Abstract
Skin manifestations are frequent among patients with primary immunodeficiency diseases (PIDs). Their prevalence varies according to the type of immunodeficiency. This review provides the reader with an up-to-date summary of the common dermatologic manifestations of PIDs among Tunisian children. We conducted a prospective study on two hundred and ninety children with immune deficiency. Demographic details (including age, sex, and consanguinity) with personal and family history were recorded. Special attention was paid to cutaneous manifestations. Dermatological involvements were grouped according to the etiology of their most prominent sign. Cutaneous manifestations were found in 164 patients (56.5%). They revealed the diagnosis of PIDs in 71 patients (24.5 %). The mean age at presentation was 21 months. Overall the most prominent cutaneous alterations were infectious. They accounted for 106 cases (36.55%). The most prevalent causes of cutaneous infections were bacterial: 93 cases (32.06%). Immuno-allergic skin diseases were among the common findings in our study. These include eczematous dermatitis found in 62 cases (21.38%). Malignancy related PIDs was seen in a boy with Wiskott Aldrich syndrome. He developed Kaposi’s sarcoma at the age of 14 months. Cutaneous changes are common among children with PIDs. In pediatric patients with failure to thrive, chronic refractory systemic manifestations often present in other family members, recurrent cutaneous infections unresponsive to adequate therapy, atypical forms of eczematous dermatitis or unusual features should arouse the suspicion of PIDs and prompt specialized immunologic consultation should be made.
Keywords: Primary immunodeficiency, Cutaneous manifestations, Cutaneous infections, Wiskott Aldrich syndrome
Introduction
Primary immunodeficiency disorders (PIDs) refer to a heterogeneous group of rare disorders characterized by poor or absent function of one or more components of the immune system. Over 270 different disorders have been identified to date, with new disorders continually being recognized.1 The clinical presentation of PIDs is highly variable; however, most disorders involve increased susceptibility to infection. Cutaneous manifestations are common in PIDs, affecting half of the pediatric cases and often precede the final diagnosis. Skin infections characterize many primary immune deficiencies, but noninfectious cutaneous involvements are frequent including allergic, inflammatory, autoimmune and malignant manifestations.2 Only few studies describing the spectrum of skin disorders in PID are available, and no similar studies have been conducted in Tunisia. This prospective study provides the reader with an up-to-date summary of the common dermatologic manifestations of primary immune deficiency diseases among Tunisian children.
Materials and Methods
We conducted a prospective study on two hundred and ninety children referrals children with suspected immune deficiency during a 10-year period (January 1, 2008, to December 31, 2017) at the Pediatric Immunohematology Department in the National Bone Marrow Transplantation Center in Tunis. Individuals with human immunodeficiency virus infection or receiving immunosuppressive therapy were excluded from the study. Demographic details (including age, sex, and consanguinity) with personal and family history were recorded. Special attention was paid to cutaneous manifestations. Cutaneous manifestations of PIDs were classified into four categories: cutaneous lesions of infectious origin, immunoallergic/autoimmune skin manifestations, pathognomonic findings related to complex phenotypes and skin cancers associated with PIDs. The diagnosis of PIDs was established according to the criteria defined by the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency.1 Informed written consent to publish images was obtained from parents.
Results
Among the two hundred and ninety children (Table 1), 111 (38%) were female, and 179 (62%) were male. Consanguineous relationships were seen in almost two-thirds of patients (65.17%). Cutaneous manifestations were found in 164 patients (56.5%). They revealed the diagnosis of PIDs in 71 patients (24.5 %). The mean age at presentation was 21 months (1 day–25 years). The most predominant cutaneous alterations were infectious in 106 cases (36.55%). The most prevalent causes of cutaneous infections were bacterial: 93 cases, (32.06%).
Table 1.
Distribution of PIDs in the studied population
| Type of PID | Total cases | % |
|---|---|---|
| Combined immunodeficiency | 92 | 31.72 |
| Well-defined immunodeficiency syndrome | 57 | 19.65 |
| Predominantly antibody deficiency | 37 | 12.76 |
| Congenital defects of phagocyte function | 86 | 29.65 |
| immune dysregulation | 16 | 5.52 |
| Other PIDs | 2 | 0.69 |
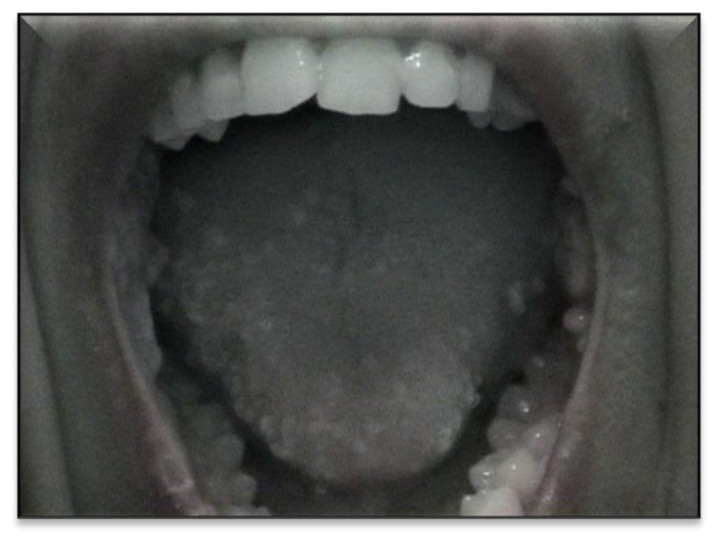
Staphylococcus aureus represented by far the most common pathogen (32 cases), and Pseudomonas was responsible for one other case. Unfortunately bacterial culture was not done in all infections. BCG infections were common among patients with combined immunodeficiency (CID) and septic granulomatous disease while anal margin abscesses were frequently seen in phagocytic disorders (Table 2). The next type of skin infection consisted of viral infections seen in 11 (3.8%) of the studied patients. Most of them had warts. Recurrent oral papillomatous lesions related to human papillomavirus were seen in 2 sisters with major histocompatibility complex class II deficiency (Figure 1). The third type was fungal skin infection (Table 2).
Table 2.
Groups of PIDs: Associated skin infections.
| Immunodeficiencies | Subtype | Mucocutaneous infections (No.%) | Bacterial type (No.) | Viral type (No.) | Mycotic type (No.) |
|---|---|---|---|---|---|
| Combined immunodeficiency | SCID | 31(11%) | BCGosis: 2 BCGitis: 5 Boils: 2 |
Warts:2 | Oral candidiasis: 20 |
| MHC Class II Deficiency | 28(10%) | BCGitis: 2 BCGosis: 1 |
Condyloma:2 | Oral candidiasis: 23 | |
| Well-defined ID syndrome | Hyper IgE Syndrome | 20(7%) | Cold abscesses:7 Impetigo:7 Pyoderma:3 Boils: 2 |
Warts:1 | 0 |
| Ataxia-telangiectasia | 4(1.3%) | 0 | Warts:4 | 0 | |
| Predominantly antibody deficiency | Agammaglobulinemia | 6(2%) | Boils: 3 Abscesses:2 Ecthyma:1 |
0 | |
| Phagocytic disorders | Septic granulomatous disease | 30(10.3%) | Abscesses:15 BCGitis:7 Boils: 5 Pyoderma:2 |
Chicken pox:1 | 0 |
| Leukocyte adhesion defect type 1 | 22(7.5%) | Omphalitis:8 Ecthyma:5 Abscesses:5 Boils:1 |
0 | Oral candidiasis: 3 | |
| Congenital neutropenia | 13(4.4%) | skin abscess/anal margin: 10 Boils:3 |
0 | 0 | |
| Mendelian mycobacteria susceptibility syndrome | - | 19(6.5%) | BCGitis:19 | 0 | 0 |
Figure 1.
Disseminated warts on oral cavity in a patient with MHC class II deficiency.
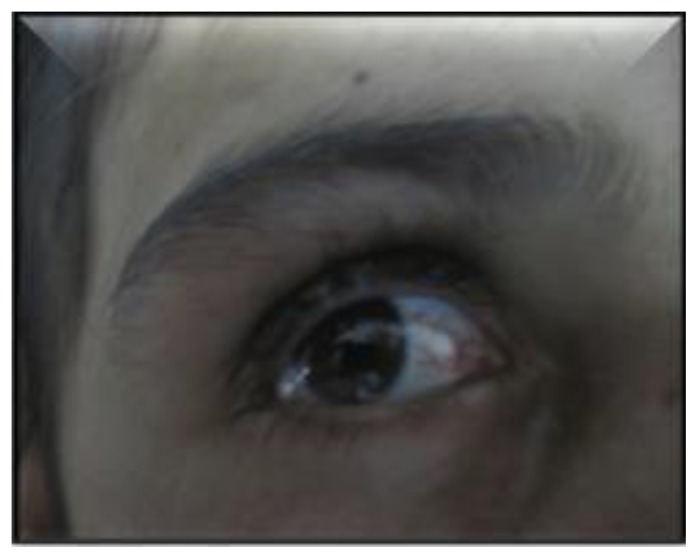
In this study, oral candidiasis was the most common infection in patients with CID, frequently associated with broncho-pulmonary infections (61 cases, 66.30%), digestive infections (56 cases, 60.9%), ear, nose, and throat infections (23 cases, 25%) and persistent diarrhea resulting in failure to thrive (39 cases, 42.4%). Mucocutaneous lesions due to candida were more frequently oral, whitish, adherent plaques and paronychia (Figure 2). Immuno-allergic skin diseases were among the common findings in our study. These include eczematous dermatitis found in 62 cases (21.38%), (Table 3). Severely generalized erythroderma with alopecia revealed Omenn syndrome in 17 cases (Figure 3 and 4). Mean patient age at the onset of these cutaneous manifestations was 6.4 months (1 day to 48 months). Other extracutaneous manifestations led to the diagnosis including recurrent infections observed in 11 patients with failure to thrive, as well as infiltration of lymphoid organs with hepatosplenomegaly. Lymphopenia was found in 15 patients (88 %) and eosinophilia in 16 cases (94%). Specific muco-cutaneous manifestations of PIDs were seen in 22 cases (7.6%) among them telangiectasia (Figure 5) revealed ataxia telangiectasia syndrome in 14 cases (100%). Among these patients, three had hypopigmented and café-au-lait macules. Cutaneous albinism was noted in 8 other cases with Chediack Higashi syndrome (five cases), Griscelli syndrome (one case) or Hermanski pudlak type 2 syndrome (two cases). Malignancy related PIDs was seen in a boy with Wiskott Aldrich syndrome. He developed Kaposi’s sarcoma at the age of 14 months (Figure 6).
Figure 2.
Oral candidiasis in a patient with SCID.
Table 3.
Groups of PIDs: Associated immunoallergic skin disorders.
| PIDs | Subtypes of PIDs | Immuno-allergic skin diseases | No. (%) |
|---|---|---|---|
| SCID | Omenn syndrome | Icthyosiform erythrodermie +/−alopécie | 17 (100%) |
| Others | Eczematiform erythroderma | 5 (19,23%) | |
| Well-defined ID syndrome | Hyper IgE Syndrome | Eczematiform erythroderma | 14 cas (73,7%) |
| Wiskott-Aldrich syndrome | Eczematiform erythroderma + purpura | 4 (100%) | |
| Immune dysregulation | X-linked lymphoproliferative syndrome | Purpura | 2 (50%) |
| Hemophagocytic lymphohistiocytosis | Purpura | 1 (25%) | |
| Phagocytic disorders | Septic granulomatous disease | Eczematiform erythroderma | 9 (28,12%) |
Figures 3 and 4.
Erythroderma with alopecia in a patient with Omenn syndrome.
Figure 5.
Conjunctival telangiectasias in a patient with ataxia telangiectasia syndrome.
Figure 6.
Kaposi’s sarcoma in a patient with Wiskott-Aldrich syndrome.
Discussion
Cutaneous manifestations may be the clue for the diagnosis of PIDs because of their early appearance, their high frequency and their easy access for examination.3 This study showed that cutaneous alterations were the presenting problem in 56.5% of cases, which are higher than what is reported by Moin et al., (32%) and Al-Herz et al., (48%).4,5 Although skin infections were the most common manifestations, the prevalence rate of infections in our series was lower than those reported in other countries.4,6 Pyogenic infections of the skin frequently occur in PIDs causing abscesses, boils, ecthyma, cellulitis, folliculitis, or impetigo (Table 2). In this study, recurrent abscesses were frequent. They predominated in phagocytic defects and hyper IgE syndrome.7 They tended to be multiple, recurrent and difficult to control. Bacteriologic cultures should be performed since the growth of unusual organisms is not infrequent. The most common organism is Staphylococcus followed by Haemophilus, Serratia, Klebsiella, Escherichia coli, and Pseudomonas. In leucocyte adhesion deficiency, recurrent skin infections, generally due to Staphylococcus aureus or Gram negative bacilli is typical. It tends to necrotize and ulcerate without pus at the wound site. Other features include delayed umbilical cord separation, omphalitis, persistent leukocytosis, and severe destructive gingivitis and periodontitis leading to tooth loss and alveolar bone resorption.8 Mycobacterium bovis in Bacille Calmette-Guérin (BCG) vaccine can cause serious complications in patients with PIDs ranging from prolonged disease with lymphadenopathy (BCGitis) to disseminated disease (BCGosis).9 These complications have been diagnosed in immunocompromised patients including combined immunodeficiency, mendelian susceptibility to mycobacterial disease, chronic granulomatous disease, complete DiGeorge syndrome, hyper-IgM and hyper-IgE syndromes.10,11 The median age of onset is three months of age. Our study showed that severe combined immunodeficiencies (SCID), MHC Class II Deficiency, CGD and mendelian susceptibility to mycobacterial disease are the most common forms of immunodeficiencies among children with BCGosis/BCGitis. A large study conducted by De Beaucoudrey et al., reported 108 cases with IL12R β1 deficiency, among them 84 cases presented BCGitis.12 Similar results were reported by Galal et al.10 In our study, 10 out of 13 patients with mendelian susceptibility to mycobacterial disease had developed suppurative BCG lymphadenitis. Since the routine national vaccination program in Tunisia includes BCG vaccine, PIDs, should be considered in any patient with prolonged BCGitis or BCGosis. Awareness of this phenomenon is important because these patients need to be treated with antitubercular drugs. Children with PID are rarely exposed to common viral skin infections as viral wart and molluscum contagiosum contrasting, thus, with their high frequency in patients with acquired immunodeficiency syndrome. Herpes simplex virus is among the most common viral skin infections encountered in T-cell deficient patients. The symptoms are often impressive and are relatively resistant to conventional treatment.4,5 In the present study, recurrent oral papillomatous lesions related to human papillomavirus were seen in two sisters with major histocompatibility complex class II deficiency.13 Warts, described as benign skin growths that develop on different parts of the body and can take on various forms, disseminated warts were found in 2 patients with WHIM syndrome and ataxia telangiectasia. Persistent mucocutaneous candidiasis may be the initial presenting sign for PID in infancy, especially in those with severe combined immunodeficiency disease.2,5 In this study, 76.92% (20/26 cases) with severe combined immunodeficiency had at least one episode of oral candidiasis. Candidiasis associated with eczema is a common feature of Hyper IgE syndrome.2 When present with mucocutaneous candidiasis, autoimmune endocrinopathies, and dystrophy of the dental enamel and nails, it is important to recall the autoimmune polyendocrinopathy- candidiasis-ectodermal dystrophy syndrome. This syndrome is caused by mutations in the autoimmune regulator gene (AIRE), resulting in autoreactive T-cells which develop autoantibodies.14 Immunodeficiency should be suspected when skin infection have a multifocal character, saprophytic or opportunistic and/or a combination of several pathogens as well as their persistence or their repetition despite a good anti-infective treatment or when there are skin changes leading to diagnosis difficulties. Eczematous dermatitis is another nonspecific cutaneous finding among several PIDs including IgA deficiency, Immune dysregulation, polyendocrinopathy and enteropathy, common variable immunodeficiency, Wiskott-Aldrich syndrome, X-linked agammaglobulinemia, hyper IgM syndromes, hyper IgE, Netherton’s syndrome and Omenn syndrome. The overall prevalence of eczema in our patient population (21.38%) was comparable with that in Mexico (22%) but higher than that reported from Libya (12%).6,15
Although widely present in PIDs, eczema was a consistent feature in patients with Wiskott-Aldrich syndrome(WAS), a complex and severe X-linked disorder characterized by microthrombocytopenia, eczema, increased susceptibility to infection, and increased risk in developing autoimmunity and lymphomas.16 Other cutaneous and mucosal manifestations and hemorrhages are frequent in WAS patients ranging from non-life-threatening (epistaxis, petechiae, purpura, oral bleeding) to severe manifestations. This heterogeneous syndrome may be complicated by autoimmune mucocutaneous manifestations including Henoch–Schönlein-like purpura, dermatomyositis and recurrent angioedema.17 In a clinical series conducted by Ouederni, eczema was observed in four patients among 35 MHC Class II Deficiency patients.18 An underlying primary immunodeficiency should also be suspected when a patient shows severe early-onset, atypical, eczematous dermatitis, refractory to therapy with a tendency to become extensive and flaring up with systemic infections chronic diarrhea and failure to thrive19 or family history suggestive of immunodeficiency in patients with severe atopic dermatitis. These features underscore the importance of eliciting a history of recurrent infections or family history suggestive of immunodeficiency in patients with severe atopic dermatitis.20
A cutaneous granuloma is a histopathological diagnosis on a tissue that is usually taken for the evaluation of the cause of an unexplained nodular swelling in the skin. Aghamohammadi reported a 27-year-old patient with CVID who presented with multiple skin granulomas on both hands. The patient had been well until the age of 20 years when she developed these skin lesions with frequent upper respiratory infections and recurrent diarrhea. Intravenous immunoglobulin therapy improved skin lesions. In our study, one patient with CVID developed annular granuloma of the right leg.
Erythroderma with diffuse alopecia was the third most common skin manifestation in children with SCID and a consistent presenting feature in infants with Omenn syndrome, a rare inherited combined immunodeficiency caused by mutations in various SCID genes. Erythroderma can occur from birth or develop during the first weeks of life. It is evocative by its infiltrated character. These patients show hypereosinophilia, associated with T cell infiltration of gut, liver, spleen, and skin leading to erythroderma, diarrhea, hepatosplenomegaly, alopecia, recurrent infection, and failure to thrive.
Our findings support the results of other studies that most PIDs have cutaneous features. These features, being their aspects typical, are highly suggestive for the diagnosis of PIDs. One of the specific cutaneous manifestations of PIDs is telangiectasia. This PID is caused by alterations in the ATM gene leading to telangiectasia, immunodeficiency with progressive ataxia and oculomotor apraxia often accompanied by extrapyramidal movement disorders.21,22 In most cases, telangiectasias first appear when the child reaches three to five years of age. Conjunctival telangiectasias are first noted in the interpalpebral bulbar conjunctiva away from the limbus. Cutaneous telangiectasias are seen on the ears, palate, bridge of the nose and later extend to the neck, the dorsum of the hands and feet. In our study all patients with ataxia telangiectasia had telangiectasia. Other skin manifestations were reported including abnormalities of pigmentation (hypopigmented and café-au-lait macules), poikiloderma, seborrheic dermatitis, and less common findings including acanthosis nigricans and hirsutism.23 Hypopigmented macules were found in 2 other patients mainly on the face, trunk, and hands. In our study, hypopigmented and café-au-lait macules were seen in three cases. Another specific feature of PIDs is partial albinism with silvery gray hair. This association is an evocative or even pathognomonic cutaneous sign of complex PIDs including Chediak Higashi, Griscelli, Hermansky-Pudlak, and MAPBP-interacting protein deficiency syndromes.24
An association of PIDs and cancers has been known for many years25 and confirmed from data collected in established registries. The overall risk for cancer developing in children with PIDs is estimated to range from 4 to 25%.26 In a recent study from the United States Immune Deficiency Network conducted among 3658 patients, 171 separate cancers were diagnosed 25 (15%) of them were skin cancers. There were 119 cancers (70%) in subjects with CVID. Thirteen cancers (9%) were observed in subjects with hypogammaglobulinema and agammaglobulinemia, and eight cancers (4.6%) were observed in subjects with WAS.27 Despite the relatively high number of chronic granulomatous disease patients in the registry (483), none were diagnosed with cancer. One of the studied patients with Wiskott Aldrich syndrome developed a Kaposi’s sarcoma at the age of 14 months. To our knowledge, this patient is the first case described in the literature.28
Conclusions
Cutaneous changes may be the presenting signs of PIDs and serve as important clues for pediatric patients with PIDs. They may also be life-saving in patients with severe PIDs. Early diagnosis and treatment will prevent associated morbidity and mortality and improve the quality of life. In pediatric patients with failure to thrive, chronic refractory systemic manifestations often present in other family members, recurrent cutaneous infections unresponsive to adequate therapy, atypical forms of eczematous dermatitis or unusual features should arouse the suspicion of PIDs and prompt specialized immunologic consultation should be made.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Picard C, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, Cunningham-Rundles C, Etzioni A, Holland SM, Klein C, Nonoyama S, Ochs HD, Oksenhendler E, Puck JM, Sullivan KE, Tang ML, Franco JL, Gaspar HB. Primary Immunodeficiency Diseases: an Update on the Classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol. 2015 Nov;35(8):696–726. doi: 10.1007/s10875-015-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehman H. Skin manifestations of primary immune deficiency. Clin Rev Allergy Immunol. 2014;46:112–119. doi: 10.1007/s12016-013-8377-8. [DOI] [PubMed] [Google Scholar]
- 3.Smitt JHS, Wulffraat NM, Kuijpers TW. The skin in primary immunodeficiency disorders. Eur J Dermatol. 2005;15:425–432. [PubMed] [Google Scholar]
- 4.Moin A, Farhoudi A, Moin M, Pourpak Z, Bazargan N. Cutaneous Manifestations of Primary Immunodeficiency Diseases in Children. Iran J Allergy Asthma Immunol. 2006;5:121–126. http://dx.doi.org/05.03/ijaai.121126. [PubMed] [Google Scholar]
- 5.Al Herz W, Nanda A. Skin manifestations in primary immunodeficient children. Pediatr Dermatol. 2011;28:494–501. doi: 10.1111/j.1525-1470.2011.01409.x. [DOI] [PubMed] [Google Scholar]
- 6.Suleman Elfaituri S, Matoug I. Cutaneous Manifestations of Primary Immunodeficiency Diseases in Libyan Children. J Clin Dermatol Ther. 2017;4:025. doi: 10.24966/CDT-8771/100025. [DOI] [Google Scholar]
- 7.Yu JE, Azar AE, Chong HJ, Jongco AM, 3rd, Prince BT. Considerations in the Diagnosis of Chronic Granulomatous Disease. J Pediatric Infect Dis Soc. 2018;7(suppl 1):S6–S11. doi: 10.1093/jpids/piy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnston SL. Clinical Immunology Review Series: An approach to the patient with recurrent superficial abscesses. Clin Exp Immunol. 2008;152(3):397–405. doi: 10.1111/j.1365-2249.2008.03640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Movahedi Z, Norouzi S, Mamishi S, Rezaei N. BCGiosis as a presenting feature of a child with chronic granulomatous disease. Braz J Infect Dis. 2011;15:83–86. doi: 10.1016/s1413-8670(11)70146-5. [DOI] [PubMed] [Google Scholar]
- 10.Galal N, Boutros J, Marsafy A, Kong XF, Feinberg J, Casanova JL, Boisson-Dupuis S, Bustamante J. Mendelian susceptibility to mycobacterial disease in egyptian children. Mediterr J Hematol Infect Dis. 2012 doi: 10.4084/MJHID.2012.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norouzi S, Aghamohammadi A, Mamishi S, et al. Bacillus Calmette-Guérin (BCG) complications associated with primary immunodeficiency diseases. J Infect. 2012;64:543–54. doi: 10.1016/j.jinf.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, et al. Revisiting human IL-12Rβ1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) 2010;89:381–402. doi: 10.1097/MD.0b013e3181fdd832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guirat-Dhouib N, Baccar Y, Mustapha IB, Ouederni M, Chouaibi S, El Fekih N, Barbouche MR, Fezaa B, Kouki R, Hmida S, Mellouli F, Bejaoui M. Oral HPV infection and MHC class II deficiency (A study of two cases with atypical outcome) Clin Mol Allergy. 2012 Apr 23;10(1):6. doi: 10.1186/1476-7961-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins SM, Dominguez M, Ilmarinen T, Costigan C, Irvine D. Dermatological manifestations of autoimmune polyendocrinopathy candidiasis ectodermal dystrophy syndrome. Br J Dermatol. 2006;154:1088–1093. doi: 10.1111/j.1365-2133.2006.07166.x. [DOI] [PubMed] [Google Scholar]
- 15.Berron-Ruiz A, Berron-Perez R, Ruiz-Maldonado R. Cutaneous markers of primary immunodeficiency diseases in children. Pediatr Dermatol. 2000 Mar;17(2):91–6. doi: 10.1046/j.1525-1470.2000.01721.x. [DOI] [PubMed] [Google Scholar]
- 16.Imai K, Morio T, Zhu Y, et al. Clinical course of patients with WASP gene mutations. Blood. 2004;103:456–464. doi: 10.1182/blood-2003-05-1480. [DOI] [PubMed] [Google Scholar]
- 17.Catucci M, Castiello MC, Pala F, Bosticardo M, Villa A. Autoimmunity in wiskott-Aldrich syndrome: an unsolved enigma. Front Immunol. 2012;3:209. doi: 10.3389/fimmu.2012.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouederni M, Vincent QB, Frange P, et al. Major histocompatibility complex class II expression deficiency caused by a RFXANK founder mutation: a survey of 35 patients. Blood. 2011;118(19):5108–5118. doi: 10.1182/blood-2011-05-352716. [DOI] [PubMed] [Google Scholar]
- 19.Szczawinska-Poplonyk A, Kycler Z, Pietrucha B, Heropolitanska-Pliszka E, Breborowicz A, et al. The hyperimmunoglobulin E syndrome - clinical manifestation diversity in primary immune deficiency. J Rare Dis. 2011;6:76. doi: 10.1186/1750-1172-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aghamohammadi A, Moghaddam ZG, Abolhassani H, Hallaji Z, Mortazavi H, Pourhamdi S, et al. Investigation of underlying primary immunodeficiencies in patients with severe atopic dermatitis. Allergol Immunopathol (Madr) 2014;42(4):336–341. doi: 10.1016/j.aller.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Chiam LY, Verhagen MM, Haraldsson A, Wulffraat N, Driessen GJ, Netea MG, Weemaes CM, Seyger MM, van Deuren M. Cutaneous granulomas in ataxia telangiectasia and other primary immunodeficiencies: Reflection of inappropriate immune regulation? Dermatology. 2011;223(1):13–19. doi: 10.1159/000330335. [DOI] [PubMed] [Google Scholar]
- 22.Carranza D, Vega AK, Torres Rusillo S, Montero E, Martinez LJ, Santamaría M, et al. Molecular and functional characterization of a cohort of Spanish patients with ataxia telangiectasia. Neuromolecular Med. 2017;19(1):161–174. doi: 10.1007/s12017-016-8440. [DOI] [PubMed] [Google Scholar]
- 23.Greenberger S, Berkun Y, Ben-Zeev B, Levi YB, Barziliai A, Nissenkorn A. Dermatologic manifestations of ataxia-telangiectasia syndrome. J Am Acad Dermatol. 2013;68(6):932–6. doi: 10.1016/j.jaad.2012.12.950. [DOI] [PubMed] [Google Scholar]
- 24.Dotta L, Parolini S, Prandini A, Tabellini G, Antolini M, Kingsmore SF, Badolato R. Clinical, laboratory and molecular signs of immunodeficiency in patients with partial oculo-cutaneous albinism. Orphanet J Rare Dis. 2013;17(8):168. doi: 10.1186/1750-1172-8-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Notarangelo LD. PIDs and cancer: an evolving story. Blood. 2010;116(8):1189–1190. doi: 10.1182/blood-2010-06-286179. [DOI] [PubMed] [Google Scholar]
- 26.Mortaz E, Tabarsi P, Mansouri D, Khosravi A, Garssen J, Velayati A, Adcock IM. Cancers Related to Immunodeficiencies: Update and Perspectives. Front Immunol. 2016;7:365. doi: 10.3389/fimmu.2016.00365. e Collection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayor PC, Eng KH, Singel KL, Abrams SI, Odunsi K, Moysich KB, Fuleihan R, Garabedian E, Lugar P, Ochs HD, Bonilla FA, Buckley RH, Sullivan KE, Ballas ZK, Cunningham-Rundles C, Segal BH. Cancer in primary immunodeficiency diseases: Cancer incidence in the United States Immune Deficiency Network Registry. J Allergy Clin Immunol. 2018;141(3):1028–1035. doi: 10.1016/j.jaci.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picard C, Mellouli F, Duprez R, Chédeville G, Neven B, Fraitag S, Delaunay J, Le Deist F, Fischer A, Blanche S, Bodemer C, Gessain A, Casanova JL, Bejaoui M. Kaposi’s sarcoma in a child with Wiskott-Aldrich syndrome. Eur J Pediatr. 2006;165(7):453–457. doi: 10.1007/s00431-006-0107-2. [DOI] [PubMed] [Google Scholar]