD2/CARD15 16q12.1-13 |
NOD2/CARD15 | AD | non-erosive granulomatous symmetric polyarthritis (referred as boggy synovitis with painless effusions and cyst-like exuberant swelling of joints), granulomatous severe panuveitis, skin granulomatous rash (frequently described as a “dirty” ichthyosiform rash) affecting trunk, buttocks and limbs | Corticosteroids, TNF-α inhibitors (infliximab), IL-1 antagonists |
| EO-IBD | IL10RA, IL10RB 11q23.3, 21q22.11 | Subunits A and B of IL-10 receptor | AD | recurrent fever, severe enterocolitis (starting within the first six years of life), enteric fistulas and perianal abscesses, failure to thrive, folliculitis, arthritides | Hematopoietic stem cell transplantation |
| ORAS |
OTULIN 5p15.2 |
Deubiquitinase (OTULIN) | AR | neonatal onset-fever, neutrophilic dermatosis, panniculitis, stunted growth | TNF-α inhibitors |
| PRAAS |
PSMB8 6p21.32 |
Proteasome subunit β8 | AR | fever, chronic atypical neutrophilic dermatosis, progressive lipodystrophy, erythema nodosum-like panniculitis, violaceous heliotrope-like eyelid swelling, abnormal growth of lips, muscular weakness and atrophy, severe joint contractures, basal ganglia calcifications, conjunctivitis, ear and nose chondritis, aseptic meningitis, hepatosplenomegaly, lymph node enlargement, arthralgias | Corticosteroids, immunosuppressive agents, anakinra, IL-6 antagonists (tocilizumab), TNF-α inhibitors, JAK inhibitors (baricitinib) |
| AGS |
TREX1, RNASEH2B, RNASEH2C, RNASEH2A, SAMHD1, ADAR, IFIH1 3p21.31, 13q14.3, 11q13.1, 19p13.13, 20q11.23, 1q21.3, 2q24.2 |
Enzymes involved in the duplication, repair and recombination of nucleic acids | AR (AD for IFIH1) | recurrent fever, subacute leukoencephalopathy (mimicking a transplacental infection with loss of white matter), cerebral and basal ganglia calcifications, dystonia, microcephaly, cognitive impairment, abnormal eye movements and nystagmus, glaucoma, livedo reticularis, digital chilblain lesions on hands and feet, cerebrospinal fluid pleocytosis, hepatosplenomegaly, jaundice, low-titer positivity of autoantibodies | Corticosteroids, intravenous immunoglobulins, reverse transcriptase inhibitors |
FMF: familial Mediterranean fever; TRAPS: tumor necrosis factor receptor-associated periodic syndrome; FCAS: familial cold autoinflammatory syndrome; MWS: Muckle-Wells syndrome; CINCA s.: chronic infantile neurologic cutaneous articular syndrome; MKD: mevalonate kinase deficiency; FCAS2: familial cold autoinflammatory syndrome 2 (NLRP12-associated autoinflammatory disorder); PAPA s.: pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) syndrome; MS: Majeed syndrome; DIRA: deficiency of IL-1 receptor antagonist; BS: Blau syndrome; EO-IBD: early onset-inflammatory bowel disease; ORAS: OTULIN-related autoinflammatory syndrome; PRAAS: proteasome-associated autoinflammatory syndrome; AGS: Aicardi-Goutières syndrome. AR: autosomic recessive; AD: autosomic dominant; TNF: tumor necrosis factor; IL-1: interleukin-1.
Distinctive Features of the Monogenic Systemic Autoinflammatory Disorders
FMF, characterized by recurrent self-limited episodes of fever and polyserositis manifesting with abdominal and/or chest pain, is the most common monogenic SAID in the world, with the highest incidence in populations living throughout areas of the Mediterranean basin, especially Turks, Arabs, non-Ashkenazi Sephardic Jews and Armenians. The gene responsible for FMF encodes for pyrin (or “marenostrin”, from the ancient Latin name of the Mediterranean sea, mare nostrum), a protein largely expressed in white blood cells and involved in the activation and processing of IL-1ß.9 Recent experimental studies have suggested that pyrin detects cytoskeletal rearrangements within cells, warranting the clinical efficacy of colchicine as a prophylactic agent in FMF.10 Specifically, pyrin role as inflammasome sensor is mediated by RhoA (Ras homolog gene family-member A) GTPases, enzymes targeted to the plasma membrane by the addition of a geranylgeranyl lipid tail: in particular, FMF-related mutations in the pyrin B30.2 domain decrease the threshold of activation of the pyrin inflammasome, normally activated by various RhoA-inhibiting toxins produced by both Gram-negative and Gram-positive bacteria. The inhibition of RhoA effector kinases leads to dephosphorylated pyrins, which in turn activate pyrin inflammasome. Colchicine, a major neutral alkaloid from Colchicum species, suppresses pyrin inflammasome activity through direct activation of RhoA, disabling host cell cytoskeletal organization and causing an anti-chemotactic effect on the polymorphonuclear cells.11 As an alternative to colchicine, more recently, the IL-1 blockade has given brilliant results in colchicine-resistant patients.12 Many organs can be involved by FMF flares and in recent years different non-classical manifestations have been associated to the disease, such as vasculitides, infantile colitis, neurologic diseases, mood or sleep disorders and sensory organ abnormalities.13 Table II shows the clinical diagnoses to consider for the discrimination of febrile attacks in children with FMF. There is no specific test for the diagnosis of FMF, which is established on the basis of clinical data and supported by the patient’s ethnic origin or family history. The most widely used diagnostic tools are the Tel Hashomer and Livneh’s criteria, which have been listed in Table III.14,15 Amyloidosis, which can impact kidney function, but also gut, liver and heart, is the most dreadful complication of late-diagnosed or untreated FMF.16 Most amyloidosis patients with renal involvement develop nephrotic syndrome and chronic renal insufficiency in the long-term.17
Table 2.
Differential Diagnosis of Familial Mediterranean Fever according to the Prominent Clinical Sign occurring in Childhood.
| Fever with abdominal pain | Fever with thoracic pain | Fever with arthralgia/arthritides | Fever with skin rash |
|---|---|---|---|
| Inflammatory bowel disease | Pleuropneumonia | Acute rheumatic fever | Hypersensitivity reaction |
| Pancreatitis | Autoimmune pleuro-pericarditis | Reactive arthritis | Crohn’s disease |
| Biliary and renal lithiasis | Recurrent benign pericarditis | Juvenile idiopathic arthritis | Cyclic neutropenia |
| Hemolytic syndromes | Idiopathic acute recurrent pericarditis | Septic arthritis | Behçet’s disease |
| Mevalonate kinase deficiency | Cholecystitis | Spondyloarthropathies | Cryopyrin-associated periodic syndrome |
| Porphyrias | Pulmonary embolism | Behçet’s disease | Mevalonate kinase deficiency |
Table 3.
Classification Criteria for the Clinical Diagnosis of Familial Mediterranean Fever (FMF). According to the Tel Hashomer criteria, diagnosis is made when 2 major criteria or 1 major and 2 minor criteria are satisfied, while the diagnosis is probable if 1 major and 1 minor criterion are present. According to Livneh’s criteria diagnosis of FMF requires ≥ 1 major criteria, or ≥ 2 minor criteria, or 1 minor criterion plus ≥5 supportive criteria (family history of FMF, appropriate ethnic origin, age less than 20 years at disease onset, severity of attacks requiring bed rest, spontaneous remission of symptoms, presence of symptom-free intervals, transient elevation of inflammatory markers, episodic proteinuria or hematuria, nonproductive laparotomy with removal of a “white” appendix, consanguinity of parents) or 1 minor criterion plus ≥ 4 of the “first” five supportive criteria.
| Tel Hashomer criteria | Livneh’s criteria |
|---|---|
| Major | Major |
| Recurrent fevers + peritonitis, synovitis, pleurisy | Typical attack of generalized peritonitis |
| AA-amyloidosis | Typical attack of unilateral pleuritis/pericarditis |
| Favorable response to colchicine | Typical attack of monoarthritis |
| Minor | Presence of fever alone (rectal temperature of 38°C or higher) |
| Recurrent febrile episodes | Minor |
| Erysipelas-like erythema | Incomplete attack involving abdomen |
| Family history of FMF in a first-degree relative | Incomplete attack involving chest |
| Incomplete attack involving one large joint | |
| Exertional leg pain | |
| Favorable response to colchicine |
Note: “Incomplete” attacks are defined as painful and recurrent flares that differ from typical attacks in 1 or 2 features, as follows: 1) normal temperature or lower than 38°C; 2) attacks longer than 1 week or shorter than 6 hours; 3) no signs of peritonitis recorded during acute abdominal complaint.
TRAPS, initially named “Hibernian fever” due to the ancient Latin name of Ireland, Hibernia, where the first patients were reported, is the most common autosomal dominant condition among SAIDs, and is related to the dysfunctional activity of the 55 kD receptor of tumor necrosis factor (TNF)-α.18 The average age at which the disease starts is around three years, but TRAPS may sometimes arise in the late adolescence or in adulthood with longlasting sudden febrile attacks, which might persist until three weeks and recur with varying intervals, generally longer than those seen in other SAIDs. Individuals with low-penetrance TRAPS-related mutations have a nonsignificant family history and a later disease onset if compared with patients carrying structural mutations.19 Amyloidosis is genotype-dependent and is frequently observed as a complication of overlooked patients with TRAPS.20 A non-amyloid recurrent self-limited acute pericardial disease is the most represented cardiovascular abnormality in both TRAPS and FMF.21 A wide genetic and clinical heterogeneity makes puzzling the management of TRAPS patients: corticosteroids can mitigate inflammatory attacks in most patients, whereas the TNF inhibitor etanercept might be successful in a minority of cases. Conversely, anti-IL-1 therapies have clearly revealed the highest efficacy regarding clinical control of all TRAPS manifestations, normalization of inflammatory markers, and even reversal of amyloidosis and are today the treatment of choice.22
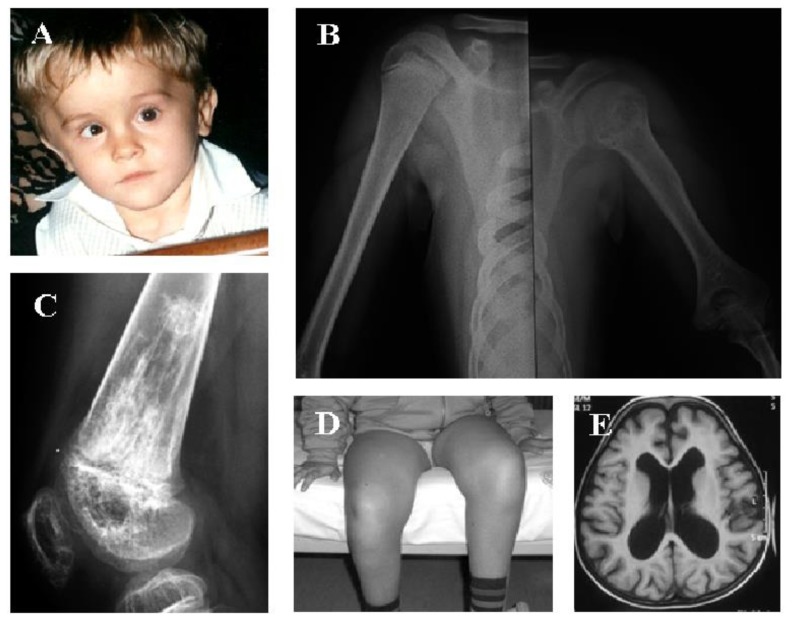
CAPS includes three phenotypes of the same autosomal-dominant disorder: FCAS, MWS and CINCA syndrome, respectively from the least to the most severe, and all involve the protein named “cryopyrin”, which is another intracellular sensor participating in inflammasome-driven innate immunity responses.23 Genotype-phenotype association studies have shown that some peculiar CAPS-related mutations are associated with early disease onset and severe organ involvement.24 All CAPS patients have abnormal body temperature patterns with flu-like muscle pain, headache, urticaria-like plaques without pruritus, fatigue and variable ocular inflammatory signs, whereas the clinical triad of skin rashes, chronic meningopathy and arhtropathy, mostly involving the knees, identifies the phenotype of CINCA syndrome (Figure 1). Additionally, a neurocognitive impairment can be found in 50% of CINCA patients.
Figure 1.
A 7-year-old boy with chronic infantile neurological cutaneous articular (CINCA) syndrome is shown, revealing a peculiar syndromic face with frontal bossing, saddle nose and midface hypoplasia (A). Epiphyses of long bones are involved with possibility of uneven bone length (B). The knee is affected with a severe typical osteopathy leading to kneecap protrusion and femoral distal epiphyseal deformity due to the presence of calcified mass-like areas (C), flexion contractures and severe disability (D). Chronic aseptic meningitis leads to ventriculomegaly, enlarged subarachnoid spaces and brain atrophy, as proven in axial T1-weighted MRI of the brain (E). A written informed consent was obtained by the patient’s parents for the publication of these pictures.
The major muscular and skeletal anomalies of CINCA syndrome are caused by premature and aberrant ossification of kneecaps, with severe abnormalities involving long bone epiphysis and hypertrophy of growth plates.25 Endocrine abnormalities might also impair the final growth of CINCA patients.26 Diagnostic criteria for CAPS, based on the elevation of inflammatory markers, which should be regarded as a mandatory criterion, have been recently validated and are listed in Table 4.27
Table 4.
Diagnostic Criteria for the Clinical Diagnosis of Cryopyrin-Associated Periodic Syndrome.
| Raised inflammatory markers (C-reactive protein/serum amyloid-A) |
| plus at least 2 or more among 6 typical signs or symptoms: |
|
|
|
|
|
|
Early and aggressive treatment is crucial to avoid end-organ damage, as most symptoms are frankly reversible if therapy is given as early as possible. For many years treatment of this condition has been supportive and use of TNF-inhibitors rather disappointing.28 After revealing that CAPS manifestations are mediated by IL-1 oversecretion a more specific treatment with IL-1 blocking therapies has produced great success in each disorder of the CAPS spectrum.29 Both the human anti-IL-1β monoclonal antibody canakinumab and IL-1 receptor antagonist anakinra are approved for treatment of CAPS.30 Long-term efficacy and sustained benefit for these drugs also on the central nervous system and skeletal disease of CINCA syndrome are expected to be proven in the near future.31,32 The development of amyloidosis occurs in 20% of cases, whereas persistent fatigue with a significant impact on the quality of life is commonly found in all CAPS patients.33
Partially related to CAPS is the NLRP12-autoinflammatory disorder, also named FCAS2 because of its clinical similarity with FCAS, an exceedingly rare autosomal dominant disease involving the protein monarch 1, which is implicated in noncanonical nuclear factor (NF)-κB signaling pathway: the disease is characterized by recurrent cold-induced episodes of fever, skin rash, abdominal pain and lymph node enlargement which are only partially responsive to the IL-1 inhibitor anakinra.34 Schnitzler syndrome is another rare disorder characterized by chronic urticaria-like rashes and monoclonal gammopathy, along with fever, arthralgia and bone pain: although the pathophysiology is presently unknown, the clinical improvement seen in patients treated with IL-1 antagonists confirms an autoinflammatory pathogenesis. The clinical onset of this syndrome resembles CAPS phenotype, though it starts at a much older age.35
MKD, also known as hyper-IgD syndrome in consequence of the frequent finding of unexplained polyclonal elevation of serum IgD in at least half of patients, is caused by deficient or aberrant activity of mevalonate kinase, the second enzyme in the metabolic pathway of cholesterol, normally supplying numerous bioactive molecules such as sterols and isoprenoids.36 An increased urinary excretion of mevalonic acid can be demonstrated only during febrile attacks and is of outstanding priority for the diagnostic confirmation. In 75% of patients, the disease starts within the first year of life with high fever, recurring every 3–4 weeks, combined with arthralgia, lymphadenopathy, gastrointestinal discomfort, oral ulcers, and heterogeneous skin signs, which might persist throughout the lifetime, though flares tend to diminish in intensity and frequency with increasing age.37 Many children have a dramatic history of recurrent fevers started in the first infancy, which might have been overlooked by general practioners.38 The prevalence of amyloidosis, which was presumed to be rare, has resulted higher than expected in the largest cohort of patients with MKD studied so far.39 Guidelines referred to the prescription of genetic testing for MKD are frequently used to support the diagnosis (Table 5).40 Therapeutic options for MKD include non-steroidal anti-inflammatory drugs and biological agents targeting IL-1, such as anakinra (also given on-demand) and canakinumab, whereas hematopoietic stem cell transplantation has been used in the very severely affected patients or in those with mevalonic aciduria, which is caused by the absolute deficiency of the enzyme mevalonate kinase.41
Table 5.
Clues Required to Suggest Genotype Analysis in Children Suspected to have Mevalonate Kinase Deficiency (MKD).
| Recurrent febrile attacks of 3-to-7-day duration for more than 6 months with disease onset before 5 years |
| plus at least 1 or more of the following signs: |
|
|
|
|
Hereditary diseases with pyogenic manifestations involving bones and skin pertain to the SAID group and are characterized by the recurrent formation of a neutrophil-rich exudate. PAPA syndrome is defined by pyogenic sterile arthritides, sterile cutaneous abscesses, disfiguring cystic acne and possibility of pyoderma gangrenosum.42
Recurrent precociously-arising multifocal osteomyelitis mostly involving the clavicles, sternum and long bones, dyserythropoietic anemia and febrile neutrophilic dermatosis ranging from palmoplantar pustular rash to Sweet syndrome and psoriasis are hallmarks of MS, which has been described in children of Arab descent.43 DIRA can be recognized by sterile multifocal osteomyelitis and skin pustulosis starting in the neonatal period, which are caused by unopposed IL-1 activity and dramatically respond to anakinra.44 In particular, the cause of PAPA syndrome is the dysfunctional protein proline-serine-threonine phosphatase-interacting protein 1 (PSTPIP1), which works as a cytoskeleton-associated adaptor,45 and diagnosis requires to rule out similar bone disorders associated with suppurative hidradenitis or psoriatic arthritis.46 Pyoderma gangrenosum belongs to the family of neutrophilic dermatoses and starts with non-infectious painful soft tissue ulcerations: systemic corticosteroids represent the cornerstone of treatment, whereas other corticosteroid-sparing drugs, including cyclosporine, azathioprine, mycophenolate mofetil or methotrexate, and TNF-α or IL-1 antagonists are useful in recalcitrant forms as second-line therapies.47 Limited experience exists with antagonists of IL-12/IL-23 or IL-17A pathways (ustekinumab or secukinumab, respectively), but the dermatologic phenotype of MS and DIRA usually responds impressively to IL-1 blockade.48 Other similar conditions are deficiency of the IL-36 receptor antagonist (also named DITRA), characterized by early-onset palmoplantar pustular rashes, joint pain and high fever, and CARD14-mediated pustular psoriasis (also named CAMPS), characterized by severe generalized pustular psoriasis.49
Known as familial juvenile granulomatosis, to differentiate its sporadic counterpart which is named “early-onset sarcoidosis”, BS is a rare autosomal dominantly-inherited disease caused by dysfunction of the apoptosis-regulating protein NOD2 (or CARD15), which is mainly involved in the intracellular recognition of bacterial peptidoglycan-conserved motifs and activation of the host innate immune response.50 Patients might present early onset-deforming polyarthritis, tan-coloured skin rash, and refractory panuveitis: the histological evaluation reveals noncaseating granulomas with multinucleated giant cells, emperipolesis (i.e., active penetration of an intact cell within the cytoplasm of another living cell), and also autophagic phenomena.51 Different studies have confirmed the association of NOD2-related mutations not only with BS, but also with Crohn’s disease, highlighting the pivotal role of the protein NOD2 in host-pathogen interactions and initiation of different inflammatory responses.52 Recent reports have also shown a very aggressive form of Crohn’s disease in children with dysfunctional subunits A or B of the IL-10 receptor, who present an aberrant IL-10-induced secretion of TNF-α and other proinflammatory cytokines: its consequence is an early onset-inflammatory bowel disease (EO-IBD), and affected children have endoscopic and histological features of Crohn’s disease, while treatment with immunomodulators, corticosteroids and TNF inhibitors might show disappointing results.53
A defect in the deubiquitination of different target molecules has been related to ORAS, a rare disease characterized by dermatosis starting in infancy and febrile panniculitis, specifically caused by dysfunction of the deubiquitinase OTULIN, leading to aberrant protein degradation within cells and secondary expression of proinflammmatory molecules.54 The brilliant response to monoclonal anti-TNF antibodies in children with ORAS confirms the seminal role of this cytokine in its pathophysiology.55 Another defect in the degradation and recycling of aberrant proteins produced by stressed cells is the cause of PRAAS, a rare early onset-disease characterized by atypical neutrophilic dermatosis resembling a “cutis leukemia”, progressive lipodystrophy and recurrent fevers, firstly identified as chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE syndrome); this disorder is specifically caused by proteasome dysfunction, and a subsequently increased type I IFN production.56 Clinical onset occurs in early infancy with fever, characteristic skin lesions and a typical facial fat loss, all conferring an unmistakable phenotype to children. Treatment of PRAAS with corticosteroids, dapsone and IFN modulators, such as the Janus kinase inhibitor baricitinib, has given interesting, though variable results.57
Further perturbations of proteins essential to IFN homeostasis can be included in the group of interferonopathies, characterized by unique expression of IFN-related genes: Aicardi-Goutières syndrome (AGS) is a clinical spectrum related to upregulated type I IFN production, which shows a noteworthy overlap with transplacental congenital infections and involves central nervous system and skin, with slight differences across numerous causing genotypes.58 The genetic causes of AGS include lost enzymatic activities important for regulating intracellular DNA and RNA metabolism, but the exact mechanism by which mutations cause this subacute encephalomyelitis is not clear: the accumulating endogenous nucleic acids might trigger nucleotide sensors and type I IFN overproduction.59 There is currently no effective treatment for AGS, though high-dose methylprednisolone, immunosuppressants and intravenous immunoglobulins have been used with inconstant results in terms of neurologic improvement.60
Discrimination between Monogenic and Polygenic Autoinflammatory Disorders
Recurrent fevers of children have an important impact both on the child and his/her family and if referred to recurrent upper respiratory infections are one of the most common reason for pediatric health assessments and hospitalization.61 The majority of these children may show a normal growth, having simply repeated benign viral or bacterial infections localized in the upper respiratory tract: only a minority might display abnormalities of the immune system. A frequent and misdiagnosed cause of recurrent fevers in the pediatric age is PFAPA syndrome: despite the many efforts made to characterize it, there are still much debate and controversies about definition of PFAPA syndrome in children and adults (Table 6), though laboratory evaluations both during and between febrile episodes together with detailed history-taking and mindful collection of physical findings during flares are useful for diagnosis. Many pros and cons related to the infectious or dysregulated immunological PFAPA origin persist.62
Table 6.
Definitions Proposed for Periodic Fever, Aphthous Stomatitis, Pharyngitis and Cervical Adenopathy (PFAPA) Syndrome in Children and Adults.
| General basic signs | Clinical signs during fever | Conditions to rule out | |
|---|---|---|---|
| Children | -Periodically recurring high fevers (with “clockwork” periodicity at intervals of 4–6 weeks) -Onset before 5 years -Child’s complete wellness between febrile episodes (with normal growth, normal development, and no sequelae) |
- At least 1 among:
|
-Cyclic neutropenia -Recurrent upper respiratory infections -Monogenic hereditary systemic autoinflammatory disorders |
| Adults (at least 16-year-old) | -Recurrent fevers -Increased inflammatory markers during febrile attacks -Symptom-free intervals |
-At least 1 between:
|
-Infections (with negative throat swab during fever and/or ineffectiveness of antibiotic therapy) -Autoimmune disorders -Monogenic hereditary systemic autoinflammatory disorders -Other polygenic systemic autoinflammatory disorders |
The syndrome is mostly observed in children less than 5 years, who are completely asymptomatic between febrile flares, with both normal growth and development, and should have no evidence of upper airways infections during fever episodes. It seems established that typical PFAPA syndrome is a benign disease, although lived with troubling perplexity by parents, and that PFAPA flares respond to intermittent low-dose corticosteroids administered at fever onset, while the role of tonsillectomy to lessen the periodic recurrence of flares is uncertain.63 The description of adults with PFAPA syndrome or the reappearance of PFAPA symptoms in adulthood contributes to the reconsideration of this condition as a non-Mendelian autoinflammatory disorder not specifically restricted to the pediatric age,64,65 though adults are characterized by a wider repertoire of clinical features.66 The genetic susceptibility of PFAPA syndrome remains to investigate, although the presence of overlapping symptoms with monogenic hereditary SAIDs, the recurrence of PFAPA symptoms in family members, the overexpression of inflammasome-associated genes during flares and, last but not least, the therapeutic efficacy of IL-1β blockade strongly indicate a potential genetic contribution to its expression.
The number of multifactorial SAIDs with a presumed polygenic basis is quickly increasing, and indeed, during the last decade, the innate immune system has been found to participate in a wide range of systemic disorders previously recognized as dysmetabolic or degenerative, including type 2 diabetes mellitus, gout, chondrocalcinosis, idiopathic recurrent acute pericarditis, storage disorders and fibrosing lung diseases. In a genuine rheumatologic context, Behçet’s disease, systemic juvenile idiopathic arthritis and adult-onset Still’s disease are at present recognized as polygenic and multifactorial autoinflammatory entities in which the innate immune system plays an active pathogenic role. The discrimination between PFAPA syndrome during childhood and early onset-Behçet’s disease, an idiopathic inflammatory disorder sharing clinical features of both SAIDs and vasculitides, may be arduous and the exact recognition of each condition challenging: Behçet’s disease involves primarily the oral and genital mucosa, but also skin and eyes; in addition, all-sized vessels can be affected.67 Behçet attacks are recurrent and self-limited, resembling PFAPA flares.68 The combination of genetic factors affecting the immune regulation and undetermined environmental triggers explains the variable disease expression of Behçet’s disease, which is also influenced by sex, country of residence and age of onset. Convincing evidence of IL-1β role in Behçet’s disease and a further proof of its potential autoinflammatory pathogenesis derive from trials related to the administration of anakinra, canakinumab and gevokizumab in patients with multi-resistant and refractory Behçet’s uveitis.69,70
Unlike other categories of juvenile idiopathic arthritis, the systemic variant of juvenile idiopathic arthritis is uniquely marked with a range of extra-articular symptoms including fever, rashes, hepatosplenomegaly, lymphadenopathy and serositis, and is thought to be an autoinflammatory condition.71 Further advances in our knowledge on the pathogenesis of many multifactorial disorders with a presumed autoinflammatory component have paved the way to the introduction of novel therapeutic modalities. For instance, urate monosodium crystals are specifically detected via intracellular inflammasomes, resulting in IL-1β overproduction and initiation of a typical gouty attack: the beneficial effects of IL-1 blockade for the treatment of patients with gout has reaffirmed the concept of this disease as belonging to the group of SAIDs.72
Moreover, type 2 diabetes mellitus is profoundly influenced by inflammasome-dependent IL-1 release, and high serum concentrations of glucose lead to increased IL-1β production in human β cells, which is followed by NF-kB activation, Fas signalling upregulation and β cell apoptosis.73 In the end, also pediatric patients with idiopathic recurrent acute pericarditis are brilliant responders to IL-1 inhibition, suggesting that a defect in inflammasome function and signaling might be associated with this mysterious pericardial disorder.74 According to these recent advances the definition of SAIDs should be revised, rethinking these diseases as clinical entities marked by abnormal inflammation mediated predominantly by cells and molecules of the innate immune system with a significant host predisposition in some patients: such definition should allow to include the Mendelian diseases that initially prompted the concept of autoinflammation as well as a broader range of common diseases with a pathogenesis linked to the innate immunity.
Acute Painful Symptoms in the Monogenic Systemic Autoinflammatory Disorders
Several organs and systems may be involved by recurrent sterile inflammation in monogenic SAIDs and severe clinical manifestations may occur unpredictably in different tissues and at different times. Almost every organ might be involved, making extremely variable the clinical sceneries of SAIDs with recurrent pain in different sites as the predominating symptom in a high number of cases.
Brief episodes of fever characterize FMF and abdominal, joint or chest pain are variably associated and frequently referred by the majority of patients: however, the clinical spectrum of this disease has expanded recently.75,76 Up to 25% of patients with FMF report muscle pain and patterns of FMF-related myalgia can be spontaneous or exercise-induced and set up a protracted febrile myalgia syndrome: this severe paralyzing myalgia is associated with fever and transient vasculitic rashes mimicking Henoch-Schönlein purpura, lasting for several weeks and usually responding to corticosteroids.77
One of the most relevant and TRAPS-specific symptoms is myalgia, which has been reported in about 60% of patients: usually, only one muscular area of the body is affected and associated with tenderness and erythematosus rashes, migrating with centrifugal mode during the inflammatory attack. In this case, a muscle-specific magnetic resonance imaging with biopsy might reveal monocytic fasciitis or lymphocytic vasculitis rather than a pure myositis as the underlying cause.78
The effect of CAPS on quality of life is significant in all phenotypes, with the limitation of ability to work and in the participation to outdoor activities, mostly in consideration of symptoms related to the musculo-skeletal system. A characteristic arthropathy caused by excessive overgrowth of kneecaps and epiphysis of the long bones may be found in 60% of patients with CINCA syndrome in combination with painful joint contractures, reduced longitudinal growth of affected bones, uneven bone length and sometimes inability to walk.79 IL-1 blockade has proven its paramount efficacy in the treatment of CAPS patients, who have reported a dramatically rapid remission of painful manifestations even after the first dose.80
Patients with MKD display severe abdominal pain during attacks, often accompanied by debilitating transient joint pain, painful cervical lymph node enlargement and severe fatigue: children with mevalonic aciduria, which is the most severe expression of MKD, or children with early onset-MKD might also display growth restriction, dysmorphic features, cerebral ventriculomegaly and skeletal abnormalities.81
Painful multifocal osteolytic lesions with periosteitis are frequently observed in children with hereditary pyogenic diseases.82 In particular, 2–4-year-aged children with PAPA syndrome might present a typical “boggy” polyarthritis.83
Conversely, patients with defects of the ubiquitin-proteasome might display a diffuse muscle involvement with a tendency to muscular atrophy, fat loss and severe joint contractures.84 Table 7 lists the potential musculo-skeletal symptoms occurring in the monogenic SAIDs and Table 8 shows the diseases most frequently characterized by acute painful symptoms related to joints and muscles, which might enter in the differential diagnosis.
Table 7.
Musculo-Skeletal Pain in the Monogenic Systemic Autoinflammatory Disorders.
| Autoinflammatory disorder | Painful manifestations in the musculo-skeletal system | Comorbid symptoms | Precipitating factors |
|---|---|---|---|
| FMF | Transient arthralgias or arthritides, exertional leg pain, enthesopathy, protracted febrile myalgia | Recurrent abdominal distress, polyserositis (with specific painful symptoms), erysipelas-like rash in the lower extremities, recurrent self-limited orchitis | Menses, stress, surgery, starvation, longlasting standing, sleeplessness, hypovitaminosis D |
| TRAPS | Monocytic fasciitis with migratory pattern and proximal-to-distal distribution, severe myalgia, muscle cramps, arthralgias, non-erosive arthritides | Recurrent abdominal distress, migratory centrifugal inflammatory skin signs varying from nonspecific rashes to cellulitis and panniculitis or angioedema, painful conjunctivitis, headache, polyserositis (with specific painful symptoms) | Unknown |
| FCAS | Arthralgias | Urticaria-like non-itchy rash, fatigue, chronic headache, mild recurrent ocular inflammatory signs | Cold exposure, stress |
| MWS | Lifolong arthralgias or arthritides | Urticaria-like rash, fatigue, headache, ocular inflammatory signs | Cold exposure, stress |
| CINCA s. | Osteoarthropathy of large joints due to enhanced chondrogenesis with deforming bony abnormal epiphyseal overgrowth | Migratory and refractory urticaria-like neutrophilic rash, chronic leptomeningitis with retinal and optic nerve changes | Cold exposure, stress |
| MKD | Arthralgias, non-erosive arthritides | Abdominal distress during febrile attacks, lymph node diffuse enlargement, heterogeneous skin rashes (from nonspecific erythematous or nummular rash to erythema nodosum, erythema elevatum diutinum and disseminated superficial actinic porokeratosis), headache, oral, genital, perianal or rectal aphthosis | Vaccinations, infections |
| FCAS2 | Diffuse musculo-skeletal pain | Recurrent fevers, fatigue, skin urticaria-like signs, early onset-abdominal pain | Cold exposure |
| PAPA s. | Sterile nonaxial destructive oligoarthritis | Pyoderma gangrenosum, severe acne, sterile abscesses at the site of needle injections | Unknown |
| MS | Chronic recurrent multifocal osteomyelitis, risk of joint contractures, skeletal disabilities | Neutrophilic dermatosis, psoriasis, odontoid process abnormalities | - |
| DIRA | Chronic recurrent multifocal osteomyelitis, osteolytic lesions, periarticular soft tissue swelling | Pustular rash, heterotopic ossification | - |
| BS | Symmetric granulomatous arthritides with noncaseating granulomas (“boggy synovitis”), joint contractures, camptodactyly | Painless synovial cyst-like swellings, lichenoid-like dermatitis with brown nodules and papules | - |
| EO-IBD | Chronic arthritides | Severe enterocolitis, bloody diarrhea, oral ulcers, recurrent folliculitis | Unknown |
| ORAS | None | Neutrophilic dermatosis, panniculitis | - |
| PRAAS | Muscular weakness and atrophy, myositis, severe joint contractures | Erythema nodosum-like panniculitis, annular erythematous plaques, progressive skin lypodystrophy | Cold, viral infections, stress |
| AGS | Spastic paraparesis, dystonic movements | Subacute encephalopathy, livedo reticularis, chilblain lesions, digital vasculitis | - |
FMF: familial Mediterranean fever; TRAPS: tumor necrosis factor receptor-associated periodic syndrome; FCAS: familial cold autoinflammatory syndrome; MWS: Muckle-Wells syndrome; CINCA s.: chronic infantile neurologic cutaneous articular syndrome; MKD: mevalonate kinase deficiency; FCAS2: familial cold autoinflammatory syndrome 2 (NLRP12-associated autoinflammatory disorder); PAPA s.: pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) syndrome; MS: Majeed syndrome; DIRA: deficiency of IL-1 receptor antagonist; BS: Blau syndrome; EO-IBD: early onset-inflammatory bowel disease; ORAS: OTULIN-related autoinflammatory syndrome; PRAAS: proteasome-associated autoinflammatory syndrome; AGS: Aicardi-Goutières syndrome.
Table 8.
General List of the Main Pediatric Diseases characterized by Acute Painful Symptoms in the Musculo-Skeletal System.
| Diseases | Description of pain | Comorbid symptoms to consider | Physical examination | Objective data to help confirm diagnosis |
|---|---|---|---|---|
| Acute rheumatic fever | Migratory arthritis of large joints, joint pain often out of proportion to physical findings | Carditis, erythema marginatum, Sydenham’s chorea | Tachycardia or heart murmurs can indicate carditis, joint small effusions | Erythrocyte sedimentation rate and C-reactive protein may be elevated at time of carditis and arthritis, anti-streptolysin O is highly increased, prolonged PR at the electrocardiogram may be found |
| Juvenile idiopathic arthritis | Pain and stiffness in one or more joints, which worse in the morning or with long stationary positions | Asymptomatic chronic uveitis | Ultrasound examination of joints to look for evidence of active inflammation | Anti-nuclear antibody positivity in a subset of patients |
| Arthritides in collagen vascular diseases | Pain and stiffness in one or more joints, finger pain with writing, temporo-mandibular joint pain with chewing | Symptoms related to collagen vascular diseases | Ultrasound examination of joints to look for evidence of active inflammation | Specific anti-extractable nuclear antigen antibody positivity |
| Pain processing disorders | Musculoskeletal pain out of proportion to physical findings | Sleep dysfunction, sensory amplification, hyperacusis, photophobia, disrupted taste or smell, abdominal pain, headache | Fibromyalgia-related pain at the pressure of “tender points”, diffuse tenderness or pain in joints, general hypersensitivity to minor trauma | None |
Hematologic Manifestations in Children with Hereditary Autoinflammatory Disorders
A hematologist should be aware of SAIDs as they share many clinical features occurring in hematologic neoplasms, like anemia, lymphadenopathy and/or splenomegaly which sometimes require that the patient should be primarily sent to hematologic wards. Furthermore, hematologic complications of SAIDs, like macrophage activation syndrome, might even display a critical role for prognosis. Laboratory investigations are useful in the diagnosis and follow-up of children with hereditary SAIDs: routine tests are useful to assess disease activity or measure the response and toxicity to treatments. Frequent is the presence of an inflammatory anemia in FMF patients, which is associated with hyposideremia and hyperferritinemia, though no studies have evaluated the potential role of hepcidin in orchestrating iron flux in blood as well as its release and mobilization from hepatocytes in FMF: colchicine prophylaxis usually has a positive effect on both FMF disease activity and anemia as well.85 Elevation of blood neutrophil-to-lymphocyte ratio may be used as a predictor of non-FMF inflammation to discriminate a true acute appendicitis in patients with FMF86, while high levels of mean platelet volume over the years might indicate a persistent subclinical inflammation with a risk of development of renal amyloidosis in adults or also a risk of early atherosclerosis in children.87,88 Furthermore, FMF patients with blood group A have a better response to colchicine administration than non-A group, and patients with blood group O are prominently associated with colchicine resistance.89
Chronic inflammation in CINCA syndrome is continuous with intermittent flares and persistent disease manifestations occurring within the central nervous system, skin, bones, eye and inner ear: these patients might present a constant elevation of acute phase reactants and white blood cells, which only reverse if the IL-1 blockade is started.90
The Schnitzler syndrome, a disease showing a noteworthy similarity with CAPS due to intermittent fevers, skin signs, musculoskeletal involvement with radiologic evidence of osteosclerosis, elevated acute phase reactants and leukocytosis, is characterized by a monoclonal IgM gammopathy combined with severe anemia of chronic diseases. Its most harmful complication is the gammopathy evolution to an authentic lymphoplasmacytic malignancy, which occurs in at least 15% of patients in the long-term.91
Dyserythropoietic anemia with extramedullary erythropoiesis and thrombocytopenia variably associated with cholestatic liver disease and recurrent sepsis-like episodes can characterize patients with mevalonic aciduria, which is the most severe and ultra-rare form of MKD, caused by the absolute deficiency of the enzyme mevalonate kinase.92
By contrast, a congenital form of microcytic dyserythropoietic anemia of variable severity has been described in children with MS, all of Middle Eastern ethnicity, starting before two years: bone marrow examination has revealed erythroid hyperplasia and dyserythropoiesis in these patients.93 At last, chronic microcytic anemia, mild leukocytosis, transient lymphopenia combined with the elevation of inflammatory markers and immunoglobulins are common in children with PRAAS. Moreover, hypertriglyceridemia and low high-density lipoprotein cholesterol can be observed in these patients, who also present a dermal infiltrate with predominantly CD68+ mononuclear cells, histiocytes, eosinophils, and neutrophils in the affected skin areas.94
Macrophage activation syndrome, an ominous complication with uncontrolled proliferation of highly activated macrophages, impaired function of natural killer and cytotoxic T cells, sepsis-like features, hemophagocytosis and hypercytokinemia, 95 has been reported in different hereditary SAIDs. All cases displayed various clinical, laboratory and histopathologic features of multi-organ failure with cytopenia affecting at least 2 of 3 lineages in the peripheral blood and overblown release of proinflammatory cytokines.95 Even if macrophage activation syndrome and SAIDs share multiple clinical features, as well as heterogeneous pathogenetic backgrounds and a potential response to anti-IL-1-targeted therapies, a prompt specific recognition of this complication in the course of SAIDs via bone marrow aspiration (revealing hemophagocytosis) should be warranted considering the profound severity and high mortality rates of this syndrome. A score based on age at onset, neutrophil and platelet count, hemoglobin, fibrinogen and splenomegaly has been created to differentiate patients who are more likely to have primary hemophagocytic lymphohistiocytosis from macrophage activation syndrome secondary to systemic juvenile idiopathic arthritis, who could be prioritized for functional and genetic testing.97 A scoring system does not exist for discriminating macrophage activation syndrome and SAIDs, but different data clearly highlight that both early suspicion and an aggressive therapeutic approach are critical points to improve the overall outcome: therefore, clinicians involved in the management of monogenic SAIDs should be aware that the combination of clinical data (multi-organ dysfunction), laboratory clues (fall in platelet count, fall in erythrocyte count, hyperferritinemia, hypertriglyceridemia, hypofibrinogenemia), and histopathology (evidence of hemophagocytosis in any involved organ) might suggest the identification of macrophage activation syndrome and start the specific treatment.98
Conclusive Remarks
A huge progress has been achieved in the recognition of non-infectious, non-rheumatic and non-tumoral causes of recurrent fevers in children:99 the continuous breakthroughs of cellular and molecular biology have shown that an impaired control of innate immune system generates the so-called SAIDs, an expanding family of diseases characterized by lifelong seemingly unprovoked attacks of systemic inflammation involving serous membranes, joints, gastroenteric tube, skin and central nervous system, while autoantibodies, autoreactive T cells or infectious antigens cannot be recognized and confirmed. Through this overview we have seen how the inflammasome works as a platform releasing bioactive IL-1β: dysregulation of this platform may be caused by mutations in the genes coding for inflammasome components and/or their interaction partners, leading to protean autoinflammatory sceneries. In addition, the contribution of deregulated inflammasomes to the pathogenesis of different inflammatory diseases with a presumed polygenic basis has been recently corroborated by the amazing efficacy of IL-1 blockade at a clinical level.100
All these increased insights related to SAIDs have allowed physicians to find out new targeted therapies against molecules responsible for the inflammatory manifestations of SAIDs and revolutionize the clinical picture and outcome of most patients. As new research will continue to unravel the innate immune system’s arsenal of sensory proteins and the family of SAIDs further expands, it is likely that the different pathways comprising IL-1 function will be better clarified and further clinical uses for IL-1 inhibitors will be defined. Different scoring systems have been proposed for assessing disease activity, establishing treatment efficacy and follow-up strategies in SAIDs,101–104 whereas an instrument to measure the chronic damage caused by SAIDs has been recently developed with the collaboration of patients.105 However, the search for clinimetric tools to compare disease outcomes in the clinical studies is still in progress.
The global severity of these inborn errors of innate immunity underscores the decisive importance of the IL-1 pathway in both physiological homeostasis as well as in the immunologic responses programmed against infections, but also against damaged or dying cells: understanding the rules of engagement of innate immunity cells, how they are influenced by both magnitude and quality of a given inflammatory trigger and how acute versus persistent stimuli influence the inflammatory equilibrium will be outstanding keys to develop novel anti-inflammatory agents and host-directed therapies for a variety of hereditary and non-hereditary SAIDs in the future. New avenues for the therapy of such disorders will be therefore paved, and our way of managing these patients will probably be overturned.
Footnotes
Competing interests: The author has declared that no competing interests exist.
References
- 1.Minasyan H. Phagocytosis and oxycytosis: two arms of human innate immunity. Immunol Res. 2018;66:271–80. doi: 10.1007/s12026-018-8988-5. [DOI] [PubMed] [Google Scholar]
- 2.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–61. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 3.Lopalco G, Cantarini L, Vitale A, et al. Interleukin-1 as a common denominator from autoinflammatory to autoimmune disorders: premises, perils, and perspectives. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/194864. 194864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodar EJ, Drenth JP, van der Meer JW, et al. Dysregulation of innate immunity: hereditary periodic fever syndromes. Br J Haematol. 2009;144:279–302. doi: 10.1111/j.1365-2141.2008.07036.x. [DOI] [PubMed] [Google Scholar]
- 5.Rigante D. New mosaic tiles in childhood hereditary autoinflammatory disorders. Immunol Lett. 2018;193:67–76. doi: 10.1016/j.imlet.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Gentileschi S, Vitale A, Frediani B, et al. Challenges and new horizons in the periodic fever, aphthous stomatitis, pharingitis and adenitis (PFAPA) syndrome. Expert Opin Orphan Drugs. 2017;5:165–71. doi: 10.1080/21678707.2017.1279049. [DOI] [Google Scholar]
- 7.Caso F, Cantarini L, Lucherini OM, et al. Working the endless puzzle of hereditary autoinflammatory disorders. Mod Rheumatol. 2014;24:381–9. doi: 10.3109/14397595.2013.843755. [DOI] [PubMed] [Google Scholar]
- 8.Vanaja SK, Rathinam VA, Fitzgerald KA. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol. 2015;25:308–15. doi: 10.1016/j.tcb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigante D, Frediani B, Galeazzi M, et al. From the Mediterranean to the sea of Japan: the transcontinental odyssey of autoinflammatory diseases. Biomed Res Int. 2013;2013 doi: 10.1155/2013/485103. 485103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rigante D, La Torraca I, Avallone L, et al. The pharmacological basis of treatment with colchicine in children with familial Mediterranean fever. Eur Rev Med Pharmacol Sci. 2006;10:173–8. [PubMed] [Google Scholar]
- 11.Park YH, Wood G, Kastner DL, et al. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol. 2016;17:914–21. doi: 10.1038/ni.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varan Ö, Kucuk H, Babaoglu H, et al. Efficacy and safety of interleukin-1 inhibitors in familial Mediterranean fever patients complicated with amyloidosis. Mod Rheumatol. 2018 Apr;27:1–4. doi: 10.1080/14397595.2018.1457469. [DOI] [PubMed] [Google Scholar]
- 13.Rigante D, Lopalco G, Tarantino G, et al. Non-canonical manifestations of familial Mediterranean fever: a changing paradigm. Clin Rheumatol. 2015;34:1503–11. doi: 10.1007/s10067-015-2916-z. [DOI] [PubMed] [Google Scholar]
- 14.Sohar E, Gafni J, Pras M, et al. Familial Mediterranean fever. A survey of 470 cases and review of the literature. Am J Med. 1967;43:227–53. doi: 10.1016/0002-9343(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 15.Livneh A, Langevitz P, Zemer D, et al. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. 1997;40:1879–85. doi: 10.1002/art.1780401023. https://doi.org/10..1002/1529-0131(199710)40. [DOI] [PubMed] [Google Scholar]
- 16.Yepiskoposyan L, Harutyunyan A. Population genetics of familial Mediterranean fever: a review. Eur J Hum Genet. 2007;15:911–6. doi: 10.1038/sj.ejhg.5201869. [DOI] [PubMed] [Google Scholar]
- 17.Rigante D. The fresco of autoinflammatory diseases from the pediatric perspective. Autoimmun Rev. 2012;11:348–56. doi: 10.1016/j.autrev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Hull KM, Drewe E, Aksentijevich I, et al. The TNF receptor-associated periodic syndrome (TRAPS): emerging concepts of an autoinflammatory disorder. Medicine (Baltimore) 2002;81:349–68. doi: 10.1097/00005792-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Cantarini L, Rigante D, Merlini G, et al. The expanding spectrum of low-penetrance TNFRSF1A gene variants in adults presenting with recurrent inflammatory attacks: clinical manifestations and long-term follow-up. Semin Arthritis Rheum. 2014;43:818–23. doi: 10.1016/j.semarthrit.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Rigante D, Lopalco G, Vitale A, et al. Key facts and hot spots on tumor necrosis factor receptor-associated periodic syndrome. Clin Rheumatol. 2014;33:1197–207. doi: 10.1007/s10067-014-2722-z. [DOI] [PubMed] [Google Scholar]
- 21.Rigante D, Cantarini L, Imazio M, et al. Autoinflammatory diseases and cardiovascular manifestations. Ann Med. 2011;43:341–6. doi: 10.3109/07853890.2010.547212. [DOI] [PubMed] [Google Scholar]
- 22.Ozen S, Demir S. Monogenic periodic fever syndromes: treatment options for the pediatric patient. Paediatr Drugs. 2017;19:303–1. doi: 10.1007/s40272-017-0232-6. [DOI] [PubMed] [Google Scholar]
- 23.Cantarini L, Lucherini OM, Frediani B, et al. Bridging the gap between the clinician and the patient with cryopyrin-associated periodic syndromes. Int J Immunopathol Pharmacol. 2011;24:827–36. doi: 10.1177/039463201102400402. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Tan X, Zhang J, et al. Gene mutations and clinical phenotypes in 15 Chinese children with cryopyrin-associated periodic syndrome (CAPS) Sci China Life Sci. 2017;60:1436–44. doi: 10.1007/s11427-017-9246-4. [DOI] [PubMed] [Google Scholar]
- 25.Rigante D. A developing portrait of hereditary periodic fevers in childhood. Expert Opin Orphan Drugs. 2018;6:47–55. doi: 10.1080/21678707.2018.1406797. [DOI] [Google Scholar]
- 26.Rigante D, Cipolla C, Rossodivita A. Recombinant human growth hormone in neonatal-onset multisystem inflammatory disease. Clin Pediatr Endocrinol. 2018;27:101–5. doi: 10.1297/cpe.27.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, et al. Diagnostic criteria for cryopyrin-associate periodic syndrome (CAPS) Ann Rheum Dis. 2017;76:942–7. doi: 10.1136/annrheumdis-2016-209686. [DOI] [PubMed] [Google Scholar]
- 28.Federico G, Rigante D, Pugliese AL, et al. Etanercept induces improvement of arthropathy in chronic infantile neurological cutaneous articular (CINCA) syndrome. Scand J Rheumatol. 2003;32:312–4. doi: 10.1080/03009740310003974. [DOI] [PubMed] [Google Scholar]
- 29.Lucherini OM, Rigante D, Sota J, et al. Updated overview of molecular pathways involved in the most common monogenic autoinflammatory diseases. Clin Exp Rheumatol. 2018;36(Suppl 110)(1):3–9. [PubMed] [Google Scholar]
- 30.Landmann EC, Walker UA. Pharmacological treatment options for cryopyrin-associated periodic syndromes. Expert Rev Clin Pharmacol. 2017;10:855–64. doi: 10.1080/17512433.2017.1338946. [DOI] [PubMed] [Google Scholar]
- 31.Rigante D, Ansuini V, Caldarelli M, et al. Hydrocephalus in CINCA syndrome treated with anakinra. Childs Nerv Syst. 2006;22:334–7. doi: 10.1007/s00381-006-1280-3. [DOI] [PubMed] [Google Scholar]
- 32.Rigante D, Manna R, Verrecchia E, et al. Resolution of femoral metaphyseal dysplasia in CINCA syndrome after long-term treatment with interleukin-1 blockade. Clin Rheumatol. 2018;37:2007–9. doi: 10.1007/s10067-018-4145-8. [DOI] [PubMed] [Google Scholar]
- 33.Ebrahimi-Fakhari D, Wahlster L, Mackensen F, et al. Clinical manifestations and longterm follow-up of a patient with CINCA/NOMID syndrome. J Rheumatol. 2010;37:2196–7. doi: 10.3899/jrheum.100290. [DOI] [PubMed] [Google Scholar]
- 34.Vitale A, Rigante D, Maggio MC, et al. Rare NLRP12 variants associated with the NLRP12-autoinflammatory disorder phenotype: an Italian case series. Clin Exp Rheumatol. 2013;31(3 Suppl 77):155–6. [PubMed] [Google Scholar]
- 35.Gusdorf L, Lipsker D. Schnitzler syndrome: a review. Curr Rheumatol Rep. 2017;19:46. doi: 10.1007/s11926-017-0673-5. [DOI] [PubMed] [Google Scholar]
- 36.Esposito S, Ascolese B, Senatore L, et al. Current advances in the understanding and treatment of mevalonate kinase deficiency. Int J Immunopathol Pharmacol. 2014;27:491–8. doi: 10.1177/039463201402700404. [DOI] [PubMed] [Google Scholar]
- 37.Frenkel J, Houten SM, Waterham HR, et al. Clinical and molecular variability in childhood periodic fever with hyperimmunoglobulinaemia D. Rheumatology (Oxford) 2001;40:579–84. doi: 10.1093/rheumatology/40.5.579. [DOI] [PubMed] [Google Scholar]
- 38.Rigante D. Autoinflammatory syndromes behind the scenes of recurrent fevers in children. Med Sci Monit. 2009;15:RA179–87. [PubMed] [Google Scholar]
- 39.Ter Haar NM, Jeyaratnam J, Lachmann HJ, et al. The phenotype and genotype of mevalonate kinase deficiency: a series of 114 cases from the Eurofever Registry. Arthritis Rheumatol. 2016;68:2795–805. doi: 10.1002/art.39763. [DOI] [PubMed] [Google Scholar]
- 40.van der Hilst JC, Bodar EJ, Barron KS, et al. Long-term follow-up, clinical features, and quality of life in a series of 103 patients with hyperimmunoglobulinemia D syndrome. Medicine (Baltimore) 2008;87:301–10. doi: 10.1097/MD.0b013e318190cfb7. [DOI] [PubMed] [Google Scholar]
- 41.Rigante D, Frediani B, Cantarini L. A comprehensive overview of the hereditary periodic fever syndromes. Clin Rev Allergy Immunol. 2018;54:446–53. doi: 10.1007/s12016-016-8537-8. [DOI] [PubMed] [Google Scholar]
- 42.Tallon B, Corkill M. Peculiarities of PAPA syndrome. Rheumatology. 2006;45:1140–3. doi: 10.1093/rheumatology/kei178. [DOI] [PubMed] [Google Scholar]
- 43.Ferguson PJ, Chen S, Tayeh MK, et al. Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome) J Med Genet. 2005;42:551–7. doi: 10.1136/jmg.2005.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jesus AA, Osman M, Silva CA, et al. A novel mutation of IL1RN in the deficiency of interleukin-1 receptor antagonist syndrome: description of two unrelated cases from Brazil. Arthritis Rheumatol. 2011;63:4007–17. doi: 10.1002/art.30588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rigante D, Vitale A, Lucherini OM, et al. The hereditary autoinflammatory disorders uncovered. Autoimmun Rev. 2014;13:892–900. doi: 10.1016/j.autrev.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Vinkel C, Thomsen SF. Autoinflammatory syndromes associated with hidradenitis suppurativa and/or acne. Int J Dermatol. 2017;56:811–8. doi: 10.1111/ijd.13603. [DOI] [PubMed] [Google Scholar]
- 47.Wollina U. Emerging treatments for pyoderma gangrenosum. Expert Opin Orphan Drugs. 2017;5:827–32. doi: 10.1080/21678707.2017.1375404. [DOI] [Google Scholar]
- 48.Rigante D, Cantarini L. Monogenic autoinflammatory syndromes at a dermatological level. Arch Dermatol Res. 2011;303:375–80. doi: 10.1007/s00403-011-1134-z. [DOI] [PubMed] [Google Scholar]
- 49.Rigante D. A systematic approach to autoinflammatory syndromes: a spelling booklet for the beginner. Expert Rev Clin Immunol. 2017;13:571–97. doi: 10.1080/1744666X.2017.1280396. [DOI] [PubMed] [Google Scholar]
- 50.Caso F, Costa L, Rigante D, et al. Caveats and truths in genetic, clinical, autoimmune and autoinflammatory issues in Blau syndrome and early onset sarcoidosis. Autoimmun Rev. 2014;13:1220–9. doi: 10.1016/j.autrev.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Janssen CE, Rose CD, De Hertogh G, et al. Morphologic and immunohistochemical characterization of granulomas in the nucleotide oligomerization domain 2-related disorders Blau syndrome and Crohn disease. J Allergy Clin Immunol. 2012;129:1076–84. doi: 10.1016/j.jaci.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Al Nabhani Z, Dietrich G, Hugot JP, et al. Nod2: The intestinal gate keeper. PLoS Pathog. 2017;13:e1006177. doi: 10.1371/journal.ppat.1006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engelhardt KR, Shah N, Faizura-Yeop I, et al. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2013;131:825–30. doi: 10.1016/j.jaci.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Q, Yu X, Demirkaya E, et al. Biallelic hypomorphic mutations in a linear deubiquitinase define otulipenia, an early-onset autoinflammatory disease. Proc Natl Acad Sci USA. 2016;113:10127–32. doi: 10.1073/pnas.1612594113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Damgaard RB, Walker JA, Marco-Casanova P, et al. The deubiquitinase OTULIN is an essential negative regulator of inflammation and autoimmunity. Cell. 2016;166:1215–30. doi: 10.1016/j.cell.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDermott A, Jacks J, Kessler M, et al. Proteasome-associated autoinflammatory syndromes: advances in pathogeneses, clinical presentations, diagnosis, and management. Int J Dermatol. 2015;54:121–9. doi: 10.1111/ijd.12695. [DOI] [PubMed] [Google Scholar]
- 57.Bienias M, Brück N, Griep C, et al. Therapeutic approaches to type I interferonopathies. Curr Rheumatol Rep. 2018;20:32. doi: 10.1007/s11926-018-0743-3. [DOI] [PubMed] [Google Scholar]
- 58.Aicardi J, Goutières F. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol. 1984;15:49–54. doi: 10.1002/ana.410150109. [DOI] [PubMed] [Google Scholar]
- 59.Crow YJ, Chase DS, Lowenstein Schmidt J, et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet A. 2015;167A:296–312. doi: 10.1002/ajmg.a.36887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crow YJ, Vanderver A, Orcesi S, et al. Therapies in Aicardi-Goutieres syndrome. Clin Exp Immunol. 2014;175:1–8. doi: 10.1111/cei.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kvaerner KJ, Nafstad P, Jaakkola JJ. Upper respiratory morbidity in preschool children: a cross-sectional study. Arch Otolaryngol Head Neck Surg. 2000;126:1201–6. doi: 10.1001/archotol.126.10.1201. [DOI] [PubMed] [Google Scholar]
- 62.Esposito S, Bianchini S, Fattizzo M, et al. The enigma of periodic fever, aphthous stomatitis, pharyngitis and adenitis syndrome. Pediatr Infect Dis J. 2014;33:650–2. doi: 10.1097/INF.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 63.Rigante D, Gentileschi S, Vitale A, et al. Evolving frontiers in the treatment of periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis (PFAPA) syndrome. Isr Med Ass J. 2017;19:444–7. [PubMed] [Google Scholar]
- 64.Cantarini L, Vitale A, Bartolomei B, et al. Diagnosis of PFAPA syndrome applied to a cohort of 17 adults with unexplained recurrent fevers. Clin Exp Rheumatol. 2012;30:269–71. [PubMed] [Google Scholar]
- 65.Cantarini L, Vitale A, Sicignano LL, et al. Diagnostic criteria for adult-onset Periodic Fever, Aphthous Stomatitis, Pharyngitis, and Cervical Adenitis (PFAPA) syndrome. Front Immunol. 2017;8:1018. doi: 10.3389/fimmu.2017.01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rigante D, Vitale A, Natale MF, et al. A comprehensive comparison between pediatric and adult patients with periodic fever, aphthous stomatitis, pharyngitis, and cervical adenopathy (PFAPA) syndrome. Clin Rheumatol. 2017;36:463–8. doi: 10.1007/s10067-016-3317-7. [DOI] [PubMed] [Google Scholar]
- 67.Koné-Paut I. Behçet’s disease in children, an overview. Pediatr Rheumatol Online J. 2016;14(1):10. doi: 10.1186/s12969-016-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cantarini L, Vitale A, Bersani G, et al. PFAPA syndrome and Behçet’s disease: a comparison of two medical entities based on the clinical interviews performed by three different specialists. Clin Rheumatol. 2016;35:501–5. doi: 10.1007/s10067-015-2890-5. [DOI] [PubMed] [Google Scholar]
- 69.Caso F, Costa L, Rigante D, et al. Biological treatments in Behçet’s disease: beyond anti-TNF-therapy. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/107421. 107421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vitale A, Rigante D, Lopalco G, et al. Interleukin-1 inhibition in Behçet’s disease. Isr Med Assoc J. 2016;18:171–6. [PubMed] [Google Scholar]
- 71.Grevich S, Shenoi S. Update on the management of systemic juvenile idiopathic arthritis and role of IL-1 and IL-6 inhibition. Adolesc Health Med Ther. 2017;8:125–35. doi: 10.2147/AHMT.S109495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deuteraiou K, Kitas G, Garyfallos A, et al. Novel insights into the role of inflammasomes in autoimmune and metabolic rheumatic diseases. Rheumatol Int. 2018;38:1345–54. doi: 10.1007/s00296-018-4074-5. [DOI] [PubMed] [Google Scholar]
- 73.Vitale A, Cantarini L, Rigante D, et al. Anakinra treatment in patients with gout and diabetes type 2. Clin Rheumatol. 2015;34:981–4. doi: 10.1007/s10067-014-2601-7. [DOI] [PubMed] [Google Scholar]
- 74.Cantarini L, Lopalco G, Selmi C, et al. Autoimmunity and autoinflammation as the yin and yang of idiopathic recurrent acute pericarditis. Autoimmun Rev. 2015;14:90–7. doi: 10.1016/j.autrev.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Rigante D, La Torraca I, Ansuini V, et al. The multi-face expression of familial Mediterranean fever in the child. Eur Rev Med Pharmacol Sci. 2006;10:163–71. [PubMed] [Google Scholar]
- 76.Soriano A, Manna R. Familial Mediterranean fever: new phenotypes. Autoimmun Rev. 2012;12:31–7. doi: 10.1016/j.autrev.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 77.Tufan G, Demir S. Uncommon clinical pattern of FMF: protracted febrile myalgia syndrome. Rheumatol Int. 2010;30:1089–90. doi: 10.1007/s00296-009-1024-2. [DOI] [PubMed] [Google Scholar]
- 78.Lachmann HJ, Papa R, Gerhold K, et al. The phenotype of TNF receptor-associated autoinflammatory syndrome (TRAPS) at presentation: a series of 158 cases from the Eurofever/EUROTRAPS international registry. Ann Rheum Dis. 2014;73:2160–7. doi: 10.1136/annrheumdis-2013-204184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harada Y, Fukiage K, Nishikomori R, et al. CINCA syndrome with surgical intervention for valgus deformity and flexion contracture of the knee joint: a case report. Mod Rheumatol. 2017;27:1098–100. doi: 10.3109/14397595.2015.1040609. [DOI] [PubMed] [Google Scholar]
- 80.Vitale A, Insalaco A, Sfriso P, et al. A snapshot on the on-label and off-label use of the interleukin-1 inhibitors in Italy among rheumatologists and pediatric rheumatologists: a nationwide multi-center retrospective observational study. Front Pharmacol. 2016;7:380. doi: 10.3389/fphar.2016.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prietsch V, Mayatepek E, Krastel H, et al. Mevalonate kinase deficiency: enlarging the clinical and biochemical spectrum. Pediatrics. 2003;111:258–61. doi: 10.1542/peds.111.2.258. [DOI] [PubMed] [Google Scholar]
- 82.Löffler W, Lohse P, Weihmayr T, et al. Pyogenic arthritis, pyoderma gangrenosum, and acne (PAPA) syndrome: differential diagnosis of septic arthritis by regular detection of exceedingly high synovial cell counts. Infection. 2017;45:395–402. doi: 10.1007/s15010-017-0996-1. [DOI] [PubMed] [Google Scholar]
- 83.Wouters CH, Maes A, Foley KP, et al. Blau syndrome, the prototypic auto-inflammatory granulomatous disease. Pediatr Rheumatol Online J. 2014;12:33. doi: 10.1186/1546-0096-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Torrelo A. CANDLE syndrome as a paradigm of proteasome-related autoinflammation. Front Immunol. 2017;8:927. doi: 10.3389/fimmu.2017.00927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Celkan T, Celik M, Kasapçopur O, et al. The anemia of familial Mediterranean fever disease. Pediatr Hematol Oncol. 2005;22:657–65. doi: 10.1080/08880010500278681. [DOI] [PubMed] [Google Scholar]
- 86.Kucuk A, Erol MF, Senel S, et al. The role of neutrophil lymphocyte ratio to leverage the differential diagnosis of familial Mediterranean fever attack and acute appendicitis. Korean J Intern Med. 2016;31:386–91. doi: 10.3904/kjim.2015.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sakallı H, Kal O. Mean platelet volume as a potential predictor of proteinuria and amyloidosis in familial Mediterranean fever. Clin Rheumatol. 2013;32:1185–90. doi: 10.1007/s10067-013-2257-8. [DOI] [PubMed] [Google Scholar]
- 88.Arıca S, Ozer C, Arıca V, et al. Evaluation of the mean platelet volume in children with familial Mediterranean fever. Rheumatol Int. 2012;32:3559–63. doi: 10.1007/s00296-011-2251-x. [DOI] [PubMed] [Google Scholar]
- 89.Erden A, Batu ED, Armagan B, et al. Blood group ‘A’ may have a possible modifier effect on familial Mediterranean fever and blood group ‘0’ may be associated with colchicine resistance. Biomark Med. 2018;12:565–72. doi: 10.2217/bmm-2017-0344. [DOI] [PubMed] [Google Scholar]
- 90.Lepore L, Paloni G, Caorsi R, et al. Follow-up and quality of life of patients with cryopyrin-associated periodic syndromes treated with anakinra. J Pediatr. 2010;157:310–5. doi: 10.1016/j.jpeds.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 91.Lipsker D, Veran Y, Grunenberger F, et al. The Schnitzler syndrome. Four new cases and review of the literature. Medicine (Baltimore) 2001;80:37–44. doi: 10.1097/00005792-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 92.Steiner LA, Ehrenkranz RA, Peterec SM, et al. Perinatal onset mevalonate kinase deficiency. Pediatr Dev Pathol. 2011;14:301–6. doi: 10.2350/11-02-0985-OA.1. [DOI] [PubMed] [Google Scholar]
- 93.Majeed HA, Kalaawi M, Mohanty D, et al. Congenital dyserythropoietic anemia and chronic recurrent multifocal osteomyelitis in three related children and the association with Sweet syndrome in two siblings. J Pediatr. 1989;115:730–4. doi: 10.1016/s0022-3476(89)80650-x. [DOI] [PubMed] [Google Scholar]
- 94.McDermott A, Jacks J, Kessler M, et al. Proteasome-associated autoinflammatory syndromes: advances in pathogeneses, clinical presentations, diagnosis, and management. Int J Dermatol. 2015;54:121–9. doi: 10.1111/ijd.12695. [DOI] [PubMed] [Google Scholar]
- 95.Stabile A, Bertoni B, Ansuini V, et al. The clinical spectrum and treatment options of macrophage activation syndrome in the pediatric age. Eur Rev Med Pharmacol Sci. 2006;10:53–9. [PubMed] [Google Scholar]
- 96.Rigante D, Emmi G, Fastiggi M, et al. Macrophage activation syndrome in the course of monogenic autoinflammatory disorders. Clin Rheumatol. 2015;34:1333–9. doi: 10.1007/s10067-015-2923-0. [DOI] [PubMed] [Google Scholar]
- 97.Minoia F, Bovis F, Davì S, et al. Development and initial validation of the macrophage activation syndrome/primary hemophagocytic lymphohistiocytosis score, a diagnostic tool that differentiates primary hemophagocytic lymphohistiocytosis from macrophage activation syndrome. J Pediatr. 2017;189:72–8. doi: 10.1016/j.jpeds.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 98.Larroche C, Mouthon L. Pathogenesis of hemophagocytic syndrome. Autoimmunity Rev. 2004;3:69–75. doi: 10.1016/S1568-9972(03)00091-0. [DOI] [PubMed] [Google Scholar]
- 99.Rigante D, Esposito S. A roadmap for fever of unknown origin in children. Int J Immunopathol Pharmacol. 2013;26:315–26. doi: 10.1177/039463201302600205. [DOI] [PubMed] [Google Scholar]
- 100.Cantarini L, Lopalco G, Cattalini M, et al. Interleukin-1: Ariadne’s thread in autoinflammatory and autoimmune disorders. Isr Med Assoc J. 2015;17:93–7. [PubMed] [Google Scholar]
- 101.Pras E, Livneh A, Balow JE, Jr, et al. Clinical differences between North African and Iraqi Jews with familial Mediterranean fever. Am J Med Genet. 1998;75:216–9. doi: 10.1002/(sici)1096-8628(19980113)75:2<216::aid-ajmg20>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 102.Cantarini L, Lucherini OM, Iacoponi F, et al. Development and preliminary validation of a diagnostic score for identifying patients affected with adult-onset autoinflammatory disorders. Int J Immunopathol Pharmacol. 2010;23:1133–4. doi: 10.1177/039463201002300417. [DOI] [PubMed] [Google Scholar]
- 103.Cantarini L, Iacoponi F, Lucherini OM, et al. Validation of a diagnostic score for the diagnosis of autoinflammatory diseases in adults. Int J Immunopathol Pharmacol. 2011;24:695–702. doi: 10.1177/039463201102400315. [DOI] [PubMed] [Google Scholar]
- 104.Piram M, Koné Paut I, Lachmann H, et al. Validation of the auto-inflammatory diseases activity index (AIDAI) for hereditary recurrent fever syndromes. Ann Rheum Dis. 2014;73:2168–73. doi: 10.1136/annrheumdis-2013-203666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ter Haar NM, Annink KV, Al-Mayouf SM, et al. Development of the autoinflammatory disease damage index (ADDI) Ann Rheum Dis. 2017;76:821–30. doi: 10.1136/annrheumdis-2016-210092. [DOI] [PubMed] [Google Scholar]